Surface Modification of Biomedical Titanium Alloy: Micromorphology, Microstructure Evolution and Biomedical Applications
Abstract
1. Introduction
2. Surface Modification Methods
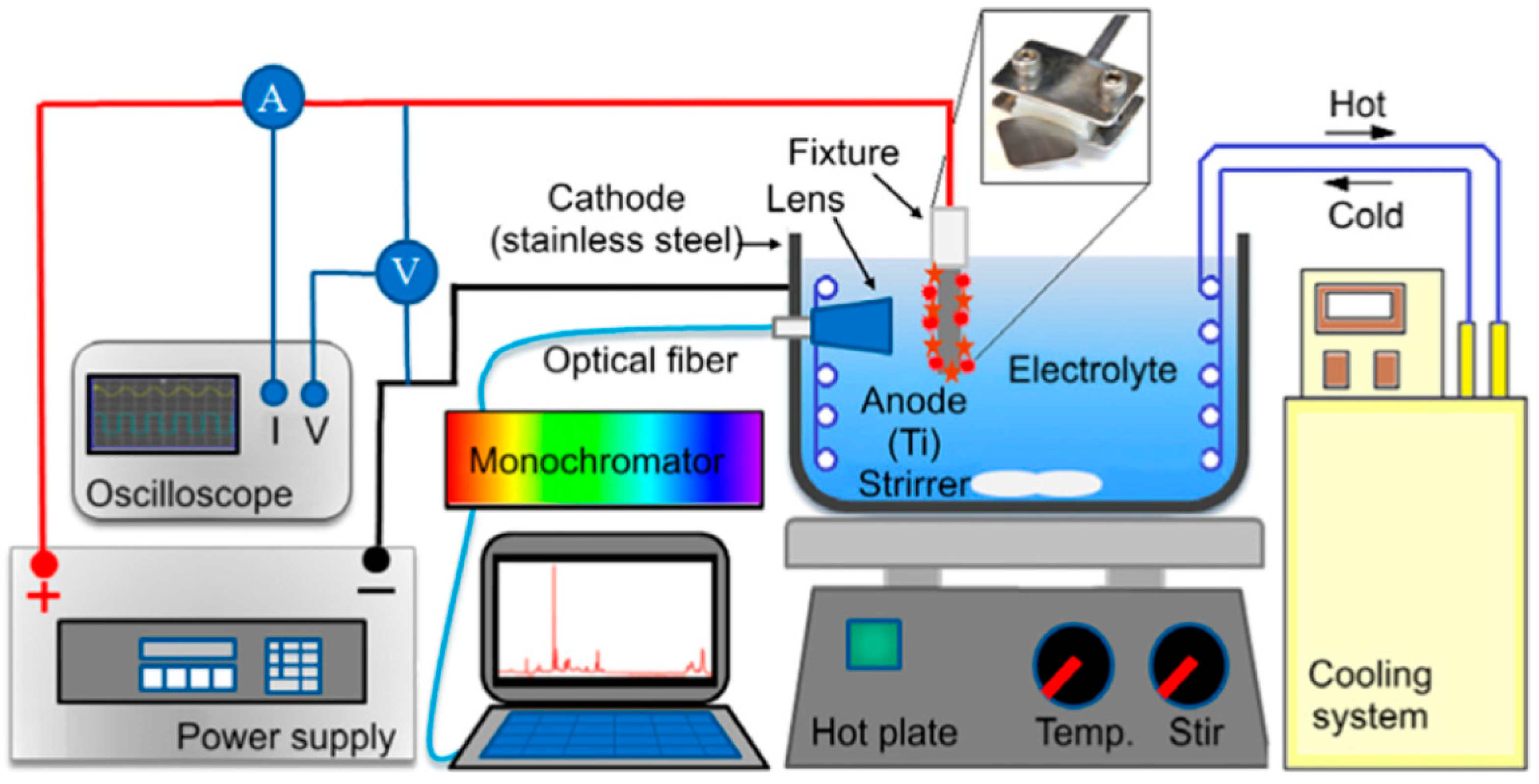
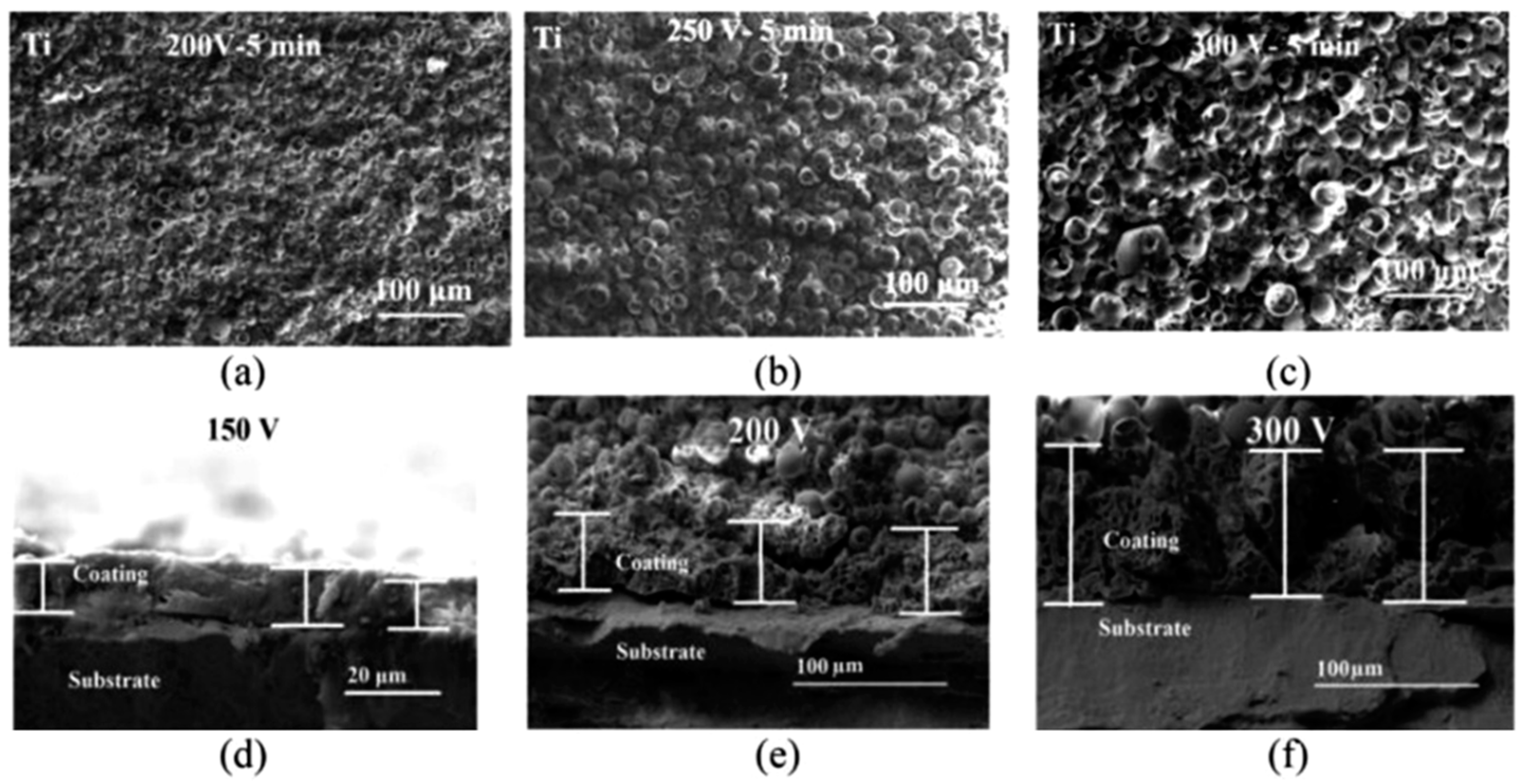
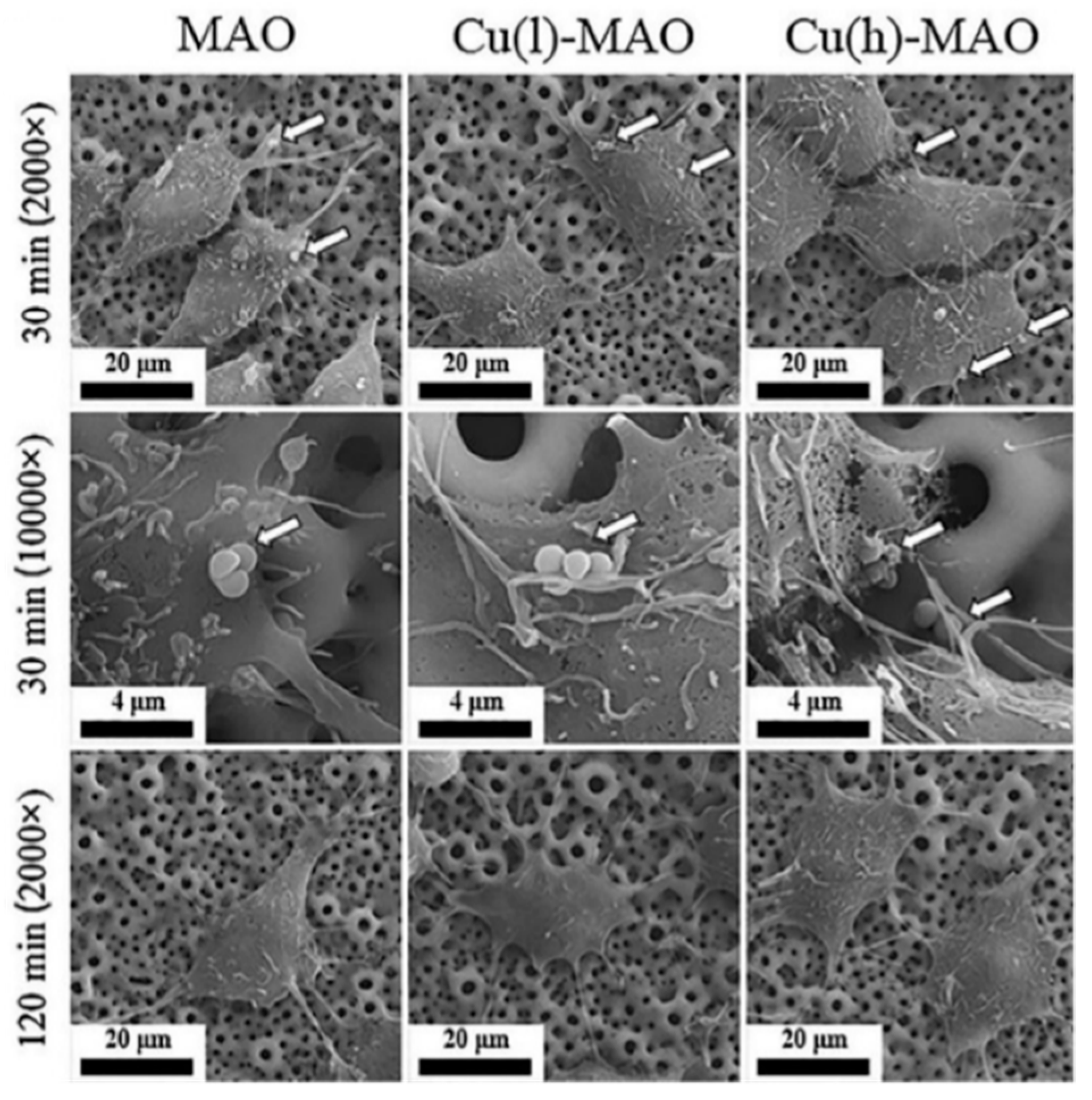
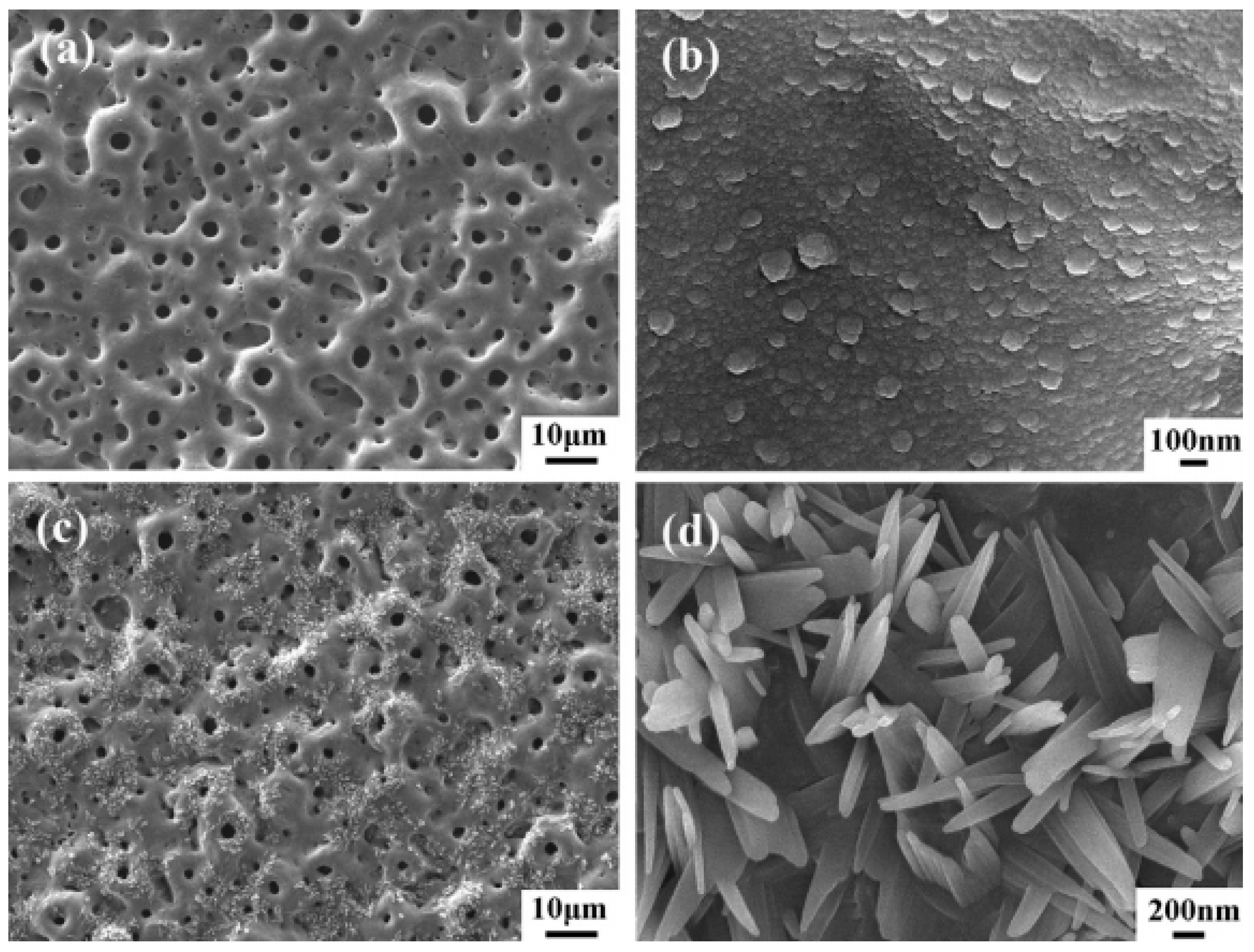
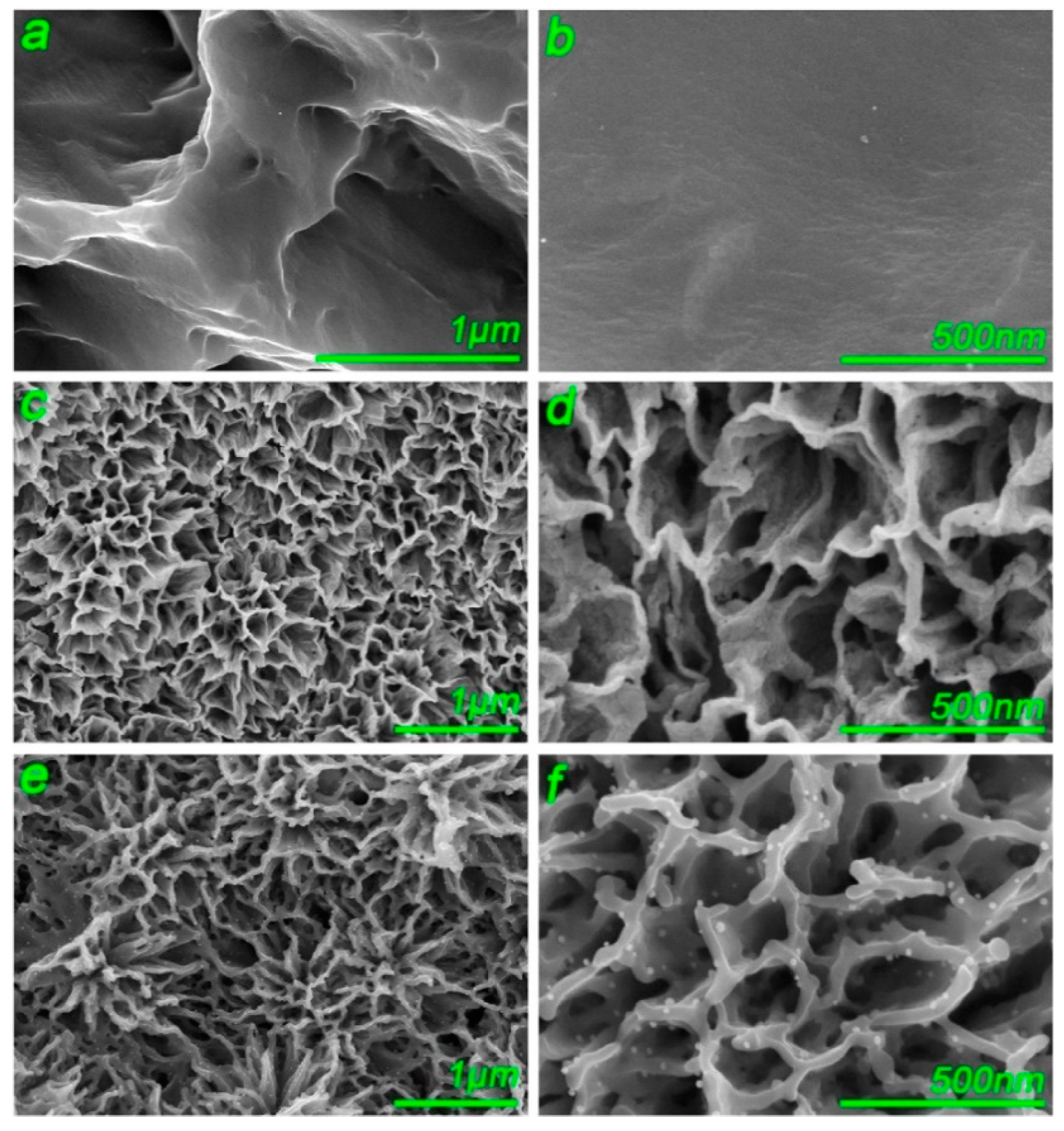
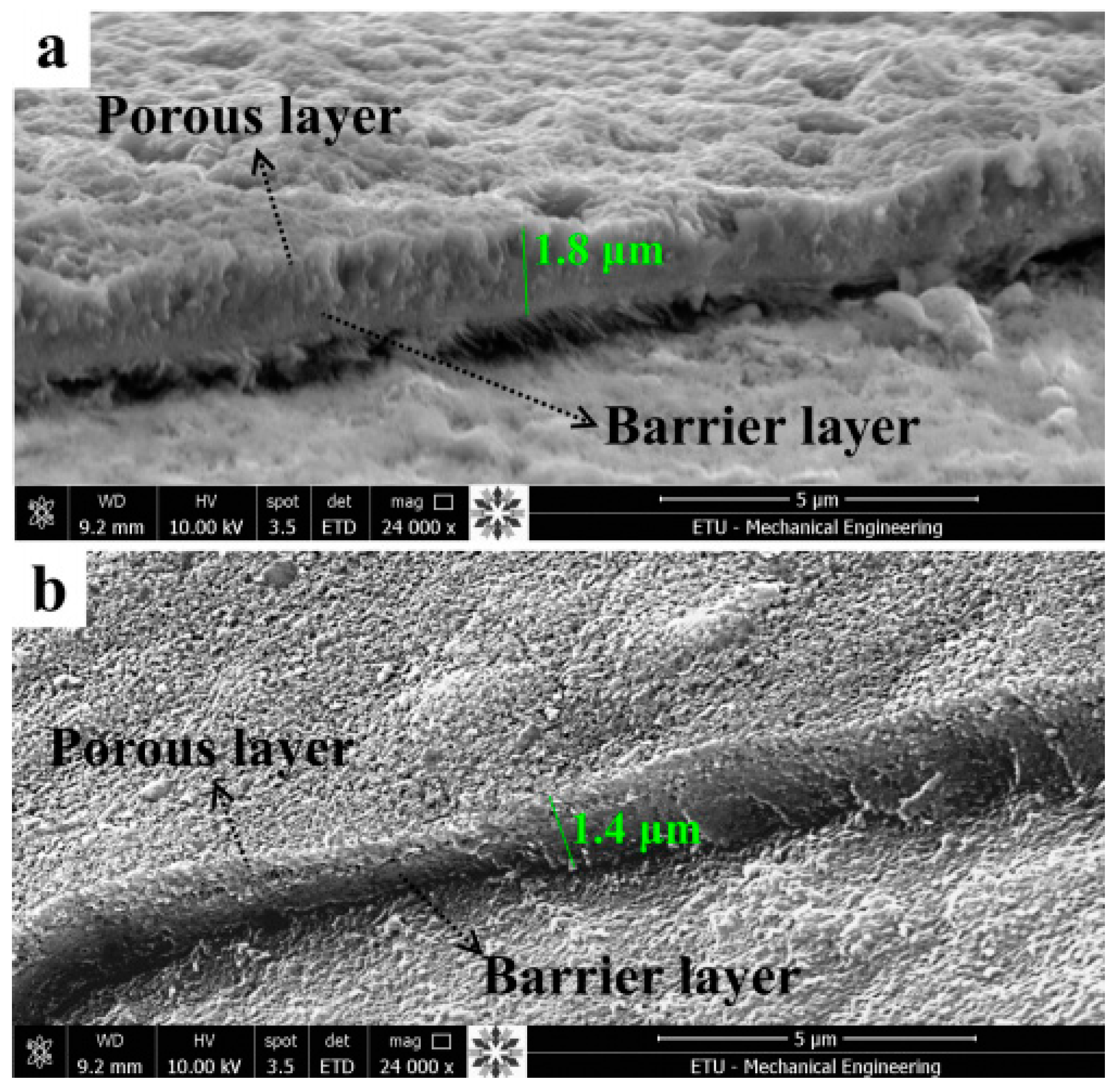
2.1. Micro-Arc Oxidation
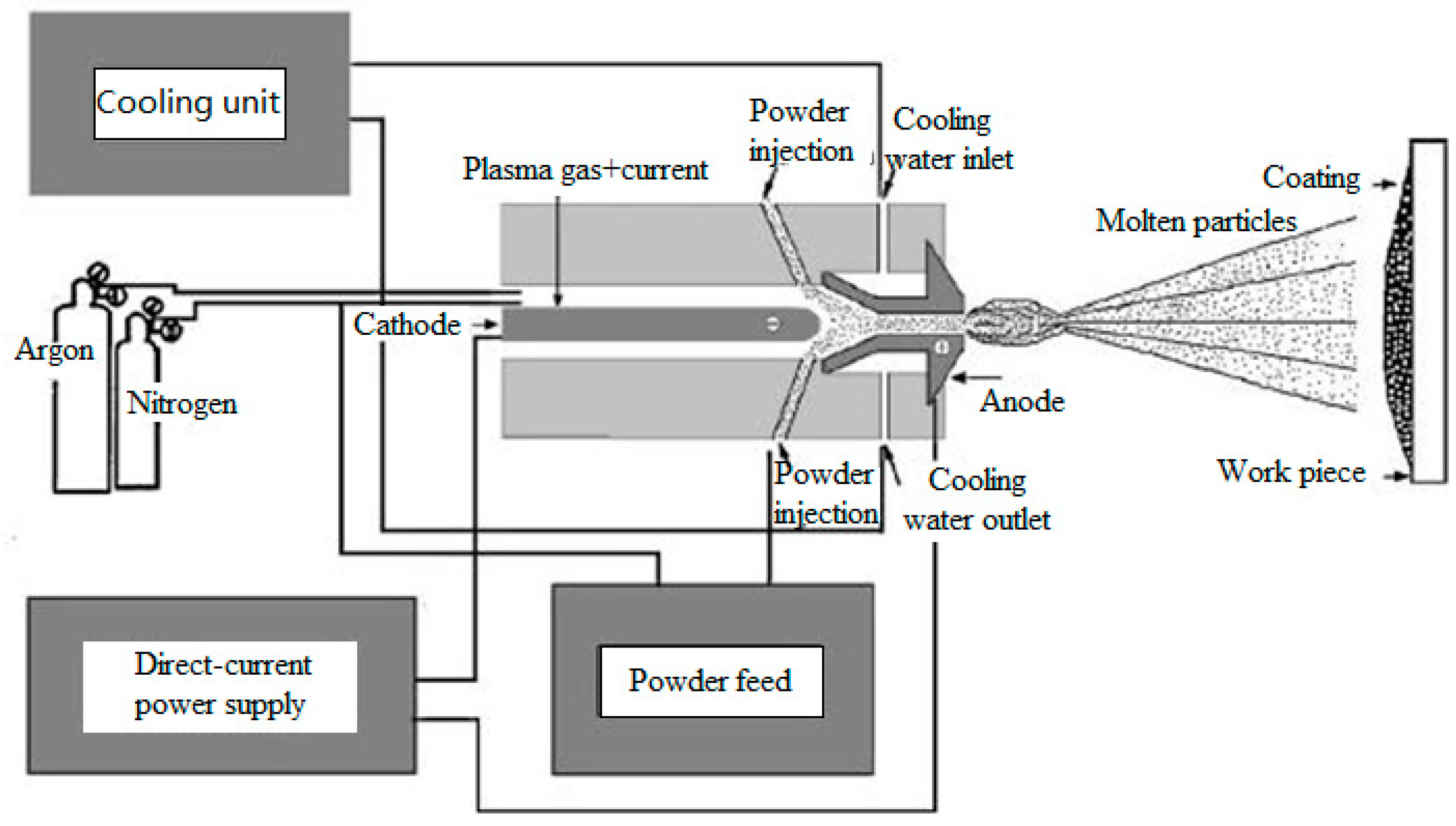
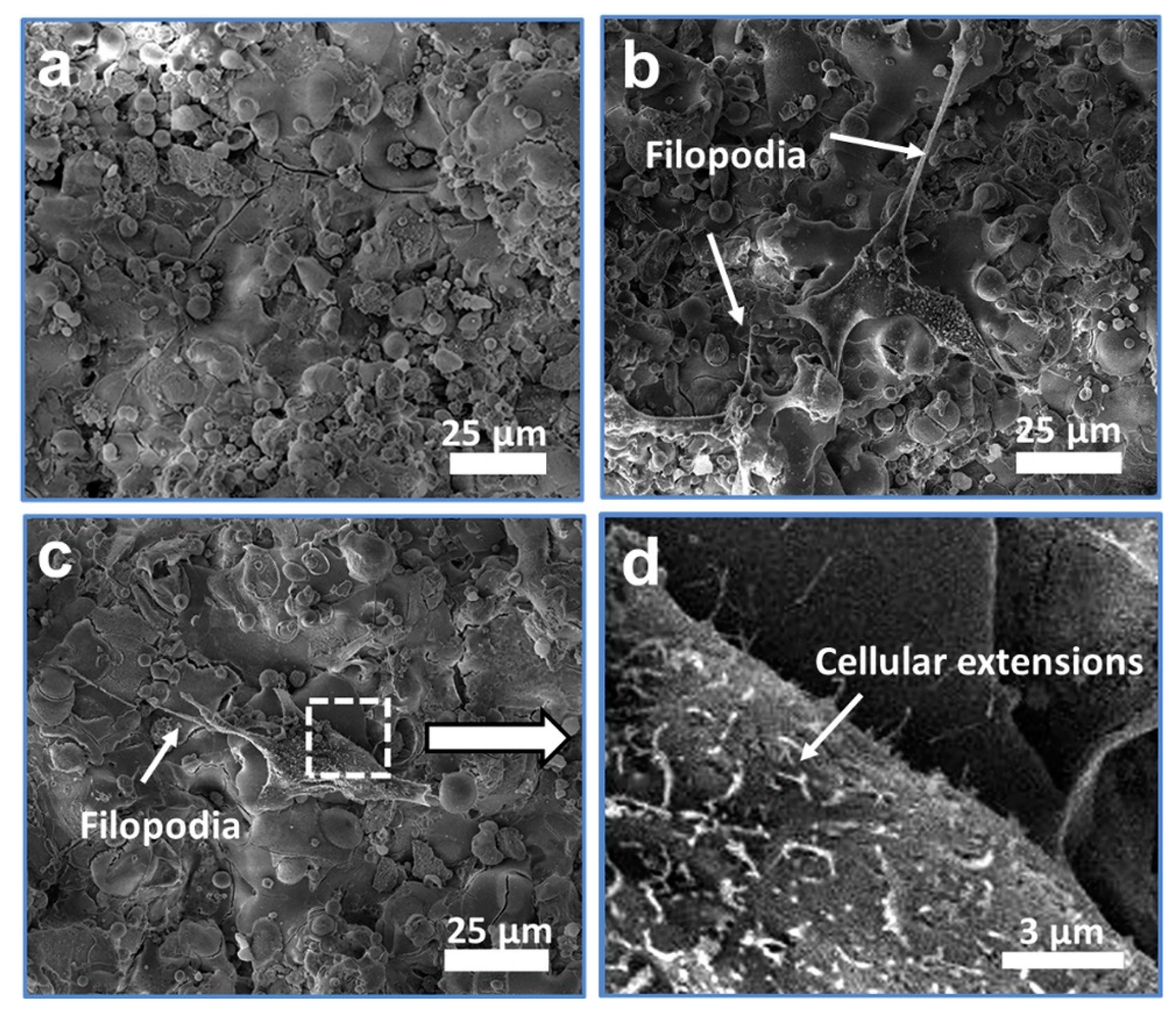
2.2. Plasma Spraying
2.3. Ion Implantation
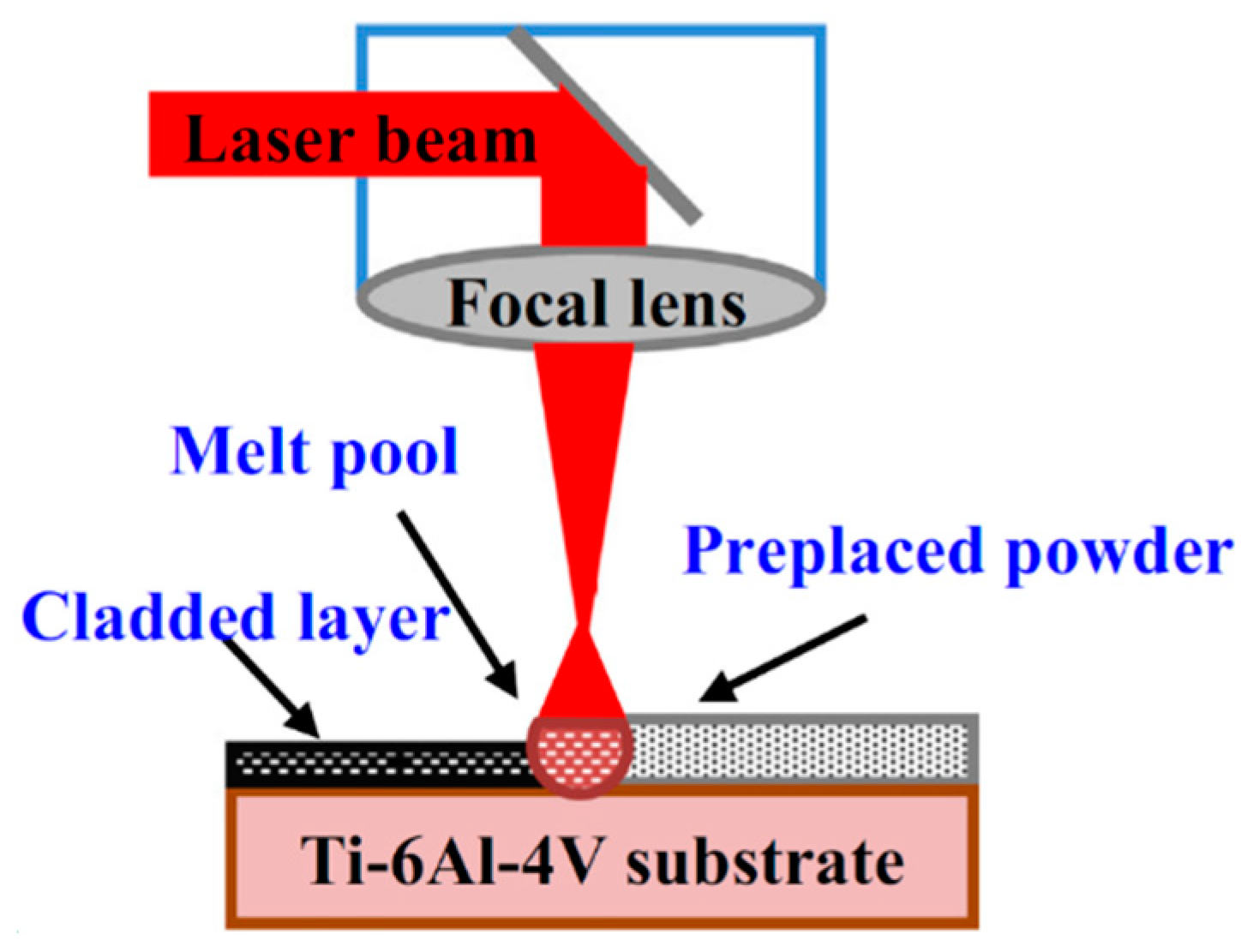
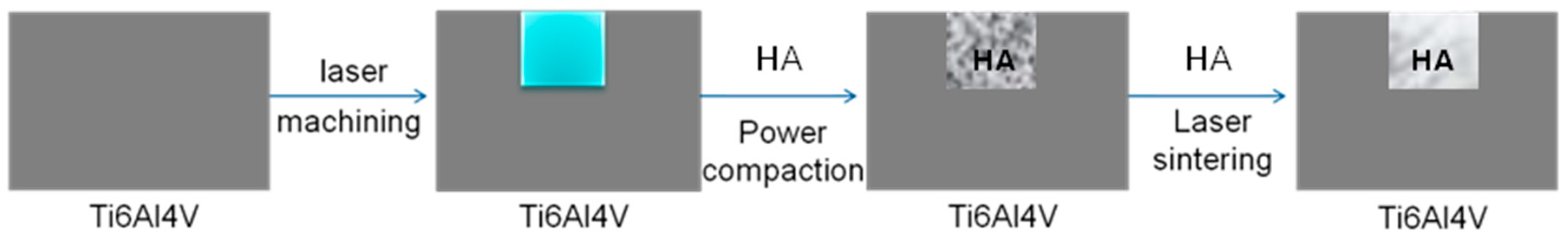
2.4. Laser Surface Modification
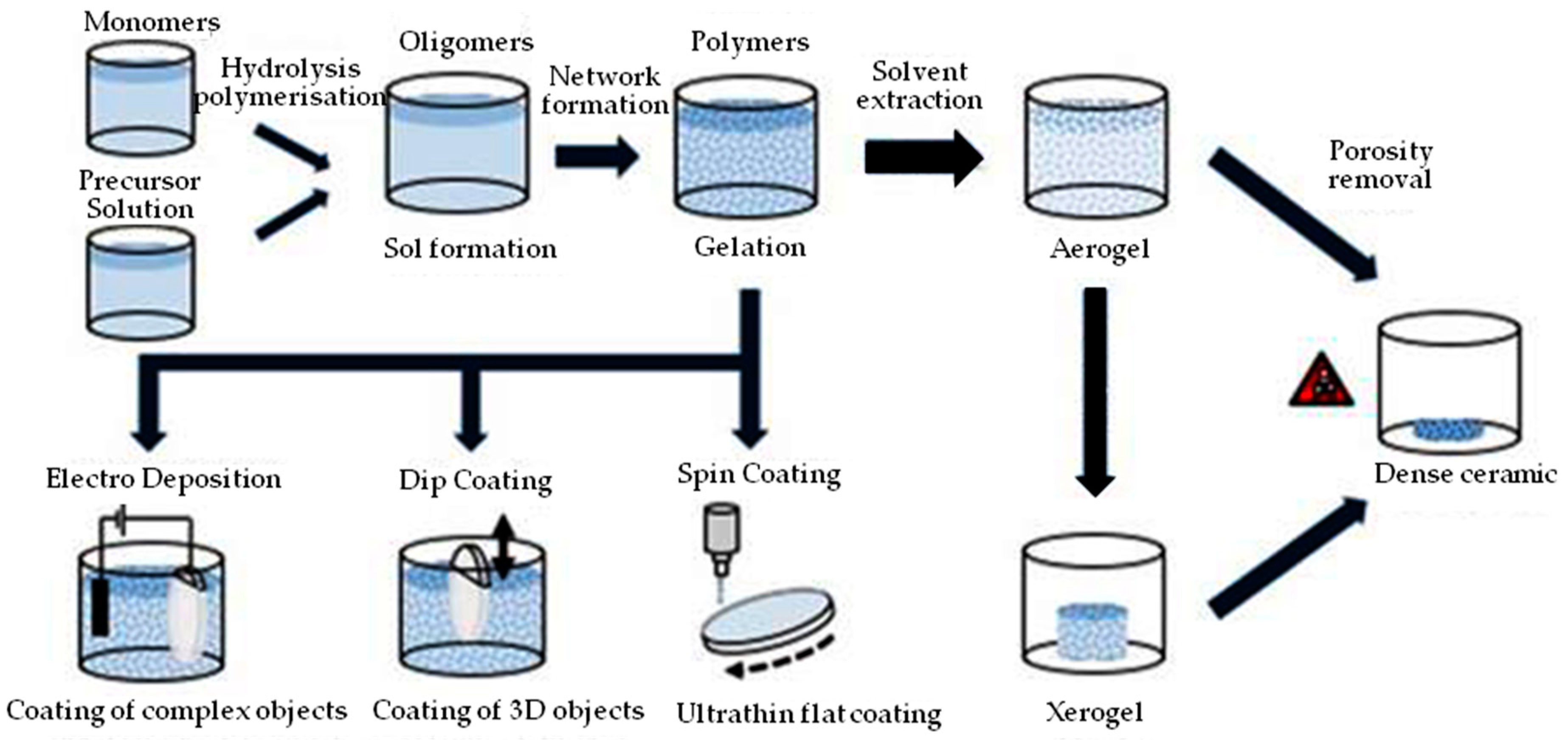
2.5. Sol-Gel
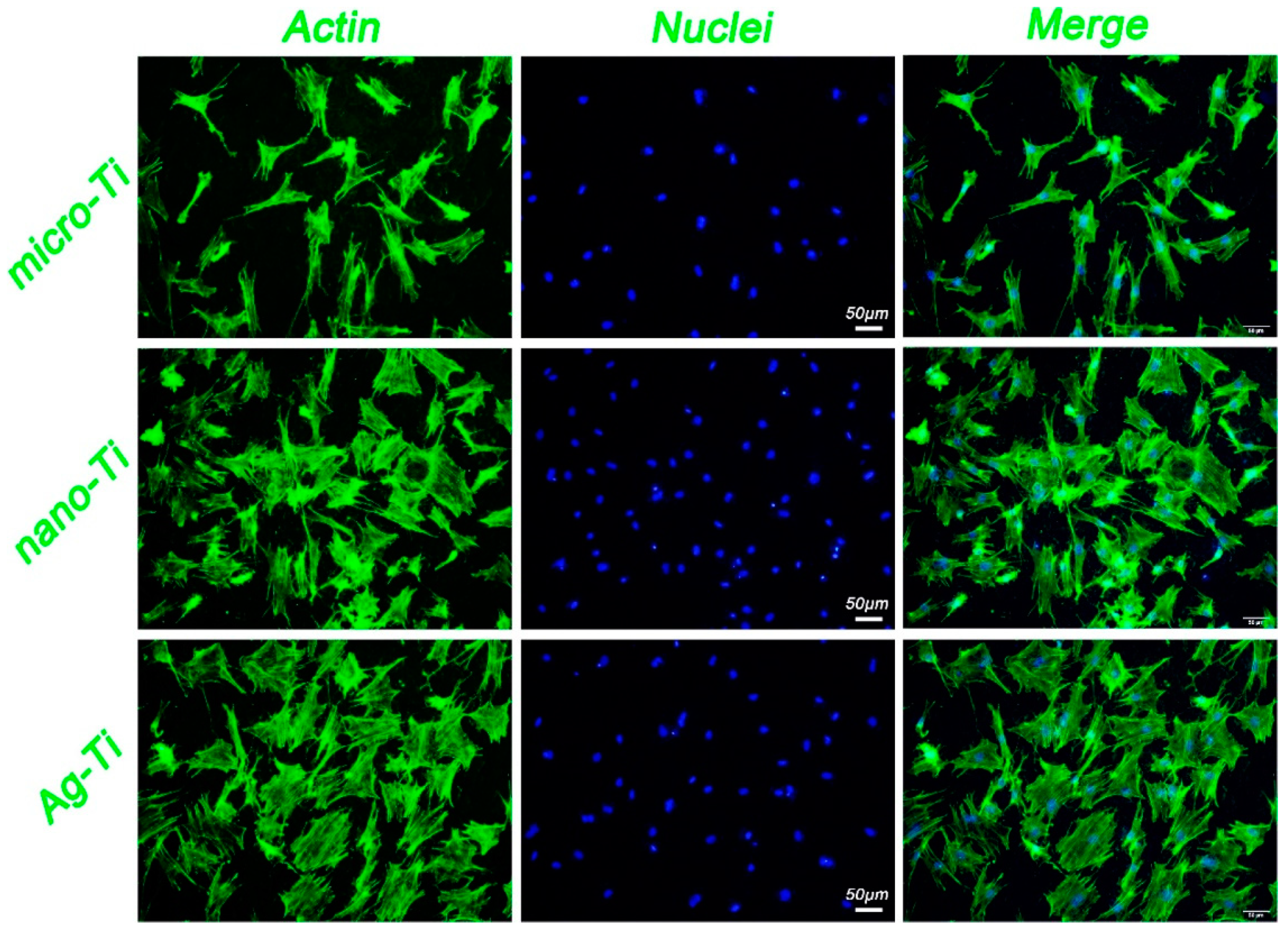
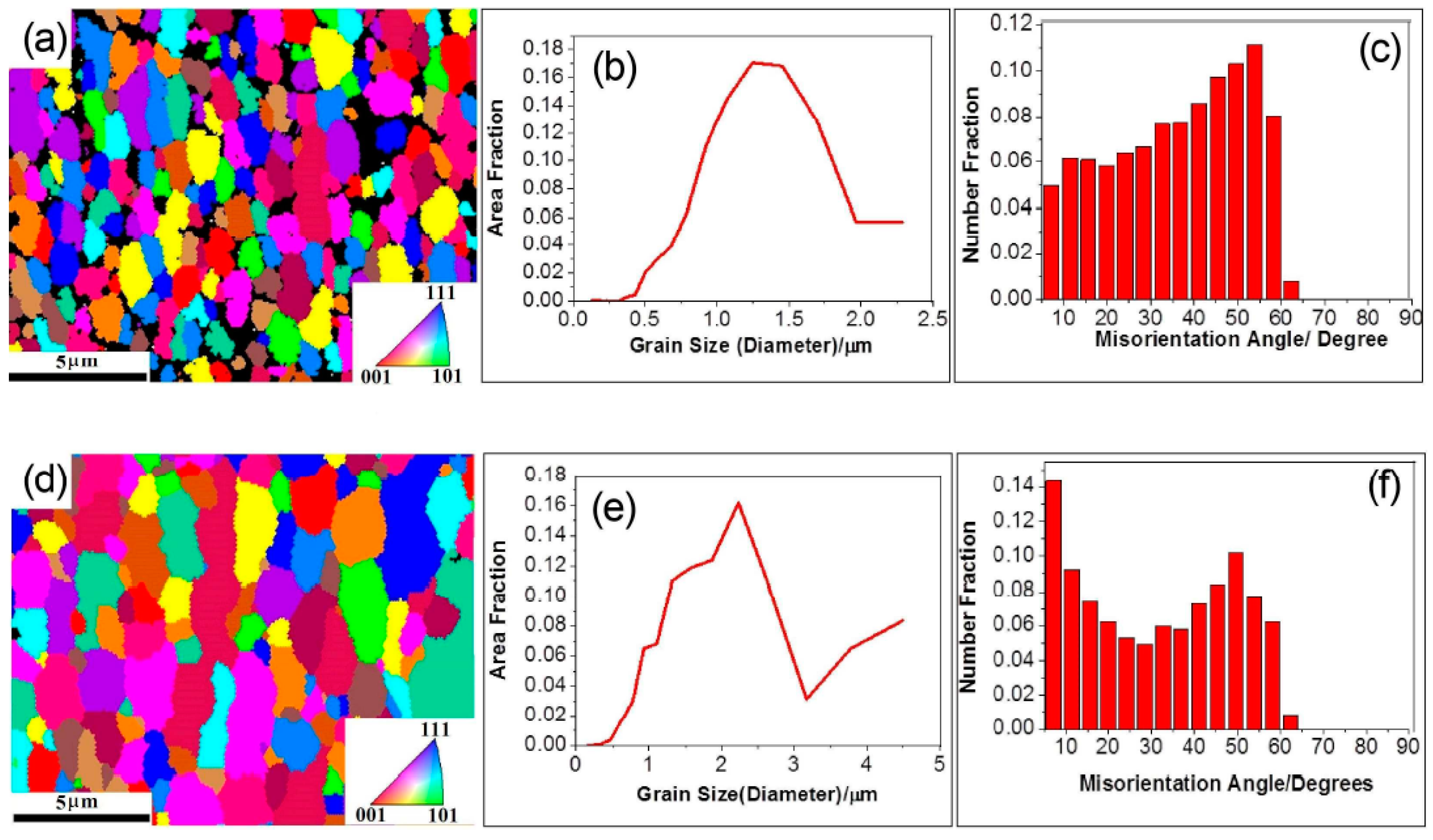
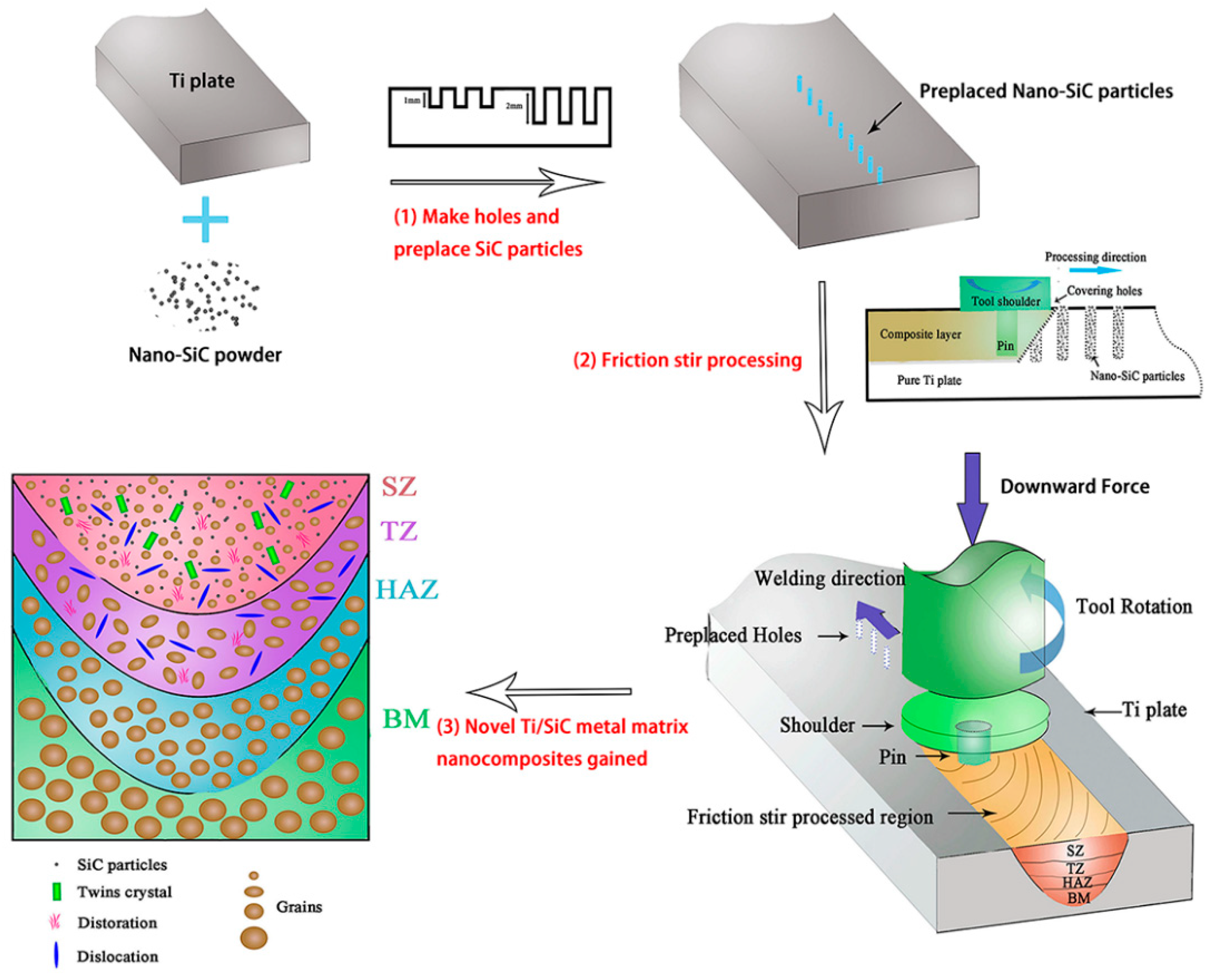
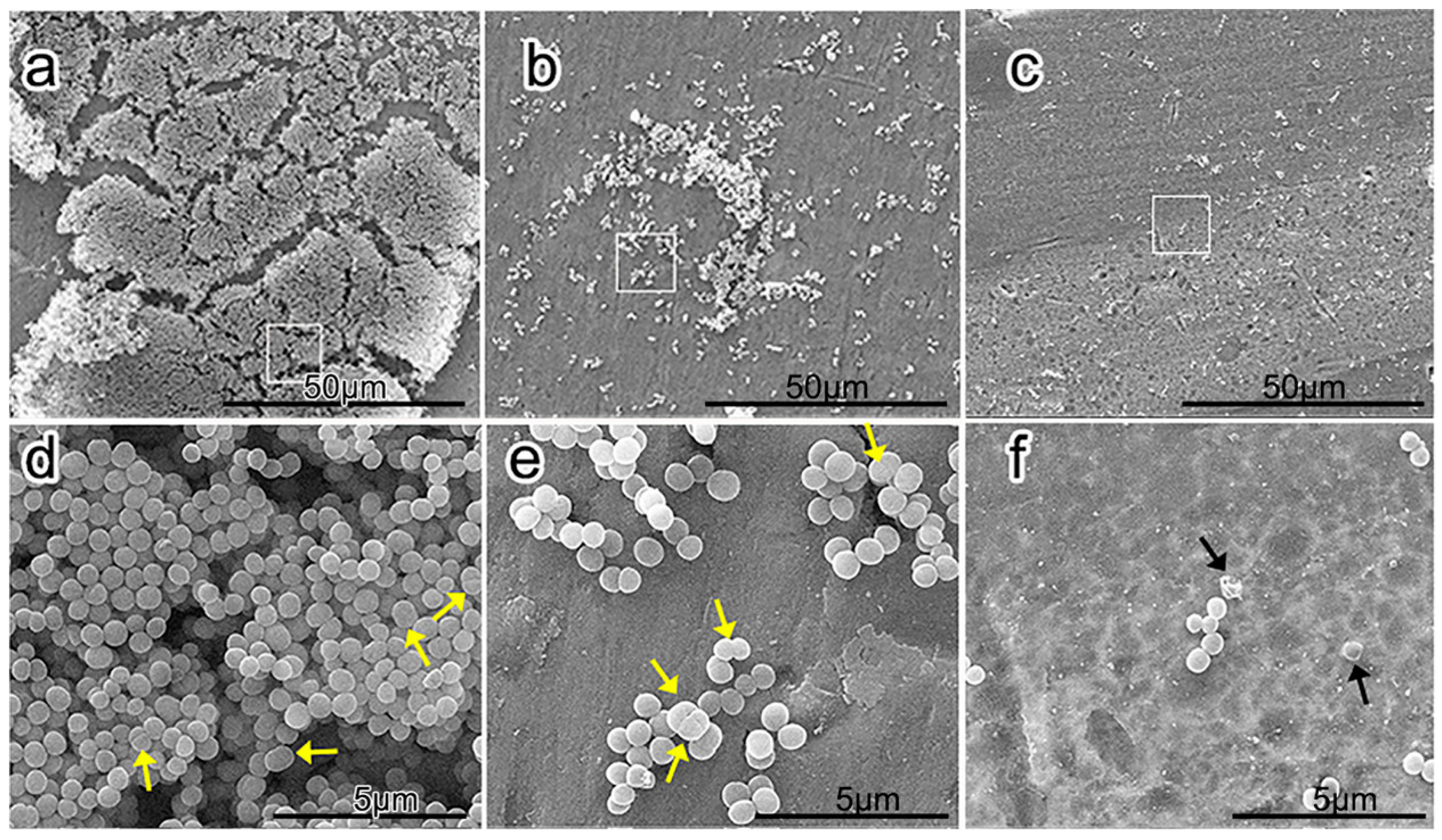
2.6. Friction Stir Processing
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurtz, S.; Mowat, F.; Ong, K. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar]
- Barrere, F.; Mahmood, T.; De Groot, K.; Van Blitterswijk, C.; Van Blitterswijk, C. Advanced biomaterials for skeletal tissue regeneration: Instructive and smart functions. Mater. Sci. Eng. R Rep. 2008, 59, 38–71. [Google Scholar] [CrossRef]
- Zethraeus, N.; Borgström, F.; Ström, O. Cost-effectiveness of the treatment and prevention of osteoporosis—A review of the literature and a reference model. Osteoporos. Int. 2007, 18, 9–23. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Hashmi, M. Implant materials and their processing technologies. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Rony, L.; Lancigu, R.; Hubert, L. Intraosseous metal implants in orthopedics: A review. Morphologie 2018, 102, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Rabadia, C.D.; Liu, Y.J.; Wang, L.; Sun, H.; Zhang, L.C. Laves phase precipitation in Ti-Zr-Fe-Cr alloys with high strength and large plasticity. Mater. Des. 2018, 154, 228–238. [Google Scholar] [CrossRef]
- Wang, L.Q.; Lu, W.J.; Qin, J.N.; Zhang, F.; Zhang, D. Microstructure and mechanical properties of cold-rolled TiNbTaZr biomedical β titanium alloy. Mater. Sci. Eng. A 2008, 490, 421–426. [Google Scholar] [CrossRef]
- Niespodziana, K.; Jurczyk, K.; Jakubowicz, J.; Jurczyk, M. Fabrication and properties of titanium–hydroxyapatite nanocomposites. Mater. Chem. Phys. 2010, 123, 160–165. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X. Activating titanium oxide coatings for orthopedic implants. Surf. Coat. Technol. 2013, 233, 57–64. [Google Scholar] [CrossRef]
- Shariq, N.; Zohaib, K.B.D.S.; Sana, Z.B.D.S.; Muhammad, S.Z.B.D.S. Bioactivity and osseointegration of PEEK are inferior to those of titanium: A systematic review. J. Oral Implantol. 2016, 42, 512–516. [Google Scholar]
- Noman, H.; Liu, S.F.; Lu, E.Y.; Wang, L.Q.; Liu, R.; Lu, W.J.; Zhang, L.C. Mechanical behavior and phase transformation of β-type Ti-35Nb-2Ta-3Zr alloy fabricated by 3D-Printing. J. Alloy. Compd. 2019, 790, 117–126. [Google Scholar]
- Rabadia, C.D.; Liu, Y.J.; Jawed, S.F.; Wang, L.; Li, Y.H.; Zhang, X.H.; Sercombe, T.B.; Sun, H.; Zhang, L.C. Improved deformation behavior in Ti-Zr-Fe-Mn alloys comprising the C14 type Laves and β phases. Mater. Des. 2018, 160, 1059–1070. [Google Scholar] [CrossRef]
- Chakraborty, R.; Shahid Raza, M.; Datta, S.; Saha, P. Synthesis and characterization of nickel free titanium–hydroxyapatite composite coating over Nitinol surface through in-situ laser cladding and alloying. Surf. Coat. Technol. 2019, 358, 539–550. [Google Scholar] [CrossRef]
- Bosco, R.; Van Den Beucken, J.; Leeuwenburgh, S.; Jansen, J. Surface engineering for bone implants: A trend from passive to active surfaces. Coatings 2012, 2, 95–119. [Google Scholar] [CrossRef]
- Aliasghari, S.; Skeldon, P.; Thompson, G. Plasma electrolytic oxidation of titanium in a phosphate/silicate electrolyte and tribological performance of the coatings. Appl. Surf. Sci. 2014, 316, 463–476. [Google Scholar] [CrossRef]
- Çelik, I.; Alsaran, A.; Purcek, G. Effect of different surface oxidation treatments on structural, mechanical and tribological properties of ultrafine-grained titanium. Surf. Coat. Technol. 2014, 258, 842–848. [Google Scholar] [CrossRef]
- Chu, H.J.; Liang, C.J.; Chen, C.H.; He, J.L. Optical emission spectroscopic determination of the optimum regions for micro-arc oxidation of titanium. Surf. Coat. Technol. 2017, 325, 166–173. [Google Scholar] [CrossRef]
- Karbowniczek, J.; Muhaffel, F.; Cempura, G.; Cimenoglu, H.; Czyrska-Filemonowicz, A. Influence of electrolyte composition on microstructure, adhesion and bioactivity of micro-arc oxidation coatings produced on biomedical Ti6Al7Nb alloy. Surf. Coat. Technol. 2017, 321, 97–107. [Google Scholar] [CrossRef]
- Ha, J.Y.; Tsutsumi, Y.; Doi, H.; Nomura, N.; Kim, K.H.; Hanawa, T. Enhancement of calcium phosphate formation on zirconium by micro-arc oxidation and chemical treatments. Surf. Coat. Technol. 2011, 205, 4948–4955. [Google Scholar] [CrossRef]
- Rao, X.; Li, J.; Feng, X.; Chu, C.L. Bone-like apatite growth on controllable macroporous titanium scaffolds coated with microporous titania. J. Mech. Behav. Biomed. 2018, 77, 225–233. [Google Scholar] [CrossRef]
- Yang, W.; Xu, D.; Wang, J.; Yao, X.; Chen, J. Microstructure and corrosion resistance of micro arc oxidation plus electrostatic powder spraying composite coating on magnesium alloy. Corros. Sci. 2018, 136, 174–179. [Google Scholar] [CrossRef]
- Liu, S.M.; Li, B.; Liang, C.H.; Wang, H.S.; Qiao, Z.X. Formation mechanism and adhesive strength of a hydroxyapatite/TiO2 composite coating on a titanium surface prepared by micro-arc oxidation. Appl. Surf. Sci. 2016, 362, 109–114. [Google Scholar] [CrossRef]
- Li, L.H.; Kong, Y.M.; Kim, H.W.; Kim, W.Y.; Kim, H.E.; Heo, S.J.; Koak, J.Y. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials 2004, 25, 2867–2875. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S.; Farooq, I.; Awais, M.; Najeeb, S.; Khurshid, Z.; Zohaib, S. Chapter 11-Bioactive surface coatings for enhancing osseointegration of dental implants. In Biomedical, Therapeutic and Clinical Applications of Bioactive Glasses; Woodhead Publishing: Cambridge, UK, 2019; pp. 313–329. [Google Scholar]
- Xu, L.; Wu, C.; Le, X.; Zhang, K.; Liu, C.; Ding, J.; Shi, X. Effect of oxidation time on cytocompatibility of ultrafine-grained pure Ti in micro-arc oxidation treatment. Surf. Coat. Technol. 2018, 342, 12–22. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Tenorio, R.G.; Mohedano, M.; Martinez-Campos, E.; Arrabal, R.; Matykina, E. Tailoring of antibacterial and osteogenic properties of Ti6Al4V by plasma electrolytic oxidation. Appl. Surf. Sci. 2018, 454, 157–172. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Duan, J.; Qi, M. A super-hydrophilic coating with a macro/micro/nano triple hierarchical structure on titanium by two-step micro-arc oxidation treatment for biomedical applications. Surf. Coat. Technol. 2017, 311, 1–9. [Google Scholar] [CrossRef]
- Wang, X.; Li, B.; Zhou, L.; Ma, J.; Zhang, X.; Li, H.; Liang, C.; Liu, S.; Wang, H. Influence of surface structures on biocompatibility of TiO2/HA coatings prepared by MAO. Mater. Chem. Phys. 2018, 215, 339–345. [Google Scholar] [CrossRef]
- Sedelnikova, M.; Komarova, E.; Sharkeev, Y.; Tolkacheva, T.; Khlusov, I.; Litvinova, L.; Yurova, K.; Shupletsova, V. Comparative investigations of structure and properties of micro-arc wollastonite-calcium phosphate coatings on titanium and zirconium-niobium alloy. Bioact. Mater. 2017, 2, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wu, T.; Peng, H.; Guo, X.Y. Effect of NaAlO2 concentrations on the properties of micro-arc oxidation coatings on pure titanium. Mater. Lett. 2016, 170, 171–174. [Google Scholar]
- Yang, W.; Xu, D.; Guo, Q.; Chen, T.; Chen, J. Influence of electrolyte composition on microstructure and properties of coatings formed on pure Ti substrate by micro arc oxidation. Surf. Coat. Technol. 2018, 349, 522–528. [Google Scholar] [CrossRef]
- Correa, D.R.N.; Rocha, L.A.; Ribeiro, A.R.; Gemini-Piperni, S.; Archanjo, B.S.; Achete, C.A.; Werckmann, J.; Afonso, C.R.M.; Shimabukuro, M.; Doi, H.; et al. Growth mechanisms of Ca- and P-rich MAO films in Ti-15Zr-xMo alloys for osseointegrative implants. Surf. Coat. Technol. 2018, 344, 373–382. [Google Scholar] [CrossRef]
- Tsai, M.T.; Chang, Y.Y.; Huang, H.L.; Wu, Y.H.; Shieh, T.M. Micro-arc oxidation treatment enhanced the biological performance of human osteosarcoma cell line and human skin fibroblasts cultured on titanium–zirconium films. Surf. Coat. Technol. 2016, 303, 268–276. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Zheng, H.; Du, C.; Ning, C.; Shi, Z.; Xu, C. Effect of frequency on the structure and cell response of Ca-and P-containing MAO films. Appl. Surf. Sci. 2010, 256, 2018–2024. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Elkhooly, T.A.; Liu, X.; Zhang, R.; Wu, H.; Feng, Q.L.; Liu, Y. The Cu-containing TiO2 coatings with modulatory effects on macrophage polarization and bactericidal capacity prepared by micro-arc oxidation on titanium substrates. Colloids Surf. B Biointerfaces 2018, 170, 242–250. [Google Scholar] [CrossRef]
- Wang, C.H.; Ma, F.C.; Liu, P.; Chen, J.; Liu, X.K.; Zhang, K.; Li, W.; Han, Q.Y. The influence of alloy elements in Ti6Al4V and Ti35Nb2Ta3Zr on the structure, morphology and properties of MAO coatings. Vacuum 2018, 157, 229–236. [Google Scholar] [CrossRef]
- Hu, H.J.; Qiao, Y.Q.; Meng, F.H.; Liu, X.Y.; Ding, C.X. Enhanced apatite-forming ability and cytocompatibility of porous and nanostructured TiO2/CaSiO3 coating on titanium. Colloids Surf. B Biointerfaces 2013, 101, 83–90. [Google Scholar] [CrossRef]
- Zhang, R.R.; Liu, X.J.; Xiong, Z.Y.; Huang, Q.L.; Yang, X.; Yan, H. Novel micro/nanostructured TiO2/ZnO coating with antibacterial capacity and cytocompatibility. Ceram. Int. 2018, 44, 9711–9719. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Hasan, S.M.; Khan, R.S. Bisphosphonate releasing dental implant surface coatings and osseointegration: A systematic review. J. Taibah Univ. Med Sci. 2017, 12, 369–375. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.M.; Yusof, N.M.; Bakhsheshi-Rad, H.R.; Hamzah, E. Deposition of duplex MAO layer/nanostructured titanium dioxide composite coatings on Mg–1%Ca alloy using a combined technique of air plasma spraying and micro arc oxidation. J. Alloy. Compd. 2015, 649, 591–605. [Google Scholar] [CrossRef]
- Heimann, R.B. Thermal spraying of biomaterials. Surf. Coat. Technol. 2006, 201, 2012–2019. [Google Scholar] [CrossRef]
- Lima, R.S.; Marple, B.R. Thermal spray coatings engineered from nano-structured ceramic agglomerated powders for structural, thermal barrier and biomedical applications: A review. Therm. Spray Technol. 2007, 16, 40–63. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, W.C.; Su, J.B. Effect of composition and annealing on the dielectric properties of ZnO/mullite composite coating. Ceram. Int. 2012, 38, 1077–1083. [Google Scholar] [CrossRef]
- Balani, K.; Anderson, R.; Laha, T. Plasma-sprayed carbon nanotube reinforced hydroxyapatite coatings and their interaction with human osteoblasts in vitro. Biomaterials 2007, 28, 618–624. [Google Scholar] [CrossRef]
- Shamray, V.; Sirotinkin, V.; Smirnov, I.; Kalita, V.; Fedotov, A.; Barinov, S.; Komlev, V.; Fedotov, A.; Komlev, V. Structure of the hydroxyapatite plasma-sprayed coatings deposited on pre-heated titanium substrates. Ceram. Int. 2017, 43, 9105–9109. [Google Scholar] [CrossRef]
- Sathish, S.; Geetha, M.; Aruna, S.; Balaji, N.; Rajam, K.; Asokamani, R. Studies on plasma sprayed bi-layered ceramic coating on bio-medical Ti–13Nb–13Zr alloy. Ceram. Int. 2011, 37, 1333–1339. [Google Scholar] [CrossRef]
- Sathish, S.; Geetha, M.; Aruna, S.; Balaji, N.; Rajam, K.; Asokamani, R. Sliding wear behavior of plasma sprayed nanoceramic coatings for biomedical applications. Wear 2011, 271, 934–941. [Google Scholar] [CrossRef]
- Kumari, R.; Majumdar, J.D. Wear Behavior of plasma spray deposited and post heat-treated hydroxyapatite (HA)-based composite coating on titanium alloy (Ti-6Al-4V) substrate. Metall. Mater. Trans. A 2018, 49, 3122–3132. [Google Scholar] [CrossRef]
- Hung, K.Y.; Lo, S.C.; Shih, C.S.; Yang, Y.C.; Feng, H.P.; Lin, Y.C. Titanium surface modified by hydroxyapatite coating for dental implants. Surf. Coat. Technol. 2013, 231, 337–345. [Google Scholar] [CrossRef]
- Yang, Y.C.; Yang, C.Y. Mechanical and histological evaluation of a plasma sprayed hydroxyapatite coating on a titanium bond coat. Ceram. Int. 2013, 39, 6509–6516. [Google Scholar] [CrossRef]
- Rahman, Z.U.; Shabib, I.; Haider, W. Surface characterization and cytotoxicity analysis of plasma sprayed coatings on titanium alloys. Mater. Sci. Eng. C 2016, 67, 675–683. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Hossein, Z.A.S.A.; Vaezi, M.R. A new double-layer hydroxyapatite/alumina-silica coated titanium implants using plasma spray technique. Surf. Coat. Technol. 2018, 352, 474–482. [Google Scholar] [CrossRef]
- Cao, L.; Ullah, I.; Li, N.; Niu, S.; Sun, R. Plasma spray of biofunctional (Mg, Sr)-substituted hydroxyapatite coatings for titanium alloy implants. J. Mater. Sci. Technol. 2018, 35, 719–726. [Google Scholar] [CrossRef]
- Walsh, W.R.; Bertollo, N.; Christou, C.; Schaffner, D.; Mobbs, R.J. Plasma-sprayed titanium coating to polyetheretherketone improves the bone-implant interface. Spine J. 2015, 15, 1041–1049. [Google Scholar] [CrossRef]
- Hickey, D.J.; Lorman, B.; Fedder, I.L. Improved response of osteoprogenitor cells to titanium plasma-sprayed PEEK surfaces. Colloids Surf. B Biointerfaces 2019, 175, 509–516. [Google Scholar] [CrossRef]
- Ke, D.; Vu, A.A.; Bandyopadhyay, A.; Bose, S. Compositionally graded doped hydroxyapatite coating on titanium using laser and plasma spray deposition for bone implants. Acta Biomater. 2019, 84, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, S.J.; Sun, F.; Ba, D.C.; Li, X.C. Surface characteristics of a dental implant modified by low energy oxygen ion implantation. Surf. Coat. Technol. 2019, 365, 208–213. [Google Scholar] [CrossRef]
- Feng, H.; Yu, Z.; Chu, P.K. Ion implantation of organisms. Mater. Sci. Eng. R 2006, 54, 49–120. [Google Scholar] [CrossRef]
- Fang, J.; Sun, Y.; Ma, H.Y.; Yu, X.L.; Ma, Y.; Ni, Y.X.; Zheng, L.; Zhou, Y.M. Biocompatibility and antibacterial properties of zinc-ion implantation on titanium. J. Hard Tissue Biol. 2014, 23, 35–43. [Google Scholar] [CrossRef]
- Rautray, T.R.; Narayanan, R.; Kim, K.H. Ion implantation of titanium based biomaterials. Prog. Mater. Sci. 2011, 56, 1137–1177. [Google Scholar] [CrossRef]
- Ming, M.; Wan, R.X.; Gong, H.H.; Lv, X.F.; Chu, S.S.; Li, D.J.; Gu, H.Q.; Cheng, P. Study on the in vitro and in vivo antibacterial activity and biocompatibility of novel TiN/Ag multilayers immobilized onto biomedical titanium. J. Nanosci. Nanotechnol. 2019, 19, 3777–3791. [Google Scholar]
- Zhu, C.; Bao, N.R.; Chen, S.; Zhao, J.N. Antimicrobial design of titanium surface that kill sessile bacteria but support stem cells adhesion. Appl. Surf. Sci. 2016, 389, 7–16. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, T.T.; Wei, S.B.; Xiang, Y.; Chen, H. Effect of Ta2O5/TiO2 thin film on mechanical properties, corrosion and cell behavior of the NiTi alloy implanted with tantalum. Mater. Sci. Eng. C 2010, 30, 1227–1235. [Google Scholar] [CrossRef]
- Xu, J.; Ding, G.; Li, J.L.; Yang, S.H.; Fang, B.S.; Sun, H.C.; Zhou, Y.M. Zinc-ion implanted and deposited titanium surfaces reduce adhesion of Streptococccus mutans. Appl. Surf. Sci. 2010, 256, 7540–7544. [Google Scholar] [CrossRef]
- Jin, G.D.; Cao, H.L.; Qiao, Y.Q.; Meng, F.H.; Zhu, H.Q.; Liu, X.Y. Osteogenic activity and antibacterial effect of zinc ion implanted titanium. Colloids Surf. B Biointerfaces 2014, 117, 158–165. [Google Scholar] [CrossRef]
- Shanaghi, A.; Chu, P.K. Enhancement of mechanical properties and corrosion resistance of NiTi alloy by carbon plasma immersion ion implantation. Surf. Coat. Technol. 2018, 365, 52–57. [Google Scholar] [CrossRef]
- Vlcak, P.; Fojt, J.; Weiss, Z.; Kopeček, J.; Perina, V. The effect of nitrogen saturation on the corrosion behaviour of Ti-35Nb-7Zr-5Ta beta titanium alloy nitrided by ion implantation. Surf. Coat. Technol. 2019, 358, 144–152. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Kim, S.; Park, C.; Cheon, K.H.; Jung, H.D.; Song, J.; Kim, H.E.; Jang, T.S. Antibacterial and bioactive properties of stabilized silver on titanium with a nanostructured surface for dental applications. Appl. Surf. Sci. 2018, 451, 232–240. [Google Scholar] [CrossRef]
- Weng, F.; Chen, C.Z.; Yu, H.J. Research status of laser cladding on titanium and its alloys: A review. Mater. Des. 2014, 58, 412–425. [Google Scholar] [CrossRef]
- Kurella, N.B.; Dahotre, A. Review paper: Surface modification for bioimplants: The role of laser surface engineering. J. Biomater. Appl. 2005, 20, 5–50. [Google Scholar] [CrossRef]
- Du Plooy, R.; Akinlabi, E.T. Analysis of laser cladding of titanium alloy. Mater. Today Proc. 2018, 5, 19594–19603. [Google Scholar] [CrossRef]
- Long, J.; Fan, P.; Gong, D.; Jiang, D.; Zhang, H.; Li, L.; Zhong, M. Super-hydrophobic surfaces fabricated by femtosecond laser with tunable water adhesion: From lotus leaf to rose petal. ACS Appl. Mater. Interfaces 2015, 7, 9858–9865. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, C.; Li, S. Research progress on laser surface modification of titanium alloys. Appl. Surf. Sci. 2005, 242, 177–184. [Google Scholar] [CrossRef]
- Antalya, H.S.L.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar]
- Das, R.K.; Zouani, O.F. A review of the effects of the cell environment physicochemical nanoarchitecture on stem cell commitment. Biomaterials 2014, 35, 5278–5293. [Google Scholar] [CrossRef]
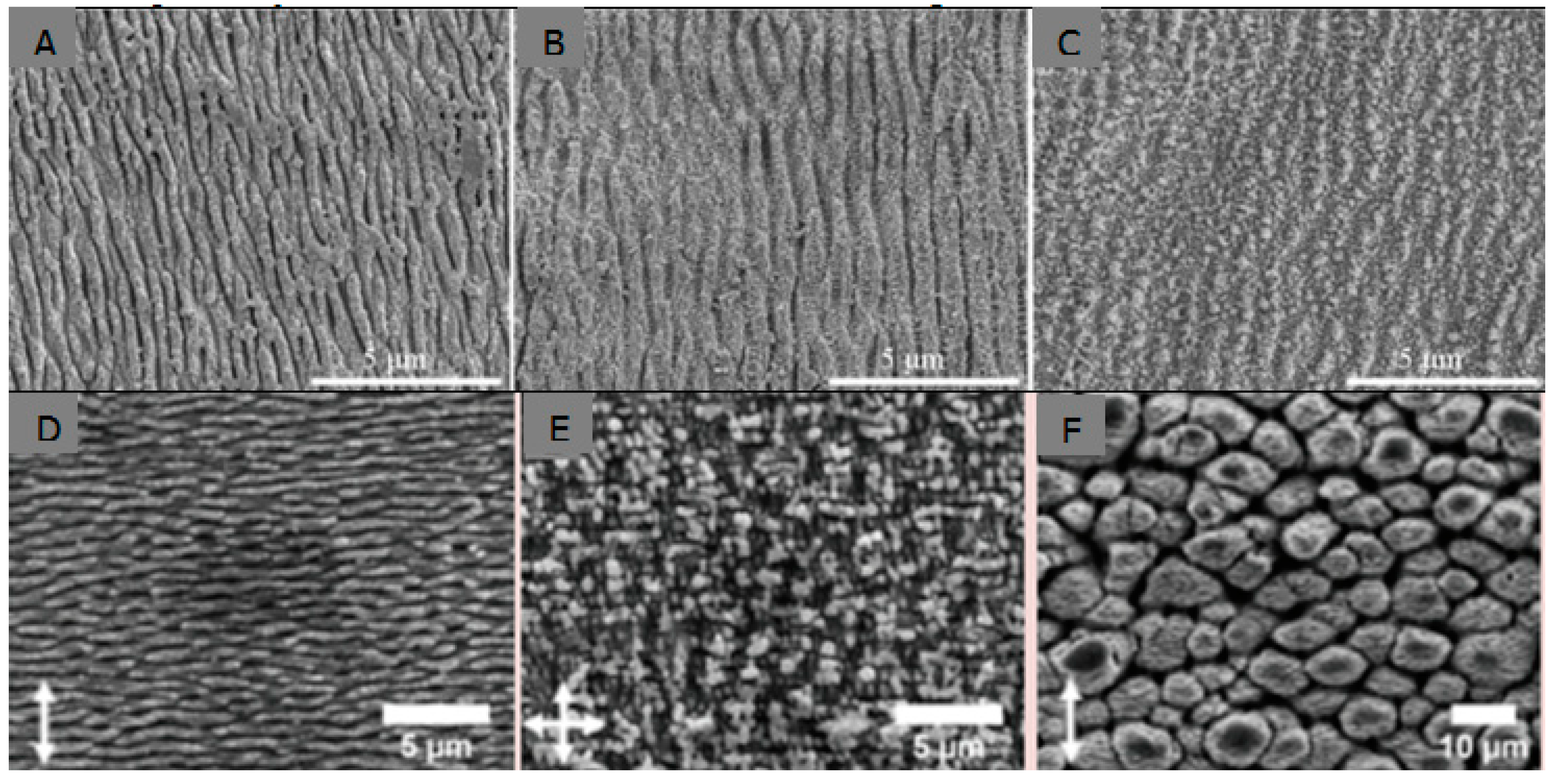
- Shinonaga, T.; Tsukamoto, M.; Kawa, T. Formation of periodic nanostructures using a femtosecond laser to control cell spreading on titanium. Appl. Phys. B. 2015, 119, 493–496. [Google Scholar] [CrossRef]
- Cunha, A.; Zouani, O.F.; Plawinski, L. Human mesenchymal stem cell behavior on femtosecond laser-textured Ti-6Al-4V surfaces. Nanomedicine 2015, 10, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.E.J.; Exir, H.; Weck, A. Characterization and evaluation of femtosecond laser-induced sub-micron periodic structures generated on titanium to improve osseointegration of implants. Appl. Surf. Sci. 2018, 441, 1034–1042. [Google Scholar] [CrossRef]
- Marticorena, M.; Corti, G.; Olmedo, D. Laser surface modification of Ti implants to improve osseointegration. J. Appl. Phys. 2007, 59, 662–665. [Google Scholar] [CrossRef]
- Trtica, M.; Stasic, J.; Batani, D.; Benocci, R.; Narayanan, V.; Ciganovic, J. Laser-assisted surface modification of Ti-implant in air and water environment. Appl. Surf. Sci. 2018, 428, 669–675. [Google Scholar] [CrossRef]
- Hsiao, W.T.; Chang, H.C.; Nanci, A.; Durand, R. Surface microtexturing of Ti–6Al–4V using an ultraviolet laser system. Mater. Des. 2016, 90, 891–895. [Google Scholar] [CrossRef]
- Kumari, R.; Scharnweber, T.; Pfleging, W.; Besser, H.; Majumdar, J.D. Laser surface textured titanium alloy (Ti–6Al–4V)–Part II–Studies on bio-compatibility. Appl. Surf. Sci. 2015, 357, 750–758. [Google Scholar] [CrossRef]
- Paital, S.R.; Balani, K.; Agarwal, A.; Dahotre, N.B. Fabrication and evaluation of a pulse laser-induced Ca–P coating on a Ti alloy for bioapplication. Biomed. Mater. 2009, 4, 015009. [Google Scholar] [CrossRef]
- Kurella, A.; Dahotre, N.B. Laser induced hierarchical calcium phosphate structures. Acta Biomater. 2006, 2, 677–683. [Google Scholar] [CrossRef]
- Bose, S.; Robertson, S.F.; Bandyopadhyay, A. Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomater. 2018, 66, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Johnson, S.; Kumar, D.; Jelinek, M.; Chrisey, D.; Doraiswamy, A. Pulsed laser deposition of hydroxyapatite thin films. Mater. Sci. Eng. C 2007, 27, 484–494. [Google Scholar] [CrossRef]
- Paital, S.R.; He, W.; Dahotre, N.B. Laser pulse dependent micro textured calcium phosphate coatings for improved wettability and cell compatibility. J. Mater. Sci. Mater. Med. 2010, 21, 2187–2200. [Google Scholar] [CrossRef]
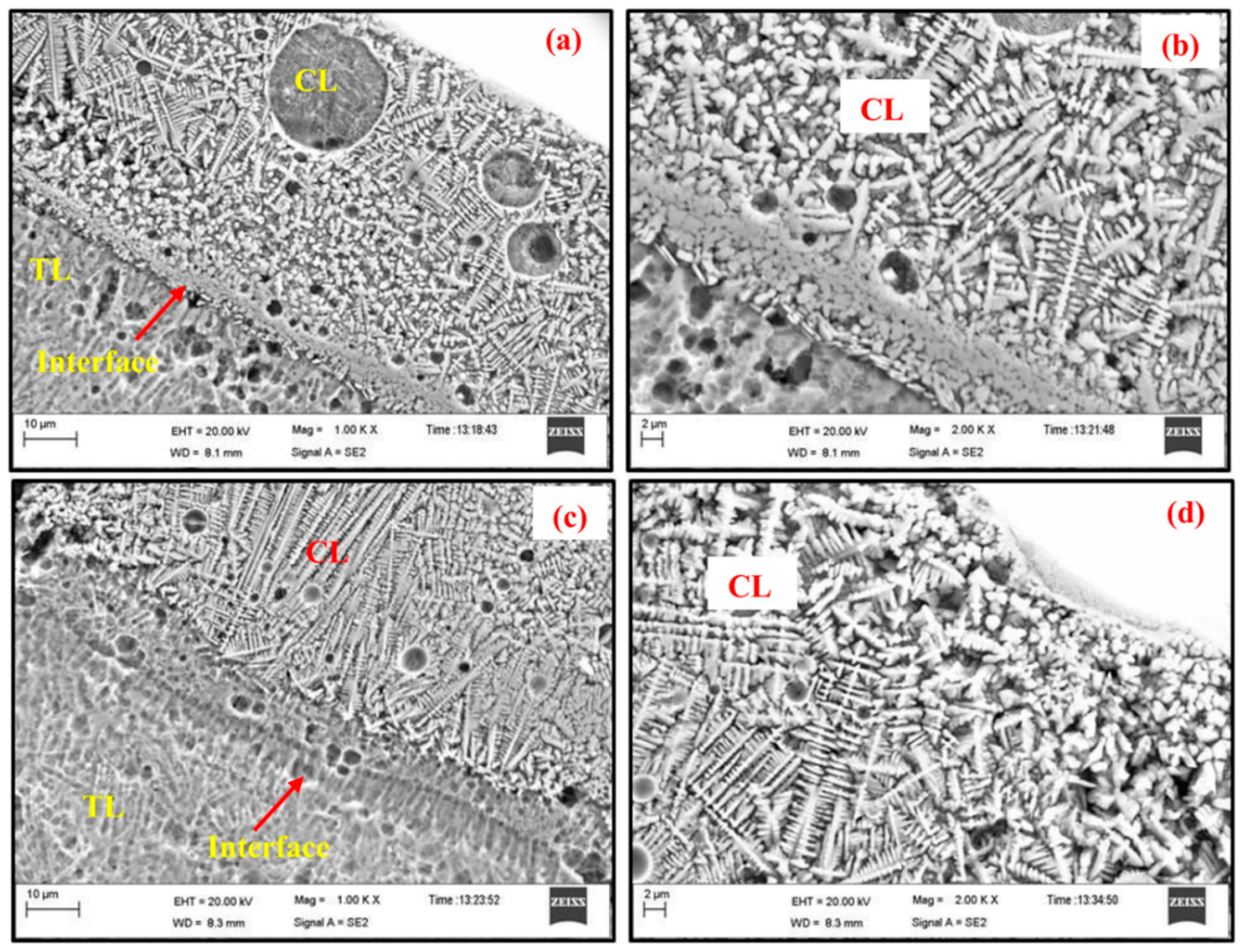
- Zheng, M.; Fan, D.; Li, X.K. Microstructure and in vitro bioactivity of laser-cladded bioceramic coating on titanium alloy in a simulated body fluid. J. Alloy. Compd. 2010, 489, 211–214. [Google Scholar] [CrossRef]
- Ranjan, B.R.; Abshar, H.; Ravi, S.M. Laser cladding with HA and functionally graded TiO2-HA precursors on Ti–6Al–4V alloy for enhancing bioactivity and cyto-compatibility. Surf. Coat. Technol. 2018, 352, 420–436. [Google Scholar]
- Bandyopadhyay, A.; Bose, S.; Roy, M. MgO-doped tantalum coating on Ti: Microstructural study and biocompatibility evaluation. ACS Appl. Mater. Interfaces 2012, 4, 577–580. [Google Scholar]
- Deng, B.W.; Bruzzaniti, A.; Cheng, G.J. Enhancement of osteoblast activity on nanostructured NiTi/hydroxyapatite coatings on additive manufactured NiTi metal implants by nanosecond pulsed laser sintering. Int. J. Nanoned. 2018, 13, 8217–8230. [Google Scholar] [CrossRef]
- Hseni, E.; Zalnezhad, E.; Bushroa, A.R. Comparative investigation on the adhesion of hydroxyapatite coating on Ti–6Al–4V implant: A review paper. Int. J. Adhes. Adhes. 2014, 48, 238–257. [Google Scholar]
- Miranda, G.; Sousa, F.; Costa, M.; Bartolomeu, F.; Silva, F.; Carvalho, O. Surface design using laser technology for Ti-6Al-4V-hydroxyapatite implants. Opt. Technol. 2019, 109, 488–495. [Google Scholar] [CrossRef]
- Liu, W.C.; Wang, H.Y.; Chen, L.C.; Huang, S.W.; Wu, C.T.; Chung, R.J. Hydroxyapatite/tricalcium silicate composites cement derived from novel two-step sol-gel process with good biocompatibility and applications as bone cement and potential coating materials. Ceram. Int. 2019, 45, 5668–5679. [Google Scholar] [CrossRef]
- Owens, G.; Singh, R.K.; Foroutan, F.; Alqaysi, M. Sol–gel based materials for biomedical applications. Prog. Mater Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Çomakli, O.; Yazici, M.; Yetim, T.; Yetim, A.F.; Çelik, A. The Effects of aging time on the structural and electrochemical properties of composite coatings on Cp-Ti substrate. J. Bionic Eng. 2017, 14, 532–539. [Google Scholar] [CrossRef]
- Çomaklı, O.; Yazıcı, M.; Kovacı, H.; Yetim, T.; Yetim, A.F.; Çelik, A. Tribological and electrochemical properties of TiO2 films produced on Cp-Ti by sol-gel and SILAR in bio-simulated environment. Surf. Coat. Technol. 2018, 352, 513–521. [Google Scholar] [CrossRef]
- Balaganapathi, T.; Kaniamuthan, B.; Vinoth, S.; Thilakan, P. PEG assisted synthesis of porous TiO2 using sol-gel processing and its characterization studies. Mater. Chem. Phys. 2017, 189, 50–55. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Veronesi, P.; Lamanna, G. Influence of PCL on mechanical properties and bioactivity of ZrO2-based hybrid coatings synthesized by sol–gel dip coating technique. Mater. Sci. Eng. C 2014, 39, 344–351. [Google Scholar] [CrossRef]
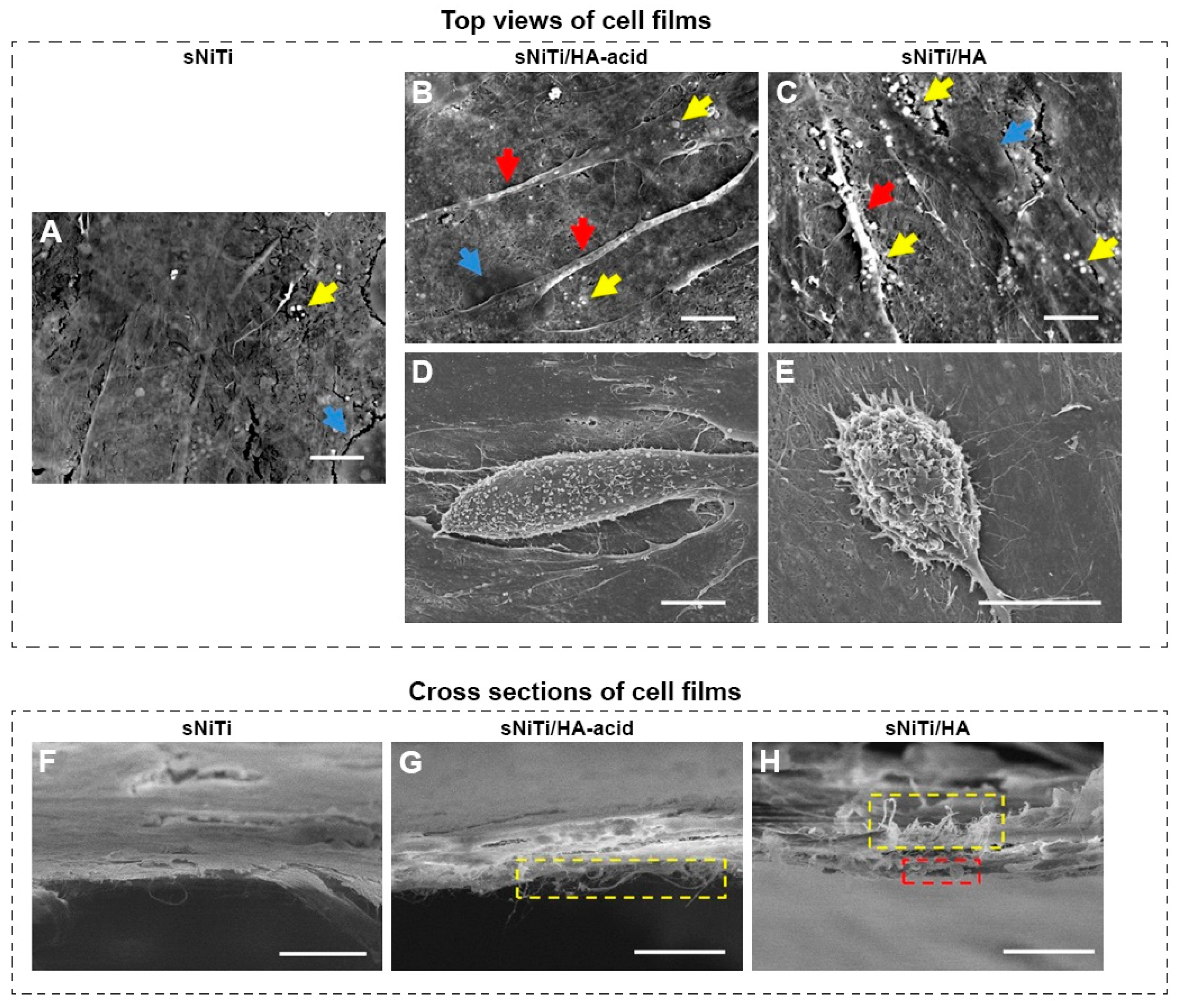
- Anjaneyulu, U.; Priyadarshini, B.; Stango, S.A.X.; Chellappa, M.; Geetha, M.; Vijayalakshmi, U. Preparation and characterisation of sol–gel-derived hydroxyapatite nanoparticles and its coatings on medical grade Ti-6Al-4V alloy for biomedical applications. Mater. Technol. 2017, 32, 800–814. [Google Scholar] [CrossRef]
- Greer, A.I.; Lim, T.S.; Brydone, A.S.; Gadegaard, N. Mechanical compatibility of sol–gel annealing with titanium for orthopaedic prostheses. Mater. Sci. Mater. Med. 2016, 27, 21–27. [Google Scholar] [CrossRef]
- Ergün, Y.; Başpınar, M.S. Effect of acid passivation and H2 sputtering pretreatments on the adhesive strength of sol-gel derived Hydroxyapatite coating on titanium surface. Int. J. Hydrogen Energy. 2017, 42, 20420–20429. [Google Scholar] [CrossRef]
- Roest, R.; Latella, B.; Heness, G.; Ben-Nissan, B. Adhesion of sol–gel derived hydroxyapatite nanocoatings on anodised pure titanium and titanium (Ti6Al4V) alloy substrates. Surf. Coat. Technol. 2011, 205, 3520–3529. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, F.; Xiao, T.; Xu, D.B.; Li, Z.; Xiao, Z.; Xiao, Z.A. Surface modification with SiO2 coating on biomedical TiNi shape memory alloy by sol−gel method. Trans. Nonferrous Met. Soc. 2015, 25, 3723–3728. [Google Scholar] [CrossRef]
- Andrea Alcantara-Garcia, A.; Garcia-Casas, A.; Jimenez-Morales, A. Electrochemical study of the synergic effect of phosphorus and cerium additions on a sol-gel coating for Titanium manufactured by powder metallurgy. Prog. Org. Coat. 2018, 124, 267–274. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F. Surface modifications of titanium implants by coating with bioactive and biocompatible poly (ɛ-caprolactone)/SiO2 hybrids synthesized via sol–gel. Asian J. Chem. 2018, 11, 1126–1133. [Google Scholar] [CrossRef]
- Sharma, V.; Prakash, U.; Kumar, B.V.M. Surface composites by friction stir processing: A review. J. Mater. Process. Technol. 2015, 224, 117–134. [Google Scholar] [CrossRef]
- Misra, R.; Thein-Han, W.; Pesacreta, T.; Hasenstein, K.; Somani, M.; Karjalainen, L. Cellular response of preosteoblasts to nanograined/ultrafine-grained structures. Acta Biomater. 2009, 5, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- McNelley, T.R. Friction stir processing (FSP): Refining microstructures and improving properties. Rev. Met. 2010, 46, 149–156. [Google Scholar] [CrossRef]
- Węglowski, M.S. Friction stir processing–State of the art. Arch. Civ. Mech. Eng. 2018, 18, 114–129. [Google Scholar] [CrossRef]
- Khodabakhshi, F.; Gerlich, A. Potentials and strategies of solid-state additive friction-stir manufacturing technology: A critical review. J. Manuf. Process. 2018, 36, 77–92. [Google Scholar] [CrossRef]
- Li, B.; Shen, Y.; Hu, W.; Luo, L. Surface modification of Ti–6Al–4V alloy via friction-stir processing: Microstructure evolution and dry sliding wear performance. Surf. Coat. Technol. 2014, 239, 160–170. [Google Scholar] [CrossRef]
- Wang, L.Q.; Xie, L.E.; Liu, Y.T.; Zhang, L.C.; Chen, L.Y.; Meng, Q.; Qu, J.; Zhang, D.; Lu, W.J. Microstructure evolution and superelastic behavior in Ti-35Nb-2Ta-3Zr alloy processed by friction stir processing. Acta Mater. 2017, 131, 499–510. [Google Scholar] [CrossRef]
- Gu, H.; Ding, Z.H.; Yang, Z.; Yu, W.Q.; Wang, L.Q. Microstructure evolution and electrochemical properties of TiO2/Ti-35Nb-2Ta-3Zr micro/nano-composites fabricated by friction stir processing. Mater. Des. 2019, 169, 10768. [Google Scholar] [CrossRef]
- Xie, L.C.; Wang, L.Q.; Wang, K.S.; Yin, G.L.; Fu, Y.F. TEM characterization on microstructure of Ti–6Al–4V/Ag nanocomposite formed by friction stir processing. Materialia 2018, 3, 139–144. [Google Scholar] [CrossRef]
- Zhu, C.; Lv, Y.; Qian, C. Proliferation and osteogenic differentiation of rat BMSCs on a novel Ti/SiC metal matrix nanocomposite modified by friction stir processing. Sci. Rep. 2016, 6, 38875. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.Q.; Shen, H.R.; Shen, Z.K.; Chen, H.Y.; Li, F. Manufacture of biodegradable magnesium alloy by high speed friction stir processing. J. Manuf. Process. 2018, 36, 22–32. [Google Scholar] [CrossRef]
- Lv, Y.T.; Ding, Z.H.; Xue, J.; Sha, G.; Wang, L.Q. Deformation mechanisms in surface nano-crystallization of low elastic modulus Ti-6Al-4V/Zn composite during severe plastic deformation. Scr. Mater. 2018, 157, 142–147. [Google Scholar] [CrossRef]
- Rahmati, R.; Khodabakhshi, F. Microstructural evolution and mechanical properties of a friction-stir processed Ti-hydroxyapatite (HA) nanocomposite. J. Mech. Behav. Biomed. Mater. 2018, 88, 127–139. [Google Scholar] [CrossRef]
- Zhang, C.J.; Ding, Z.H.; Xie, L.H.; Zhang, L.C.; Wu, L.Z.; Fu, Y.F.; Wang, L.Q.; Lu, W.J. Electrochemical and in vitro behavior of the nanosized composites of Ti-6Al-4V and TiO2 fabricated by friction stir process. Appl. Surf. Sci. 2017, 423, 331–339. [Google Scholar] [CrossRef]
- Yang, Z.; Gu, H.; Sha, G.; Lu, W.J.; Yu, W.Q.; Zhang, W.J.; Fu, Y.J.; Wang, K.S.; Wang, L.Q. TC4/Ag metal matrix nanocomposites modified by friction stir processing: Surface characterization, antibacterial property, and cytotoxicity in vitro. ACS Appl. Mater. Interfaces 2018, 10, 41155–41166. [Google Scholar] [CrossRef]
- Wang, L.Q.; Qu, J.; Chen, L.Y.; Meng, Q.; Zhang, L.C.; Qin, J.N. Investigation of deformation mechanisms in beta-type Ti-35Nb-2Ta-3Zr alloy via FSP leading to surface strengthening. Metall. Mater. Trans. A 2015, 46A, 4813–4818. [Google Scholar] [CrossRef]
- Ding, Z.H.; Zhang, C.J.; Xie, L.C.; Zhang, L.C.; Wang, L.Q.; Lu, W.J. Effects of friction stir processing on the phase transformation and microstructure of TiO2-compounded Ti-6Al-4V alloy. Metall. Mater. Trans. A 2016, 47A, 5675–5679. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Liu, S.; Wang, L. Surface Modification of Biomedical Titanium Alloy: Micromorphology, Microstructure Evolution and Biomedical Applications. Coatings 2019, 9, 249. https://doi.org/10.3390/coatings9040249
Liu W, Liu S, Wang L. Surface Modification of Biomedical Titanium Alloy: Micromorphology, Microstructure Evolution and Biomedical Applications. Coatings. 2019; 9(4):249. https://doi.org/10.3390/coatings9040249
Chicago/Turabian StyleLiu, Wei, Shifeng Liu, and Liqiang Wang. 2019. "Surface Modification of Biomedical Titanium Alloy: Micromorphology, Microstructure Evolution and Biomedical Applications" Coatings 9, no. 4: 249. https://doi.org/10.3390/coatings9040249
APA StyleLiu, W., Liu, S., & Wang, L. (2019). Surface Modification of Biomedical Titanium Alloy: Micromorphology, Microstructure Evolution and Biomedical Applications. Coatings, 9(4), 249. https://doi.org/10.3390/coatings9040249




