Effect of Nano-Edible Coating Based on Beeswax Solid Lipid Nanoparticles on Strawberry’s Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Strawberries
2.3. BSLN Preparation
2.4. Determination of Particle Size (PS), the Polydispersion Index (PDI), and Zeta Potential (ζ)
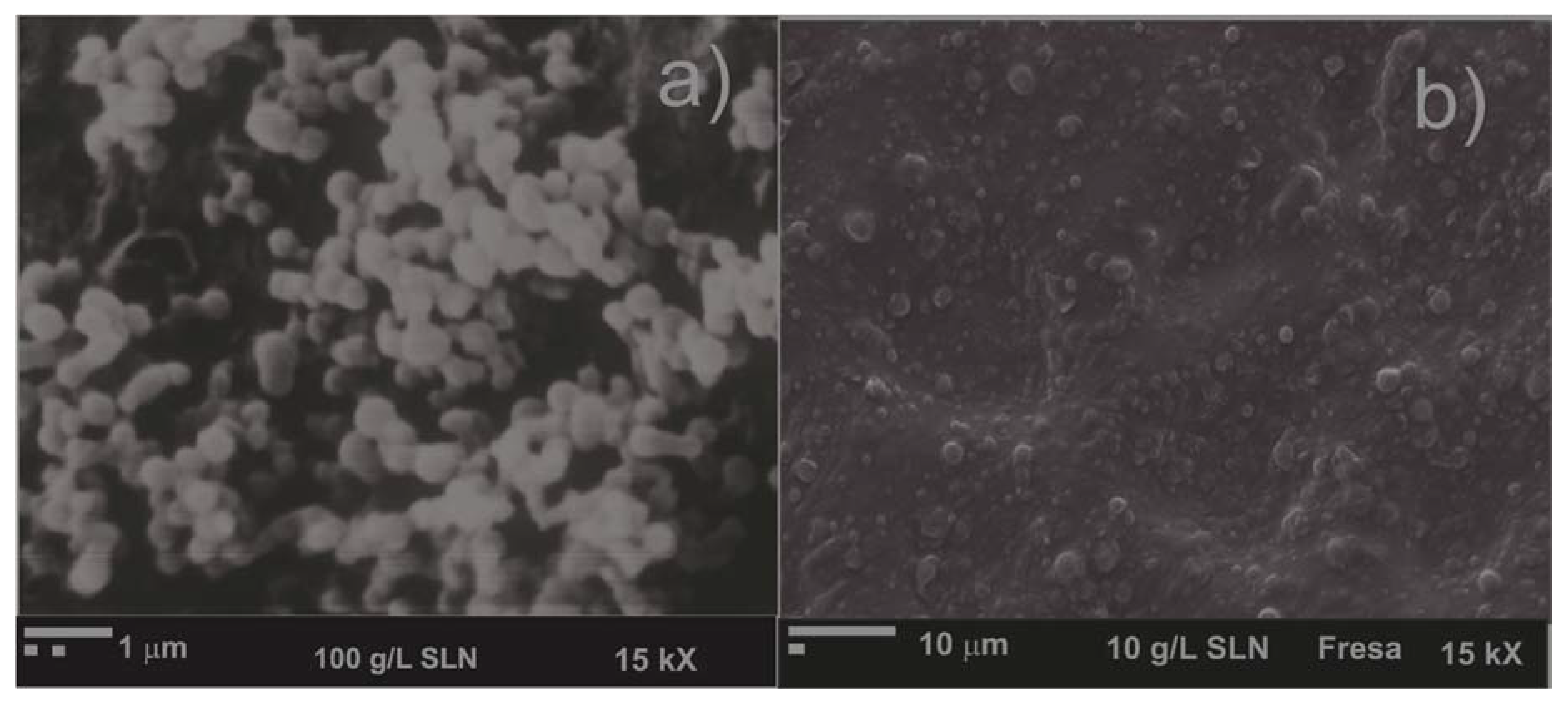
2.5. Scanning Electron Microscopy (SEM)
2.6. Preparation of the Film-Forming Dispersions
2.7. Coating Application on Strawberries
2.8. Oxygen Consumption
2.9. Skin Color Development
2.10. Firmness Measurement
2.11. Physical and Chemical Changes
2.12. Juiciness
2.13. Decay Index
2.14. Statistical Analysis
3. Results and Discussion
3.1. Characterization of SLN with Beeswax
3.2. Morphological Studies
3.3. Respiration Rate as Function Treatment
3.4. Color Development
3.5. Firmness and Decay Index
3.6. Physicochemical Changes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eroglu, E.; Torun, M.; Dincer, C.; Topuz, A. influence of pullulan-based edible coating on some quality properties of strawberry during cold storage. Packag. Technol. Sci. 2014, 27, 831–838. [Google Scholar] [CrossRef]
- Garcia, L.C.; Pereira, L.M.; de Luca Sarantópoulos, C.I.G.; Hubinger, M.D. Selection of an edible starch coating for minimally processed strawberry. Food Bioprocess Technol. 2010, 3, 834–842. [Google Scholar] [CrossRef]
- Formiga, A.S.; Pinsetta, J.S.; Pereira, E.M.; Cordeiro, I.N.F.; Mattiuz, B.H. Use of edible coatings based on hydroxypropyl methylcellulose and beeswax in the conservation of red guava ‘Pedro Sato’. Food Chem. 2019, 290, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Zaragoza, M.L.; González-Reza, R.; Mendoza-Muñoz, N.; Miranda-Linares, V.; Bernal-Couoh, T.F.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Nanosystems in edible coatings: A novel strategy for food preservation. Int. J. Mol. Sci. 2018, 19, 705. [Google Scholar] [CrossRef]
- González-Reza, R.; García-Betanzos, C.; Sánchez-Valdes, L.; Quintanar-Guerrero, D.; Cornejo-Villegas, M.; Zambrano-Zaragoza, M. The functionalization of nanostructures and their potential applications in edible coatings. Coatings 2018, 8, 160. [Google Scholar] [CrossRef]
- Cakmak, H.; Kumcuoglu, S.; Tavman, S. Electrospray coating of minimally processed strawberries and evaluation of the shelf-life quality properties. J. Food Process Eng. 2019, 42. [Google Scholar] [CrossRef]
- Treviño-Garza, M.Z.; García, S.; Flores-González, M.S.; Arévalo-Niño, K. Edible active coatings based on pectin, pullulan, and chitosan increase quality and shelf life of strawberries (fragaria ananassa). J. Food Sci. 2015, 80, M1823–M1830. [Google Scholar] [CrossRef]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv Camarosa) under commercial storage conditions. LWT Food Sci. Technol. 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Nadim, Z.; Ahmadi, E.; Sarikhani, H.; Amiri Chayjan, R. Effect of methylcellulose-based edible coating on strawberry fruit’s quality maintenance during storage. J. Food Process. Preserv. 2015, 39, 80–90. [Google Scholar] [CrossRef]
- Musyanovych, A.; Landfester, K. Polymer micro- and nanocapsules as biological carriers with multifunctional properties. Macromol. Biosci. 2014, 14, 458–477. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. Recent advances in edible polymer based hydrogels as a sustainable alternative to conventional polymers. J. Agric. Food Chem. 2018, 66, 6940–6967. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Chen, J.; Xie, D.; Xia, W.; Xu, W.; Xiong, Y.L. Effect of xanthan gum/soybean fiber ratio in the batter on oil absorption and quality attributes of fried breaded fish nuggets. J. Food Sci. 2018, 83, 1832–1838. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Varshosaz, J.; Mohebbi, M.; Shahidi, F. Hesperetin-loaded solid lipid nanoparticles and nanostructure lipid carriers for food fortification: Preparation, Characterization, and Modeling. Food Bioprocess Technol. 2013, 6, 1464–1475. [Google Scholar] [CrossRef]
- García-Betanzos, C.I.; Hernández-Sánchez, H.; Quintanar-Guerrero, D.; Del Real, A.L.; Zambrano-Zaragoza, M.L. The evaluation of mechanical, thermal, optical and microstructural properties of edible films with solid lipid nanoparticles-xanthan gum stored at different temperatures and relative humidities. Food Bioprocess Technol. 2016, 9, 1756–1768. [Google Scholar] [CrossRef]
- Tumbarski, Y.; Nikolova, R.; Petkova, N.; Ivanov, I.; Lante, A. Biopreservation of fresh strawberries by carboxymethyl cellulose edible coatings enriched with a bacteriocin from Bacillus methylotrophicus BM47. Food Technol. Biotechnol. 2019, 57, 230–237. [Google Scholar] [CrossRef]
- González-Reza, R.M.; Pérez-Olivier, M.S.; Miranda-Linares, V.; Zambrano-Zaragoza, M.L. Effect of solid lipid nanoparticles coating on shelf life of refrigerated fresh-cut guava. Acta Hortic. 2018, 1194, 553–559. [Google Scholar] [CrossRef]
- Miranda-Linares, V.; Escamilla-Rendón, P.; Del Real-López, A.; González-Reza, R.M.; Zambrano-Zaragoza, M.L. Solid lipid nanoparticles based edible coating for saladette tomato preservation. Acta Hortic. 2018, 1194, 305–312. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Mercado-Silva, E.; Ramirez-Zamorano, P.; Cornejo-Villegas, M.A.; Gutiérrez-Cortez, E.; Quintanar-Guerrero, D. Use of solid lipid nanoparticles (SLNs) in edible coatings to increase guava (Psidium guajava L.) shelf-life. Food Res. Int. 2013, 51, 946–953. [Google Scholar] [CrossRef]
- González-Reza, R.M.; Quintanar-Guerrero, D.; Del Real-López, A.; Piñon-Segundo, E.; Zambrano-Zaragoza, M.L. Effect of sucrose concentration and pH onto the physical stability of β-carotene nanocapsules. LWT Food Sci. Technol. 2018, 90, 354–361. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Gutiérrez-Cortez, E.; Del Real, A.; González-Reza, R.M.; Galindo-Pérez, M.J.; Quintanar-Guerrero, D. Fresh-cut Red Delicious apples coating using tocopherol/mucilage nanoemulsion: Effect of coating on polyphenol oxidase and pectin methylesterase activities. Food Res. Int. 2014, 62, 974–983. [Google Scholar] [CrossRef]
- Galindo-Pérez, M.J.; Quintanar-Guerrero, D.; Mercado-Silva, E.; Real-Sandoval, S.A.; Zambrano-Zaragoza, M.L. The effects of tocopherol nanocapsules/xanthan gum coatings on the preservation of fresh-cut apples: Evaluation of phenol metabolism. Food Bioprocess Technol. 2015, 8, 1791–1799. [Google Scholar] [CrossRef]
- Bierhals, V.S.; Chiumarelli, M.; Hubinger, M.D. Effect of cassava starch coating on quality and shelf life of fresh-cut pineapple (ananas comosus l. merril cv “pérola”). J. Food Sci. 2011, 76, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Cano-Sarmiento, C.; Téllez-Medina, D.I.; Viveros-Contreras, R.; Cornejo-Mazón, M.; Figueroa-Hernández, C.Y.; García-Armenta, E.; Alamilla-Beltrán, L.; García, H.S.; Gutiérrez-López, G.F. Zeta Potential of Food Matrices. Food Eng. Rev. 2018, 10, 113–138. [Google Scholar] [CrossRef]
- Barrios, S.; Lema, P.; Lareo, C. Modeling respiration rate of strawberry (cv. San Andreas) for modified atmosphere packaging design. Int. J. Food Prop. 2014, 17, 2039–2051. [Google Scholar] [CrossRef]
- Eshghi, S.; Hashemi, M.; Mohammadi, A.; Badii, F.; Mohammadhoseini, Z.; Ahmadi, K. Effect of nanochitosan-based coating with and without copper loaded on physicochemical and bioactive components of fresh strawberry fruit (fragaria x ananassa duchesne) during storage. Food Bioprocess Technol. 2014, 7, 2397–2409. [Google Scholar] [CrossRef]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan-lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Pinzon, M.I.; Sanchez, L.T.; Garcia, O.R.; Gutierrez, R.; Luna, J.C.; Villa, C.C. Increasing shelf life of strawberries (Fragaria ssp) by using a banana starch-chitosan-Aloe vera gel composite edible coating. Int. J. Food Sci. Technol. 2019, 55, 92–98. [Google Scholar] [CrossRef]
- Gol, N.B.; Patel, P.R.; Rao, T.V.R. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Hussain, P.R.; Dar, M.A.; Wani, A.M. Effect of edible coating and gamma irradiation on inhibition of mould growth and quality retention of strawberry during refrigerated storage. Int. J. Food Sci. Technol. 2012, 47, 2318–2324. [Google Scholar] [CrossRef]
- Martínez-gonzález, M.C.; Bautista-baños, S.; Correa-pacheco, Z.N.; Corona-rangel, M.L.; Ventura-aguilar, R.I.; Río-garcía, J.C.; Ramos-garcía, M.D.L.; Nutrición, F.D.; Autónoma, U.; Los, C. Effect of nanostructured chitosan/propolis coatings on the quality and antioxidant capacity of strawberries during storage. Coatings 2020, 10, 90. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Drouiche, N.; Lounici, H.; Pauss, A.; Mameri, N. Effect of shrimp chitosan coatings as affected by chitosan extraction processes on postharvest quality of strawberry. J. Food Meas. Charact. 2013, 7, 215–221. [Google Scholar] [CrossRef]
 Control,
Control,  XG,
XG,  10BSLN,
10BSLN,  20BSLN,
20BSLN,  30BSLN. Error bars represent the standard deviation of the triplicate results.
30BSLN. Error bars represent the standard deviation of the triplicate results.
 Control,
Control,  XG,
XG,  10BSLN,
10BSLN,  20BSLN,
20BSLN,  30BSLN. Error bars represent the standard deviation of the triplicate results.
30BSLN. Error bars represent the standard deviation of the triplicate results.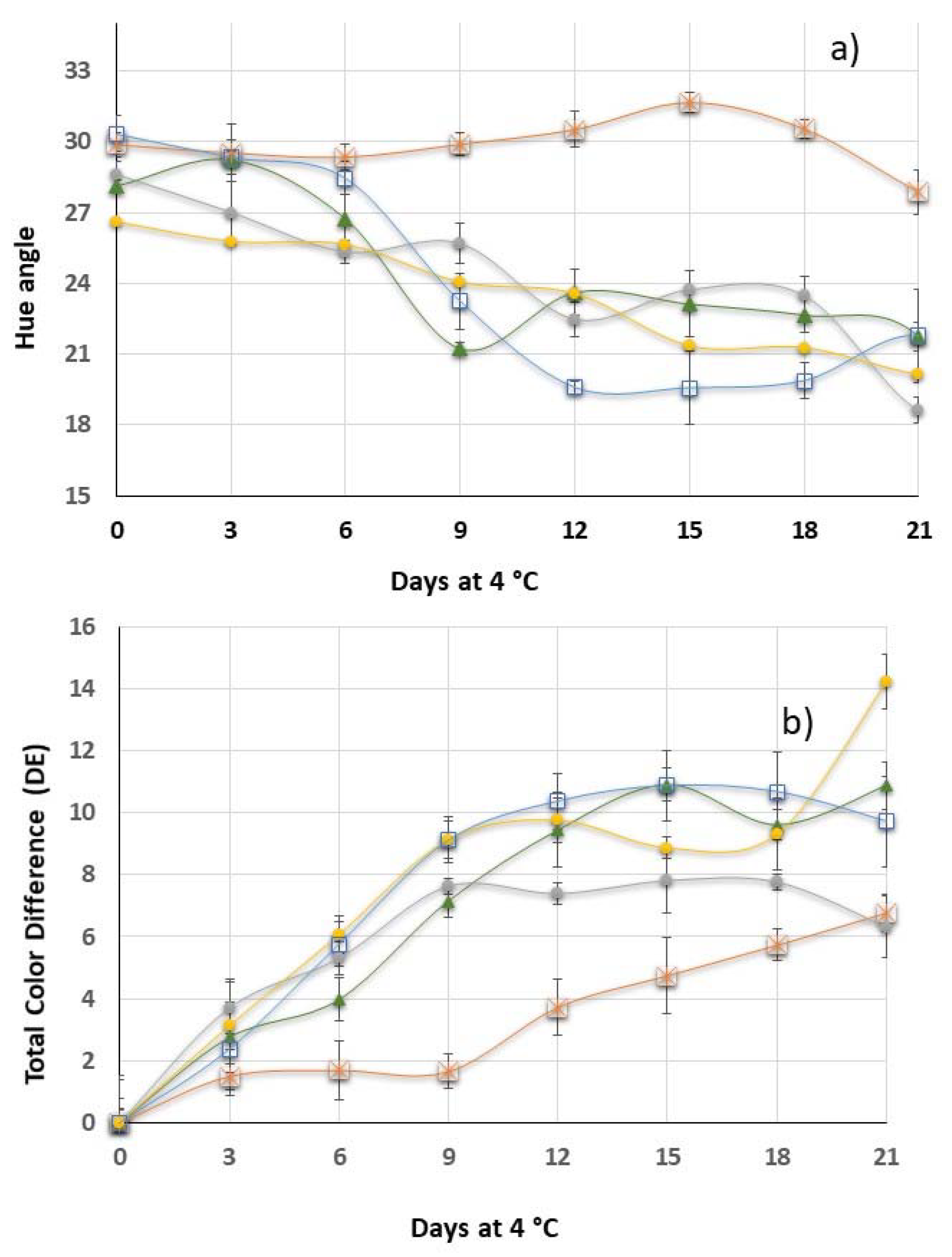
 10 g/L of BSLN,
10 g/L of BSLN,  20 g/L of BSLN,
20 g/L of BSLN,  30 g/L of BSLN,
30 g/L of BSLN,  Control,
Control,  XG; and (b) decay index
XG; and (b) decay index  Control,
Control,  XG,
XG,  10BSLN,
10BSLN,  20BSLN,
20BSLN,  30BSLN. Error bars represent the standard deviation of the triplicate results.
30BSLN. Error bars represent the standard deviation of the triplicate results.
 10 g/L of BSLN,
10 g/L of BSLN,  20 g/L of BSLN,
20 g/L of BSLN,  30 g/L of BSLN,
30 g/L of BSLN,  Control,
Control,  XG; and (b) decay index
XG; and (b) decay index  Control,
Control,  XG,
XG,  10BSLN,
10BSLN,  20BSLN,
20BSLN,  30BSLN. Error bars represent the standard deviation of the triplicate results.
30BSLN. Error bars represent the standard deviation of the triplicate results.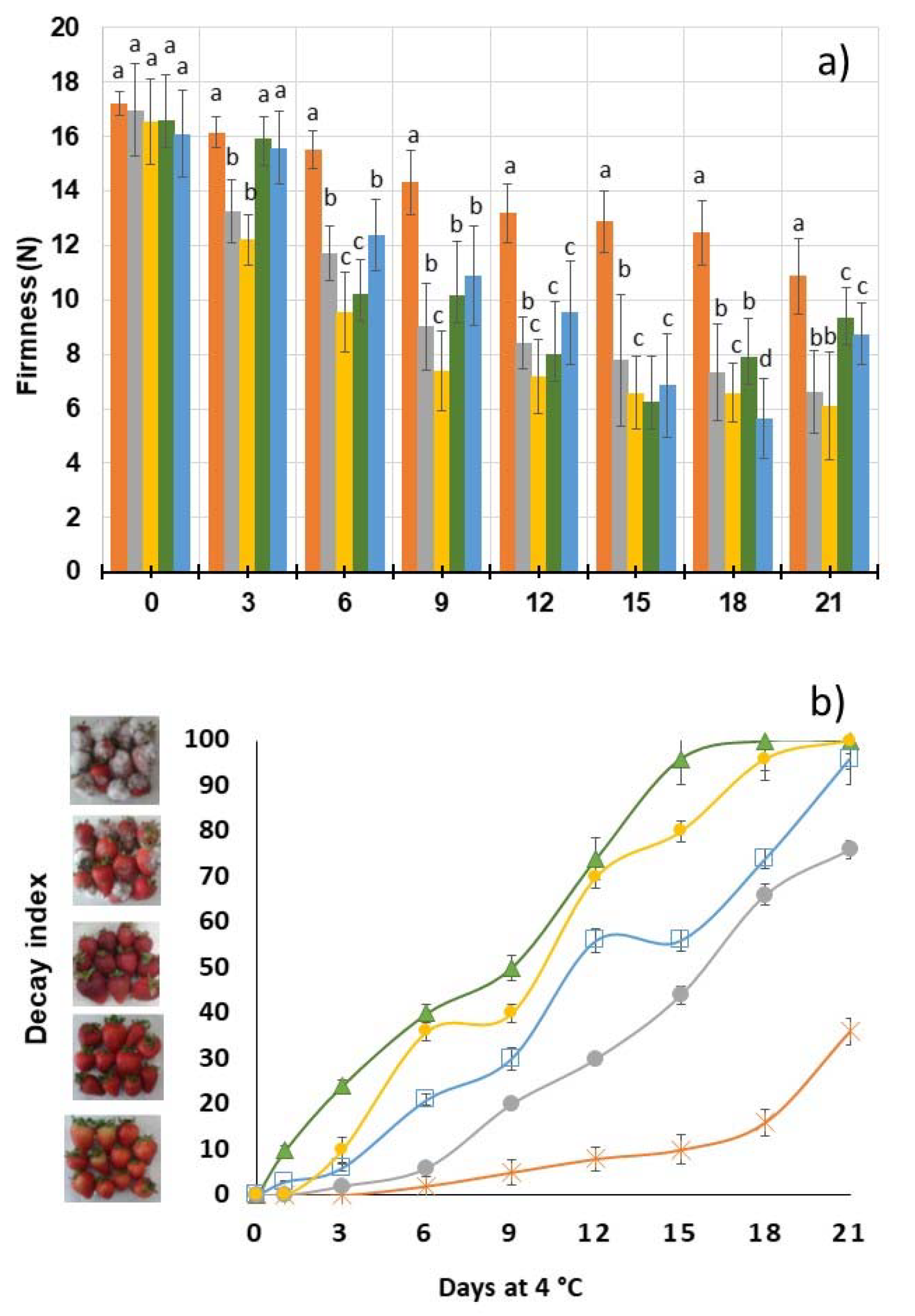
| Coating | Days | Weight Loss (%) | Juiciness (%) | pH | SSC | TTA (%) |
|---|---|---|---|---|---|---|
| 10BSLN | 0 | 0.00 ± 0.00 | 59.58 ± 1.84 | 3.83 ± 0.06 | 7.97 ± 0.64 | 0.29 ± 0.05 |
| 3 | 0.89 ± 0.038 | 59.72 ± 1.56 | 3.70 ± 0.10 | 7.67 ± 0.51 | 0.22 ± 0.03 | |
| 9 | 1.96 ± 0.034 | 60.61 ± 2.69 | 3.87 ± 0.12 | 7.70 ± 0.98 | 0.20 ± 0.09 | |
| 15 | 3.53 ± 0.03 | 56.94 ± 3.05 | 3.43 ± 0.06 | 7.40 ± 0.39 | 0.25 ± 0.03 | |
| 21 | 6.15 ± 0.57 | 32.33 ± 2.22 | 3.43 ± 0.06 | 8.47 ± 0.29 | 0.19 ± 0.02 | |
| 20BSLN | 0 | 0.00 ± 0.00 | 64.44 ± 4.57 | 3.33 ± 0.15 | 7.20 ± 1.04 | 0.25 ± 0.04 |
| 3 | 2.22 ± 0.22 | 60.45 ± 1.81 | 3.67 ± 0.06 | 7.60 ± 0.45 | 0.23 ± 0.04 | |
| 9 | 3.38 ± 0.59 | 54.79 ± 1.79 | 3.80 ± 0.10 | 7.37 ± 0.55 | 0.24 ± 0.06 | |
| 15 | 6.44 ± 0.41 | 43.03 ± 4.06 | 3.60 ± 0.26 | 8.33 ± 0.06 | 0.26 ± 0.02 | |
| 21 | 9.71 ± 0.21 | 25.31 ± 1.96 | 3.37 ± 0.06 | 8.43 ± 0.45 | 0.26 ± 0.02 | |
| 30 BSLN | 0 | 0.00 ± 0.00 | 61.94 ± 2.17 | 3.20 ± 0.10 | 6.67 ± 0.12 | 0.28 ± 0.07 |
| 3 | 2.20 ± 0.25 | 51.98 ± 8.10 | 3.83 ± 0.26 | 8.43 ± 0.81 | 0.30 ± 0.06 | |
| 9 | 4.28 ± 1.08 | 37.55 ± 13.51 | 3.70 ± 0.06 | 8.50 ± 0.44 | 0.30 ± 0.01 | |
| 15 | 13.33 ± 2.13 | 28.97 ± 5.02 | 3.97 ± 0.10 | 8.57 ± 0.25 | 0.31 ± 0.03 | |
| 21 | 17.21 ± 0.35 | 24.32 ± 1.18 | 3.10 ± 0.12 | 8.47 ± 0.15 | 0.43 ± 0.02 | |
| CONTROL | 0 | 0.00 ± 0.00 | 61.30 ± 5.80 | 3.10 ± 0.10 | 7.40 ± 0.12 | 0.42 ± 0.08 |
| 3 | 3.41 ± 1.27 | 59.87 ± 3.95 | 3.03 ± 0.15 | 7.83 ± 0.72 | 0.30 ± 0.02 | |
| 9 | 2.78 ± 0.19 | 34.53 ± 2.61 | 3.87 ± 0.06 | 8.00 ± 0.98 | 0.24 ± 0.04 | |
| 15 | 11.54 ± 3.21 | 23.02 ± 1.73 | 3.70 ± 0.06 | 8.20 ± 0.10 | 0.16 ± 0.02 | |
| 21 | 19.85 ± 2.23 | 24.36 ± 3.70 | 3.70 ± 0.25 | 8.17 ± 0.06 | 0.14 ± 0.03 | |
| XG | 0 | 0.00 ± 0.00 | 59.24 ± 0.97 | 3.57 ± 0.06 | 7.33 ± 0.70 | 0.30 ± 0.08 |
| 3 | 1.62 ± 0.35 | 53.05 ± 1.21 | 3.67 ± 0.10 | 8.50 ± 1.04 | 0.32 ± 0.03 | |
| 9 | 7.89 ± 0.41 | 49.35 ± 1.57 | 3.10 ± 0.10 | 8.33 ± 0.58 | 0.44 ± 0.02 | |
| 15 | 13.78 ± 0.49 | 35.24 ± 2.40 | 3.60 ± 0.06 | 8.90 ± 0.36 | 0.25 ± 0.03 | |
| 21 | 16.73 ± 1.98 | 25.85 ± 1.09 | 3.53 ± 0.06 | 8.13 ± 0.21 | 0.26 ± 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; Del Real, A.; González-Reza, R.M.; Cornejo-Villegas, M.A.; Gutiérrez-Cortez, E. Effect of Nano-Edible Coating Based on Beeswax Solid Lipid Nanoparticles on Strawberry’s Preservation. Coatings 2020, 10, 253. https://doi.org/10.3390/coatings10030253
Zambrano-Zaragoza ML, Quintanar-Guerrero D, Del Real A, González-Reza RM, Cornejo-Villegas MA, Gutiérrez-Cortez E. Effect of Nano-Edible Coating Based on Beeswax Solid Lipid Nanoparticles on Strawberry’s Preservation. Coatings. 2020; 10(3):253. https://doi.org/10.3390/coatings10030253
Chicago/Turabian StyleZambrano-Zaragoza, Maria L., David Quintanar-Guerrero, Alicia Del Real, Ricardo M. González-Reza, Maria A. Cornejo-Villegas, and Elsa Gutiérrez-Cortez. 2020. "Effect of Nano-Edible Coating Based on Beeswax Solid Lipid Nanoparticles on Strawberry’s Preservation" Coatings 10, no. 3: 253. https://doi.org/10.3390/coatings10030253
APA StyleZambrano-Zaragoza, M. L., Quintanar-Guerrero, D., Del Real, A., González-Reza, R. M., Cornejo-Villegas, M. A., & Gutiérrez-Cortez, E. (2020). Effect of Nano-Edible Coating Based on Beeswax Solid Lipid Nanoparticles on Strawberry’s Preservation. Coatings, 10(3), 253. https://doi.org/10.3390/coatings10030253







