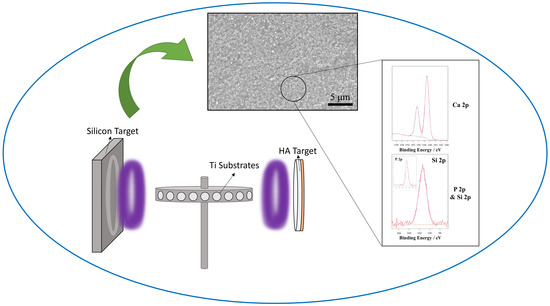
Production of High Silicon-Doped Hydroxyapatite Thin Film Coatings via Magnetron Sputtering: Deposition, Characterisation, and In Vitro Biocompatibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Preparation
2.2. Target Preparation
2.3. RF/Pulsed DC Magnetron Sputtering
2.4. Post Deposition Heat Treatments
2.5. Materials Characterisation
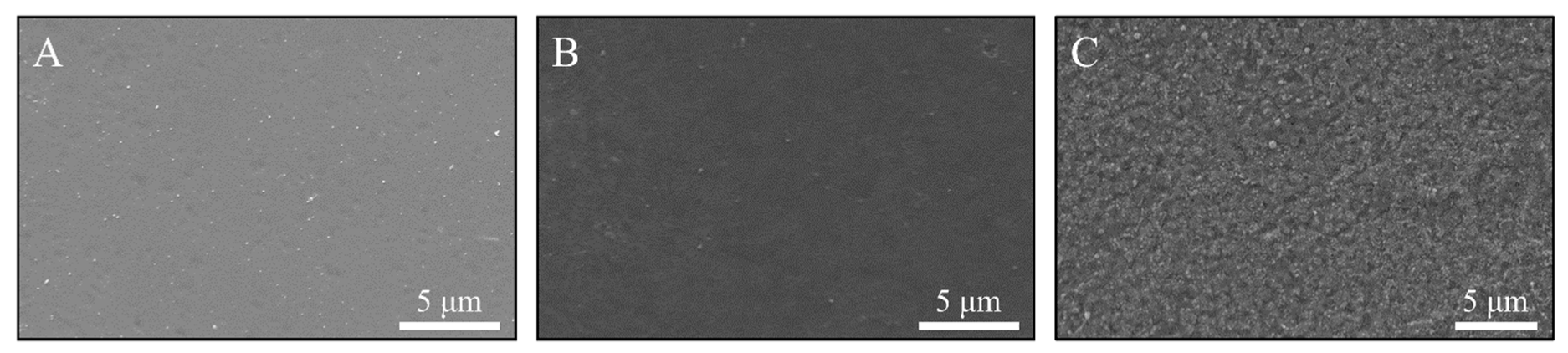
2.5.1. Scanning Electron Microscopy (SEM) and Energy Dispersive X-Ray Spectroscopy (EDX)
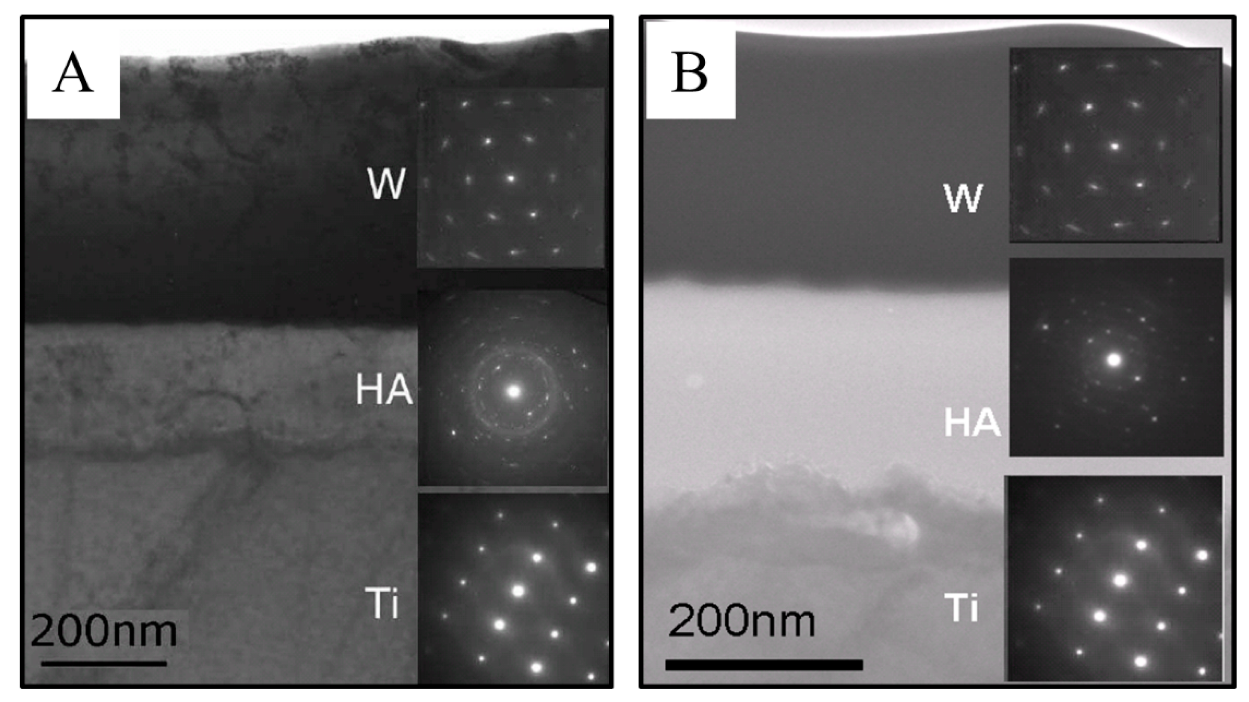
2.5.2. Focused Ion Beam SEM (FIB-SEM) and Transmission Electron Microscopy (TEM)
2.5.3. X-Ray Diffraction (XRD)
2.5.4. Reflective High-Energy Electron Diffraction
2.5.5. X-Ray Photoelectron Spectroscopy (XPS)
2.5.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.7. Surface Roughness – Optical Profilometry
2.5.8. Surface Wettability—Sessile Drop/Contact Angle
2.6. In Vitro Biocompatibility
2.6.1. Alamarblue™ Assay
2.6.2. Alkaline Phosphatase Assay
2.6.3. DNA Hoechst Staining Assay
2.6.4. SEM Sample Preparation
3. Results
3.1. Chemical and Structural Characterisation
3.1.1. Film Morphology and Thickness
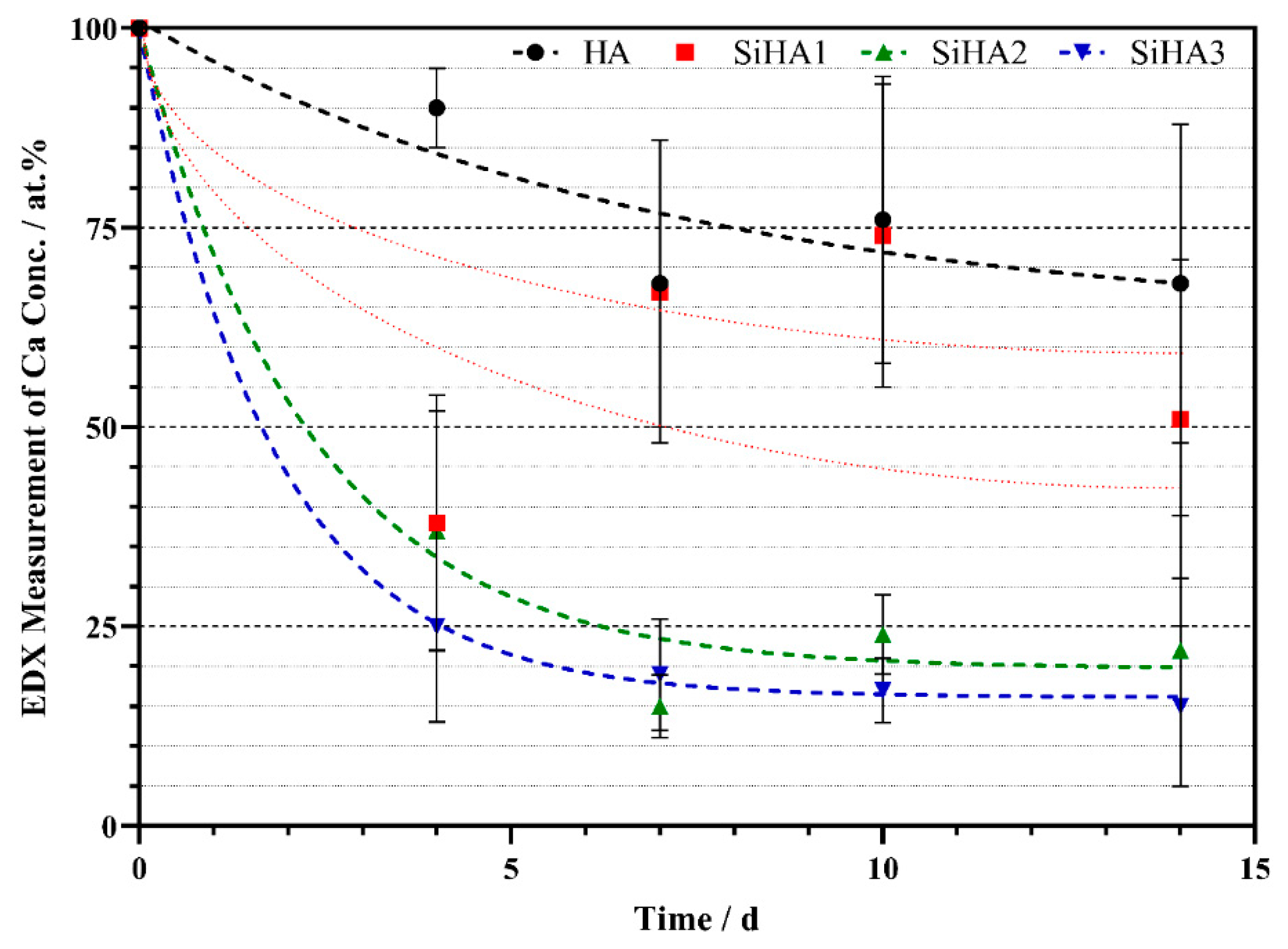
3.1.2. Energy Dispersive X-Ray Analysis (EDX)
3.1.3. X-Ray Diffraction (XRD) Analysis
3.1.4. RHEED Analysis
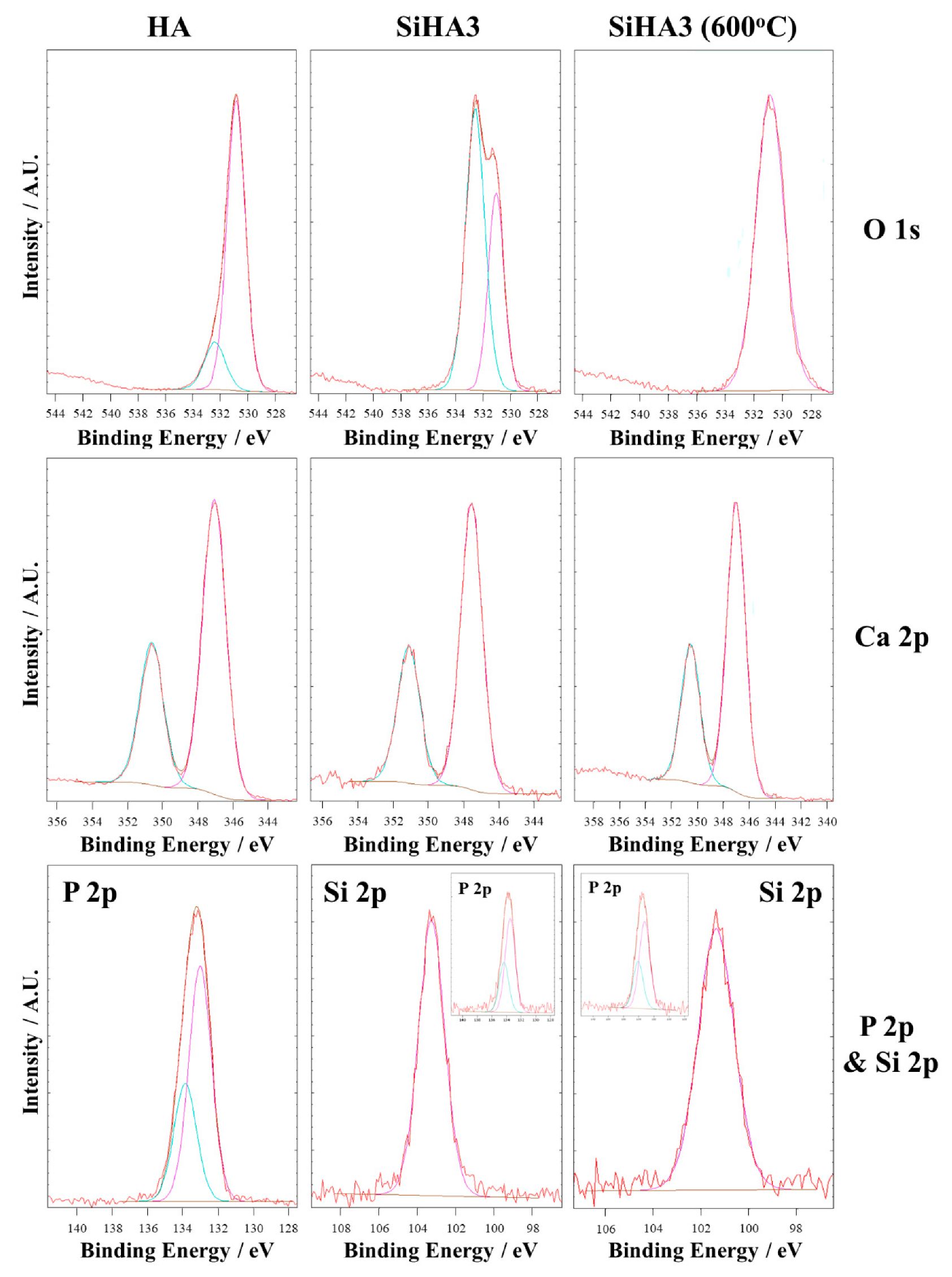
3.1.5. X-Ray Photoelectron Spectroscopy (XPS)
3.1.6. Fourier Transform Infrared Spectroscopy (FTIR)
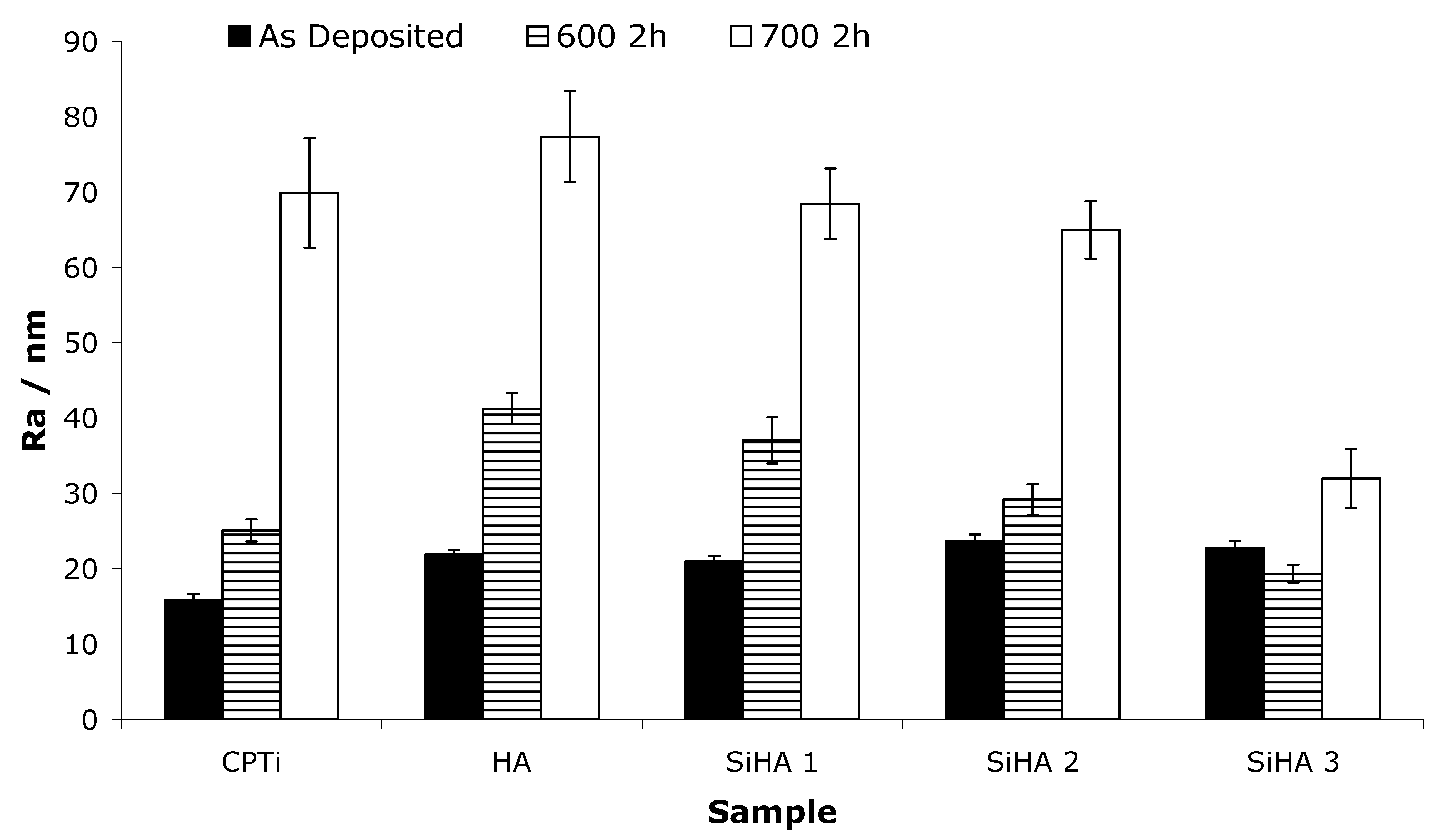
3.1.7. Surface Roughness
3.1.8. Film Wettability
3.2. In Vitro Cytocompatibility Testing
3.2.1. Elusion Testing – Metabolic Activity, DNA Content and Morphology
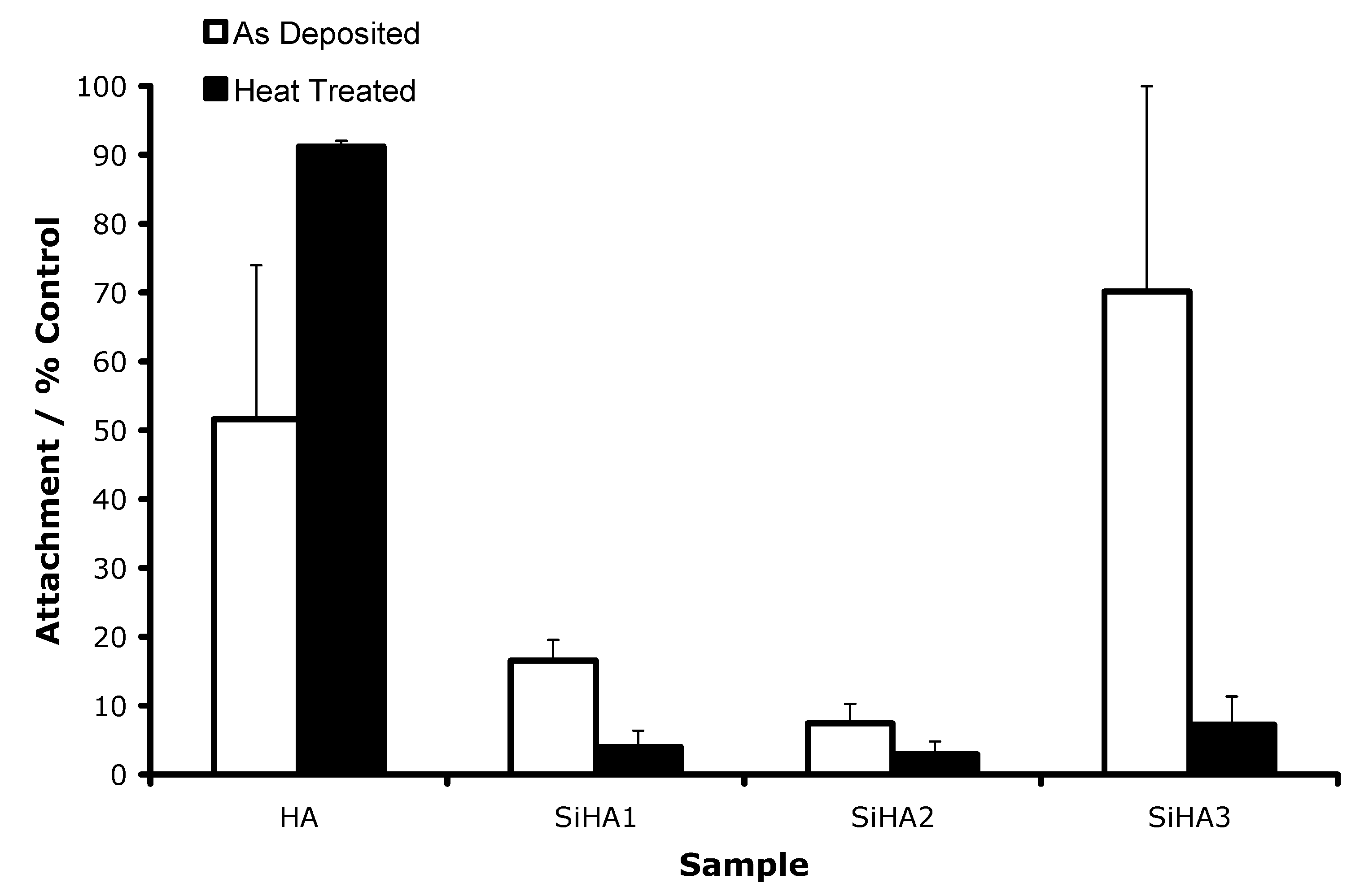
3.2.2. Initial Attachment
3.2.3. Proliferation and Differentiation—Cellular Activity, ALP, and Morphology
4. Discussion
4.1. Composition and Topographical Analysis
4.2. In Vitro Cytocompatibility
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Cao, W.P.; Hench, L.L. Bioactive materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar] [CrossRef]
- Narayanan, R.; Seshadri, S.K.; Kwon, T.Y.; Kim, K.H. Calcium phosphate-based coatings on titanium and its alloys. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 85, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.W.; Gimeno-Fabra, M.; Segal, J.; Ahmed, I.; Grant, D.M. Mechanical, structural and dissolution properties of heat treated thin-film phosphate based glasses. Appl. Surf. Sci. 2017, 416, 605–617. [Google Scholar] [CrossRef]
- Schrooten, J.; Helsen, J.A. Adhesion of bioactive glass coating to Ti6Al4V oral implant. Biomaterials 2000, 21, 1461–1469. [Google Scholar] [CrossRef]
- Chung, C.J.; Long, H.Y. Systematic strontium substitution in hydroxyapatite coatings on titanium via micro-arc treatment and their osteoblast/osteoclast responses. Acta Biomater. 2011, 7, 4081–4087. [Google Scholar] [CrossRef]
- Lim, J.Y.; Donahue, H.J. Cell sensing and response to micro- and nanostructured surfaces produced by chemical and topographic patterning. Tissue Eng. 2007, 13, 1879–1891. [Google Scholar] [CrossRef]
- Suchanek, W.; Yoshimura, M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- Nawawi, N.; Alqap, A.S.F.; Sopyan, I. Recent progress on hydroxyapatite-based dense biomaterials for load bearing bone substitutes. Recent Pat. Mater. Sci. 2011, 4, 63–80. [Google Scholar] [CrossRef]
- Mohseni, E.; Zalnezhad, E.; Bushroa, A.R. Comparative investigation on the adhesion of hydroxyapatite coating on Ti–6Al–4V implant: A review paper. Int. J. Adhes. Adhes. 2014, 48, 238–257. [Google Scholar] [CrossRef]
- Wadge, M.D.; Stuart, B.W.; Thomas, K.G.; Grant, D.M. Generation and characterisation of gallium titanate surfaces through hydrothermal ion-exchange processes. Mater. Des. 2018, 155, 264–277. [Google Scholar] [CrossRef]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J. Biomed. Mater. Res. 2001, 58, 570–592. [Google Scholar] [CrossRef] [PubMed]
- Cheang, P.; Khor, K.A. Addressing processing problems associated with plasma spraying of hydroxyapatite coatings. Biomaterials 1996, 17, 537–544. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Chang, E. Influence of residual stress on bonding strength and fracture of plasma-sprayed hydroxyapatite coatings on Ti–6Al–4V substrate. Biomaterials 2001, 22, 1827–1836. [Google Scholar] [CrossRef]
- Mattox, D.M. Handbook of Physical Vapor Deposition (PVD) Processing; William Andrew: Norwich, NY, USA, 2010. [Google Scholar]
- Wadge, M.D.; Turgut, B.; Murray, J.W.; Stuart, B.W.; Felfel, R.M.; Ahmed, I.; Grant, D.M. Developing highly nanoporous titanate structures via wet chemical conversion of DC magnetron sputtered titanium thin films. J. Colloid Interface Sci. 2020, 566, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Pichugin, V.F.; Surmenev, R.A.; Shesterikov, E.V.; Ryabtseva, M.; Eshenko, E.; Tverdokhlebov, S.I.; Prymak, O.; Epple, M. The preparation of calcium phosphate coatings on titanium and nickel–titanium by rf-magnetron-sputtered deposition: Composition, structure and micromechanical properties. Surf. Coat. Technol. 2008, 202, 3913–3920. [Google Scholar] [CrossRef]
- van Dijk, K.; Schaeken, H.G.; Wolke, J.G.; Jansen, J.A. Influence of annealing temperature on RF magnetron sputtered calcium phosphate coatings. Biomaterials 1996, 17, 405–410. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, K.-H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process—an alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef]
- Jeong, Y.-H.; Choe, H.-C.; Brantley, W.A. Hydroxyapatite-silicon film deposited on Ti–Nb–10Zr by electrochemical and magnetron sputtering method. Thin Solid Film. 2016, 620, 114–118. [Google Scholar] [CrossRef]
- Kozelskaya, A.; Kulkova, S.; Fedotkin, A.; Bolbasov, E.; Zhukov, Y.; Stipniece, L.; Bakulin, A.; Useinov, A.; Shesterikov, E.; Locs, J. Radio frequency magnetron sputtering of Sr-and Mg-substituted β-tricalcium phosphate: Analysis of the physicochemical properties and deposition rate of coatings. Appl. Surf. Sci. 2019, 144763. [Google Scholar] [CrossRef]
- Graziani, G.; Bianchi, M.; Sassoni, E.; Russo, A.; Marcacci, M. Ion-substituted calcium phosphate coatings deposited by plasma-assisted techniques: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 219–229. [Google Scholar] [CrossRef]
- Thian, E.S.; Huang, J.; Best, S.M.; Barber, Z.H.; Bonfield, W. Silicon-substituted hydroxyapatite: The next generation of bioactive coatings. Mater. Sci. Eng. C 2007, 27, 251–256. [Google Scholar] [CrossRef]
- Thian, E.S.; Huang, J.; Vickers, M.E.; Best, S.M.; Barber, Z.H.; Bonfield, W. Silicon-substituted hydroxyapatite (SiHA): A novel calcium phosphate coating for biomedical applications. J. Mater. Sci. 2006, 41, 709–717. [Google Scholar] [CrossRef]
- Thian, E.S.; Huang, J.; Best, S.M.; Barber, Z.H.; Bonfield, W. Novel silicon-doped hydroxyapatite (Si-HA) for biomedical coatings: An in vitro study using acellular simulated body fluid. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 76, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Surmeneva, M.A.; Mukhametkaliyev, T.M.; Tyurin, A.I.; Teresov, A.D.; Koval, N.N.; Pirozhkova, T.S.; Shuvarin, I.A.; Shuklinov, A.V.; Zhigachev, A.O.; Oehr, C.; et al. Effect of silicate doping on the structure and mechanical properties of thin nanostructured RF magnetron sputter-deposited hydroxyapatite films. Surf. Coat. Technol. 2015, 275, 176–184. [Google Scholar] [CrossRef]
- Qi, J.; Chen, Z.; Han, W.; He, D.; Yang, Y.; Wang, Q. Effect of deposition parameters and heat-treatment on the microstructure, mechanical and electrochemical properties of hydroxyapatite/titanium coating deposited on Ti6Al4V by RF-magnetron sputtering. Mater. Res. Express 2017, 4, 096409. [Google Scholar] [CrossRef]
- Thian, E.S.; Huang, J.; Best, S.M.; Barber, Z.H.; Bonfield, W. A new way of incorporating silicon in hydroxyapatite (Si-HA) as thin films. J. Mater. Sci. Mater. Med. 2005, 16, 411. [Google Scholar] [CrossRef]
- San Thian, E.; Huang, J.; Best, S.M.; Barber, Z.H.; Brooks, R.A.; Rushton, N.; Bonfield, W. The response of osteoblasts to nanocrystalline silicon-substituted hydroxyapatite thin films. Biomaterials 2006, 27, 2692–2698. [Google Scholar] [CrossRef]
- Agyapong, D.A.Y.; Zeng, H.; Du, W.; Acquah, I.; Liu, Y. Comparative analysis of the structural and physical properties of magnetron Co-sputtered Ag-doped and Si-doped hydroxyapatite coatings on titanium substrates. Integr. Ferroelectr. 2017, 180, 69–76. [Google Scholar] [CrossRef]
- Boyd, A.R.; Meenan, B.J.; Leyland, N.S. Surface characterisation of the evolving nature of radio frequency (RF) magnetron sputter deposited calcium phosphate thin films after exposure to physiological solution. Surf. Coat. Technol. 2006, 200, 6002. [Google Scholar] [CrossRef]
- Boyd, A.; Akay, M.; Meenan, B.J. Influence of target surface degredation on the properties of r.f. magnetron-sputtered calcium phosphate coatings. Surf. Interface Anal. 2003, 35, 188–198. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Tanaka, Y.; Ide-Ektessabi, A. Fabrication of hydroxyapatite thin films for biomedical applications using RF magnetron sputtering. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2006, 249, 723. [Google Scholar] [CrossRef]
- Wagner, C.D.; Naumkin, A.V.; Kraut-Vass, A.; Allison, J.W.; Powell, C.J., Jr. NIST X-Ray Photoelectron Spectroscopy Database; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Gibson, I.R.; Best, S.M.; Bonfield, W. Chemical characterization of silicon-substituted hydroxyaptite. J. Biomed. Mater. Res. 1999, 44, 422–428. [Google Scholar] [CrossRef]
- Gibson, I.R.; Hing, K.; Revell, P.A.; Santos, J.D.; Best, S.M.; Bonfield, W. Enhanced in vivo response to silicate-substituted hydroxyapaptite. Key Eng. Mater. 2002, 218-220, 203–206. [Google Scholar] [CrossRef]
- Qadir, M.; Li, Y.; Wen, C. Ion-substituted calcium phosphate coatings by physical vapor deposition magnetron sputtering for biomedical applications: A review. Acta Biomater. 2019, 89, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Thian, S.; Huang, J.; Barber, Z.H.; Best, S.M.; Bonfield, W. Surface modification of magnetron-sputtered hydroxyapatite thin films via silicon substitution for orthopaedic and dental applications. Surf. Coat. Technol. 2011, 205, 3472–3477. [Google Scholar] [CrossRef]
- Thian, E.S.; Haung, J.; Best, S.M.; Barber, Z.H.; Bonfield, W. Surface wettability enhances osteoblast adhesion on silicon-substituted hydroxyapatite thin films. Key Eng. Mater. 2007, 330-332, 877–880. [Google Scholar] [CrossRef]
- Thian, E.S.; Huang, J.; Best, S.M.; Barber, Z.H.; Bonfield, W. Silicon-substituted hydroxyapatite thin films: Effect of annealing temperature on coating stability and bioactivity. J. Biomed. Mater. Res. 2006, 78, 121–128. [Google Scholar] [CrossRef]
- Porter, A.E.; Rea, S.M.; Galtrey, M.; Best, S.M.; Barber, Z.H. Production of thin film silicon-doped hydroxyapatite via sputter deposition. J. Mater. Sci. 2004, 39, 1895–1898. [Google Scholar] [CrossRef]
- Zou, S.; Huang, J.; Best, S.M.; Bonfield, W. Crystal imperfection studies of pure and silicon substituted hydroxyapatite using Raman and XRD. J. Mater. Sci. Mater. Med. 2005, 16, 1143–1148. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Chaikina, M.V.; Zaikovskiy, V.I.; Pichugin, V.F.; Buck, V.; Prymak, O.; Epple, M.; Surmenev, R.A. The structure of an RF-magnetron sputter-deposited silicate-containing hydroxyapatite-based coating investigated by high-resolution techniques. Surf. Coat. Technol. 2013, 218, 39–46. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Surmenev, R.A.; Pichugin, V.F.; Chernousova, S.S.; Epple, M. In-vitro investigation of magnetron-sputtered coatings based on silicon-substituted hydroxyapatite. J. Surf. Investig. X-Ray Synchrotron Neutron Tech. 2011, 5, 1202–1207. [Google Scholar] [CrossRef]
- Pichugin, V.F.; Surmeneva, M.A.; Surmenev, R.A.; Khlusov, I.A.; Epple, M. Study of physicochemical and biological properties of calcium phosphate coatings prepared by RF magnetron sputtering of silicon-substituted hydroxyapatite. J. Surf. Investig. X-Ray Synchrotron Neutron Tech. 2011, 5, 863–869. [Google Scholar] [CrossRef]
- Surmeneva, M.; Tyurin, A.; Mukhametkaliyev, T.; Teresov, A.; Koval, A.; Pirozhkova, T.; Shuvarin, I.; Chudinova, E.; Surmenev, R. The effect of Si content on structure and mechanical features of silicon-containing calcium-phosphate-based films deposited by RF-magnetron sputtering on titanium substrate treated by pulsed electron beam. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Tambov, Russia, 21–22 May 2015; p. 012028. [Google Scholar]
- Wang, J.; Xue, C.; Zhu, P. Hydrothermal synthesis and structure characterization of flower-like self assembly of silicon-doped hydroxyapatite. Mater. Lett. 2017, 196, 400–402. [Google Scholar] [CrossRef]
- Bohdansky, J. A Universal Relation for the Sputtering Yield of Monatomic Solids at Normal Ion Incidence. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. At. 1984, 2, 587–591. [Google Scholar] [CrossRef]
- Balas, F.; Perez-Pariente, J.; Vallet-Regi, M. In Vitro bioactivity of silicon-substituted hydroxyapatites. J. Biomed. Mater. Res. 2003, 66, 364–375. [Google Scholar] [CrossRef]
- Okada, K.; Kameshima, Y.; Yasumori, A. Chemical shifts of silicon x-ray photoelectron spectra by polymerization structures of silicates. J. Am. Ceram. Soc. 1998, 81, 1970–1972. [Google Scholar] [CrossRef]
- Steveson, M.; Arora, P.A.; Smart, R.S.C. XPS studies of low temperature plasma-produced graded oxide-silicate-silica layers on titanium. Surf. Interface Anal. 1998, 26, 1027–1034. [Google Scholar] [CrossRef]
- Botelho, C.M.; Lopes, M.A.; Gibson, I.R.; Best, S.M.; Santos, J.D. Structural analysis of Si-substituted hydrooxyapatite:zeta potential and X-ray photoeletron spectroscopy. J. Mater. Sci. Mater. Med. 2002, 13, 1123–1127. [Google Scholar] [CrossRef]
- Arcos, D.; Sanchez-Salcedo, S.; Izquierdo-Barba, I.; Ruiz, L.; Gonzalez-Calbet, J.; Vallet-Regi, M. Crystallochemistry, textural properties, and in vitro biocompatability of diffferent silicon-doped calcium phoshates. J. Biomed. Mater. Res. 2006, 78, 762–771. [Google Scholar] [CrossRef]
- Long, J.D.; Xu, S.; Cai, J.W.; Jiang, N.; Lu, J.H.; Ostrikov, K.N.; Diong, C.H. Structure, bonding state and in-vitro study of Ca-P-Ti film deposited on Ti6Al4V by RF magnetron sputtering. Mater. Sci. Eng. C 2002, 20, 175. [Google Scholar] [CrossRef]
- Van Raemdonck, W.; Ducheyne, P.; De Meester, P. Metal and Ceramic Biomaterials; CRC Press: Boca Raton, FL, USA, 1984; pp. 143–146. [Google Scholar]
- Lo, W.J.; Grant, D.M. Hydroxyapatite thin films deposited onto uncoated and (Ti,Al,V)N-coated Ti alloys. J. Biomed. Mater. Res. 1999, 46, 408–417. [Google Scholar] [CrossRef]
- Boyd, A.R.; Burke, G.A.; Duffy, H.; Cairns, M.L.; O′Hare, P.; Meenan, B.J. Characterisation of calcium phosphate/titanium dioxide hybrid coatings. J. Mater. Sci. Mater. Med. 2007, 19, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Astala, R.; Calderin, L.; Yin, X.; Stott, M.J. Ab initio simulation of Si-doped hydroxyapatite. Chem. Mater. 2006, 18, 413–422. [Google Scholar] [CrossRef]
- Pan, Y.; Fleet, M.E. Substitution Mechanisms and Controlling Factors. In Reviews in Mineralogy and Geochemistry -Phosphates -Geochemical, Geobiological and Materials Importance; Kohn, M.J., Hughes, J.M., Eds.; American Chemical Society: Washington, DC, USA, 2002; p. 742. [Google Scholar]
- Vandiver, J.; Dean, D.D.; Patel, N.; Botelho, C.M.; Best, S.M.; Santos, J.D.; Lopes, M.A.; Bonfield, W.; Ortiz, C. Silicon addition to hydroxyapatite increases nanoscale electrostatic, van der Waals and adhesive interactions. J. Biomed. Mater. Res. 2006, 78, 352–363. [Google Scholar] [CrossRef]
- Takeda, S.; Fukawa, M. Role of surface OH groups in the surface chemical properties of metal oxide films. Mater. Sci. Eng. B 2005, 119, 265–267. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Courtney, H.S.; Bettenga, M.; Argrawal, C.M.; Bumgardner, J.D.; Ong, J.L. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 2006, 27, 5512–5517. [Google Scholar] [CrossRef]
- Pietak, A.M.; Reid, J.W.; Stott, M.J.; Sayer, M. Silicon substitution in the calcium phosphate bioceramics. Biomaterials 2007, 28, 4023–4032. [Google Scholar] [CrossRef]
- Gao, J.; Wang, M.; Shi, C.; Wang, L.; Wang, D.; Zhu, Y. Synthesis of trace element Si and Sr codoping hydroxyapatite with non-cytotoxicity and enhanced cell proliferation and differentiation. Biol. Trace Elem. Res. 2016, 174, 208–217. [Google Scholar] [CrossRef]
- Reffitt, D.M.; Ogston, N.; Jugdaohsingh, R.; Cheung, H.F.J.; Evans, B.A.J.; Thompson, R.P.H.; Powell, J.J.; Hampson, G.N. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast like cells in vitro. Bone 2003, 32, 127–135. [Google Scholar] [CrossRef]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.K.; Hench, L.; Polak, J.M. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass 45S5 dissolution. J. Mater. Res. 2001, 55, 151–157. [Google Scholar] [CrossRef]
- Burling, L. Novel Phosphate Glasses for Bone Regeneration Applications; The University of Nottingham: Nottingham, UK, 2005. [Google Scholar]
- Knabe, C.; Klar, F.; Fitzner, R.; Radlanski, R.J.; Gross, U. In vitro investigation of titanium and hydroxyapatite dental implant surfaces using a rat bone marrow stromal cell culture system. Biomaterials 2002, 23, 3235–3245. [Google Scholar] [CrossRef]
- Stupack, D.G.; Cheresh, D.A. Get a ligand, get a life: Integrins, signalling and cell survival. J. Cell Sci. 2002, 115, 3729–3738. [Google Scholar] [CrossRef] [PubMed]
- Miranti, C.K.; Brugge, J.S. Sensing the environment: A historical perspective on integrin signal transduction. Nat. Cell Biol. 2002, 4, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Bigerelle, M.; Noel, B.; Iost, A.; Hardouin, P. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J. Biomed. Mater. Res. 2000, 49, 155–166. [Google Scholar] [CrossRef]
- Porter, A.E.; Patel, N.; Skepper, J.N.; Best, S.M.; Bonfield, W. Comparison of in vivo dissolution processes in hydroxyapatite and silicon-substituted hydroxyapatite bioceramics. Biomaterials 2003, 24, 4609. [Google Scholar] [CrossRef]
- Thian, E.S.; Huang, J.; Best, S.M.; Barber, Z.H.; Bonfield, W. Magnetron co-sputtered silicon-containing hydroxyapatite thin films--an in vitro study. Biomaterials 2005, 26, 2947–2956. [Google Scholar] [CrossRef]
- Thian, E.S.; Huang, J.; Best, S.M.; Bonfield, W. Fabrication of hydroxyapatite ceramics via a modified slip casting route. Key Eng. Mater. 2004, 254, 111–114. [Google Scholar] [CrossRef]
- Hing, K.A.; Revell, P.A.; Smith, N.; Buckland, T. Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials 2006, 27, 5014. [Google Scholar] [CrossRef]
- Porter, A.E.; Botelho, C.M.; Lopes, M.A.; Santos, J.D.; Best, S.M.; Bonfield, W. Ultrastructural comparison of dissolution and apatite precipitation on hydroxyapatite and silicon-substituted hydroxyaptite in vitro and in vivo. J. Biomed. Mater. Res. 2004, 69, 670–679. [Google Scholar] [CrossRef]
- Lo, W.J.; Grant, D.M.; Ball, M.D.; Welsh, B.S.; Howdle, S.M.; Antonov, E.N.; Bagratashvili, V.N.; Popov, W.K. Physical, chemical, and biological characterization of pulsed laser deposited and plasma sputtered hydroxyapatite thin films on titanium alloy. J. Biomed. Mater. Res. 2000, 50, 536–545. [Google Scholar] [CrossRef]
- Biomaterials Science: An Introduction to Materials in Medicine; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Scotchford, C.A.; Ball, M.; Winkelmann, M.; Voros, J.; Csucs, C.; Brunette, D.M.; Danuser, G.; Textor, M. Chemically patterned, metal-oxide-based surfaces produced by photolithographic techniques for studying protein- and cell-interactions. II: Protein adsorption and early cell interactions. Biomaterials 2003, 24, 1147. [Google Scholar] [CrossRef]
- Dalby, M.J.; Pasqui, D.; Affrossman, S. Cell response to nano-islands produced by polymer demixing: A breif review. IEE Proc. Nanobiotechnol. 2004, 151, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.; Wilkinson, C. Nanotechniques and approaches in biotechnology. Trends Biotechnol. 2001, 19, 97–101. [Google Scholar] [CrossRef]
- Kahn, S.P.; Auner, G.G.; Newaz, G.M. Influence of nanoscale surface roughness on neural cell attachement on silicon. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 125–129. [Google Scholar] [CrossRef]
- Ward, B.C.; Webster, T.J. The effect of nanotopography on calcium and phosphorus deposition on metallic materials in vitro. Biomaterials 2006, 27, 3064–3074. [Google Scholar] [CrossRef]
- Dalby, M.J.; Giannaras, D.; Riehle, M.O.; Gadegaard, N.; Affrossman, S.; Curtis, A.S.G. Rapid fibroblast adhesion to 27 nm high polymer demixing nano-topography. Biomaterials 2004, 25, 77–83. [Google Scholar] [CrossRef]
- Dalby, M.J.; Childs, S.; Riehle, M.O.; Johnstone, H.J.H.; Affrossman, S.; Curtis, A.S.G. Fibroblast reaction to island topography:changes in cytoskeleton and morphology with time. Biomaterials 2003, 24, 927–935. [Google Scholar] [CrossRef]
- Ong, J.L.; Prince, C.W.; Lucas, L.C. Cellular reposonse to well-characterized calcium phosphate coatings and titanium surfaces in vitro. J. Biomed. Mater. Res. 1995, 29, 165–172. [Google Scholar] [CrossRef]
- Wang, C.; Duan, Y.; Markovic, B.; Barbara, J.; Howlett, C.R.; Zhang, X.; Zreiquat, H. Phenotypic expression of bone-related genes in osteoblasts grown on calcium phosphate ceramics with different pase compositions. Biomaterials 2004, 25, 2507–2514. [Google Scholar] [CrossRef]







| Sample | Silicon Target Density/Wcm−2 | Ca/P Ratio (EDX) | Ca/P Ratio (XPS) | Silicon Content/wt.% |
|---|---|---|---|---|
| Stoichiometric HA | N/A | 1.67 | 1.67 | N/A |
| Plasma Sprayed HA Target | N/A | 1.90 ± 0.02 | N/A | 0.0 ± 0.1 |
| HA | N/A | 1.76 ± 0.03 | 1.43 ± 0.03 | 0.0 ± 0.1 |
| SiHA1 | 6.6 × 10−4 | 1.74 ± 0.03 | 1.23 ± 0.05 | 1.8 ± 0.3 |
| SiHA2 | 1.6×10−3 | 1.79 ± 0.08 | 1.16 ± 0.06 | 4.2 ± 0.7 |
| SiHA3 | 3.3×10−3 | 1.68 ± 0.09 | 1.03 ± 0.13 | 13.4 ± 1.4 |
| Sample | Heat Treatment Temperature/°C | |
|---|---|---|
| 600 | 700 | |
| HA | 78 ± 16 | 89 ± 16 |
| SiHA1 | 65 ± 14 | 71 ± 14 |
| SiHA2 | 71 ± 23 | 86 ± 31 |
| SiHA3 | N/A | N/A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coe, S.C.; Wadge, M.D.; Felfel, R.M.; Ahmed, I.; Walker, G.S.; Scotchford, C.A.; Grant, D.M. Production of High Silicon-Doped Hydroxyapatite Thin Film Coatings via Magnetron Sputtering: Deposition, Characterisation, and In Vitro Biocompatibility. Coatings 2020, 10, 190. https://doi.org/10.3390/coatings10020190
Coe SC, Wadge MD, Felfel RM, Ahmed I, Walker GS, Scotchford CA, Grant DM. Production of High Silicon-Doped Hydroxyapatite Thin Film Coatings via Magnetron Sputtering: Deposition, Characterisation, and In Vitro Biocompatibility. Coatings. 2020; 10(2):190. https://doi.org/10.3390/coatings10020190
Chicago/Turabian StyleCoe, Samuel C., Matthew D. Wadge, Reda M. Felfel, Ifty Ahmed, Gavin S. Walker, Colin A. Scotchford, and David M. Grant. 2020. "Production of High Silicon-Doped Hydroxyapatite Thin Film Coatings via Magnetron Sputtering: Deposition, Characterisation, and In Vitro Biocompatibility" Coatings 10, no. 2: 190. https://doi.org/10.3390/coatings10020190
APA StyleCoe, S. C., Wadge, M. D., Felfel, R. M., Ahmed, I., Walker, G. S., Scotchford, C. A., & Grant, D. M. (2020). Production of High Silicon-Doped Hydroxyapatite Thin Film Coatings via Magnetron Sputtering: Deposition, Characterisation, and In Vitro Biocompatibility. Coatings, 10(2), 190. https://doi.org/10.3390/coatings10020190