The Pulsed Electron Deposition Technique for Biomedical Applications: A Review
Abstract
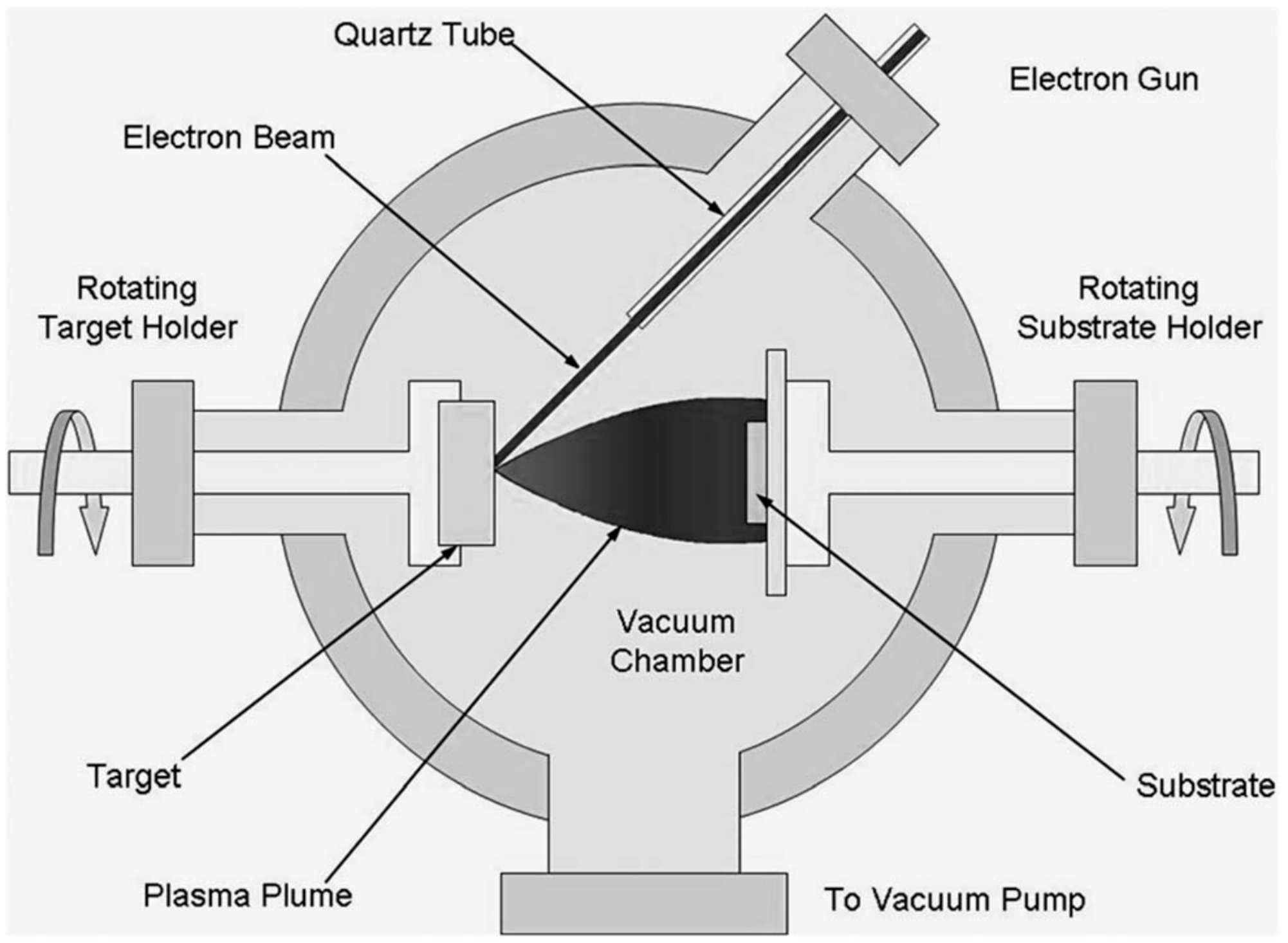
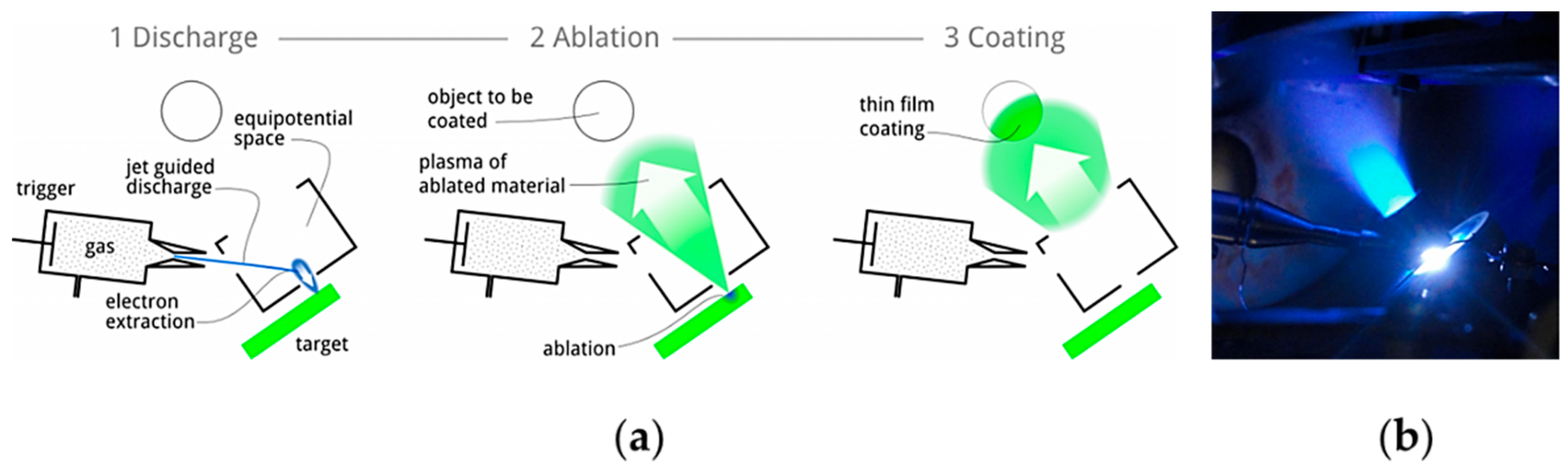
1. Introduction
2. Scope and Methodology of the Review
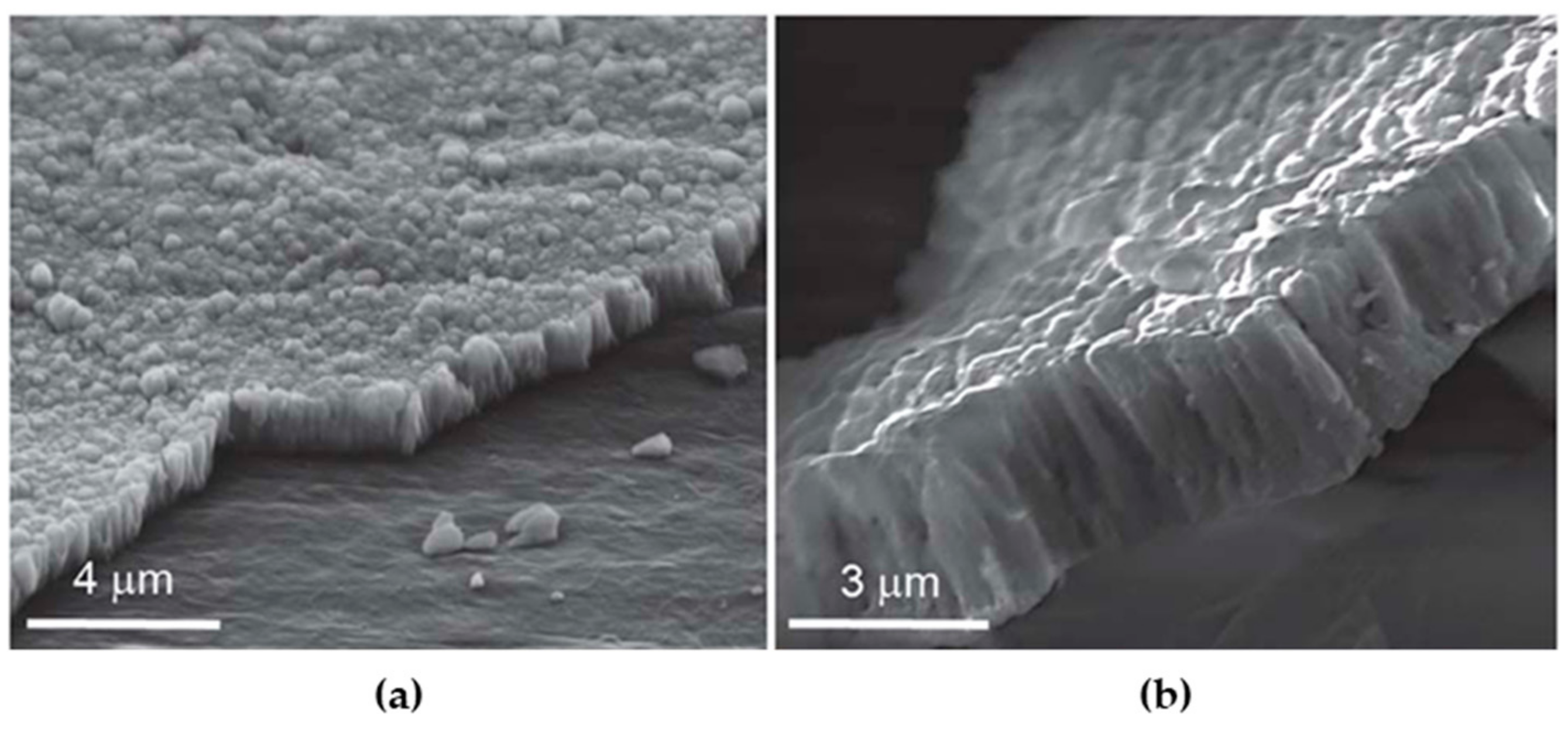
3. Hard Ceramic Coatings for Articulating Surfaces in Joint Arthroplasty
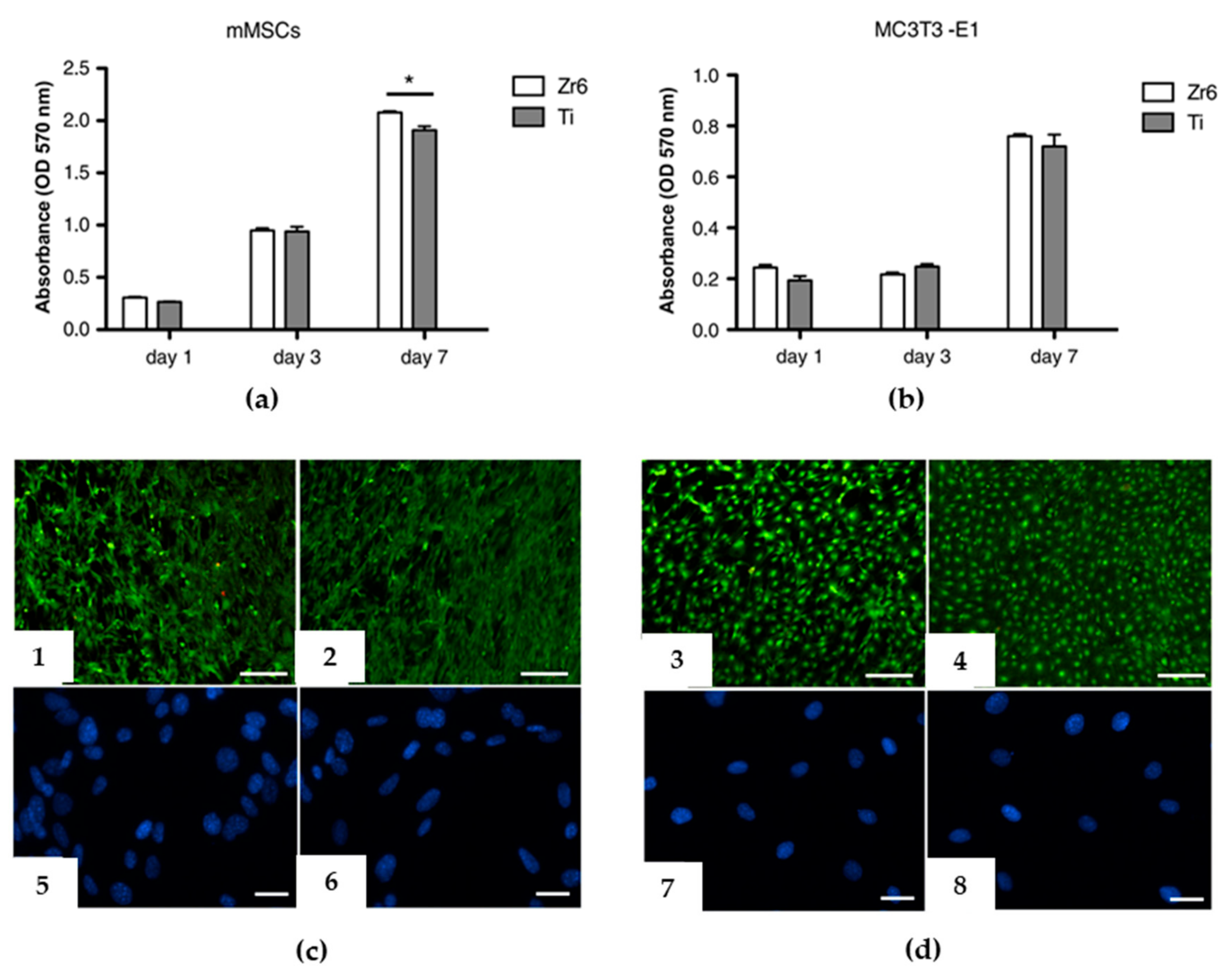
4. Bioactive Coatings and Thin Films by PED
5. Antibacterial Thin Films by PED
6. Summary and Future Perspectives
7. Conclusions
- The possibility to vary the depositions of a wide range of materials in a broad spectrum of operating independent conditions;
- The possibility to efficiently operate at low temperatures thanks to the high plasma density;
- The high fidelity in stoichiometry transfer from the target to the coating;
- The technological maturity and the competitive upscaling costs compared to similar techniques, such as PLD.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wan, R.; Yang, M.; Zhou, Q.; Zhang, Q. Transparent conductive indium zinc oxide films prepared by pulsed plasma deposition. J. Vac. Sci. Technol. A 2012, 30, 61508. [Google Scholar] [CrossRef]
- Arisi, E.; Bergenti, I.; Cavallini, M.; Murgia, M.; Riminucci, A.; Ruani, G.; Dediu, V. Room temperature deposition of magnetite thin films on organic substrate. J. Magn. Magn. Mater. 2007, 316, 410–412. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Q.; Li, G. Transparent conductive tungsten-doped tin oxide polycrystalline films prepared on quartz substrates. Semicond. Sci. Technol. 2009, 24, 15003. [Google Scholar] [CrossRef]
- Huang, Y.W.; Li, G.F.; Feng, J.H.; Zhang, Q. Investigation on structural, electrical and optical properties of tungsten doped tin oxide thin films. Thin Solid Films 2010, 518, 1892–1896. [Google Scholar] [CrossRef]
- Yao, Q.J.; Li, S.X.; Zhang, Q. Influences of channel metallic composition on indium zinc oxide thin-film transistor performance. Semicond. Sci. Technol. 2011, 26, 85011. [Google Scholar] [CrossRef]
- Huang, Y.W.; Zhang, Q.; Xi, J.H.; Ji, Z.G. Transparent conductive p-type lithium-doped nickel oxide thin films deposited by pulsed plasma deposition. Appl. Surf. Sci. 2012, 258, 7435–7439. [Google Scholar] [CrossRef]
- Yang, M.; Pu, H.F.; Zhou, Q.F.; Zhang, Q. Transparent p-type conducting K-doped NiO films deposited by pulsed plasma deposition. Thin Solid Films 2012, 520, 5884–5888. [Google Scholar] [CrossRef]
- Witke, T.; Lenk, A.; Siemroth, P. Channel spark discharges for thin film technology. In Proceedings of the 17th International Symposium on Discharges and Electrical Insulation in Vacuum, Berkeley, CA, USA, 21–26 July 1996. [Google Scholar]
- Witke, T.; Ziegele, H. Plasma state in pulsed arc, laser and electron deposition. Surf. Coat. Technol. 1997, 97, 414–419. [Google Scholar] [CrossRef]
- Dediu, V.I.; Jiang, J.D.; Matacotta, F.C.; Scardi, P.; Lazzarino, M.; Nieva, G.; Civale, L. Deposition of MBa2Cu3O7-x thin films by channel-spark method. Supercond. Sci. Technol. 1995, 8, 160–164. [Google Scholar] [CrossRef]
- Henda, R.; Wilson, G.; Gray-Munro, J.; Alshekhli, O.; McDonald, A.M. Preparation of polytetrafluoroethylene by pulsed electron ablation: Deposition and wettability aspects. Thin Solid Films 2012, 520, 1885–1889. [Google Scholar] [CrossRef]
- Taliani, C.; Nozar, P. Method for Depositing Metal Oxide Films. U.S. Patent No. 12/809,326, 3 March 2011. [Google Scholar]
- Lotti, R.; Nozar, P.; Taliani, C. Device for Generating Plasma and for Directing a Flow of Electrons towards a Target. U.S. Patent No. 8803425B2, 12 August 2014. [Google Scholar]
- Bianchi, M.; Russo, A.; Taliani, C.; Marcacci, M. Pulsed Plasma Deposition (PPD) Technique, in Dekker Encyclopedia of Nanoscience and Nanotechnology, 3rd ed.; Taylor and Francis: New York, NY, USA, 2015; pp. 1–7. [Google Scholar]
- Bianchi, M.; Russo, A.; Lopomo, N.; Boi, M.; Maltarello, M.C.; Sprio, S.; Baracchi, M.; Marcacci, M. Pulsed plasma deposition of zirconia thin films on UHMWPE: Proof of concept of a novel approach for joint prosthetic implants. J. Mater. Chem. B 2013, 1, 310–318. [Google Scholar] [CrossRef]
- Harshavardhan, K.S.; Strikovski, M. Second-Generation HTS Conductors; Goyal, A., Ed.; Springer: New York, NY, USA, 2005; pp. 109–133. [Google Scholar]
- Henda, R.; Al-Shareeda, O.; McDonald, A.; Pratt, A. Deposition of iron pyrite via pulsed electron ablation. Appl. Phys. A 2012, 108, 967–974. [Google Scholar] [CrossRef]
- Strikovski, M.; Harshavardhan, K.S. Parameters that control pulsed electron beam ablation of materials and film deposition processes. Appl. Phys. Lett. 2003, 82, 853–855. [Google Scholar] [CrossRef]
- Chandra, V.; Manoharan, S.S. Pulsed electron beam deposition of highly oriented thin films of polytetrafluoroethylene. Appl. Surf. Sci. 2008, 254, 4063–4066. [Google Scholar] [CrossRef]
- Mathis, J.E.; Christen, H.M. Factors that influence particle formation during pulsed electron deposition of YBCO precursors. Physica C 2007, 459, 47–51. [Google Scholar] [CrossRef]
- Christen, H.M.; Lee, D.F.; List, F.A.; Cook, S.W.; Leonard, K.J.; Heatherly, L.; Martin, P.M.; Paranthaman, P.; Goyal, A.; Rouleau, C.M. Pulsed electron deposition of fluorine-based precursors for YBa2Cu3O7-x-coated conductors. Supercond. Sci. Technol. 2005, 18, 1168–1175. [Google Scholar] [CrossRef]
- Skocdopolova, L. Device for Generating Plasma and Directing an Electron Beam towards a Target. WO Patent 2013/186697, 25 December 2013. [Google Scholar]
- Mazzer, M.; Rampino, S.; Gombia, E.; Bronzoni, M.; Bissoli, F.; Pattini, F.; Calicchio, M.; Kingma, A.; Annoni, F.; Calestani, D.; et al. Progress on low-temperature pulsed electron deposition of CuInGaSe2 solar cells. Energies 2016, 9, 207–218. [Google Scholar] [CrossRef]
- Menossi, D.; Di Mare, S.; Rimmaudo, I.; Artegiani, E.; Tedeschi, G.; Pena, J.L.; Piccinell, F.; Salavei, F.; Romeo, A. SnS by Ionized Jet Deposition for photovoltaic applications. In Proceedings of the IEEE 44th Photovoltaic Specialist Conference (PVSC), Washington, DC, USA, 25–30 June 2017. [Google Scholar]
- Skocdopole, J.; Aversa, L.; Golan, M.; Schenk, A.; Baldi, G.; Kratochvilova, I.; Kalvoda, L.; Nozar, P. Preparing of the chameleon coating by the ion jet deposition method. Acta Polytech. CTU Proc. 2017, 9, 19–25. [Google Scholar] [CrossRef]
- Skocdopole, J.; Kalvoda, L.; Nozar, P.; Netopilik, M. Preparation of polymeric coatings by ionized jet deposition method. Chem. Pap. 2018, 72, 1735–1739. [Google Scholar] [CrossRef]
- Zenker, R. Electron Beam Surface Technologies; Wang, Q.J., Chung, Y.W., Eds.; Springer: Boston, MA, USA, 2013. [Google Scholar]
- Duta, L.; Popescu, A.C. Current status on pulsed laser deposition of coatings from animal-origin calcium phosphate sources. Coatings 2019, 9, 335–376. [Google Scholar] [CrossRef]
- Shia, J.Z.; Chena, C.Z.; Yub, H.J.; Zhang, S.J. The effect of process conditions on the properties of bioactive films prepared by magnetron sputtering. Vacuum 2008, 83, 249–256. [Google Scholar] [CrossRef]
- Pelletier, J.; Anders, A. Plasma-based ion implantation and deposition: A review of physics, technology, and applications. IEEE Trans. Plasma Sci. 2005, 33, 1944–1959. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, Y.; Jin, S.; Wang, J.R.; Liu, R.; Liu, H.; Shi, W.; Kolawole, K.S.; Ren, L.; Yu, B.; et al. Antibacterial TiCu/TiCuN multilayer films with good corrosion resistance deposited by axial magnetic field-enhanced arc ion plating. ACS Appl. Mater. Interfaces 2019, 11, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Döring, J.; Crackau, M.; Nestler, C.; Welzel, F.; Bertrand, J.; Lohmann, C.H. Characteristics of different cathodic arc deposition coatings on CoCrMo for biomedical applications. J. Mech. Behav. Biomed. Mater. 2019, 97, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Sobieraj, M.C.; Rimnac, C.M. Ultra high molecular weight polyethylene: Mechanics, morphology, and clinical behavior. J. Mech. Behav. Biomed. Mater. 2009, 2, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Dumbleton, J.H.; Manley, M.T.; Edidin, A.A. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J. Arthroplast. 2002, 17, 649–661. [Google Scholar] [CrossRef]
- Ingham, E.; Fisher, J. The role of macrophages in osteolysis of total joint replacement. Biomaterials 2005, 26, 1271–1286. [Google Scholar] [CrossRef]
- Drees, P.; Eckardt, A.; Gay, R.E.; Gay, S.; Huber, L.C. Mechanisms of disease: Molecular insights into aseptic loosening of orthopedic implants. Nat. Clin. Pract. Rheumatol. 2007, 3, 165–171. [Google Scholar] [CrossRef]
- Kaufman, A.M.; Alabre, C.I.; Rubash, H.E.; Shanbhag, A.S. Human macrophage response to UHMWPE, TiAlV, CoCr, and alumina particles: analysis of multiple cytokines using protein arrays. J. Biomed. Mater. Res. Part A 2008, 84, 464–474. [Google Scholar] [CrossRef]
- Labek, G.; Thaler, M.; Janda, W.; Agreiter, M.; Stöckl, B. Revision rates after total joint replacement: Cumulative, Cumulative results from worldwide joint register datasets. J. Bone Joint Surg. Br. 2011, 93, 293–297. [Google Scholar] [CrossRef]
- Russo, A.; Bianchi, M.; Lopomo, N.; Boi, M.; Ortolani, A.; Marchiori, G.; Gambardella, A.; Maltarello, M.C.; Visani, A.; Marcacci, M. Hard ceramic thin films on UHMWPE insert: A novel approach for longlasting joint implants. Orthop. Proc. 2016, 98, 10. [Google Scholar]
- Bianchi, M.; Boi, M.; Lopomo, N. Nanomechanical characterization of zirconia thin films deposited on UHMWPE by pulsed plasma deposition. J. Mech. Med. Biol. 2015, 15, 1550070–1550085. [Google Scholar] [CrossRef]
- Marchiori, G.; Lopomo, N.; Boi, M.; Berni, M.; Bianchi, M.; Gambardella, A.; Visani, A.; Russo, A.; Marcacci, M. Optimizing thickness of ceramic coatings on plastic components for orthopedic applications: A finite element analysis. Mater. Sci. Eng. C 2016, 58, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Berni, M.; Marchiori, G.; Gambardella, A.; Boi, M.; Bianchi, M.; Russo, A.; Visani, A.; Marcacci, M.; Pavanc, P.G.; Lopomo, N.F. Effects of working gas pressure on zirconium dioxide thin film prepared by pulsed plasma deposition: Roughness, wettability, friction and wear characteristics. J. Mech. Behav. Biomed. Mater 2017, 72, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Berni, M.; Lopomo, N.; Marchiori, G.; Gambardella, A.; Boi, M.; Bianchi, M.; Visani, A.; Pavan, P.; Russo, A.; Marcacci, M. Tribological characterization of zirconia coatings deposited on Ti6Al4V components for orthopedic applications. Mater. Sci. Eng. 2016, 62, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Lopomo, N.; Boi, M.; Gambardella, A.; Marchiori, G.; Berni, M.; Pavan, P.; Marcacci, M.; Russo, A. Ceramic thin films realized by means of pulsed plasma deposition technique: Applications for orthopedics. J. Mech. Med. Biol. 2015, 15, 1540002–1540009. [Google Scholar] [CrossRef]
- Bianchi, M.; Gambardella, A.; Berni, M.; Panseri, S.; Montesi, M.; Lopomo, N.; Tampieri, A.; Marcacci, M.; Russo, A. Surface morphology, tribological properties and in vitro biocompatibility of nanostructured zirconia thin films. J. Mater. Sci. Mater. Med. 2016, 27, 95–105. [Google Scholar] [CrossRef]
- Dong, H.; Shi, W.; Bell, T. Potential of improving tribological performance of UHMWPE by engineering the Ti6Al4V counterfaces. Wear 1999, 225, 146–153. [Google Scholar] [CrossRef]
- Xiong, D.; Ge, S. Friction and wear properties of UHMWPE/Al2O3 ceramic under different lubricating conditions. Wear 2001, 250, 242–245. [Google Scholar] [CrossRef]
- Gambardella, A.; Berni, M.; Russo, A.; Bianchi, M. A comparative study of the growth dynamics of zirconia thin films deposited by ionized jet deposition onto different substrates. Surf. Coat. Technol. 2018, 337, 306–312. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Kocagoz, S.; Arnholt, C.; Huet, R.; Ueno, M.; Walter, W.L. Advances in zirconia toughened alumina biomaterials for total joint replacement. J. Mech. Behav. Biomed. Mater. 2014, 31, 107–116. [Google Scholar] [CrossRef] [PubMed]
- De Aza, A.H.; Chevalier, J.; Fantozzi, G.; Schehl, M.; Torrecillas, R. Crack growth resistance of alumina, zirconia and zirconia toughened alumina ceramics for joint prostheses. Biomaterials 2002, 23, 937–945. [Google Scholar] [CrossRef]
- Russo, A.; Lopomo, N.; Bianchi, M.; Boi, M.; Ortolani, A.; Gambardella, A.; Marchiori, G.; Maltarello, M.C.; Visani, A.; Marcacci, M. Protective zirconia-toughened-alumina films deposited on the UHMWPE insert by the pulsed plasma deposition method: Preliminary results. Orthop. Proc. 2018, 98, 10. [Google Scholar]
- Kaciulis, S.; Mezzi, A.; Bianchi, M.; Gambardella, A.; Boi, M.; Liscio, F.; Marcacci, M.; Russo, A. Ceramic coatings for orthopaedic implants: Preparation and characterization. Surf. Interface Anal. 2015, 48, 616–620. [Google Scholar] [CrossRef]
- Junker, R.; Dimakis, A.; Thoneick, M.; Jansen, J.A. Effects of implant surface coatings and composition on bone integration: A systematic review. Clin. Oral Implants Res. 2009, 20, 185–206. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 57, 49–121. [Google Scholar] [CrossRef]
- Nijhuis, A.W.; Leeuwenburgh, S.C.; Jansen, J.A. Wet-chemical deposition of functional coatings for bone implantology. Macromol. Biosci. 2010, 10, 1316–1329. [Google Scholar] [CrossRef]
- Surmenev, R.A. A review of plasma-assisted methods for calcium phosphate-based coatings fabrication. Surf. Coat. Technol. 2012, 206, 2035–2056. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis—A review. Acta Biomater. 2014, 10, 557–579. [Google Scholar] [CrossRef]
- Boi, M.; Bianchi, M.; Gambardella, A.; Liscio, F.; Kaciulis, S.; Visani, A.; Barbalinardo, M.; Valle, F.; Iafisco, M.; Lungaro, L.; et al. Tough and adhesive nanostructured calcium phosphate thin films deposited by the pulsed plasma deposition method. RSC Adv. 2015, 5, 78561–78571. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.W.; Zhang, L.N.; Hou, Z.T.; Ye, X.; Gu, H.S.; Yan, G.P.; Shang, P. Physical modification of polyetheretherketone for orthopedic implants. Front. Mater. Sci. 2014, 8, 313–324. [Google Scholar] [CrossRef]
- Toth, J.M.; Wang, M.; Estes, B.T.; Scifert, J.L.; Seim Iii, H.B.; Turner, A.S. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials 2006, 27, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F.; McNamara, A.; Turner, R.M. Potential of polyetheretherketone (PEEK) and carbon-fibre-reinforced PEEK in medical applications. J. Mater. Sci. Lett. 1987, 6, 188–190. [Google Scholar] [CrossRef]
- Zhao, M.; An, M.; Wang, Q.; Liu, X.; Lai, W.; Zhao, X.; Wei, S.; Ji, J. Quantitative proteomic analysis of human osteoblast-likeMG-63 cells in response to bioinert implant material titanium and polyetheretherketone. J. Proteome 2012, 75, 3560–3573. [Google Scholar] [CrossRef]
- Sagomonyants, K.B.; Jarman-Smith, M.L.; Devine, J.N.; Aronow, M.S.; Gronowicz, G.A. The in vitro response of human osteoblasts to polyetheretherketone (PEEK) substrates compared to commercially pure titanium. Biomaterials 2008, 29, 1563–1572. [Google Scholar] [CrossRef]
- Bianchi, M.; Degli Esposti, L.; Ballardini, A.; Liscio, F.; Berni, M.; Gambardella, A.; Leeuwenburghd, S.G.C.; Sprio, S.; Tampieri, A.; Iafisco, M. Strontium doped calcium phosphate coatings on poly(etheretherketone) (PEEK) by pulsed electron deposition. Surf. Coat. Technol. 2017, 319, 191–199. [Google Scholar] [CrossRef]
- Marie, P.J. Strontium as therapy for osteoporosis. Curr. Opin. Pharmacol. 2005, 5, 633–636. [Google Scholar] [CrossRef]
- Braceras, I.; Vera, C.; Izquierdo, A.A.; Muñoz, R.; Lorenzo, J.; Alvareza, N.; Maeztu, M.A. Ion implantation induced nanotopography on titanium and bone cell adhesion. Appl. Surf. Sci. 2014, 310, 24–30. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2000, 22, 87–96. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Bianchi, M.; Sassoni, E.; Russo, A.; Marcacci, M. Ion-substituted calcium phosphate coatings deposited by plasma-assisted techniques: A review. Mater. Sci. Eng. C 2017, 74, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Boi, M.; Bianchi, M. A review on ionic substitutions in hydroxyapatite thin films: Towards complete biomimetism. Coatings 2018, 8, 269–285. [Google Scholar] [CrossRef]
- Rau, J.V.; Cacciotti, I.; Laureti, S.; Fosca, M.; Varvaro, G.; Latini, A. Bioactive, nanostructured Si-substituted hydroxyapatite coatings on titanium prepared by pulsed laser deposition. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Gambardella, A.; Graziani, G.; Liscio, F.; Maltarello, M.C.; Boi, M.; Berni, M.; Bellucci, D.; Marchiori, G.; Valle, F.; et al. Plasma-assisted deposition of bone apatite-like thin films from natural apatite. Mater. Lett. 2017, 199, 32–36. [Google Scholar] [CrossRef]
- Graziani, G.; Berni, M.; Gambardella, A.; De Carolis, M.; Cristina Maltarello, M.; Boi, M.; Carnevale, G.; Bianchi, M. Fabrication and characterization of biomimetic hydroxyapatite thin films for bone implants by direct ablation of a biogenic source. Mater. Sci. Eng. C 2019, 99, 853–862. [Google Scholar] [CrossRef]
- Miller, L.M.; Vairavamurthy, V.; Chance, M.R.; Mendelsohn, R.; Paschalis, E.P.; Betts, F.; Boskey, A.L. In situ analysis of mineral content and crystallinity in bone using infrared micro-spectroscopy of the ν4 PO43− vibration. Biochim. Biophys. Acta 2001, 1527, 11–19. [Google Scholar] [CrossRef]
- Bianchi, M.; Pisciotta, A.; Bertoni, L.; Berni, M.; Gambardella, A.; Visani, A.; Russo, A.; de Pol, A.; Carnevale, G. Osteogenic differentiation of hDPSCs on biogenic bone apatite thin films. Stem Cells Int. 2017, 2017, 1–10. [Google Scholar]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Bellucci, D.; Bianchi, M.; Graziani, G.; Gambardella, A.; Berni, M.; Russo, A.; Cannillo, V. Pulsed electron deposition of nanostructured bioactive glass coatings for biomedical applications. Ceram. Int. 2017, 43, 15862–15867. [Google Scholar] [CrossRef]
- Vafa, H.M.; Johansson, D.; Wallen, H.; Sanchez, J. Improved ex vivo blood compatibility of central venous catheter with noble metal alloy coating. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. H 2014, 228, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, M.; Elsner, J.J. Antibiotic-eluting medical devices for various applications. J. Control. Release 2008, 130, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Alt, V. Antimicrobial coated implants in trauma and orthopaedics-A clinical review and risk-benefit analysis. Injury 2017, 48, 599–607. [Google Scholar] [CrossRef]
- Blaker, J.J.; Nazhat, S.N.; Boccaccini, A.R. Development and characterisation of silver-doped bioactive glass-coated sutures for tissue engineering and wound healing applications. Biomaterials 2004, 25, 1319–1329. [Google Scholar] [CrossRef]
- Branemark, P. Tissue-Integrated Prostheses. Osseointegration in Clinical Dentistry. In Introduction to Osseointegration; Branemark, P.I., Zarb, C., Albrektsson, T., Eds.; Quintessence Publishing Co.: Chicago, IL, USA, 1985; pp. 11–76. [Google Scholar]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef]
- Xie, C.M.; Lu, X.; Wang, K.F.; Meng, F.Z.; Jiang, O.; Zhang, H.P.; Zhi, W.; Fang, L.M. Silver nanoparticles and growth factors incorporated hydroxyapatite coatings on metallic implant surfaces for enhancement of osteoinductivity and antibacterial properties. ACS Appl. Mater. Interfaces 2014, 6, 8580–8589. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Różycka, D. Substituted hydroxyapatites with antibacterial properties. Biomed. Res. Int. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Melaiye, A.; Youngs, W.J. Silver and its application as an antimicrobial agent. Expert Opin. Ther. Pat. 2005, 15, 125–130. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, M.; Galdiero, M. Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef]
- Lansdown, A.B.G. A pharmacological and toxicological profile of Ag as an antimicrobial agent in medical devices. Adv. Phar. Sci. 2010, 2010, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pauksch, L.; Hartmann, S.; Rohnk, M.; Szalay, G.; Alt, V.; Schnettler, R.; Lips, K.S. Biocompatibility of Ag nanoparticles and Ag ions in primary human mesenchymal stem cells and osteoblasts. Acta Biomater. 2014, 10, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of Ag nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Gambardella, A.; Berni, M.; Graziani, G.; Kovtun, A.; Liscio, A.; Russo, A.; Visani, A.; Bianchi, M. Nanostructured Ag thin films deposited by pulsed electron ablation. Appl. Surf. Sci. 2019, 475, 917–925. [Google Scholar] [CrossRef]
- Li, B.; Webster, T. Orthopedic Biomaterials: Advances and Applications; Springer: Berlin, Germany, 2018. [Google Scholar]
- Ficai, A.; Grumezescu, A. Nanostructures for Antimicrobial Therapy, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Thompson, C.V. Solid-state dewetting of thin films. Annu. Rev. Mater. Res. 2012, 42, 399–434. [Google Scholar] [CrossRef]
- Jacquet, P.; Podor, R.; Ravaux, J.; Teisseire, J.; Gozhyk, I.; Jupille, J.; Lazzari, R. Grain growth: The key to understand solid-state dewetting of silver thin films. Scripta Mater. 2016, 115, 128–132. [Google Scholar] [CrossRef]
- Leroy, F.; Borowik, L.; Cheynis, F.; Almadori, Y.; Curiotto, S.; Trautmann, M.; Barbè, J.C.; Muller, P. How to control solid state dewetting: A short review. Surf. Sci. Rep. 2016, 71, 391–409. [Google Scholar] [CrossRef]
- Berni, M.; Carrano, I.; Kovtun, A.; Russo, A.; Visani, A.; Dionigi, C.; Liscio, A.; Valle, F.; Gambardella, F. Monitoring morphological and chemical properties during silver solid-state dewetting. Appl. Surf. Sci. 2019, 498, 1–8. [Google Scholar] [CrossRef]
- Taylor, E.N.; Webster, T.J. The use of superparamagnetic nanoparticles for prosthetic biofilm prevention. Int. J. Nanomed. 2009, 4, 145–152. [Google Scholar]
- Gambardella, A.; Bianchi, M.; Kaciulis, S.; Mezzi, A.; Brucale, M.; Cavallini, M.; Herrmannsdoerfer, T.; Chanda, G.; Uhlarz, M.; Cellini, A.; et al. Magnetic hydroxyapatite coatings as a new tool in medicine: A scanning probe investigation. Mater. Sci. Eng. C 2016, 62, 444–449. [Google Scholar] [CrossRef]
- Amberg, M.; Grieder, K.; Barbadoro, P.; Heuberger, M.; Hegemann, D. Electromechanical behavior of nanoscale silver coatings on PET fibers. Plasma Process. Polym. 2008, 5, 874–880. [Google Scholar] [CrossRef]
- Atwa, Y.; Maheshwari, N.; Goldthorpe, I.A. Silver nanowire coated threads for electrically conductive textiles. J. Mater. Chem. C 2015, 3, 3908–3912. [Google Scholar] [CrossRef]
- Ortolani, A.; Bianchi, M.; Mosca, M.; Caravelli, S.; Fuiano, M.; Marcacci, M.; Russo, A. The prospective opportunities offered by magnetic scaffolds for bone tissue engineering: A review. Joints 2016, 4, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Vandrovcova, M.; Douglasb, T.E.L.; Mróz, W.; Musial, O.; Schaubroeck, D.; Budner, B.; Syroka, R.; Dubruel, P.; Bacakova, L. Pulsed laser deposition of magnesium-doped calcium phosphate coatings on porous polycaprolactone scaffolds produced by rapid prototyping. Mater. Lett. 2015, 148, 178–183. [Google Scholar] [CrossRef]
- Carli, S.; Bianchi, M.; Zucchini, E.; Di Lauro, M.; Prato, M.; Murgia, M.; Fadiga, L.; Biscarini, F. Electrodeposited PEDOT: Nafion composite for neural recording and stimulation. Adv. Healthc. Mater. 2019, 8, 1900765–1900775. [Google Scholar] [CrossRef]
- Di Lauro, M.; Benaglia, S.; Berto, M.; Bortolotti, C.A.; Zoli, M.; Biscarini, F. Exploiting interfacial phenomena in organic bioelectronics: Conformable devices for bidirectional communication with living systems. Colloids Surf. B Biointerfaces 2018, 168, 143–147. [Google Scholar] [CrossRef]
| Hard Coatings for Articulating Surfaces | |||
| Target | Substrate | Coating’s Main Features | References |
| ZrO2 stabilized with Y2O3 | UHMWPE | Fully cubic crystalline phase; excellent adhesion to the plastic substrate; high strength to normal plastic deformation. | [31,39,40] |
| ZrO2 stabilized with Y2O3 | AISI 316-L | Low roughness and high thickness; reduction of the wear rate of polymeric counterpart; steady friction coefficient during the tribological test. | [42] |
| ZrO2 stabilized with Y2O3 | Titanium and titanium alloys | Significant reduction of UHMWPE wear rate; prevention of the formation of abrasion scratches on the substrate; no cytotoxic effects. | [43,44,45] |
| ZrO2 stabilized with Y2O3 | Glass, silicon, titanium, PEEK | Morphology independent from the substrate in case of flat substrates; morphology resembling the one of the substrate in case of rough substrates. | [48] |
| 75% Al2O3/25% ZrO2 | AISI 420 | High adhesion to the substrate; increase of the surface roughness along with the thickness. | [51,52] |
| Bioactive Coatings and Thin Films | |||
| Target | Substrate | Coating’s Main Features | References |
| Sintered HA | Titanium alloy | Transition from amorphous to crystalline coatings by means of the thermal treatment of the deposited coating; cell adhesion and proliferation on both as-deposited and annealed coatings. | [58] |
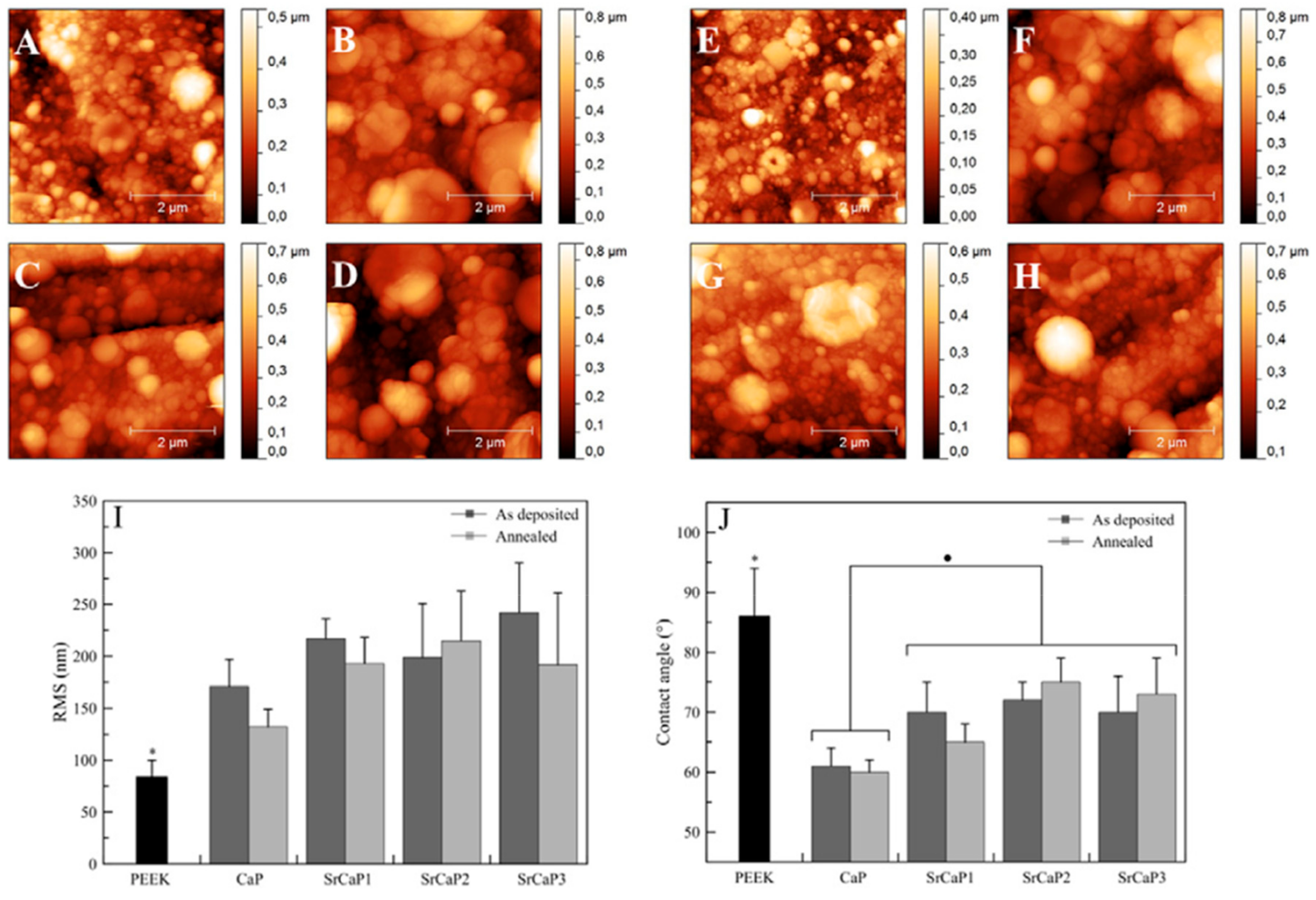
| CaP target doped with Sr2+ | PEEK | Same stoichiometry of the target; dense and uniform packing of spherical-like grains; Sr2+ in the coating increased the surface roughness and decreased the water contact angle. | [65] |
| Biogenic HA (deproteinized bovine bone shaft) | Titanium | Composition well mimicking that of the target; increase in crystallinity up to that typical of biogenic apatite through the thermal treatment of the as deposited films; high adhesion on the surface of 3D objects. | [73,74] |
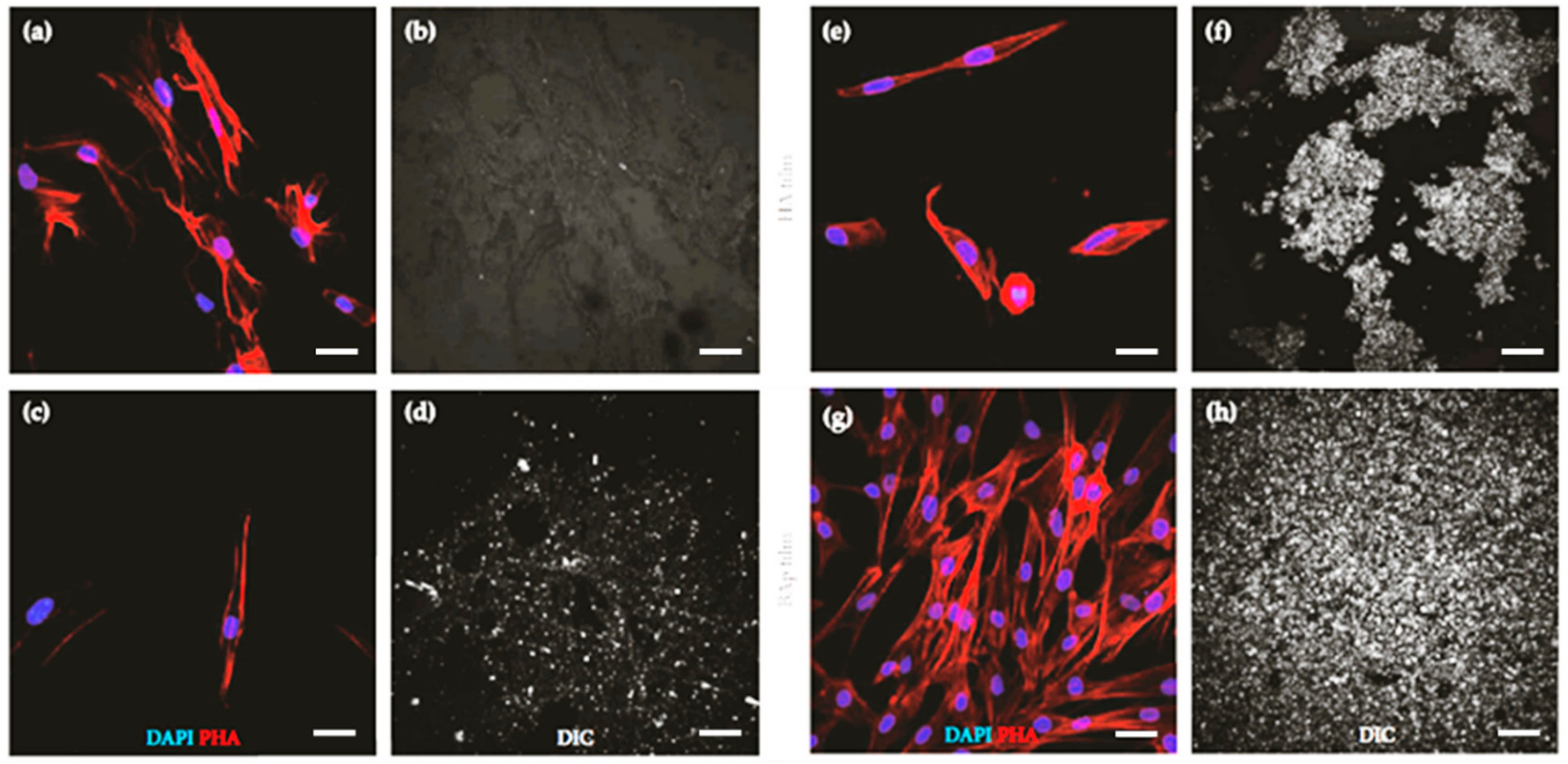
| Biogenic HA (deproteinized bovine bone shaft) | Glass | hDPSCs cultured on annealed films preserved their morphology and homogeneously proliferated through the whole surface; cells grown in an osteogenic medium on annealed thin films expressed osteogenic markers. | [76] |
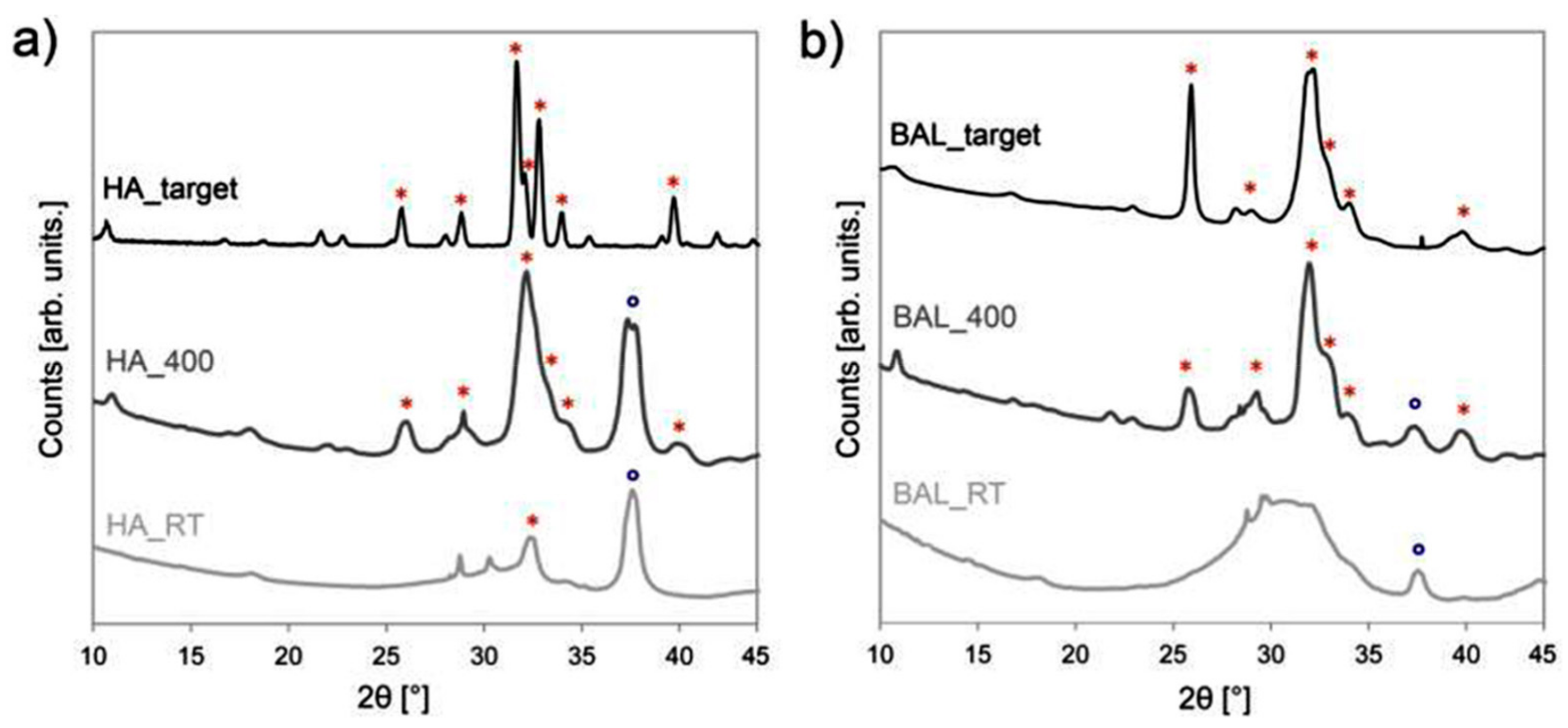
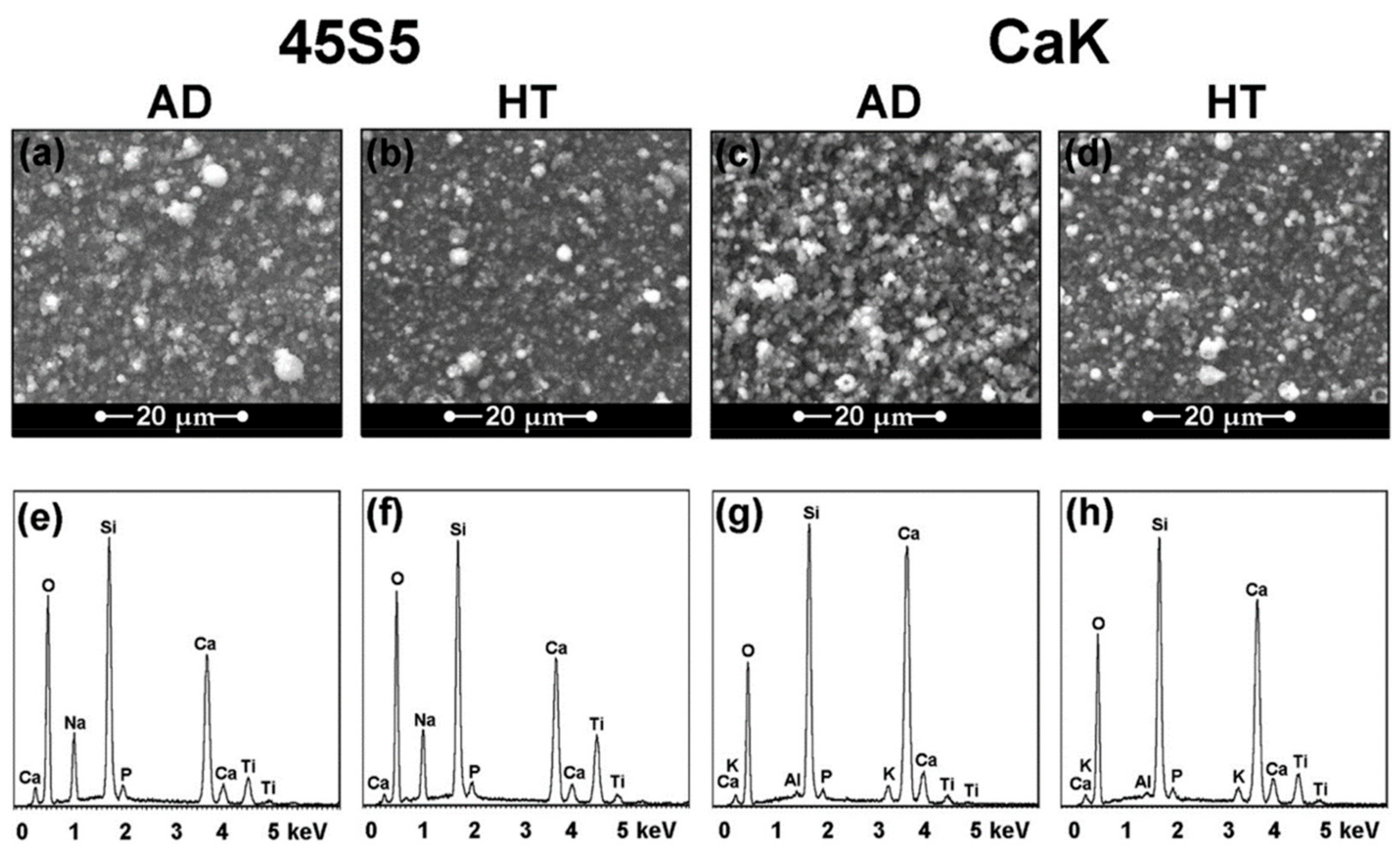
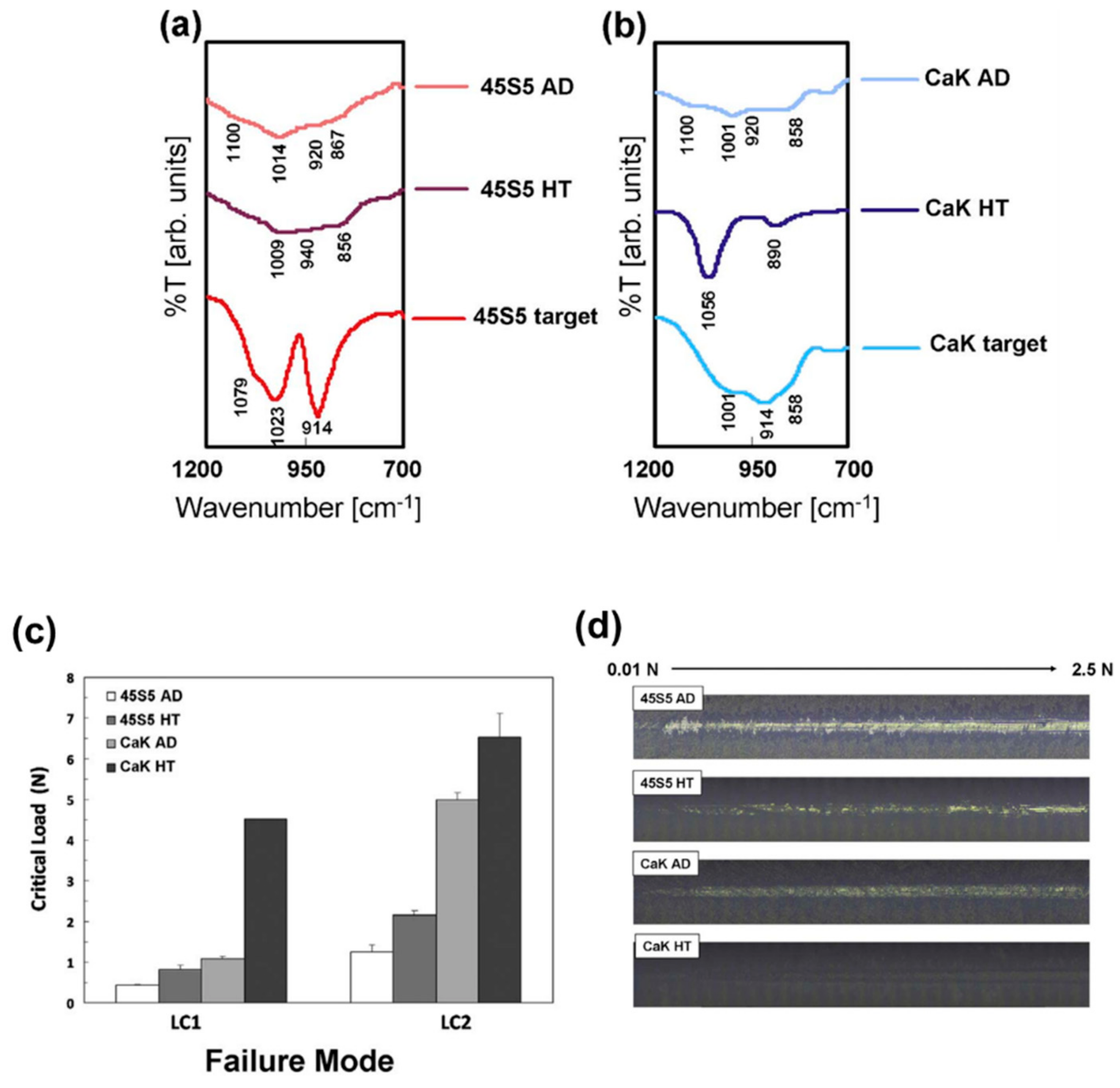
| 45S5 and CaK bioglasses | Titanium alloy | Spherical aggregates on both as-deposited and annealed coatings; chemical composition analogous to that of the starting bioactive glasses; resistance to delamination closely correlated to the crystallinity: the higher the crystallinity of the coating the higher the resistance. | [78] |
| Antibacterial Thin Films | |||
| Target | Substrate | Coating’s Main Features | References |
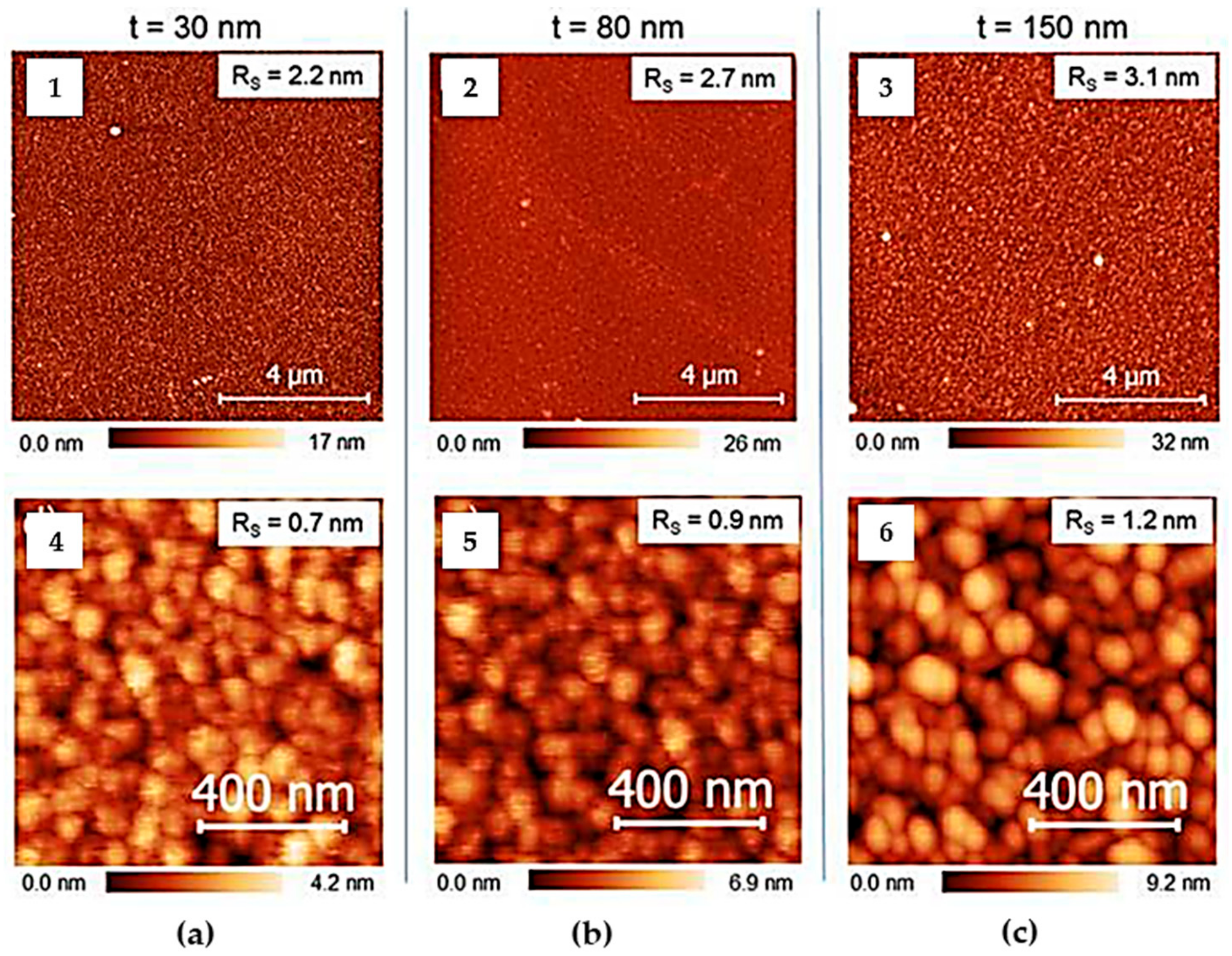
| Ag | Titanium alloy and silicon dioxide | Composition resembling the one of the target; homogeneity in thickness; presence of grains composed by small subunits with a size of few nm; no variation of the grains’ diameter with the increase of the film thickness. | [93] |
| Ag | Silicon dioxide | Formation and separation of 3D islands from the coating when subjected to a dewetting process carried out at 600 °C for 1 h. | [99] |
| HA/magnetite | Silicon wafers | Composition resembling the one of the target, despite the random and inhomogeneous distribution of the magnetic particles on the surface; the coatings hindered the E. coli adhesion. | [101] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liguori, A.; Gualandi, C.; Focarete, M.L.; Biscarini, F.; Bianchi, M. The Pulsed Electron Deposition Technique for Biomedical Applications: A Review. Coatings 2020, 10, 16. https://doi.org/10.3390/coatings10010016
Liguori A, Gualandi C, Focarete ML, Biscarini F, Bianchi M. The Pulsed Electron Deposition Technique for Biomedical Applications: A Review. Coatings. 2020; 10(1):16. https://doi.org/10.3390/coatings10010016
Chicago/Turabian StyleLiguori, Anna, Chiara Gualandi, Maria Letizia Focarete, Fabio Biscarini, and Michele Bianchi. 2020. "The Pulsed Electron Deposition Technique for Biomedical Applications: A Review" Coatings 10, no. 1: 16. https://doi.org/10.3390/coatings10010016
APA StyleLiguori, A., Gualandi, C., Focarete, M. L., Biscarini, F., & Bianchi, M. (2020). The Pulsed Electron Deposition Technique for Biomedical Applications: A Review. Coatings, 10(1), 16. https://doi.org/10.3390/coatings10010016







