Plasma Spray Coatings of Natural Ores From Structural, Mechanical, Thermal, and Dielectric Viewpoints
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
2.2.1. Phase Composition
2.2.2. Thermal Analyses
2.2.3. Microstructure
2.2.4. Porosity, Microhardness, and Roughness
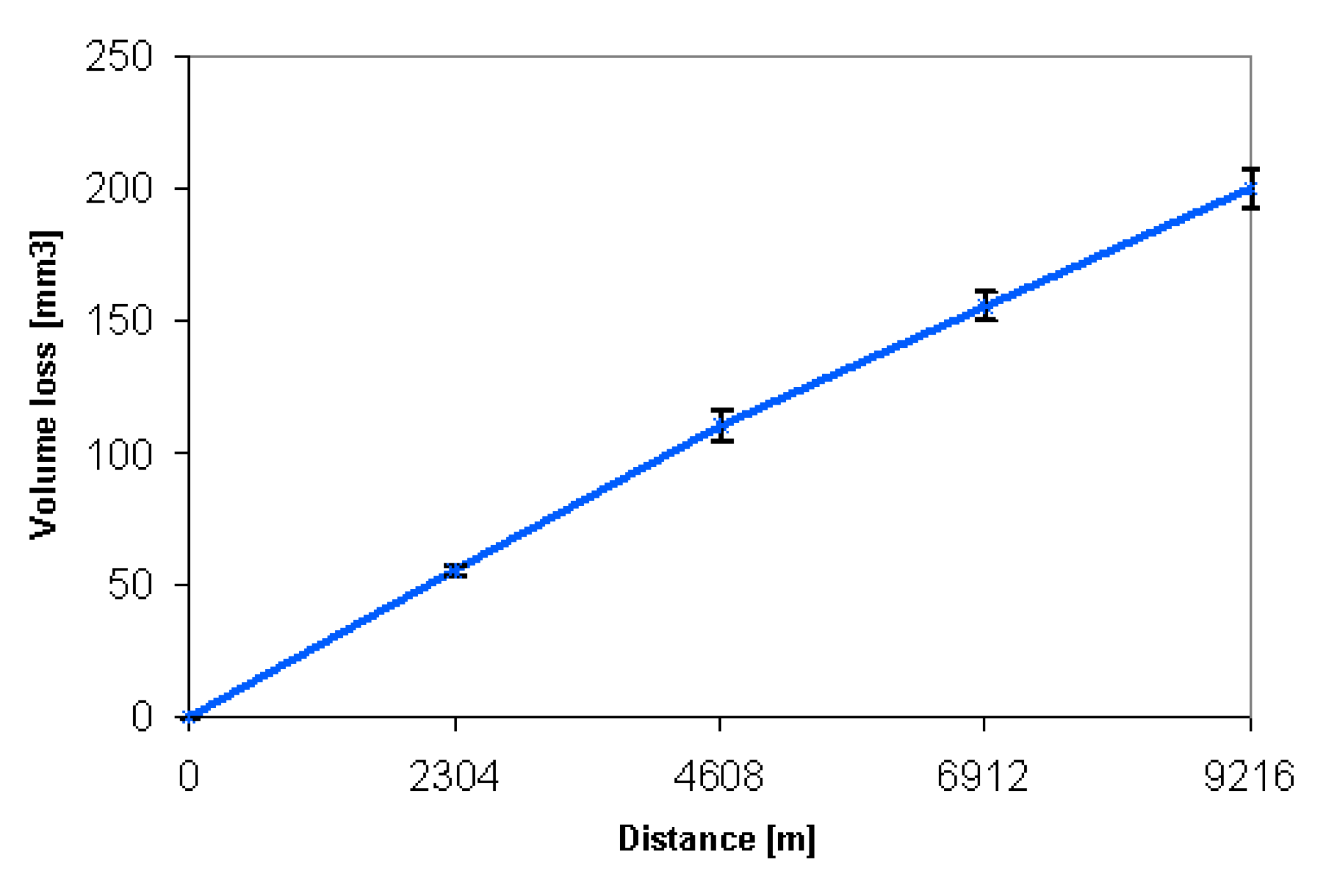
2.2.5. Wear Resistance
2.2.6. Dielectric Measurements
2.2.7. Reflectance
3. Results and Discussion
3.1. Microstructure and Porosity
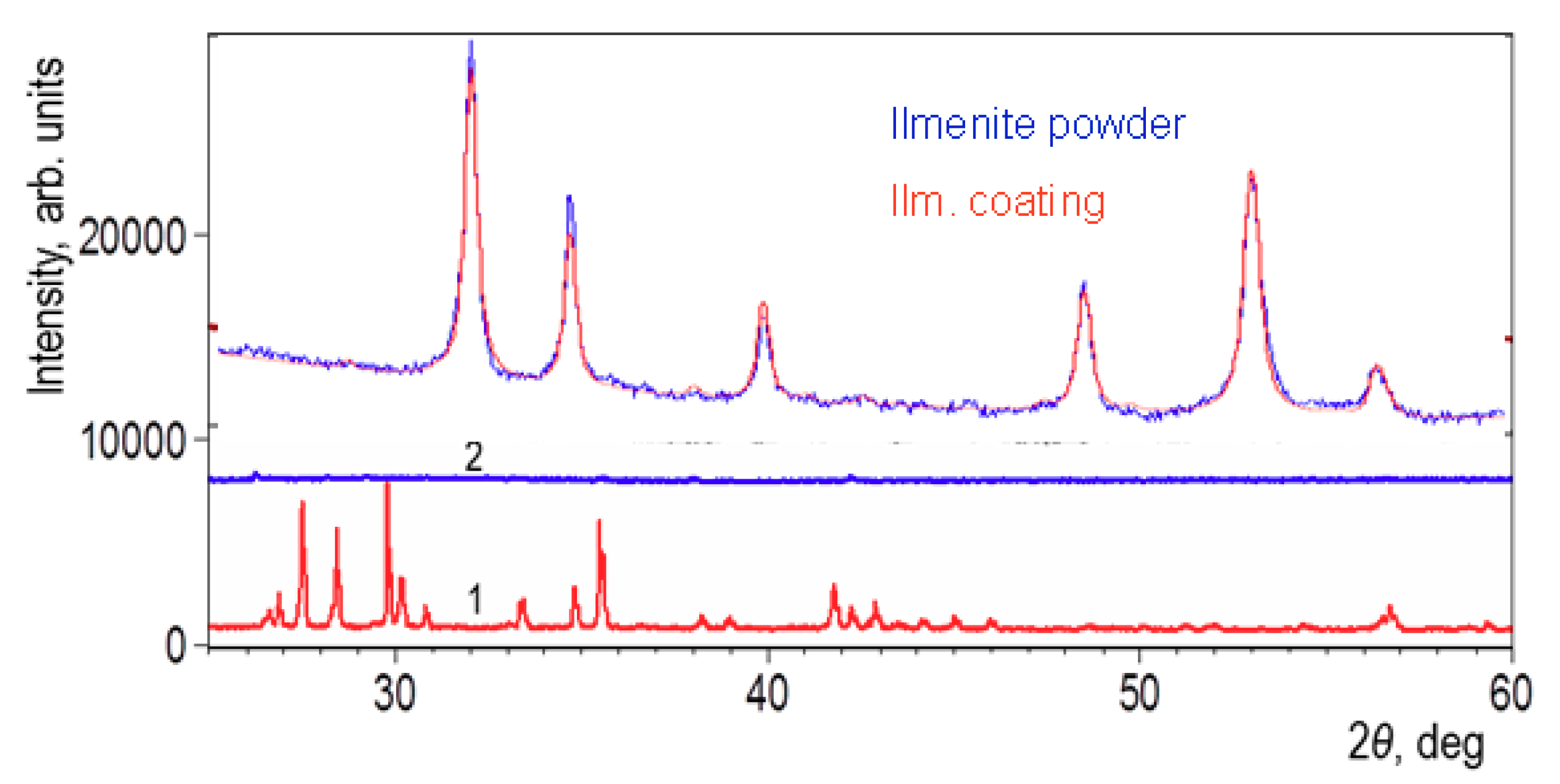
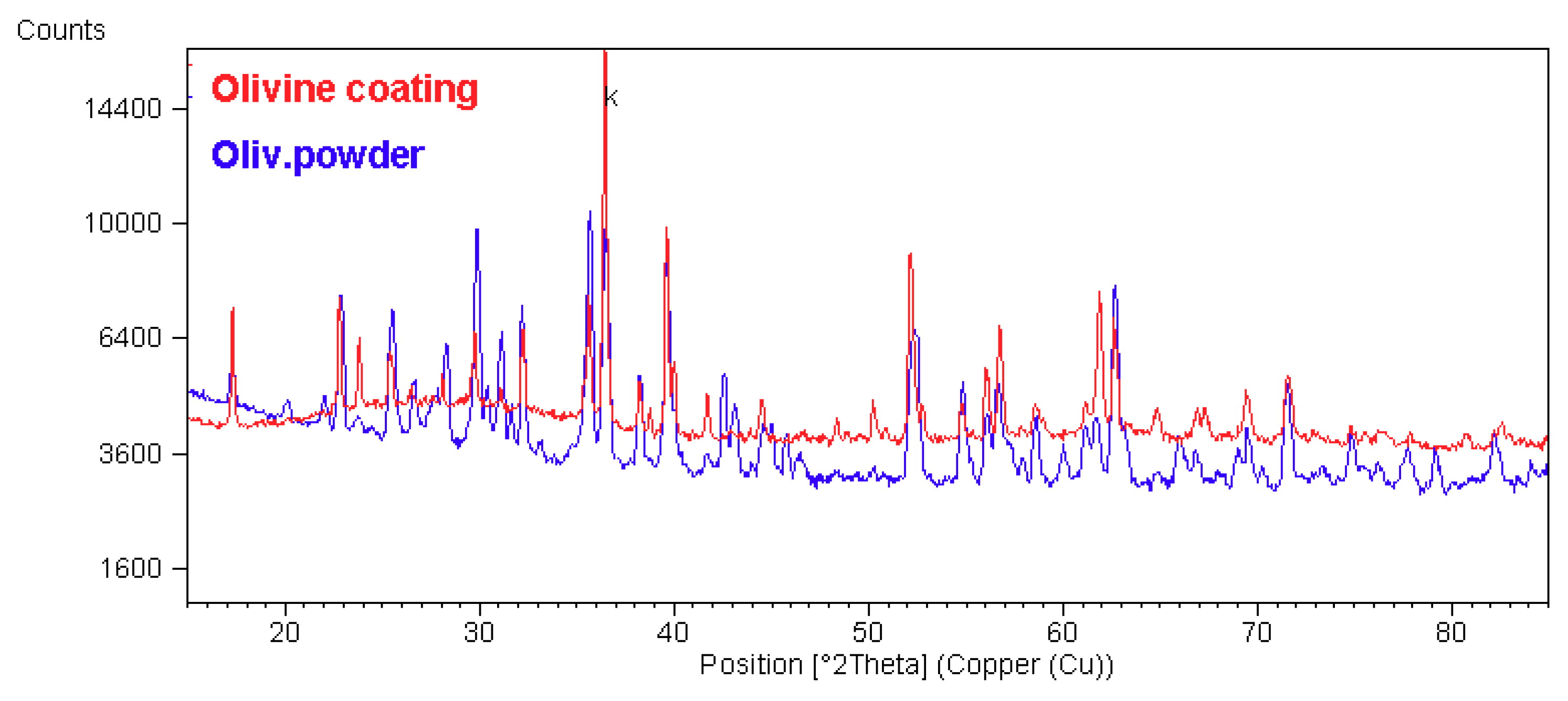
3.2. Phase Composition
3.3. Mechanical Properties
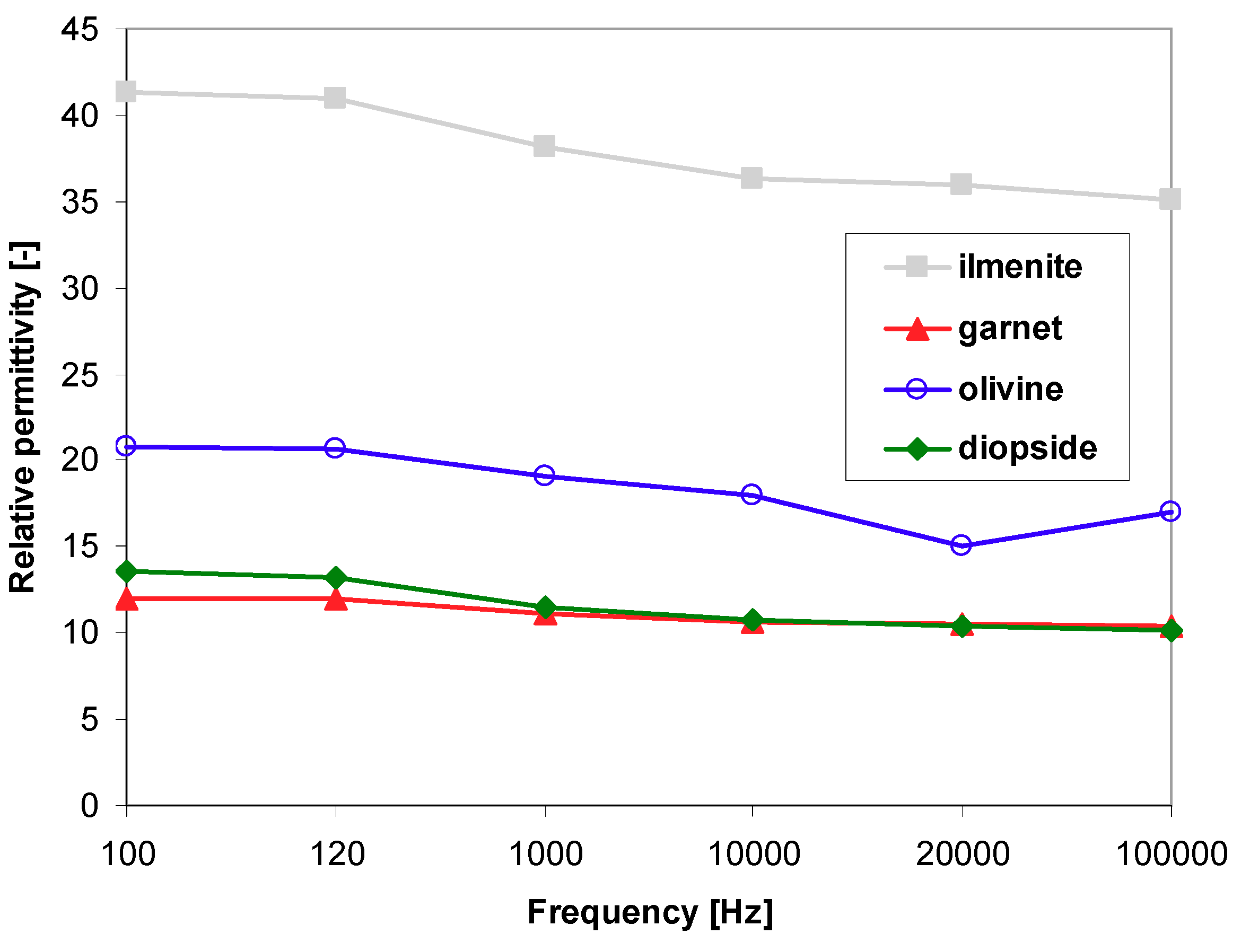
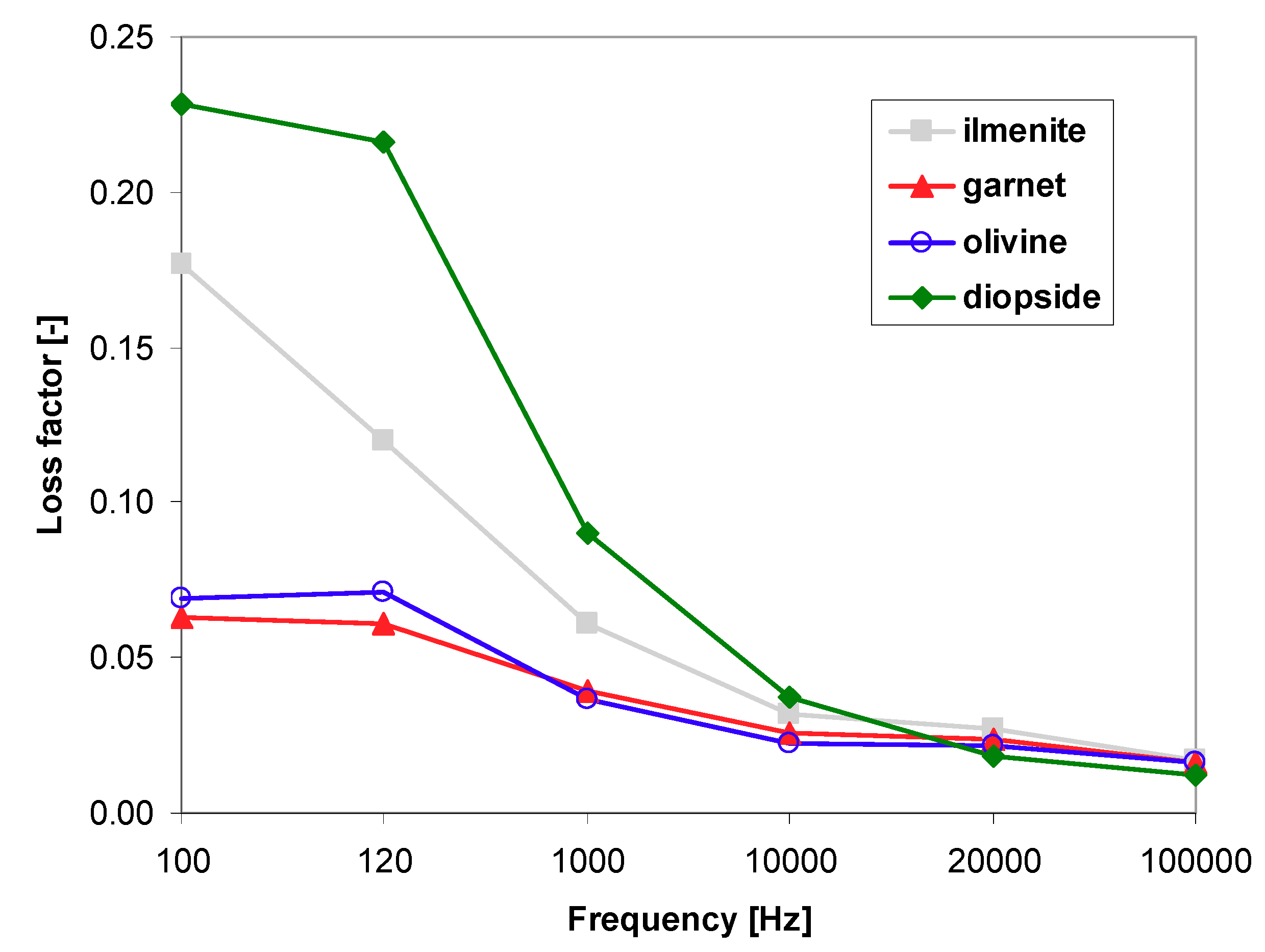
3.4. Dielectric Properties
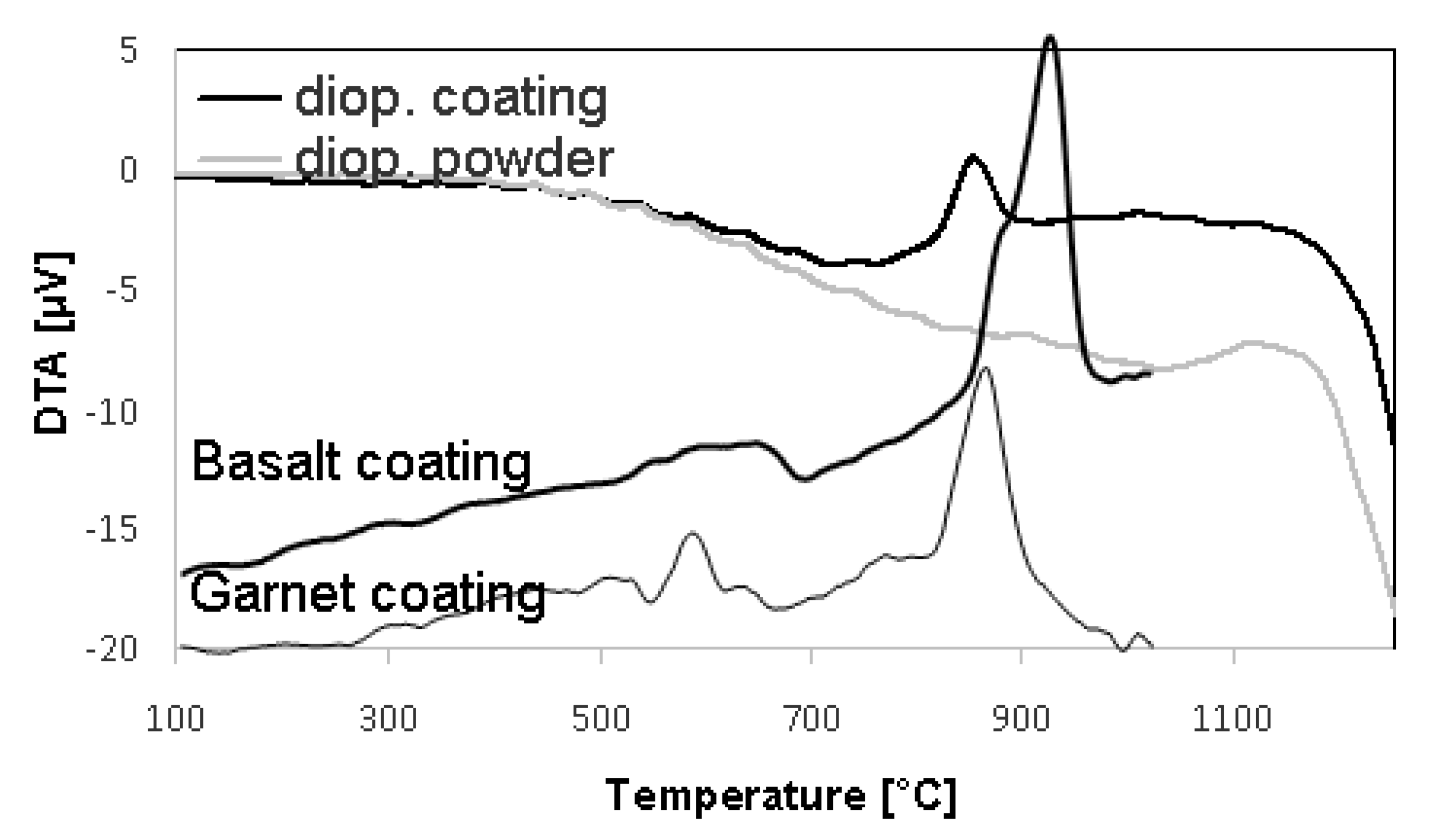
3.5. Thermal Properties
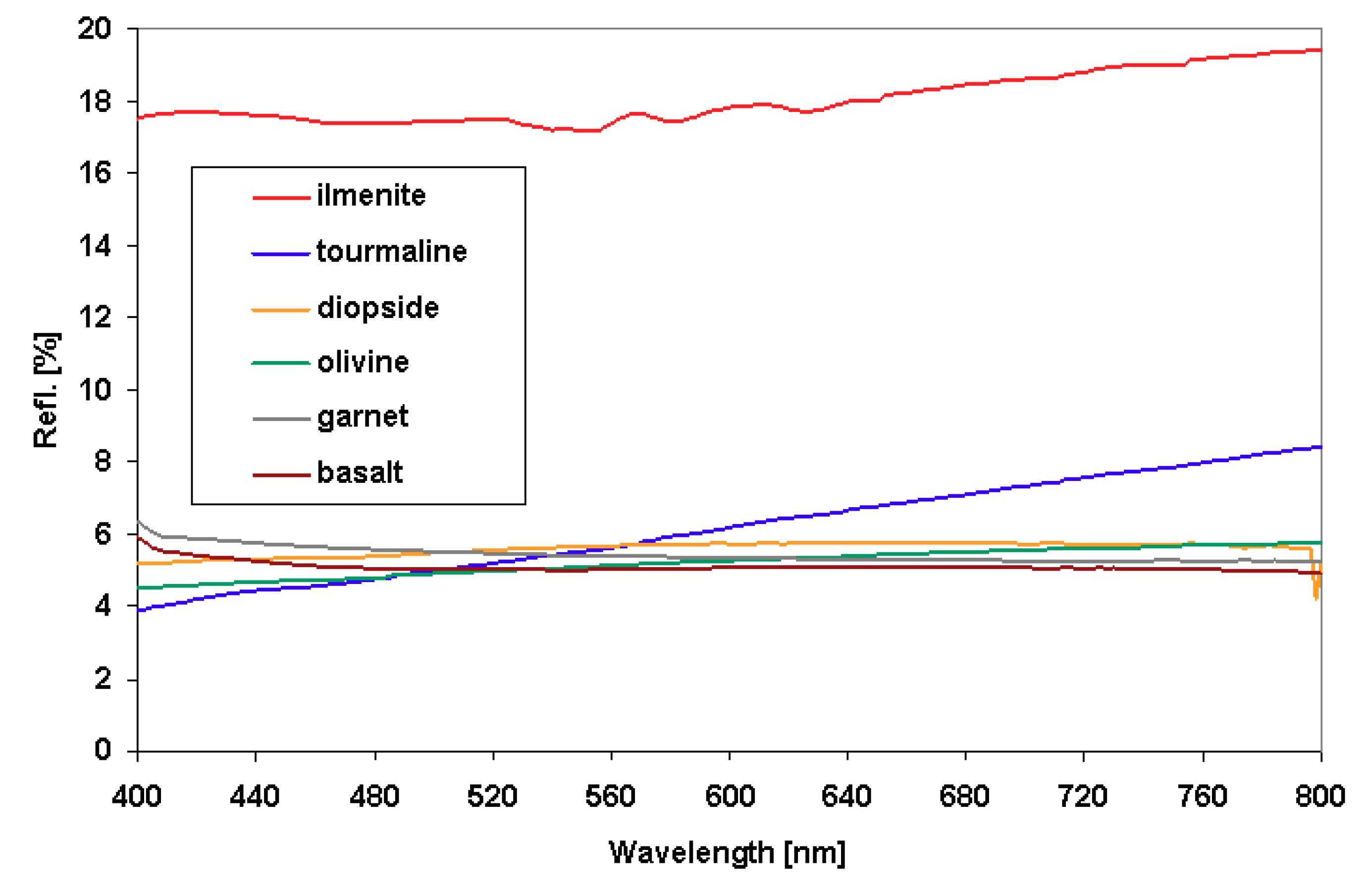
3.6. Optical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shankland, T.J. Synthetic forsterite. Bull. Am. Ceram. Soc. 1967, 46, 1160–1168. [Google Scholar]
- Bouhifd, M.A.; Andrault, A.; Fiques, G.; Richet, P. Thermal expansion of forsterite upto the melting point. Geophys. Res. Lett. 1996, 23, 1143–1146. [Google Scholar] [CrossRef]
- Cojocaru, C.V.; Lamarre, J.M.; Legoux, J.G.; Marple, B.R. Atmospheric Plasma-sprayed Forsterite (Mg2SiO4) Coatings: An Investigation of the Processing-Microstructure-Performance Relationship. J. Therm. Spray Technol. 2013, 22, 145–150. [Google Scholar] [CrossRef]
- Pospisil, Z.; Koller, A. Fine Ceramics, 1st ed.; SNTL: Prague, Czech, 1981. [Google Scholar]
- Ohsato, H.; Tsunooka, T.; Sugiyama, T.; Kakimoto, K.; Ogawa, H. Forsterite ceramics for millimeterwave dielectrics. J. Electroceram. 2006, 17, 445–450. [Google Scholar] [CrossRef]
- Han, C.S.; Mohanty, B.C.; Choi, H.R.; Cho, Y.S. Surface scaling evolution and dielectric properties of sputter-deposited low loss Mg2SiO4 thin films. Surf. Coat. Technol. 2013, 231, 229–233. [Google Scholar] [CrossRef]
- Xie, Y.; Zhai, W.; Chen, L.; Chang, J.; Zheng, X.; Ding, C. Preparation and in vitro evaluation of plasma-sprayed Mg2SiO4 coating on titanium alloy. Acta. Biomater. 2009, 5, 2331–2337. [Google Scholar] [CrossRef]
- Lee, R.B.; Juan, J.C.; Lai, C.W.; Lee, K.M. Ilmenite: Properties and photodegradation kinetic on Reactive Black 5 dye. Chin. Chem. Lett. 2017, 28, 1613–1618. [Google Scholar] [CrossRef]
- Gao, B.; Kim, Y.J.; Chakraborty, A.K.; Lee, W.I. Efficient decomposition of organic compounds with FeTiO3/TiO2 heterojunction under visible light irradiation. Appl. Catal. B 2008, 83, 202–207. [Google Scholar] [CrossRef]
- Cano, N.F.; Yauri, J.M.; Watanabe, S.; Mittani, J.C.R.; Blak, A. Thermoluminescence of natural and synthetic diopside. J. Lumin. 2008, 128, 1185–1190. [Google Scholar] [CrossRef]
- Ito, M.; Morioka, M. Growth of diopside (CaMgSi2O6) single crystal by the Czochralski technique. Geochem. J. 2006, 40, 625–629. [Google Scholar] [CrossRef][Green Version]
- Romero, M.; Rincon, A.; Acosta, A. Effect of iron oxide content on the crystallization of a diopside glass-ceramic glaze. J. Europ. Ceram. Soc. 2002, 22, 883–890. [Google Scholar] [CrossRef]
- Bozadjiev, L.S.; Bozadjiev, R.L.; Georgiev, G.T.; Doncheva, L.S. Diopside Porcelain Tile. Bull. Am. Ceram. Soc. 2006, 85, 12–9101. [Google Scholar]
- Kostikov, K.S.; Pogrebenkov, V.M.; Vereshchagin, V.I. Diopside—the prospective compound of ceramics. In Proceedings of the KORUS 01—Proceedings of the Fifth Russian-Korean International Symposium on Science and Technology, Tomsk, Russia, 16–20 June 2001; Volume 2. [Google Scholar]
- Razavi, M.; Fathi, M.; Savabi, O.; Vashaee, D.; Tayebi, L. In vitro study of nanostructured diopside coating on Mg alloy orthopedic implants. Mater. Sci. Eng. C 2014, 41, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Liu, X.; Zheng, X.; Ding, C. Plasma-sprayed diopside coatings for biomedical applications. Surf. Coat. Technol. 2004, 185, 340–345. [Google Scholar] [CrossRef]
- Maldiney, T.; Lecointre, A.; Viana, B.; Bessiere, A.; Gourier, D.; Bessodes, M.; Cyrille, R.; Sherman, D. Trap depth optimization to improve optical properties of diopside-based nanophosphors for medical imaging. In Proceedings of the Oxide-based Materials and Devices III, San Francisco, CA, USA, 29 February 2012. [Google Scholar] [CrossRef]
- Choi, B.K.; Sun, G.N.; Kim, E.S. Microwave dielectric properties of diopside glass-ceramics. Ceram. Int. 2013, 39, 677–680. [Google Scholar] [CrossRef]
- Feng, K.C.; Chou, C.C.; Chu, L.W.; Chen, H. Zirconia nucleating agent on microstructural and electrical properties of a CaMgSi2O6 diopside glass-ceramic for microwave dielectrics. Mater. Res. Bull. 2012, 47, 2851–2855. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.; Peng, B.; Xie, Z.; Zhang, X.; Yue, Z. Microwave Dielectric Properties and Thermally Stimulated Depolarization Currents of MgF2-Doped Diopside Ceramics. J. Am. Ceram. Soc. 2014, 97, 3537–3543. [Google Scholar] [CrossRef]
- Eoh, Y.J.; Kim, E.S. Effect of heat-treatment on the dielectric properties of CaMgSi2O6 glass-ceramics with Cr2O3-Fe2O3-TiO2. Jpn. J. Appl. Phys. 2014, 53, 08NB01. [Google Scholar] [CrossRef]
- Shannon, R.D.; Dickinson, J.E.; Rossman, G.R. Dielectric constants of crystalline and amorphous spodumene, anorthite and diopside and the oxide additivity rule. Phys. Chem. Miner. 1992, 19, 148–156. [Google Scholar] [CrossRef]
- Cojocaru, C.V.; Lévesque, D.; Moreau, C.; Lima, R.S. Performance of thermally sprayed Si/mullite/BSAS environmental barrier coatings exposed to thermal cycling in water vapor environment. Surf. Coat. Technol. 2013, 216, 215–223. [Google Scholar] [CrossRef]
- Salimijazi, H.; Hossejni, M.; Mostaghimi, J.; Pershin, L.; Coyle, T.; Samadi, H.; Shafeyi, A. Plasma-sprayed Coating Using Mullite and Mixed Alumina/Silica Powders. J. Therm. Spray Technol. 2012, 21, 825–830. [Google Scholar] [CrossRef]
- Wang, W.; Liang, J.; Guo, X.; Xuan, F.Z.; Hong, H. Mechanical Properties and Dissolution Behavior of Plasma-sprayed Wollastonite Coatings Deposited at Different Substrate Temperatures. J. Therm. Spray Technol. 2012, 21, 496–504. [Google Scholar] [CrossRef]
- Henderson, E. Chemical and Physical Properties of Tourmaline. Ph.D. Thesis, Plymouth Polytechnic, PLYMOUTH, UK, 15 March 1971. [Google Scholar]
- Liu, S.M.; Li, D.C.; Hu, W.T.; Qin, G.Q.; Li, L.F. Ion-beam deposition of tourmaline film on glass. J. Non-Cryst. Solids 2008, 354, 1444–1446. [Google Scholar] [CrossRef]
- Ercenk, E.; Sen, U.; Yilmaz, S. Structural characterization of plasma-sprayed basalt–SiC glass–ceramic coatings. Ceram. Int. 2011, 37, 883–889. [Google Scholar] [CrossRef]
- Ageorges, H.; Medarhri, Z.; Ctibor, P.; Fauchais, P. Plasma-sprayed basalt/chromium oxide coatings. High Temp. Mater. Processes. 2007, 11, 71–81. [Google Scholar] [CrossRef]
- Bayrak, G.; Yilmaz, S. Crystallization kinetics of plasma-sprayed basalt coatings. Ceram. Int. 2006, 32, 441–446. [Google Scholar] [CrossRef]
- Ctibor, P.; Nevrlá, B.; Pala, Z.; Sedláček, J.; Soumar, J.; Kubatík, T.; Neufuss, K.; Vilémová, M.; Medřický, J. Study on the plasma-sprayed amorphous diopside and annealed fine-grained crystalline diopside. Ceram. Int. 2015, 41, 10578–10586. [Google Scholar] [CrossRef]
- Ctibor, P.; Neufuss, K.; Pala, Z.; Kotlan, J.; Soumar, J. Dielectric and mechanical properties of plasma-sprayed olivine. Rom. Rep. Phys. 2015, 67, 600–617. [Google Scholar]
- Ctibor, P.; Nevrlá, B.; Pala, Z.; Vrtiška, L. Natural tourmaline as an efficient alternative to ceramic-type material for plasma spraying. J. South Afr. Inst. Min. Metall. 2018, 118, 387–393. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Goland, A.; Herman, H.; Allen, A.J.; Ilavsky, J.; Long, G.G.; Johnson, C.A.; Ruud, J.A. Microstructure–Property Correlations in Industrial Thermal Barrier Coatings. J. Am. Ceram. Soc. 2004, 87, 1294–1300. [Google Scholar] [CrossRef]
- ASTMG 75-95: Standard Test Method for Determination of Slurry Abrasivity (Miller number) and Slurry Abrasion Response of Materials (SAR Number); ASTM International: West Conshohocken, PA, USA, 1995.
- Kalashnikova, I.A.; Kalashnikov, A.V. A technology for producing basalt-based powders for thermal spraying of wear-resistant coatings. Weld. Int. 2010, 24, 26–29. [Google Scholar] [CrossRef]
- Oberste Berghaus, J.; Chráska, T.; Neufuss, K.; Moreau, C.; Chráska, P. Thermal spraying of basalt for abrasion protective coatings using WSP, HVOF and APS. In Proceedings of the International Thermal Spray Conference, Osaka, Japan, 10–12 May 2004. [Google Scholar]
- Kovařík, O.; Siegl, J.; Procházka, Z. Fatigue Behavior of Bodies with Thermally Sprayed Metallic and Ceramic Deposits. J. Therm. Spray Technol. 2008, 17, 525–532. [Google Scholar] [CrossRef]
- Froehlich, F.H. Theory of Dielectrics; Clarendon Press: Oxford, UK, 1949. [Google Scholar]
- Ctibor, P.; Sedlacek, J.; Neufuss, K.; Dubsky, J.; Chraska, P. Dielectric properties of plasma-sprayed silicates. Ceram. Int. 2005, 31, 315–321. [Google Scholar] [CrossRef]
- Andeozzi, G.B.; Cellucci, F.; Gozzi, D. High-temperature electrical conductivity of FeTiO3 and ilmenite. J. Mater. Chem. 1996, 6, 987–991. [Google Scholar] [CrossRef]
- Samadi, H.; Pershin, L.; Coyle, T.W. Effect of in-flight particle properties on deposition of air plasma-sprayed forsterite. Surf. Coat. Technol. 2010, 204, 3300–3306. [Google Scholar] [CrossRef]
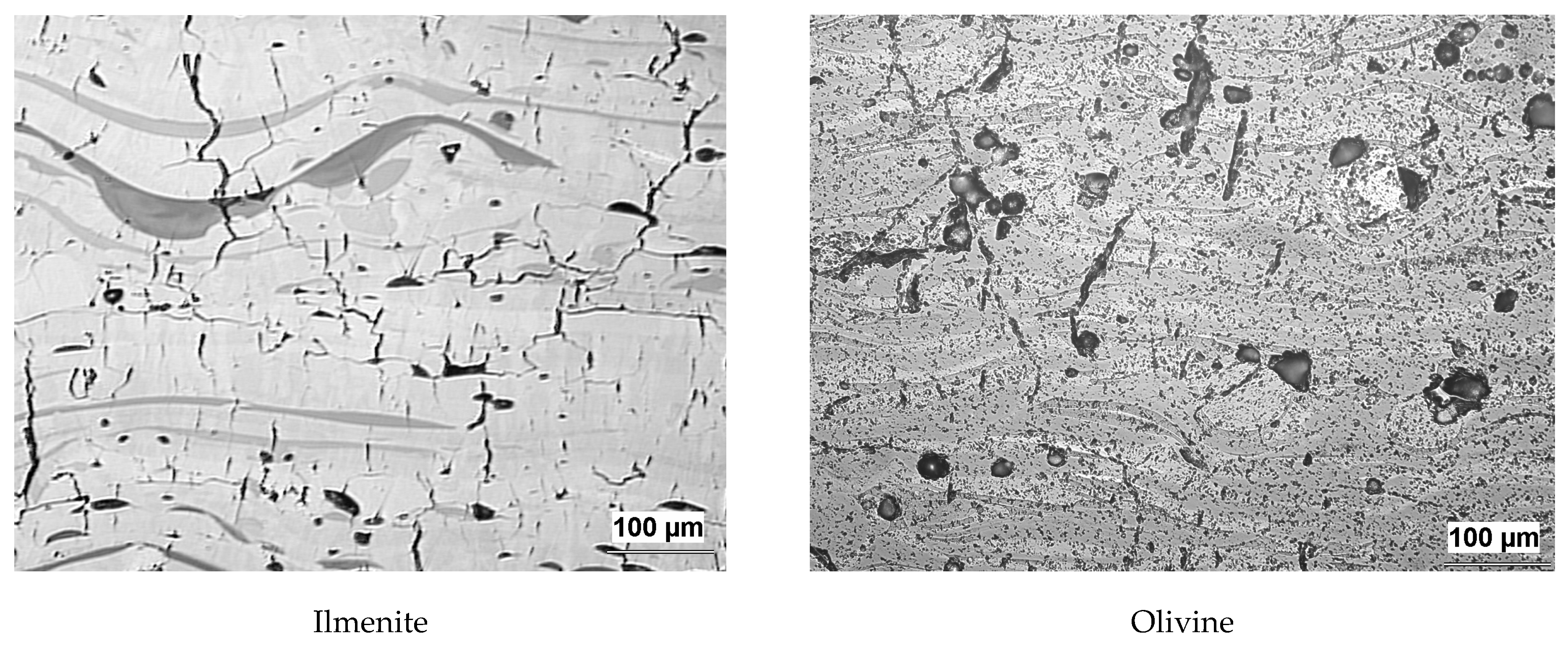
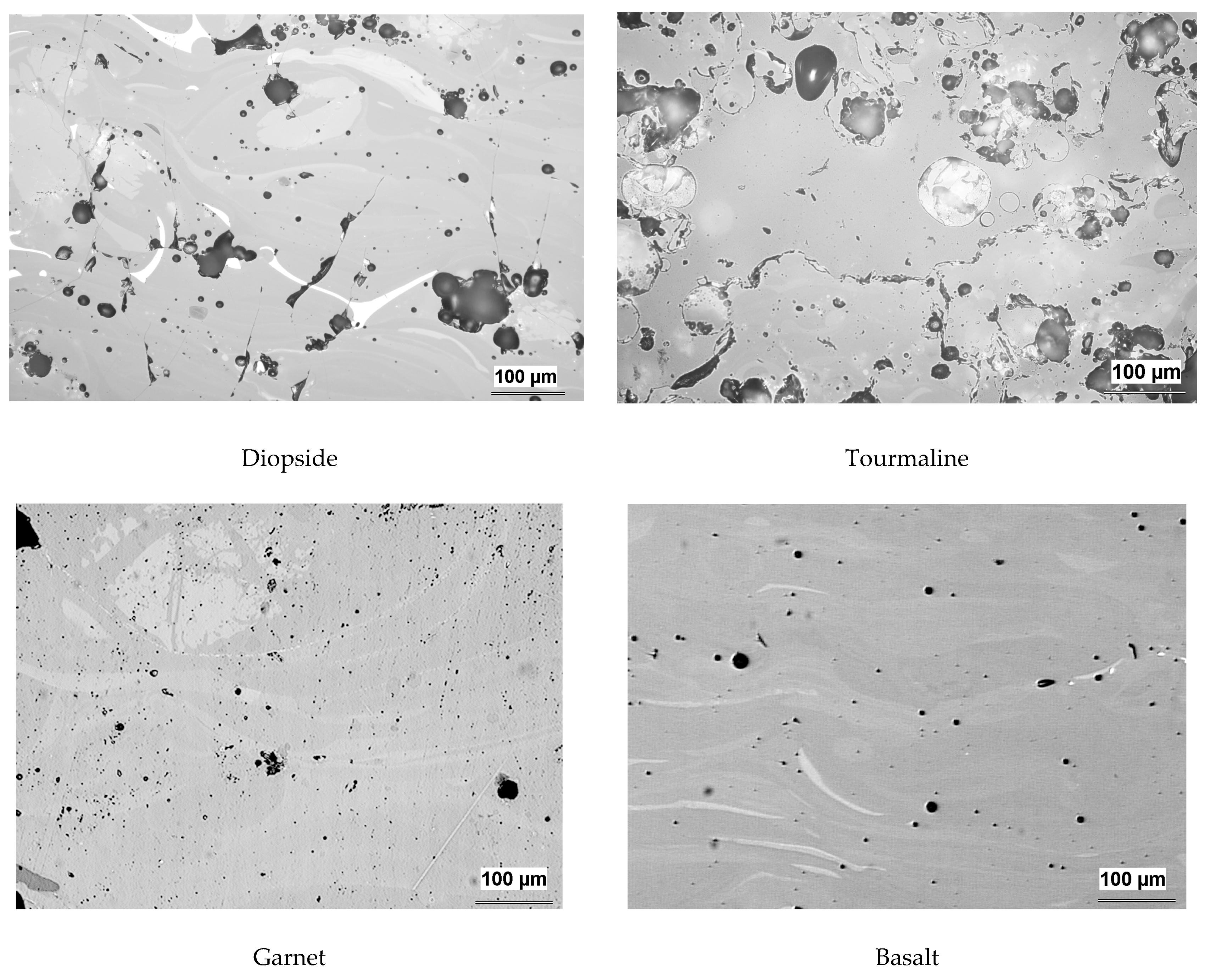

| Coating | Olivine | Ilmenite | Diopside | Garnet | Tourmaline | Basalt |
|---|---|---|---|---|---|---|
| Powder [µm] | 63–125 | 63–125 | 100–180 | 100–180 | 63–125 | 63–125 |
| Technique | WSP | WSP | WSPH | WSP | WSPH | WSP |
| Substrate | carbon steel | stainless steel | stainless steel | carbon steel | stainless steel | carbon steel |
| Thickness [mm] | 1.5 | 3.0 | 2.5 | 3.0 | 2.0 | 1.5 |
| Feeding Distance [mm]* | 90 | 33 | 65 | 65 | 80 | 22 |
| Spray Distance [mm]** | 450 | 415 | 380 | 370 | 250 | 400 |
| Feeding gas | air | air | air | air | air | air |
| Power [kW] | 150 | 150 | 150 | 160 | 160 | 150 |
| Preheat [°C] | 250 | 300 | 300 | 280 | 300 | 250 |
| Parameter | Porosity [%] | NV per mm2 | ED [µm] | Circularity |
|---|---|---|---|---|
| Ilmenite | 3.9 | 2363 | 7.1 | 0.541 |
| Diopside | 8.8 | 523 | 10.4 | 0.610 |
| Tourmaline | 15.8 | 1145 | 8.7 | 0.426 |
| Olivine | 14.2 | 991 | 7.3 | 0.633 |
| Garnet | 2* | - | - | - |
| Basalt | 3* | - | - | - |
| Material | Phase 1 | Content [%] | Phase 2 | Content [%] |
|---|---|---|---|---|
| Olivine Powder | enstatite | n.a. | quartz SiO2 | n.a. |
| Olivine Coating | enstatite | 44.7 | amorphous | 55.3 |
| Ilmenite Powder | ilmenite | 100 | - | - |
| Ilmenite Coating | ilmenite | 100 | - | - |
| Diopside Powder | diopside | 78.5 | clinoenstatite | 16.5 |
| Diopside Coating | amorphous | 99.5 | - | - |
| Tourmaline Powder | tourmaline | 97.2 | rutile TiO2 | 1.3 |
| Tourmaline Coating | tourmaline | 55.0 | amorphous | 45.0 |
| Garnet Powder | almandine | n.a. | quartz SiO2 | n.a. |
| Garnet Coating | almandine | n.a. | quartz SiO2, amorphous | n.a. |
| Basalt Powder | amorphous | 100 | - | - |
| Basalt Coating | amorphous | 100 | - | - |
| Parameter | Microhardness [GPa] | IWR [m/mm3] | Roughness Ra [µm] |
|---|---|---|---|
| Ilmenite WSP | 8.39 ± 1.11 | 46.1 | 8 ± 1 |
| Diopside WSP | 8.72 ± 1.24 | 35.6 | 50 ± 6 |
| Tourmaline WSPH | 8.67 ± 1.35 | 15.0 | 35 ± 5 |
| Olivine WSP | 10.65 ± 1.43 | 62.8 | 14 ± 1 |
| Garnet WSP | 8.8* | 45.0 | 31 ± 2 |
| Basalt WSP | 6.07 ± 1.28 | 41.8 | 29 ± 1 |
| Material | Resistivity [Ωm] |
|---|---|
| Ilmenite | * |
| Diopside | 5.57 × 109 |
| Tourmaline | ** |
| Olivine | 1.34 × 1010 |
| Garnet | 1.16 × 1012 |
| Basalt | * |
| Material | DTA | Dilatometry |
|---|---|---|
| Olivine Powder | n.a. | - |
| Olivine Coating | crystallizes above 890 °C | TEC (200–600 °C), 10.68 × 10−6 /K |
| Ilmenite Powder | n.a. | TEC (200–600 °C), 9.47 × 10−6 /K |
| Ilmenite Coating | n.a. | TEC (30–600 °C), 6.47 × 10−6 /K |
| Diopside Powder | reaction above 1100 °C | - |
| Diopside Coating | reaction above 800 °C | TEC (30–600 °C), 6.72 × 10−6 /K |
| Tourmaline Powder | reaction above 890 °C | - |
| Tourmaline Coating | crystallizes above 890 °C | TEC (r.t.), 1 × 10−6 /K |
| Garnet powder | n.a. | - |
| Garnet coating | crystallizes above 800 °C | TEC (150–500 °C), 4.5 to 5.9 × 10−6 /K |
| Basalt powder | n.a. | - |
| Basalt coating | reaction above 650 °C, crystallizes above 850 °C, contraction of 9 % (i.e., large) | TEC (150–500 °C), 7.32 × 10−6 /K |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ctibor, P.; Nevrlá, B.; Neufuss, K.; Petrášek, J.; Sedláček, J. Plasma Spray Coatings of Natural Ores From Structural, Mechanical, Thermal, and Dielectric Viewpoints. Coatings 2020, 10, 3. https://doi.org/10.3390/coatings10010003
Ctibor P, Nevrlá B, Neufuss K, Petrášek J, Sedláček J. Plasma Spray Coatings of Natural Ores From Structural, Mechanical, Thermal, and Dielectric Viewpoints. Coatings. 2020; 10(1):3. https://doi.org/10.3390/coatings10010003
Chicago/Turabian StyleCtibor, Pavel, Barbara Nevrlá, Karel Neufuss, Jan Petrášek, and Josef Sedláček. 2020. "Plasma Spray Coatings of Natural Ores From Structural, Mechanical, Thermal, and Dielectric Viewpoints" Coatings 10, no. 1: 3. https://doi.org/10.3390/coatings10010003
APA StyleCtibor, P., Nevrlá, B., Neufuss, K., Petrášek, J., & Sedláček, J. (2020). Plasma Spray Coatings of Natural Ores From Structural, Mechanical, Thermal, and Dielectric Viewpoints. Coatings, 10(1), 3. https://doi.org/10.3390/coatings10010003




