Guideline Adherence in Antibiotic Prescribing to Patients with Respiratory Diseases in Primary Care: Prevalence and Practice Variation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nivel Primary Care Database
2.2. Study Sample
- acute cough (recorded using ICPC-codes: R05—acute cough; R71—whooping cough; R77—laryngitis/trachitis; and R78—acute bronchitis/bronchiolitis);
- allergic and non-allergic rhinitis (recorded using ICPC-codes: R07—sneezing/nasal congestion; R08—nose symptom/complaint other; and R97—allergic rhinitis),
- acute rhinosinusitis (recorded using ICPC-codes: R09—sinus symptom/complaint; R74—upper respiratory infection acute; and R75—sinusitis acute/chronic)
- acute sore throat (recorded using: R21—throat symptom/complaint; R22—tonsils symptom/complaint; R72—strep throat/scarlet fever; R76—tonsillitis acute).
2.3. Measurements
2.3.1. Antibiotics Indication
2.3.2. Antibiotics Prescriptions (Dependent Variable)
2.4. Statistical Analyses
3. Results
3.1. Antibiotic Prescribing
3.2. Practice Variation
4. Discussion
5. Strengths and Limitations
6. Conclusions and Implications
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [Green Version]
- Goossens, H.; Ferech, M.; Vander Stichele, R.; Elseviers, M.; ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef]
- Smith, S.M.; Fahey, T.; Smucny, J.; A Becker, L. Antibiotics for acute bronchitis. Cochrane Database Syst. Rev. 2017, 2017, CD000245. [Google Scholar] [CrossRef] [PubMed]
- Lemiengre, M.B.; Van Driel, M.L.; Merenstein, D.; Liira, H.; Mäkelä, M.; De Sutter, A. Antibiotics for acute rhinosinusitis in adults. Cochrane Database Syst. Rev. 2018, 9, CD006089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanovska, V.; Hek, K.; Van Dijk, L.; Mantel-Teeuwisse, A.K.; Leufkens, H.G.M.; Nielen, M.M.J. Antibiotic prescribing for children in primary care and adherence to treatment guidelines. J. Antimicrob. Chemother. 2016, 71, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Hope, E.C.; Crump, R.E.; Hollingsworth, T.D.; Smieszek, T.; Robotham, J.V.; Pouwels, K.B. Identifying English Practices that Are High Antibiotic Prescribers Accounting for Comorbidities and Other Legitimate Medical Reasons for Variation. EClinicalMedicine 2018, 6, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Dekker, A.R.J.; Verheij, T.J.M.; Van Der Velden, A.W. Inappropriate antibiotic prescription for respiratory tract indications: Most prominent in adult patients. Fam. Pact. 2015, 32, 401–407. [Google Scholar] [CrossRef]
- WHO. WHO Report on Surveillance of Antibiotic Consumption: 2016–2018 Early Implementation; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Nivel. Nivel Primary Care Database. Available online: http://nivel.nl/en/dossier/nivel-primary-care-database (accessed on 10 July 2020).
- Lamberts, H.; Wood, M. ICPC International Classification of Primary Care; Oxford University Press: Oxford, UK, 1987. [Google Scholar]
- Nielen, M.; Spronk, I.; Davids, R.; Korevaar, J.C.; Poos, M.J.J.C.; Hoeymans, N.; Opstelten, W.; Sande, M.; Biermans, M.; Schellevis, F.; et al. A new method for estimating morbidity rates based on routine electronic medical records in primary care. In Proceedings of the 21st WONCA Europe Conference, Rio de Janeiro, Brazil, 15–18 June 2016. [Google Scholar]
- Diening, J.A.A. Dutch Civil Law. Reason. Liabil. 1982, 7, 39–60. [Google Scholar] [CrossRef]
- NHG. NHG-Standaard Acuut Hoesten [NHG Guideline Acute Coughing]. 2011. Available online: https://www.nhg.org/standaarden/volledig/nhg-standaard-acuut-hoesten (accessed on 19 January 2016).
- NHG. NHG-standaard Acute Rhinosinusitis [NHG Guidelines Acute Rhinosinusitis]. 2014. Available online: https://www.nhg.org/standaarden/volledig/nhg-standaard-acute-rhinosinusitis (accessed on 19 January 2016).
- NHG. NHG-Standaard Acute Keelpijn (NHG-Guideline Sore Throat). 2015. Available online: https://richtlijnen.nhg.org/standaarden/acute-keelpijn (accessed on 19 January 2016).
- NHG. NHG-Standaard Allergische en Niet-Allergische Rinitis (NHG-Guideline Allergic and Non-Allergic Rhinitis). 2006. Available online: https://richtlijnen.nhg.org/standaarden/allergische-en-niet-allergische-rinitis (accessed on 19 January 2016).
- Saust, L.T.; Bjerrum, L.; Siersma, V.; Arpi, M.; Hansen, M.P. Quality assessment in general practice: Diagnosis and antibiotic treatment of acute respiratory tract infections. Scand. J. Prim. Health Care 2018, 36, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Gulliford, M.; Dregan, A.; Moore, M.V.; Ashworth, M.; Van Staa, T.P.; McCann, G.; Charlton, J.; Yardley, L.; Little, P.; McDermott, L. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: Survey of 568 UK general practices. BMJ Open 2014, 4, e006245. [Google Scholar] [CrossRef]
- Pouwels, K.B.; Dolk, F.C.K.; Smith, D.R.M.; Robotham, J.V.; Smieszek, T. Actual versus ‘ideal’ antibiotic prescribing for common conditions in English primary care. J. Antimicrob. Chemother. 2018, 73, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Vervloet, M.; A Meulepas, M.; Cals, J.W.L.; Eimers, M.; Van Der Hoek, L.S.; Van Dijk, L. Reducing antibiotic prescriptions for respiratory tract infections in family practice: Results of a cluster randomized controlled trial evaluating a multifaceted peer-group-based intervention. NPJ Prim. Care Respir. Med. 2016, 26, 15083. [Google Scholar] [CrossRef] [PubMed]
- Juszczyk, D.; Charlton, J.; McDermott, L.; Soames, J.; Sultana, K.; Ashworth, M.; Fox, R.; Hay, A.D.; Little, P.; Moore, M.V.; et al. Electronically delivered, multicomponent intervention to reduce unnecessary antibiotic prescribing for respiratory infections in primary care: A cluster randomised trial using electronic health records—REDUCE Trial study original protocol. BMJ Open 2016, 6, e010892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonagh, M.S.; Peterson, K.; Winthrop, K.; Cantor, A.; Lazur, B.H.; Buckley, D.I. Interventions to reduce inappropriate prescribing of antibiotics for acute respiratory tract infections: Summary and update of a systematic review. J. Int. Med Res. 2018, 46, 3337–3357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borek, A.J.; Wanat, M.; Sallis, A.; Ashiru-Oredope, D.; Atkins, L.; Beech, E.; Hopkins, S.; Jones, L.; McNulty, C.; Shaw, K.; et al. How Can National Antimicrobial Stewardship Interventions in Primary Care Be Improved? A Stakeholder Consultation. Antibiotics 2019, 8, 207. [Google Scholar] [CrossRef] [Green Version]
- Esch, T.E.M.V.; Brabers, A.E.; Hek, K.; Van Dijk, L.; Verheij, R.A.; De Jong, J.D. Does shared decision-making reduce antibiotic prescribing in primary care? J. Antimicrob. Chemother. 2018, 73, 3199–3205. [Google Scholar] [CrossRef] [Green Version]
- Aabenhus, R.; Siersma, V.; Sandholdt, H.; Køster-Rasmussen, R.; Hansen, M.P.; Bjerrum, L. Identifying practice-related factors for high-volume prescribers of antibiotics in Danish general practice. J. Antimicrob. Chemother. 2017, 72, 2385–2391. [Google Scholar] [CrossRef] [Green Version]
- Coenen, S.; Francis, N.; Kelly, M.; Hood, K.; Nuttall, J.; Little, P.; Verheij, T.J.M.; Melbye, H.; Goossens, H.; Butler, C.C.; et al. Are Patient Views about Antibiotics Related to Clinician Perceptions, Management and Outcome? A Multi-Country Study in Outpatients with Acute Cough. PLoS ONE 2013, 8, e76691. [Google Scholar] [CrossRef] [Green Version]
- Biezen, R.; Grando, D.; Mazza, D.; Brijnath, B. Dissonant views—GPs’ and parents’ perspectives on antibiotic prescribing for young children with respiratory tract infections. BMC Fam. Pact. 2019, 20, 46. [Google Scholar] [CrossRef] [Green Version]
- McKay, R.; Mah, A.; Law, M.R.; McGrail, K.; Patrick, D.M. Systematic Review of Factors Associated with Antibiotic Prescribing for Respiratory Tract Infections. Antimicrob. Agents Chemother. 2016, 60, 4106–4118. [Google Scholar] [CrossRef] [Green Version]
- Machowska, A.; Lundborg, C.S. Drivers of Irrational Use of Antibiotics in Europe. Int. J. Environ. Res. Public Health 2018, 16, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Bij, S.; Khan, N.; Veen, P.T.; De Bakker, D.H.; Verheij, R.A. Improving the quality of EHR recording in primary care: A data quality feedback tool. J. Am. Med. Inform. Assoc. 2016, 24, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuart, B.; Brotherwood, H.; Hoff, C.V.; Brown, A.; Bruel, A.V.D.; Hay, A.D.; Moore, M.; Little, P. Exploring the appropriateness of antibiotic prescribing for common respiratory tract infections in UK primary care. J. Antimicrob. Chemother. 2019, 75. [Google Scholar] [CrossRef] [PubMed]
| Guideline | ||||
|---|---|---|---|---|
| Acute Cough, (M78, 2011) [14] | Allergic and non-Allergic Rhinitis (M48, 2006) [16] | Acute Rhinosinusitis, (M33, 2014) [14] | Acute Sore Throat (M11, 2015) [15] | |
| Diagnoses included in the guideline (ICPC) | Acute cough (R05), Whooping cough (R71), Laryngitis/tracheitis acute (R77), Acute bronchitis/bronchiolitis (R78) | Sneezing/nasal congestion (R07), Nose symptom/complaint other (R08) Allergic rhinitis (R97) | Sinus symptom/complaint (R09), Upper respiratory infection acute (R74), Sinusitis acute/chronic (R75) | Throat symptom/complaint (R21), Tonsils symptom/complaint (R22), Strep throat/scarlet fever (R72), Tonsillitis acute (R76) |
| Antibiotics recommen-dations in guideline | No antibiotics if pneumonia is not considered likely. Exceptions in which antibiotics should be considered are patients with one or more risk factors: • Age < 3 months or > 75 years • Relevant comorbidity: heart failure, severe COPD, diabetes mellitus (in particular when using insulin), neurological diseases, severe kidney diseases. • Poor immune response • CRP in adults: <20 mg/L no indication for antibiotics, 20–100 mg/L indication for antibiotics depends on the clinical presentation, >100 mg/L indication for antibiotics. | Antibiotics are not mentioned in the guideline. | In principle, no antibiotics. Antibiotics are indicated in patients who are seriously ill. Antibiotics can be considered in patients with poor immune response: • Chronic use of corticosteroids or other immunosuppressive medicines • HIV infection with a reduced number of T-cells • Chemotherapy or radiotherapy • Immune disorders • Frail elderly who are sick • Patients with diabetes mellitus Antibiotics can be considered for patients who have had fever for more than 5 days, or for patients who have recurrent fever after a few fever-free days within one episode of rhinosinusitis. | In principle, no antibiotics. Antibiotics are indicated • in seriously ill patients • if advised by public health services in the rare case of scarlet fever clusters in a closed community. Antibiotics can be considered in patients with an increased risk of complications, e.g., in case of: • Chronic use of corticosteroids or other immunosuppressive medicines • HIV infection with a reduced number of T-cells • Chemotherapy or radiotherapy • Cancer • Immune disorders • Diabetes mellitus • Rheumatic fever • Severe alcohol abuse • Drug abuse • Functional asplenic • sickle cell disease |
| Study definitions Antibiotics not indicated | in patients with cough (R05, R77, R78) between three months and 75 years, without indications for poor immune response *, with CRP <20 and without relevant comorbidity. | in all patients | in patients with sinus complaints (R09, R74, R75) without indications for poor immune response *. | in patients with sore throat complaints (R21, R22, R72, R76) without indications for poor immune response * and without rheumatic fever in their medical history. |
| Study definitions Antibiotics possibly indicated | in patients with cough (R05, R77, R78) younger than three months or over 75 years, or with indications for poor immune response *, or with CRP >20 or with relevant comorbidity and in patients with whooping cough (R71). | not applicable | in patients with sinus complaints (R09, R74, R75) with an indication for poor immune response *. | in patients with sore throat complaints (R21, R22, R72, R76) with an indication for poor immune response * or with rheumatic fever in their medical history. |
| Study definitions Remarks | Not all measured CRP values are recorded. CRP limits for indications are only applied if CRP values were recorded. Relevant comorbidity includes: heart failure, COPD, neurological diseases and severe kidney diseases. | not applicable | Being seriously ill and having prolonged or recurrent fever cannot be retrieved from Nivel Primary Care Database and are consequently not taken into account. | Being seriously ill cannot be retrieved from Nivel Primary Care Database and is consequently not taken into account. The same holds for scarlet fever clusters in a closed community. |
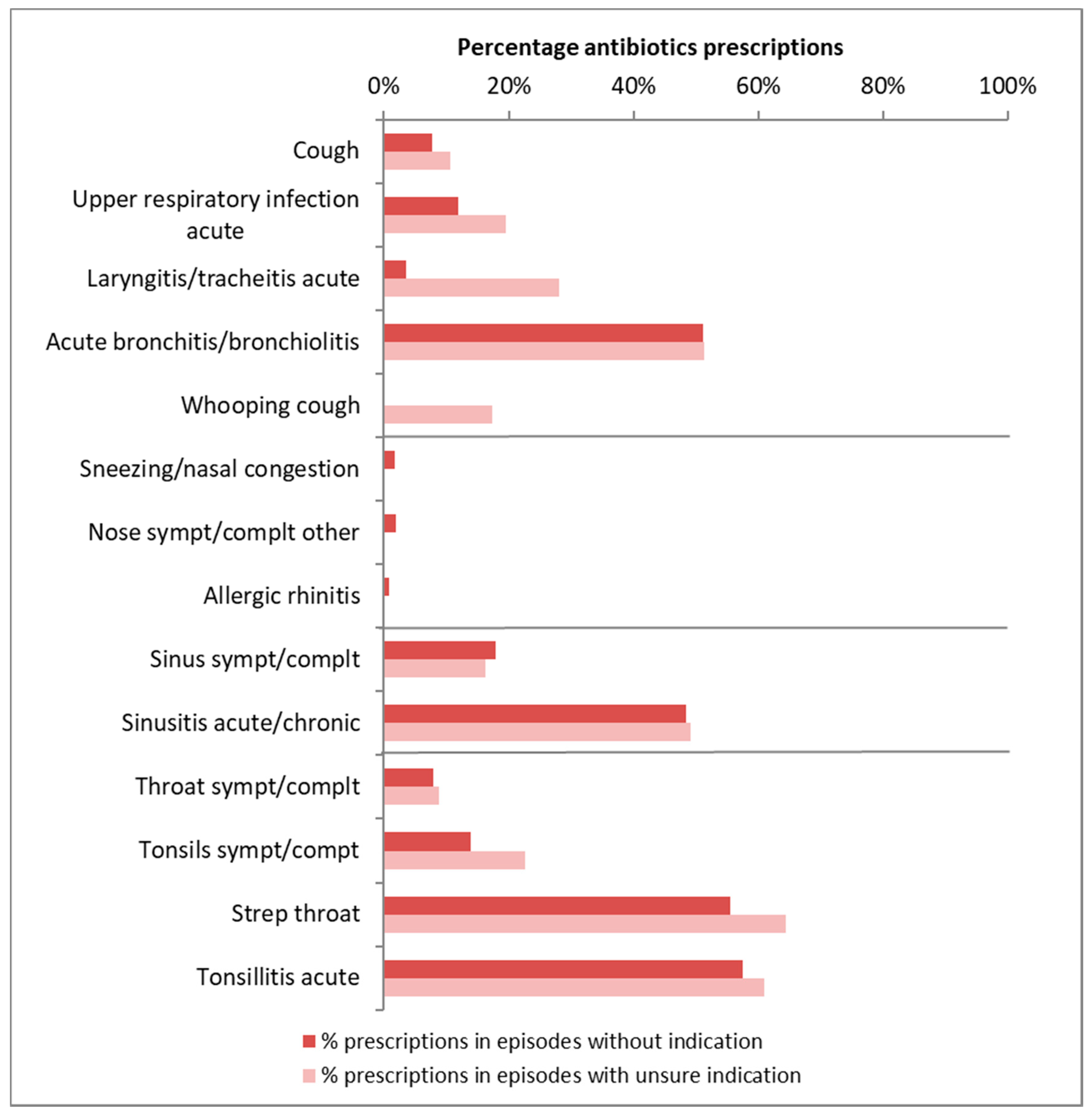
| ICPC | Antibiotics Indicated | Number of Episodes | Number of Practices | Mean % with AB Prescription | % with AB Prescription (95% Range) |
|---|---|---|---|---|---|
| Guideline acute cough | |||||
| Cough (R05) | no | 33,571 | 307 | 8% | 2–25% |
| unsure | 18,714 | 307 | 11% | 3–29% | |
| Upper respiratory infection acute (R74) | no | 45,717 | 307 | 12% | 3–36% |
| unsure | 17,287 | 307 | 20% | 6–48% | |
| Laryngitis/tracheitis acute (R77) | no | 1653 | 283 | 4% | 0–27% |
| unsure | 484 | 158 | 28% | 3–84% | |
| Acute bronchitis/bronchiolitis (R78) | no | 9910 | 306 | 51% | 18–83% |
| unsure | 8176 | 307 | 51% | 23–79% | |
| Whooping cough (R71) | unsure | 666 | 190 | 17% | 1–83% |
| Guideline allergic and non allergic rhinitis | |||||
| Sneezing/nasal congestion (R07) | no | 5331 | 303 | 2% | 0–6% |
| Nose sympt/complt other (R08) | no | 5977 | 306 | 2% | 1–6% |
| Allergic rhinitis (R97) | no | 42,599 | 307 | 1% | 0–2% |
| Guideline acute rhinosinusitis | |||||
| Sinus sympt/complt (R09) | no | 2176 | 285 | 18% | 4–51% |
| unsure | 532 | 182 | 16% | 2–66% | |
| Sinusitis acute/chronic (R75) | no | 17,360 | 307 | 48% | 23–75% |
| unsure | 4316 | 306 | 49% | 24–74% | |
| Guideline sore throat | |||||
| Throat sympt/complt (R21) | no | 17,889 | 307 | 8% | 2–29% |
| unsure | 3549 | 307 | 9% | 2–32% | |
| Tonsils sympt/compt (R22) | no | 1368 | 283 | 14% | 3–50% |
| unsure | 119 | 23% | - | ||
| Strep throat/scarlet fever (R72) | no | 1457 | 259 | 56% | 24–83% |
| unsure | 166 | 64% | - | ||
| Tonsillitis acute (R76) | no | 9297 | 307 | 57% | 32–79% |
| unsure | 582 | 210 | 61% | 36–81% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hek, K.; van Esch, T.E.M.; Lambooij, A.; Weesie, Y.M.; van Dijk, L. Guideline Adherence in Antibiotic Prescribing to Patients with Respiratory Diseases in Primary Care: Prevalence and Practice Variation. Antibiotics 2020, 9, 571. https://doi.org/10.3390/antibiotics9090571
Hek K, van Esch TEM, Lambooij A, Weesie YM, van Dijk L. Guideline Adherence in Antibiotic Prescribing to Patients with Respiratory Diseases in Primary Care: Prevalence and Practice Variation. Antibiotics. 2020; 9(9):571. https://doi.org/10.3390/antibiotics9090571
Chicago/Turabian StyleHek, Karin, Thamar E.M. van Esch, Anke Lambooij, Yvette M. Weesie, and Liset van Dijk. 2020. "Guideline Adherence in Antibiotic Prescribing to Patients with Respiratory Diseases in Primary Care: Prevalence and Practice Variation" Antibiotics 9, no. 9: 571. https://doi.org/10.3390/antibiotics9090571





