Characteristics and Management of Candidaemia Episodes in an Established Candida auris Outbreak
Abstract
1. Introduction
2. Results
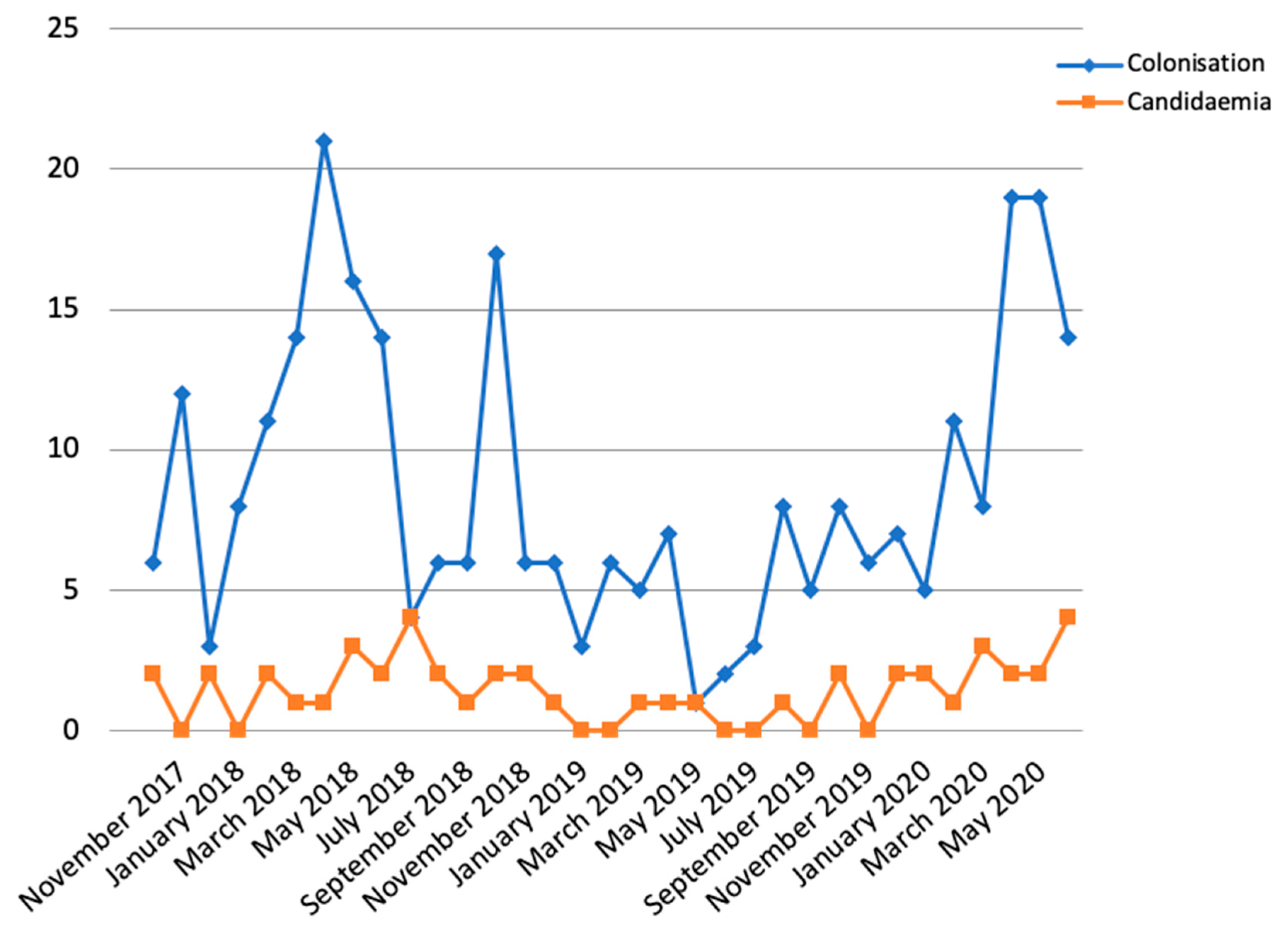
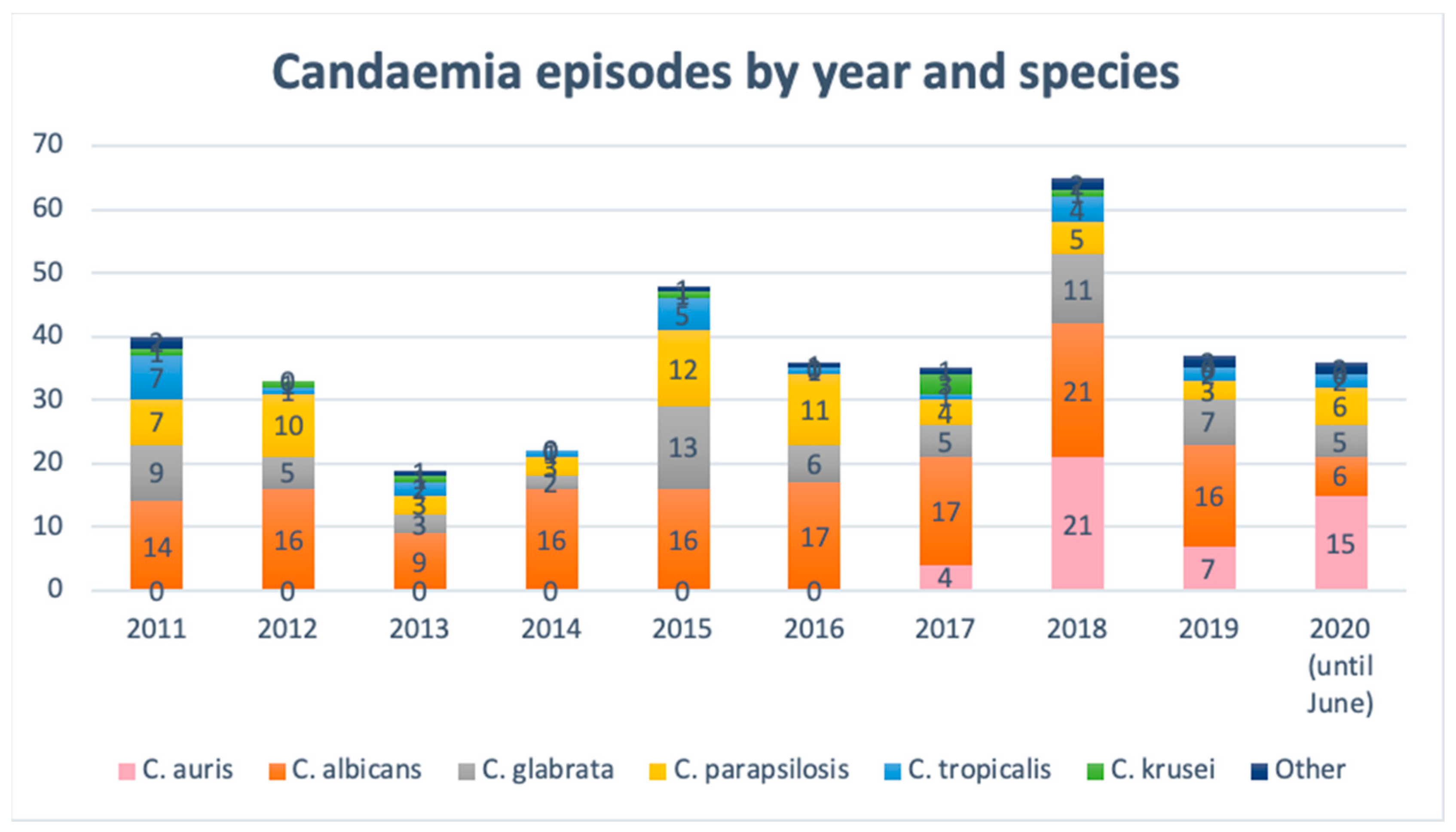
2.1. Description of the Outbreak
2.2. Characteristics of C. auris Candidaemias
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van Schalkwyk, E.; Mpembe, R.S.; Thomas, J.; Shuping, L.; Ismail, H.; Lowman, W.; Karstaedt, A.S.; Chibabhai, V.; Wadula, J.; Avenant, T.; et al. Epidemiologic Shift in Candidemia Driven by Candida auris, South Africa, 2016–2017. Emerg. Infect. Dis. 2019, 25, 1698–1707. [Google Scholar] [CrossRef]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef] [PubMed]
- CDC. Tracking Candida auris. Available online: https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html (accessed on 27 June 2020).
- Forsberg, K.; Woodworth, K.; Walters, M.; Berkow, E.L.; Jackson, B.; Chiller, T.; Vallabhaneni, S. Candida auris: The recent emergence of a multidrug-resistant fungal pathogen. Med. Mycol. 2018, 57, 1–12. [Google Scholar] [CrossRef] [PubMed]
- CDC. Candida auris. Information for Laboratorians and Health Professionals. Available online: https://www.cdc.gov/fungal/candida-auris/health-professionals.html (accessed on 28 June 2020).
- European Centre for Disease Prevention and Control. Candida auris in Healthcare Settings—Europe; First update, 23 April 2018; ECDC: Stockholm, Sweden, 2018.
- Salvador García, C.; Tormo Palop, N.; Mulet Bayona, J.V.; Melero García, M.; Navalpotro Rodríguez, D.; Belda Álvarez, M.; del Remedio Guna Serrano, M.; Gimeno, C. Candida auris: Descripción de un brote. Enferm. Infecc. Microbiol. Clin. 2020, 38 (Suppl. 1), 39–44. [Google Scholar]
- Meis, J.F.; Chowdhary, A. Candida auris: A global fungal public health threat. Lancet Infect. Dis. 2018, 18, 1298–1299. [Google Scholar] [CrossRef]
- Kumar, J.; Eilertson, B.; Cadnum, J.L.; Whitlow, C.S.; Jencson, A.L.; Safdar, N.; Krein, S.L.; Tanner, W.D.; Mayer, J.M.; Samore, M.H.; et al. Environmental Contamination with Candida Species in Multiple Hospitals Including a Tertiary Care Hospital with a Candida auris Outbreak. Pathog. Immun. 2019, 4, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Piedrahita, C.T.; Cadnum, J.L.; Jencson, A.L.; Shaikh, A.A.; Ghannoum, M.A.; Donskey, C.J. Environmental Surfaces in Healthcare Facilities are a Potential Source for Transmission of Candida auris and Other Candida Species. Infect. Control Hosp. Epidemiol. 2017, 38, 1107–1109. [Google Scholar] [CrossRef]
- Ruiz-Gaitán, A.; Moret, A.M.; Tasias-Pitarch, M.; Aleixandre-López, A.I.; Martínez-Morel, H.; Calabuig, E.; Salavert-Lletí, M.; Ramírez, P.; López-Hontangas, J.L.; Hagen, F.; et al. An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses 2018, 61, 498–505. [Google Scholar] [CrossRef]
- Jeffery-Smith, A.; Taori, S.K.; Schelenz, S.; Jeffery, K.; Johnson, E.M.; Borman, A.; Candida auris Incident Management Team; Manuel, R.; Brown, C.S. Candida auris: A review of the literature. Clin. Microbiol. Rev. 2018, 31, 1–18. [Google Scholar]
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob. Resist. Infect. Control 2016, 5, 35. [Google Scholar] [CrossRef]
- Castanheira, M. Fungemia Surveillance in Denmark Demonstrates Emergence of Non-albicans Candida Species and Higher Antifungal Usage and Resistance Rates than in Other Nations. J. Clin. Microbiol. 2018, 56, e01907-17. [Google Scholar] [CrossRef] [PubMed]
- Horn, D.L.; Neofytos, D.; Anaissie, E.J.; Fishman, J.A.; Steinbach, W.J.; Olyaei, A.J.; Marr, K.A.; Pfaller, M.; Chang, C.-H.; Webster, K.M. Epidemiology and Outcomes of Candidemia in 2019 Patients: Data from the Prospective Antifungal Therapy Alliance Registry. Clin. Infect. Dis. 2009, 48, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L.; Calandra, T. Changes in the epidemiological landscape of invasive candidiasis. J. Antimicrob. Chemother. 2018, 73 (suppl. 1), i4–i13. [Google Scholar] [CrossRef]
- Puig-Asensio, M.; Padilla, B.; Garnacho-Montero, J.; Zaragoza, O.; Aguado, J.M.; Zaragoza, R.; Montejo, M.; Muñoz, P.; Ruiz-Camps, I.; Cuenca-Estrella, M.; et al. Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: A population-based surveillance in Spain. Clin. Microbiol. Infect. 2014, 20, O245–O254. [Google Scholar] [CrossRef] [PubMed]
- Yapar, N. Epidemiology and risk factors for invasive candidiasis. Ther. Clin. Risk Manag. 2014, 10, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Lausch, K.R.; Søgaard, M.; Rosenvinge, F.S.; Johansen, H.K.; Boysen, T.; Røder, B.; Mortensen, K.L.; Nielsen, L.; Lemming, L.; Olesen, B.; et al. High incidence of candidaemia in a nationwide cohort: Underlying diseases, risk factors and mortality. Int. J. Infect. Dis. 2018, 76, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, S.M.; Chakrabarti, A.; Paul, R.A.; Sood, P.; Kaur, H.; Capoor, M.R.; Kindo, A.J.; Marak, R.S.K.; Arora, A.; Sardana, R.; et al. Candida auris candidaemia in Indian ICUs: Analysis of risk factors. J. Antimicrob. Chemother. 2017, 72, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Breazzano, M.P.; Tooley, A.A.; Godfrey, K.J.; Iacob, C.E.; Yannuzzi, N.A.; Flynn, H.W. Candida auris and endogenous panophthalmitis: Clinical and histopathological features. Am. J. Ophthalmol. Case Rep. 2020, 19, 100738. [Google Scholar] [CrossRef]
- Taori, S.K.; Khonyongwa, K.; Hayden, I.; Athukorala, G.D.A.; Letters, A.; Fife, A.; Desai, N.; Borman, A.M. Candida auris outbreak: Mortality, interventions and cost of sustaining control. J. Infect. 2019, 79, 601–611. [Google Scholar] [CrossRef]
- Sayeed, M.A.; Farooqi, J.; Jabeen, K.; Awan, S.; Mahmood, S.F. Clinical spectrum and factors impacting outcome of Candida auris: A single center study from Pakistan. BMC Infect. Dis. 2019, 19, 384. [Google Scholar] [CrossRef]
- Calvo, B.; Melo, A.S.A.; Perozo-Mena, A.; Hernandez, M.; Francisco, E.C.; Hagen, F.; Meis, J.F.; Colombo, A.L. First report of Candida auris in America: Clinical and microbiological aspects of 18 episodes of candidemia. J. Infect. 2016, 73, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Sarma, D.S.; Kumar, N.; Sharma, S.; Govil, D.; Ali, T.; Mehta, Y.; Rattan, A. Candidemia caused by amphotericin B and Fluconazole resistant Candida auris. Indian J. Med. Microbiol. 2013, 31, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.G.; Shin, J.H.; Uh, Y.; Kang, M.G.; Kim, S.H.; Park, K.H.; Jang, H.-C. First three reported cases of nosocomial fungemia caused by Candida auris. J. Clin. Microbiol. 2011, 49, 3139–3142. [Google Scholar] [CrossRef]
- Vallabhaneni, S.; Kallen, A.T.S.; Chow, N.; Welsh, R. Investigation of the First Seven Reported Cases of Candida auris, a Globally Emerging Invasive, Multidrug-Resistant Fungus—United States, May 2013–August 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1234–1237. [Google Scholar] [CrossRef]
- CDC. Antifungal Susceptibility Testing and Interpretation. Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html (accessed on 6 July 2020).
- Ostrowsky, B.; Greenko, J.; Adams, E.; Quinn, M.; O’Brien, B.; Chaturvedi, V.; Berkow, E.; Vallabhaneni, S.; Forsberg, K.; Chaturvedi, S.; et al. Candida auris Isolates Resistant to Three Classes of Antifungal Medications—New York, 2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Ademe, M.; Girma, F. Candida auris: From multidrug resistance to pan-resistant strains. Infect. Drug Resist. 2020, 13, 1287–1294. [Google Scholar]
- Sekyere, J.O. Candida auris: A systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. Microbiologyopen 2018, 7, e00578. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | N = 47 |
|---|---|
| Age, mean (SD) | 61 (16.3) |
| Male | 35 (74.5%) |
| APACHE II, mean (SD) | 15.1 (7.0) |
| Candida score, mean (SD) | 1.48 (1.18) |
| No risk | 9 (19.2%) |
| Low risk | 23 (48.9%) |
| High risk | 15 (31.9%) |
| Charlson comorbidity index, mean (SD) | 3.0 (2.1) |
| Underlying disease | |
| Abdominal surgery | 14 (29.8%) |
| Digestive disease, not surgical | 9 (19.1%) |
| Cardiovascular disease | 7 (14.9%) |
| Neurovascular disease | 5 (10.6%) |
| COVID-19 | 5 (10.6%) |
| Respiratory disease, not COVID-19 | 3 (6.4%) |
| Otorhinolaringologic disease | 2 (4.3%) |
| Politrauma | 2 (4.3%) |
| Risk factors | |
| Diabetes | 13 (27.7%) |
| Malignancy | 12 (25.5%) |
| Immunosuppression | 2 (4.3%) |
| Renal replacement therapy | 8 (17.0%) |
| Total parenteral nutrition | 7 (14.9%) |
| Sepsis | 10 (21.3%) |
| Surgery (<30 days before candidaemia) | 24 (51.1%) |
| Mechanical ventilation | 29 (61.7%) |
| Central venous catheter | 39 (83.0%) |
| Urinary catheter | 38 (80.9%) |
| ICU stay more than two weeks | 33 (70.2%) |
| Previous antibiotic treatment | 43 (91.5%) |
| Days administered: | |
| <7 days | 3 (6.4%) |
| 7 to 14 days | 13 (27.7%) |
| >15 days | 27 (57.4%) |
| Carbapenem use | 25 (53.2%) |
| Previous antifungal treatment (administered for more than 5 days) | 14 (29.8%) |
| Fluconazole | 2 (4.3%) |
| Voriconazole | 1 (2.1%) |
| Echinocandin | 11 (23.4%) |
| Therapeutic measures | |
| Antifungal treatment | 44 (93.6%) |
| Echinocandin in monotherapy | 22 (46.8%) |
| Echinocandin plus Amphotericin B | 12 (25.6%) |
| Echinocandin plus Isavuconazole | 10 (21.3%) |
| Other measures | |
| Central venous catheter removal | 33 (70.2%) |
| Urinary catheter replacement or removal | 3 (6.4%) |
| Outcome | |
| 30-day mortality | 11 (23.4%) |
| Complications | |
| Endophtalmitis | 2 (4.3%) |
| Recurrence of candidaemia | 7 (14.9%) |
| Persistence of candidaemia | 6 (12.8%) |
| Antifungal | FLZ | AFG | MCF | CFG | AMB | PSC | VRC | ITC | 5FC | ISA |
|---|---|---|---|---|---|---|---|---|---|---|
| Isolates tested | 47 | 47 | 47 | 47 | 47 | 30 | 36 | 28 | 19 | 15 |
| Range | 256 | 0.06–0.25 | 0.03–0.5 | 0.03–0.25 | 0.125–1 | 0.015–0.5 | 0.5–8 | 0.06–0.5 | 0.06–0.25 | 0.03–0.5 |
| MIC50 | 256 | 0.125 | 0.06 | 0.125 | 0.5 | 0.125 | 4 | 0.25 | 0.125 | 0.25 |
| MIC90 | 256 | 0.25 | 0.125 | 0.25 | 1 | 0.25 | 8 | 0.5 | 0.25 | 0.5 |
| GM | 256 | 0.144 | 0.068 | 0.102 | 0.606 | 0.123 | 2.939 | 0.269 | 0.110 | 0.170 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulet Bayona, J.V.; Tormo Palop, N.; Salvador García, C.; Herrero Rodríguez, P.; Abril López de Medrano, V.; Ferrer Gómez, C.; Gimeno Cardona, C. Characteristics and Management of Candidaemia Episodes in an Established Candida auris Outbreak. Antibiotics 2020, 9, 558. https://doi.org/10.3390/antibiotics9090558
Mulet Bayona JV, Tormo Palop N, Salvador García C, Herrero Rodríguez P, Abril López de Medrano V, Ferrer Gómez C, Gimeno Cardona C. Characteristics and Management of Candidaemia Episodes in an Established Candida auris Outbreak. Antibiotics. 2020; 9(9):558. https://doi.org/10.3390/antibiotics9090558
Chicago/Turabian StyleMulet Bayona, Juan V., Nuria Tormo Palop, Carme Salvador García, Paz Herrero Rodríguez, Vicente Abril López de Medrano, Carolina Ferrer Gómez, and Concepción Gimeno Cardona. 2020. "Characteristics and Management of Candidaemia Episodes in an Established Candida auris Outbreak" Antibiotics 9, no. 9: 558. https://doi.org/10.3390/antibiotics9090558
APA StyleMulet Bayona, J. V., Tormo Palop, N., Salvador García, C., Herrero Rodríguez, P., Abril López de Medrano, V., Ferrer Gómez, C., & Gimeno Cardona, C. (2020). Characteristics and Management of Candidaemia Episodes in an Established Candida auris Outbreak. Antibiotics, 9(9), 558. https://doi.org/10.3390/antibiotics9090558




