Ibrexafungerp: A Novel Oral Triterpenoid Antifungal in Development for the Treatment of Candida auris Infections
Abstract
:1. Introduction
2. Ibrexafungerp
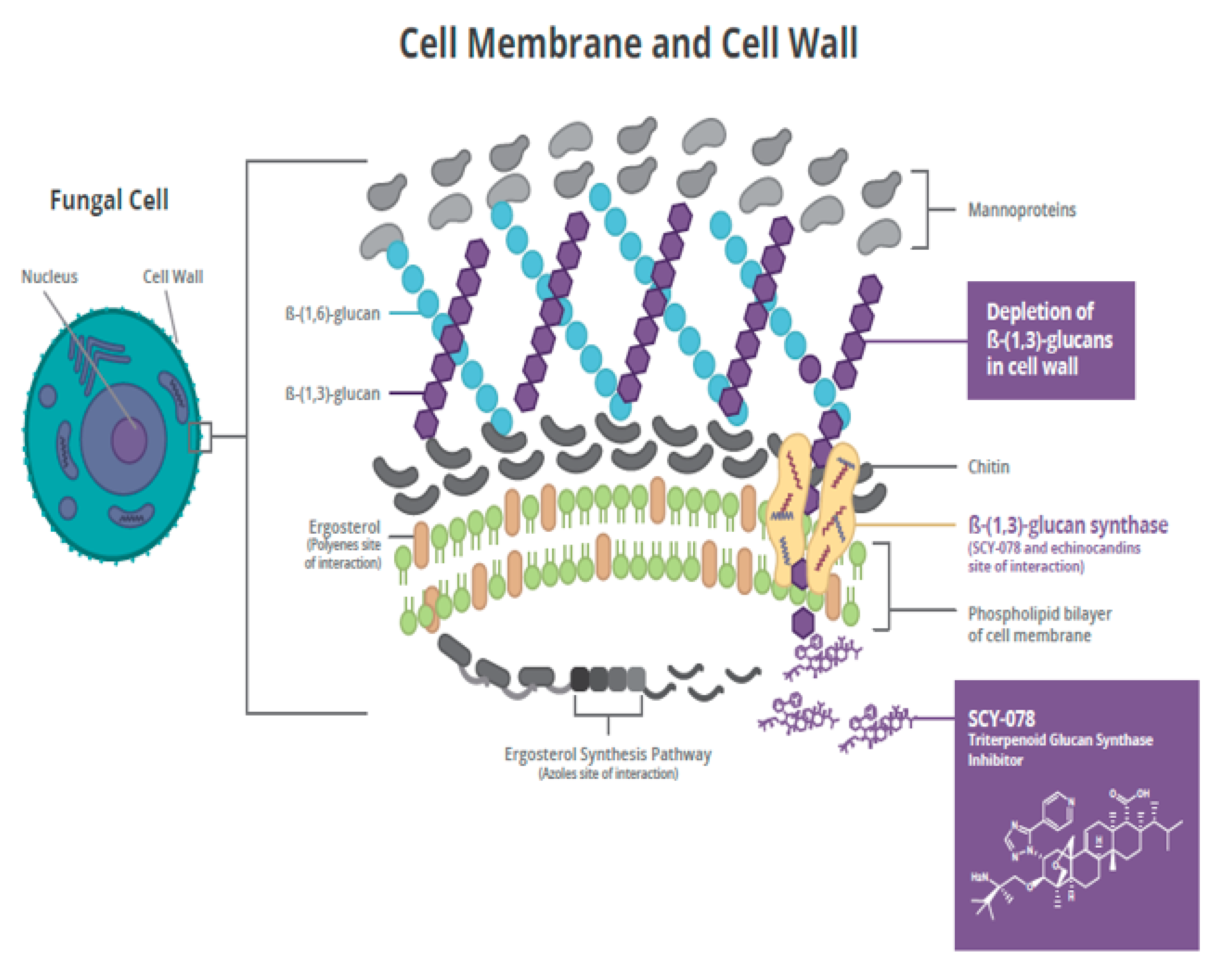
2.1. Mechanism of Action
2.2. In Vitro and In Vivo Activity
2.3. Pharmacokinetics
2.4. Clinical Development
3. Ibrexafungerp for Candida auris
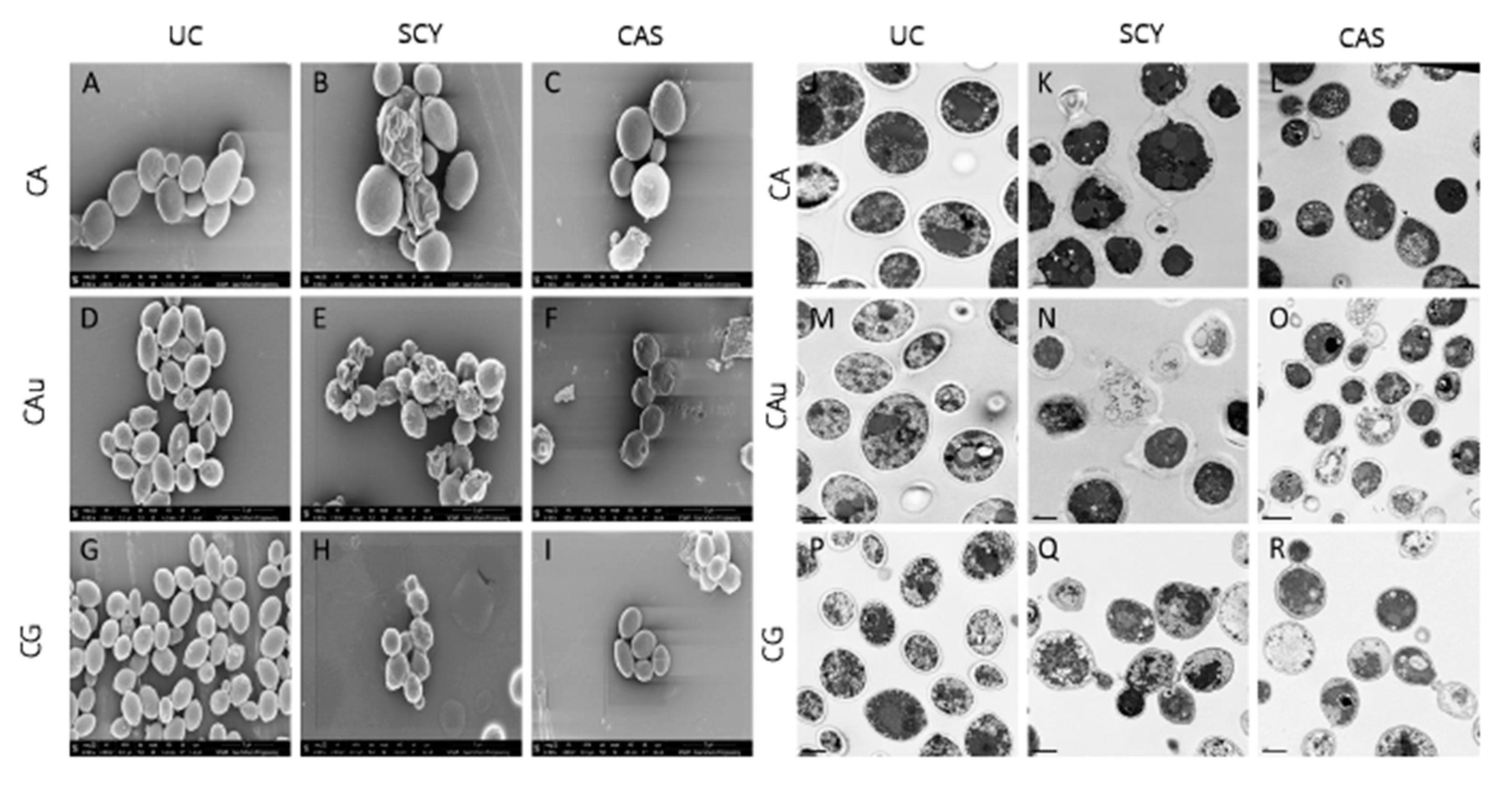
3.1. In Vitro Activity
3.2. In Vivo Activity
3.3. Clinical Experience
3.4. Echinocandin Resistance and C. auris
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Wickes, B.L. Analysis of a Candida auris Outbreak Provides New Insights into an Emerging Pathogen. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Tracking Candida Auris. Available online: https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html (accessed on 30 June 2020).
- Clancy, C.J.; Nguyen, M.H. Emergence of Candida auris: An International Call to Arms. Clin. Infect. Dis. 2016, 64, 141–143. [Google Scholar] [CrossRef] [Green Version]
- Chowdhary, A.; Sharma, C.; Meis, J.F. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLOS Pathog. 2017, 13, e1006290. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; O’Brien, B.; Leach, L.; Clarke, A.; Bates, M.; Adams, E.; Ostrowsky, B.; Quinn, M.; Dufort, E.; Southwick, K.; et al. Laboratory Analysis of an Outbreak of Candida auris in New York from 2016 to 2018: Impact and Lessons Learned. J. Clin. Microbiol. 2019, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabino, R.F.P.; Veríssimo, C.; Pereira, Á.A.; Antunes, F. Candida auris, An Agent of Hospital-Associated Outbreaks: Which Challenging Issues Do We Need to Have in Mind? Microorganisms 2020, 8, 181. [Google Scholar] [CrossRef] [Green Version]
- Ademe, M.; Girma, F. Candida auris: From Multidrug Resistance to Pan-Resistant Strains. Infect. Drug Resist. 2020, 13, 1287–1294. [Google Scholar] [CrossRef]
- Lockhart, S.R. Candida auris and multidrug resistance: Defining the new normal. Fungal Genet. Boil. 2019, 131, 103243. [Google Scholar] [CrossRef]
- Chaabane, F.; Graf, A.; Jequier, L.; Coste, A.T. Review on Antifungal Resistance Mechanisms in the Emerging Pathogen Candida auris. Front. Microbiol. 2019, 10, 2788. [Google Scholar] [CrossRef]
- Forsberg, K.; Woodworth, K.; Walters, M.; Berkow, E.L.; Jackson, B.; Chiller, T.; Vallabhaneni, S. Candida auris: The recent emergence of a multidrug-resistant fungal pathogen. Med. Mycol. 2018, 57, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cortegiani, A.; Misseri, G.; Giarratano, A.; Bassetti, M.; Eyre, D. The global challenge of Candida auris in the intensive care unit. Crit. Care 2019, 23, 150. [Google Scholar] [CrossRef] [Green Version]
- Caceres, D.H.; Forsberg, K.; Welsh, R.M.; Sexton, D.J.; Lockhart, S.R.; Jackson, B.R.; Chiller, T. Candida auris: A Review of Recommendations for Detection and Control in Healthcare Settings. J. Fungi 2019, 5, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, E.; Quinn, M.; Tsay, S.; Poirot, E.; Chaturvedi, S.; Southwick, K.; Greenko, J.; Fernandez, R.; Kallen, A.; Vallabhaneni, S.; et al. Candida auris in Healthcare Facilities, New York, NY, USA, 2013–2017. Emerg. Infect. Dis. 2018, 24, 1816–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, B.; Chaturvedi, S.; Chaturvedi, V. In Vitro Evaluation of Antifungal Drug Combinations against Multidrug-Resistant Candida auris Isolates from New York Outbreak. Antimicrob. Agents Chemother. 2020, 64, e02195-19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Barat, S.A.; Borroto-Esoda, K.; Angulo, D.; Chaturvedi, S.; Chaturvedi, V. Pan-resistant Candida auris isolates from the outbreak in New York are susceptible to ibrexafungerp (a glucan synthase inhibitor). Int. J. Antimicrob. Agents 2020, 55, 105922. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Arensman, K.; Miller, J.L.; Chiang, A.; Mai, N.; Levato, J.; Lachance, E.; Anderson, M.; Beganovic, M.; Dela-Pena, J. Clinical Outcomes of Patients Treated for Candida auris Infections in a Multisite Health System, IL, USA. Emerg. Infect. Dis. 2020, 26, 876–880. [Google Scholar] [CrossRef]
- Ostrowsky, B.; Greenko, J.; Adams, E.; Quinn, M.; O’Brien, B.; Chaturvedi, V.; Berkow, E.; Vallabhaneni, S.; Forsberg, K.; Chaturvedi, S.; et al. Candida auris Isolates Resistant to Three Classes of Antifungal Medications—New York. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 6–9. [Google Scholar] [CrossRef] [Green Version]
- Heasley, B.; Pacofsky, G.J.; Mamai, A.; Liu, H.; Nelson, K.; Coti, G.; Peel, M.R.; Balkovec, J.M.; Greenlee, M.L.; Liberator, P.; et al. Synthesis and biological evaluation of antifungal derivatives of enfumafungin as orally bioavailable inhibitors of β-1,3-glucan synthase. Bioorg. Med. Chem. Lett. 2012, 22, 6811–6816. [Google Scholar] [CrossRef]
- Davis, M.R.; Donnelley, M.A.; Thompson, G.R. Ibrexafungerp: A novel oral glucan synthase inhibitor. Med. Mycol. 2020, 58, 579–592. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Messer, S.A.; Motyl, M.R.; Jones, R.N.; Castanheira, M. In Vitro Activity of a New Oral Glucan Synthase Inhibitor (MK-3118) Tested against Aspergillus spp. by CLSI and EUCAST Broth Microdilution Methods. Antimicrob. Agents Chemother. 2013, 57, 1065–1068. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Ortigosa, C.; Perez, W.B.; Angulo, D.; Borroto-Esoda, K.; Perlin, D.S. De Novo Acquisition of Resistance to SCY-078 in Candida glabrata Involves FKS Mutations That both Overlap and Are Distinct from Those Conferring Echinocandin Resistance. Antimicrob. Agents Chemother. 2017, 61, e00833-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaller, M.A.; Messer, S.A.; Rhomberg, P.R.; Borroto-Esoda, K.; Castanheira, M. Differential Activity of the Oral Glucan Synthase Inhibitor SCY-078 against Wild-Type and Echinocandin-Resistant Strains of Candida Species. Antimicrob. Agents Chemother. 2017, 61, e00161-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkow, E.L.; Angulo, D.; Lockhart, S.R. In Vitro Activity of a Novel Glucan Synthase Inhibitor, SCY-078, against Clinical Isolates of Candida auris. Antimicrob. Agents Chemother. 2017, 61, e00435-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghannoum, M.; Long, L.; Isham, N.; Hager, C.; Wilson, R.; Borroto-Esoda, K.; Barat, S.; Angulo, D. Activity of a Novel 1,3-Beta-D-Glucan Synthase Inhibitor, Ibrexafungerp (Formerly SCY-078), against Candida glabrata. Antimicrob. Agents Chemother. 2019, 63, AAC.01510-19. [Google Scholar] [CrossRef]
- Ghannoum, M.; Long, L.; Larkin, E.L.; Isham, N.; Sherif, R.; Borroto-Esoda, K.; Barat, S.; Angulo, D. Evaluation of the Antifungal Activity of the Novel Oral Glucan Synthase Inhibitor SCY-078, Singly and in Combination, for the Treatment of Invasive Aspergillosis. Antimicrob. Agents Chemother. 2018, 62, e00244-18. [Google Scholar] [CrossRef] [Green Version]
- Larkin, E.L.; Hager, C.; Chandra, J.; Mukherjee, P.K.; Retuerto, M.; Salem, I.; Long, L.; Isham, N.; Kovanda, L.; Borroto-Esoda, K.; et al. The Emerging Pathogen Candida auris: Growth Phenotype, Virulence Factors, Activity of Antifungals, and Effect of SCY-078, a Novel Glucan Synthesis Inhibitor, on Growth Morphology and Biofilm Formation. Antimicrob. Agents Chemother. 2017, 61, e02396-16. [Google Scholar] [CrossRef] [Green Version]
- Marcos-Zambrano, L.J.; Gómez-Perosanz, M.; Escribano, P.; Bouza, E.; Guinea, J. The novel oral glucan synthase inhibitor SCY-078 shows in vitro activity against sessile and planktonic Candida spp. J. Antimicrob. Chemother. 2017, 72, 1969–1976. [Google Scholar] [CrossRef] [Green Version]
- Nunnally, N.S.; Etienne, K.A.; Angulo, D.; Lockhart, S.R.; Berkow, E.L. In Vitro Activity of Ibrexafungerp, a Novel Glucan Synthase Inhibitor against Candida glabrata Isolates with FKS Mutations. Antimicrob. Agents Chemother. 2019, 63, E01692-19. [Google Scholar] [CrossRef] [Green Version]
- Rautermaa-Richardson, R.; Moore, C.B.; Rawson, K. Aspergillus isolates from patients with chronic pulmonary aspergillosis mycologically and clinically resistant to azole antifungals are sensitive to ibrexafungerp (SCY-078). Presented at the 9th Congress on Trends in Medical Mycology, Nice, France, 11–14 October 2019. [Google Scholar]
- Scorneaux, B.; Angulo, D.; Borroto-Esoda, K.; Ghannoum, M.; Peel, M.; Wring, S.A. SCY-078 Is Fungicidal against Candida Species in Time-Kill Studies. Antimicrob. Agents Chemother. 2017, 61, e01961-16. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.C.; Barat, S.A.; Borroto-Esoda, K.; Angulo, D.; Chaturvedi, S.; Chaturvedi, V. In vitro Efficacy of Novel Glucan Synthase Inhibitor, Ibrexafungerp (SCY-078), Against Multidrug- and Pan-resistant Candida auris Isolates from the Outbreak in New York. BioRxiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Arendrup, M.; Jørgensen, K.M.; Hare, R.K.; Chowdhary, A. In Vitro Activity of Ibrexafungerp (SCY-078) against Candida auris Isolates as Determined by EUCAST Methodology and Comparison with Activity against C. albicans and C. glabrata and with the Activities of Six Comparator Agents. Antimicrob. Agents Chemother. 2020, 64, e02136-19. [Google Scholar] [CrossRef] [PubMed]
- Barat, S.; Borroto-Esoda, A.D. Ibrexafungerp (SCY-078): A first-in-class, orally-bioavailable, glucan synthase inhibitor has fungicidal activity against C. auris, an emerging multidrug-resistant pathogen. Presented at the 2018 Mycoses Study Group Education and Research Consortium, Big Sky, MT, USA, 25–28 September 2018. [Google Scholar]
- Wiederhold, N.P.; Najvar, L.K.; Jaramillo, R.; Olivo, M.; Pizzini, J.; Catano, G.; Patterson, T.F. Oral glucan synthase inhibitor SCY-078 is effective in an experimental murine model of invasive candidiasis caused by WT and echinocandin-resistant Candida glabrata. J. Antimicrob. Chemother. 2018, 73, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.; Isham, N.; Angulo, D.; Borroto-Esoda, K.; Barat, S.; Long, L. Efficacy of Ibrexafungerp (SCY-078) Against Candida auris in an In Vivo Guinea Pig Cutaneous Infection Model. Antimicrob. Agents Chemother. 2020, AAC.00854-20. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.; Long, L.; Hager, C.; Borroto-Esoda, K.; Barat, S.; Angulo, D. Efficacy of oral ibrexafungerp (IBX, Formerly SCY-078) in the treatment of Candida auris infection in a murine disseminated model. ASM Microbe 2019. [Google Scholar] [CrossRef]
- Lepak, A.J.; Marchillo, K.; Andes, D.R. Pharmacodynamic Target Evaluation of a Novel Oral Glucan Synthase Inhibitor, SCY-078 (MK-3118), Using anIn VivoMurine Invasive Candidiasis Model. Antimicrob. Agents Chemother. 2015, 59, 1265–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkin, E.L.; Long, L.; Isham, N.; Borroto-Esoda, K.; Barat, S.; Angulo, D.; Wring, S.; Ghannoum, M. A Novel 1,3-Beta-D-Glucan Inhibitor, Ibrexafungerp (Formerly SCY-078), Shows Potent Activity in the Lower pH Environment of Vulvovaginitis. Antimicrob. Agents Chemother. 2019, 63, e02611-18. [Google Scholar] [CrossRef] [Green Version]
- Wring, S.A.; Randolph, R.; Park, S.; Abruzzo, G.; Chen, Q.; Flattery, A.; Garrett, G.; Peel, M.; Outcalt, R.; Powell, K.; et al. Preclinical Pharmacokinetics and Pharmacodynamic Target of SCY-078, a First-in-Class Orally Active Antifungal Glucan Synthesis Inhibitor, in Murine Models of Disseminated Candidiasis. Antimicrob. Agents Chemother. 2017, 61, e02068-16. [Google Scholar] [CrossRef] [Green Version]
- Wring, S.; Borroto-Esoda, K.; Solon, E.; Angulo, D. SCY-078, a Novel Fungicidal Agent, Demonstrates Distribution to Tissues Associated with Fungal Infections during Mass Balance Studies with Intravenous and Oral [14C]SCY-078 in Albino and Pigmented Rats. Antimicrob. Agents Chemother. 2019, 63, e02119-18. [Google Scholar] [CrossRef] [Green Version]
- Wring, S.; Murphy, G.; Atiee, G.; Corr, C.; Hyman, M.; Willett, M.; Angulo, D. Clinical Pharmacokinetics and Drug-Drug Interaction Potential for Coadministered SCY-078, an Oral Fungicidal Glucan Synthase Inhibitor, and Tacrolimus. Clin. Pharmacol. Drug Dev. 2018, 8, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Trucksis, M.; Garrett, G.; Heirman, I. A phase I single rising dose study evaluating the safety, tolerability and pharmacokinetics of an oral glucan synthase inhibitor in healthy male volunteers. In Proceedings of the 50th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Boston, MA, USA, 12–15 September 2010. [Google Scholar]
- Murphy, G.; Darpo, B.; Marbury, T. Lack of an effect of SCY-078 a novel antifungal agent on QTc interval in healthy subjects. In Abstracts of ASM Microbe; ASM Press: Washington, DC, USA, 2017; Abstract 172. [Google Scholar]
- Wring, S.; Murphy, G.; Atiee, G.; Corr, C.; Hyman, M.; Willett, M.; Angulo, D. Lack of Impact by SCY-078, a First-in-Class Oral Fungicidal Glucan Synthase Inhibitor, on the Pharmacokinetics of Rosiglitazone, a Substrate for CYP450 2C8, Supports the Low Risk for Clinically Relevant Metabolic Drug-Drug Interactions. J. Clin. Pharmacol. 2018, 58, 1305–1313. [Google Scholar] [CrossRef]
- Murphy, G.; Hyman, M.; Willett, M. CYP-mediated drug interaction profile of SCY-078, a novel glucan synthase inhibitor (GSI). In Abstracts of ASM Microbe; ASM Press: Washington, DC, USA, 2017; Abstract 173. [Google Scholar]
- Barat, S.; Borroto-Esoda, A.D. Ibrexafungerp demonstrates potent and consistent in vitro activity against >400 global Candida auris isolates, including isolates with elevated MIC’s to echinocandins. In Proceedings of the ECCMID, Paris, France, 18–21 April 2020. [Google Scholar]
- Sayeed, M.A.; Farooqi, J.; Jabeen, K.; Mahmood, S.F. Comparison of risk factors and outcomes of Candida auris candidemia with non-Candida auris candidemia: A retrospective study from Pakistan. Med Mycol. 2019, 58, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Akrabarti, A. Candida auris candidaemia in an intensive care ujit-prospective observational study to evaluate epidemiology, risk factors, and outcome. J. Crit. Care 2020, 57, 42–48. [Google Scholar] [CrossRef]
- Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. In vitro growth and analysis of Candida biofilms. Nat. Protoc. 2008, 3, 1909–1924. [Google Scholar] [CrossRef]
- Hager, C.L.; Sun, P.-L.; Fujioka, H. Morphological effect of SCY-078 and caspofungin on different caspofungin-resistant Candida species. Presented at ASM Microbe, Atlanta, GA, USA, 8–10 June 2018. [Google Scholar]
- Juneja, D.; Singh, O.; Tarai, B.; Angulo, D. Sucessful Treatment of Two patienTs with Candida Auris Candidemia with the Investigational Agent, Oral Ibrexafungerp (formerly SCY-078), from the CARES Study; European Congress of Clinical Microbiology and Infectious Diseases (ECCMID): Amsterdam, The Netherlands, 2019; Poster no. L0028. [Google Scholar]
- Chowdhary, A.; Prakash, A.; Sharma, C.; Kordalewska, M.; Kumar, A.; Sarma, S.; Tarai, B.; Singh, A.; Upadhyaya, G.; Upadhyay, S.; et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009-17) in India: Role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J. Antimicrob. Chemother. 2018, 73, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Khan, Z.; Al-Sweih, N.; Alfouzan, W.; Joseph, L. Candida auris in various hospitals across Kuwait and their susceptibility and molecular basis of resistance to antifungal drugs. Mycoses 2020, 63, 104–112. [Google Scholar] [CrossRef]
- Kordalewska, M.; Lee, A.; Park, S.; Berrio, I.; Chowdhary, A.; Zhao, Y.; Perlin, D.S. Understanding Echinocandin Resistance in the Emerging Pathogen Candida auris. Antimicrob. Agents Chemother. 2018, 62, e00238-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biagi, M.J.; Wiederhold, N.P.; Gibas, C.; Wickes, B.L.; Lozano, V.; Bleasdale, S.C.; Danziger, L. Development of High-Level Echinocandin Resistance in a Patient with Recurrent Candida auris Candidemia Secondary to Chronic Candiduria. Open Forum Infect. Dis. 2019, 6. [Google Scholar] [CrossRef]
- Rhodes, J.; Abdolrasouli, A.; Farrer, R.A. Genomic epidemiology of the UK outbreak of the emerging human fungal pathogen Candida auris. Emerg. Microbes Infect. 2018, 7, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petraitis, V.; Petraitiene, R.; Katragkou, A.; Maung, B.B.W.; Naing, E.; Kavaliauskas, P.; Barat, S.; Borroto-Esoda, K.; Azie, N.; Angulo, D.; et al. Combination Therapy with Ibrexafungerp (Formerly SCY-078), a First-in-Class Triterpenoid Inhibitor of (1→3)-β-D-Glucan Synthesis, and Isavuconazole for Treatment of Experimental Invasive Pulmonary Aspergillosis. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Spec, A.; Pullman, J.; Thompson, G.R., III; Powderly, W.G.; Tobin, E.H.; Vazquez, J.; Wring, S.A.; Angulo, D.; Helou, S.; Mycoses Study Group; et al. MSG-10: A Phase 2 study of oral ibrexafungerp (SCY-078) following initial echinocandin therapy in non-neutropenic patients with invasive candidiasis. J. Antimicrob. Chemother. 2019, 74, 3056–3062. [Google Scholar] [CrossRef] [Green Version]

| Drug (No. of Isolates) | MIC50 a | Modal MIC | MIC Range |
|---|---|---|---|
| Ibrexafungerp (n = 122) | 0.5 | 0.5 | 0.06–2 |
| Anidulafungin | 0.125 | 0.06 | 0.016–>32 |
| Micafungin | 0.125 | 0.125 | 0.03–>32 |
| Amphotericin B | 1 | 1 | 0.5–1 |
| Fluconazole | ≥64 | ≥64 | 0.5–≥64 |
| Voriconazole | 0.5 | Bimodal | ≤0.004–4 |
| Isavuconazole | 0.125 | Trimodal | ≤0.004–2 |
| Reference | No. of Isolates | MIC, μg/mL | |||
|---|---|---|---|---|---|
| MIC50 | MIC90 | Mode | MIC Range | ||
| Berkow et al., 2017 [24] | 107 | 1 | 1 | 1 | 0.0625–2 |
| Larkin et al., 2017 [27] | 16 | 1 | 1 | 1 | 0.5–1 |
| Zhu et al., 2020 [32] | 200 | 0.5 | 1 | 0.5 | 0.0625–8 |
| Arendrup et al., 2020 [33] | 122 | 0.5 | 1 | 0.5 | 0.0625–2 |
| Overall | 445 | 0.5 | 1 | 0.5 | 0.625–8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghannoum, M.; Arendrup, M.C.; Chaturvedi, V.P.; Lockhart, S.R.; McCormick, T.S.; Chaturvedi, S.; Berkow, E.L.; Juneja, D.; Tarai, B.; Azie, N.; et al. Ibrexafungerp: A Novel Oral Triterpenoid Antifungal in Development for the Treatment of Candida auris Infections. Antibiotics 2020, 9, 539. https://doi.org/10.3390/antibiotics9090539
Ghannoum M, Arendrup MC, Chaturvedi VP, Lockhart SR, McCormick TS, Chaturvedi S, Berkow EL, Juneja D, Tarai B, Azie N, et al. Ibrexafungerp: A Novel Oral Triterpenoid Antifungal in Development for the Treatment of Candida auris Infections. Antibiotics. 2020; 9(9):539. https://doi.org/10.3390/antibiotics9090539
Chicago/Turabian StyleGhannoum, Mahmoud, Maiken Cavling Arendrup, Vishnu P. Chaturvedi, Shawn R. Lockhart, Thomas S. McCormick, Sudha Chaturvedi, Elizabeth L. Berkow, Deven Juneja, Bansidhar Tarai, Nkechi Azie, and et al. 2020. "Ibrexafungerp: A Novel Oral Triterpenoid Antifungal in Development for the Treatment of Candida auris Infections" Antibiotics 9, no. 9: 539. https://doi.org/10.3390/antibiotics9090539
APA StyleGhannoum, M., Arendrup, M. C., Chaturvedi, V. P., Lockhart, S. R., McCormick, T. S., Chaturvedi, S., Berkow, E. L., Juneja, D., Tarai, B., Azie, N., Angulo, D., & Walsh, T. J. (2020). Ibrexafungerp: A Novel Oral Triterpenoid Antifungal in Development for the Treatment of Candida auris Infections. Antibiotics, 9(9), 539. https://doi.org/10.3390/antibiotics9090539








