Controlled Fermentation Using Autochthonous Lactobacillus plantarum Improves Antimicrobial Potential of Chinese Chives against Poultry Pathogens
Abstract
1. Introduction
2. Results
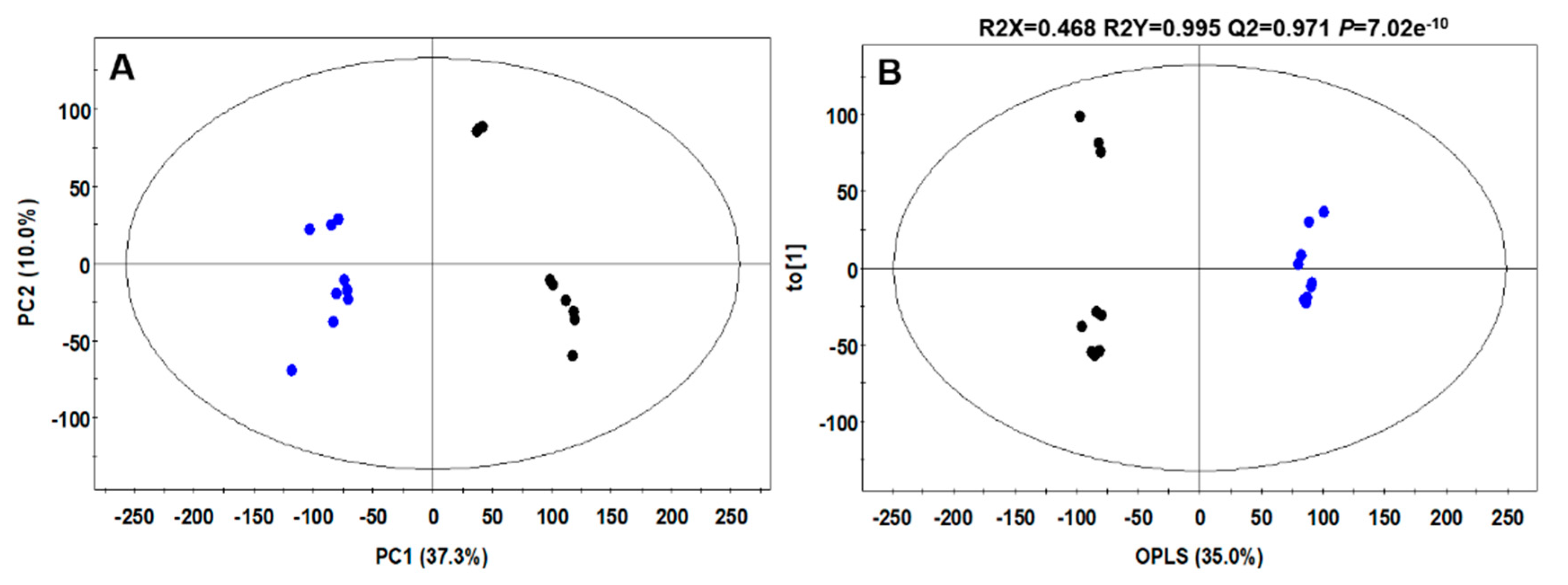
2.1. Selection of Autochthonous Starter Culture
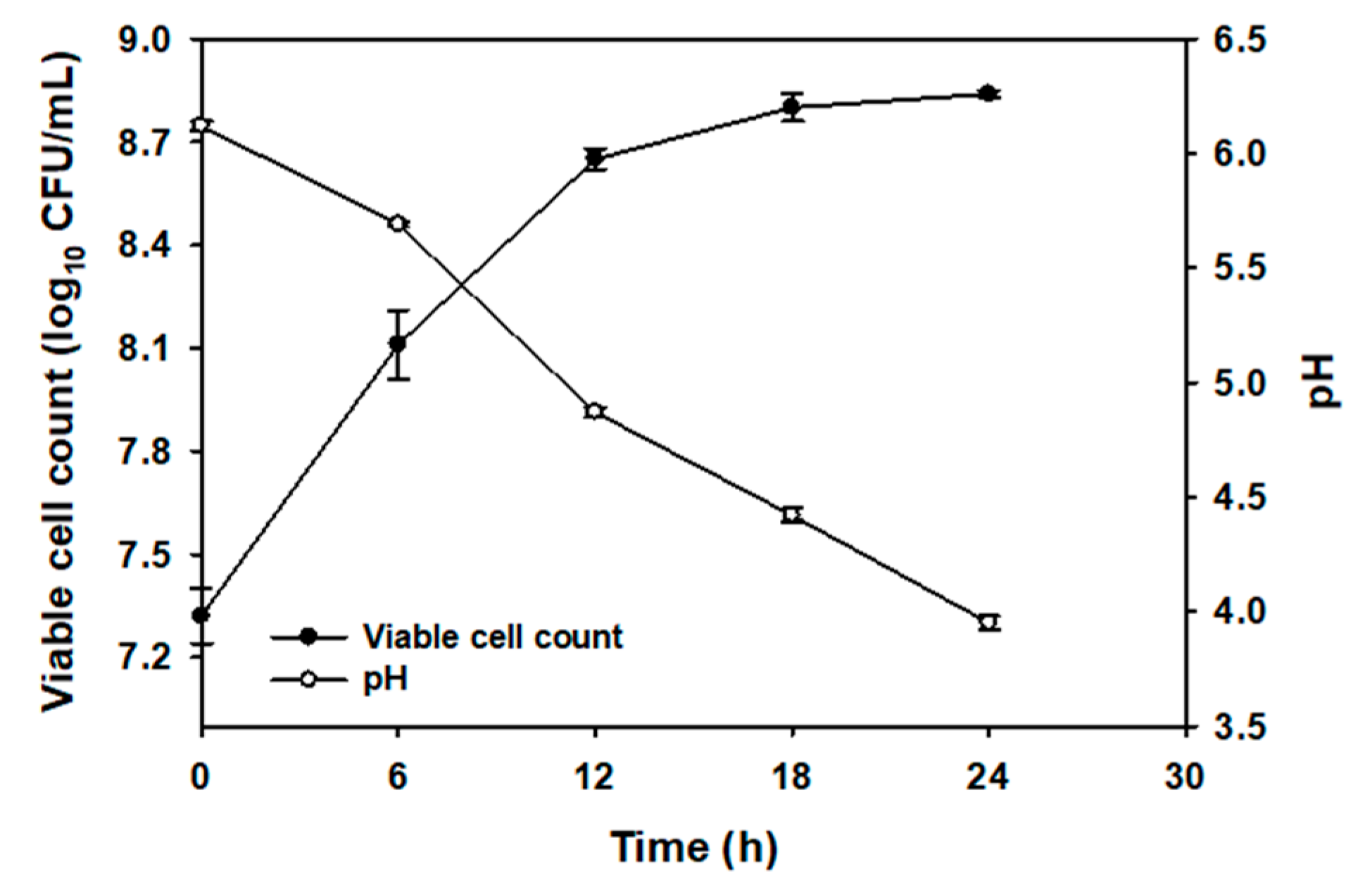
2.2. Microbial Population and pH Changes During Fermentation of CC Juice
2.3. Changes in the Bioactivities of CC Juice Following the L. plantarum Mediated Fermentation
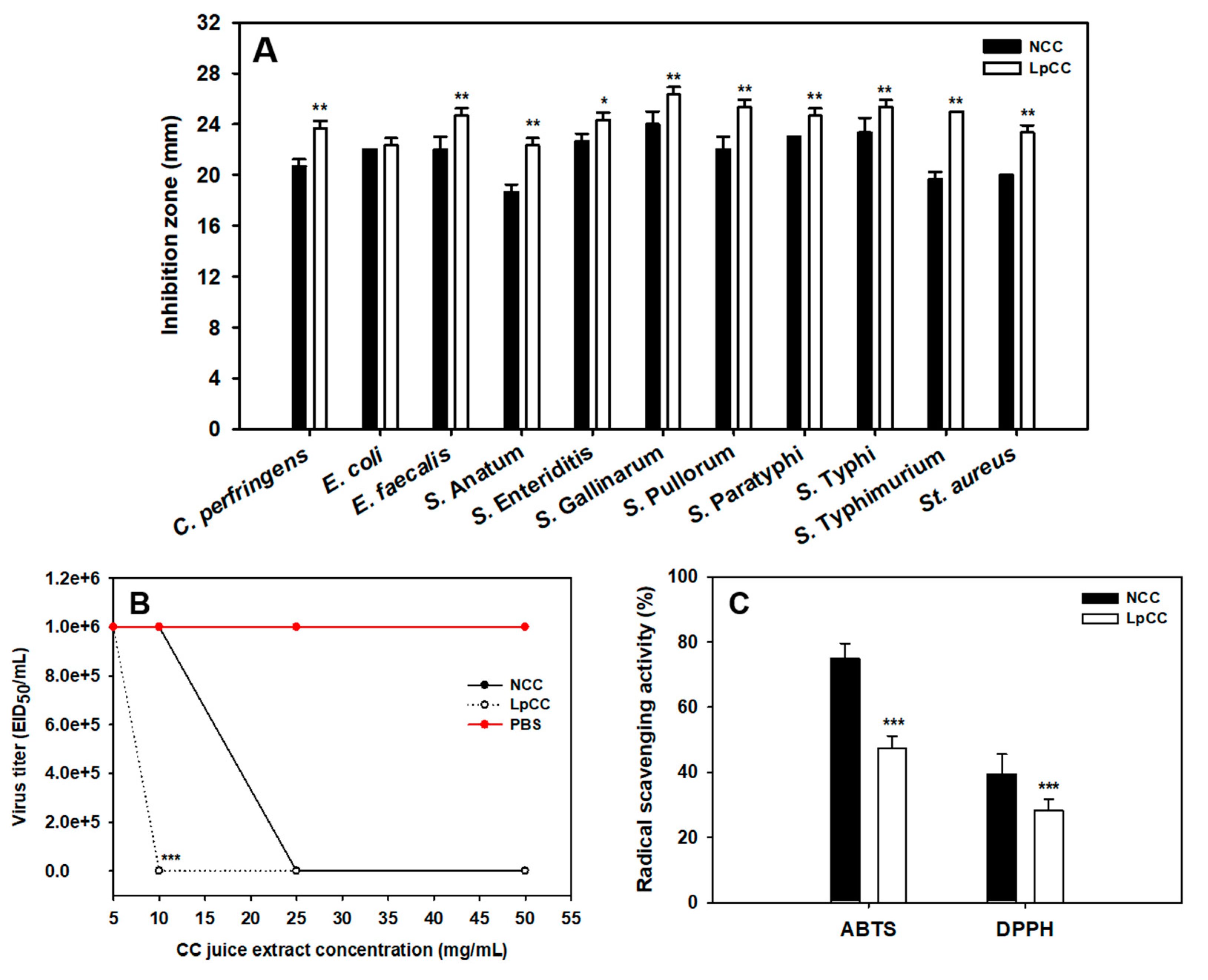
2.3.1. Antibacterial Activity
2.3.2. Antiviral Activity
2.3.3. Antioxidant Activity
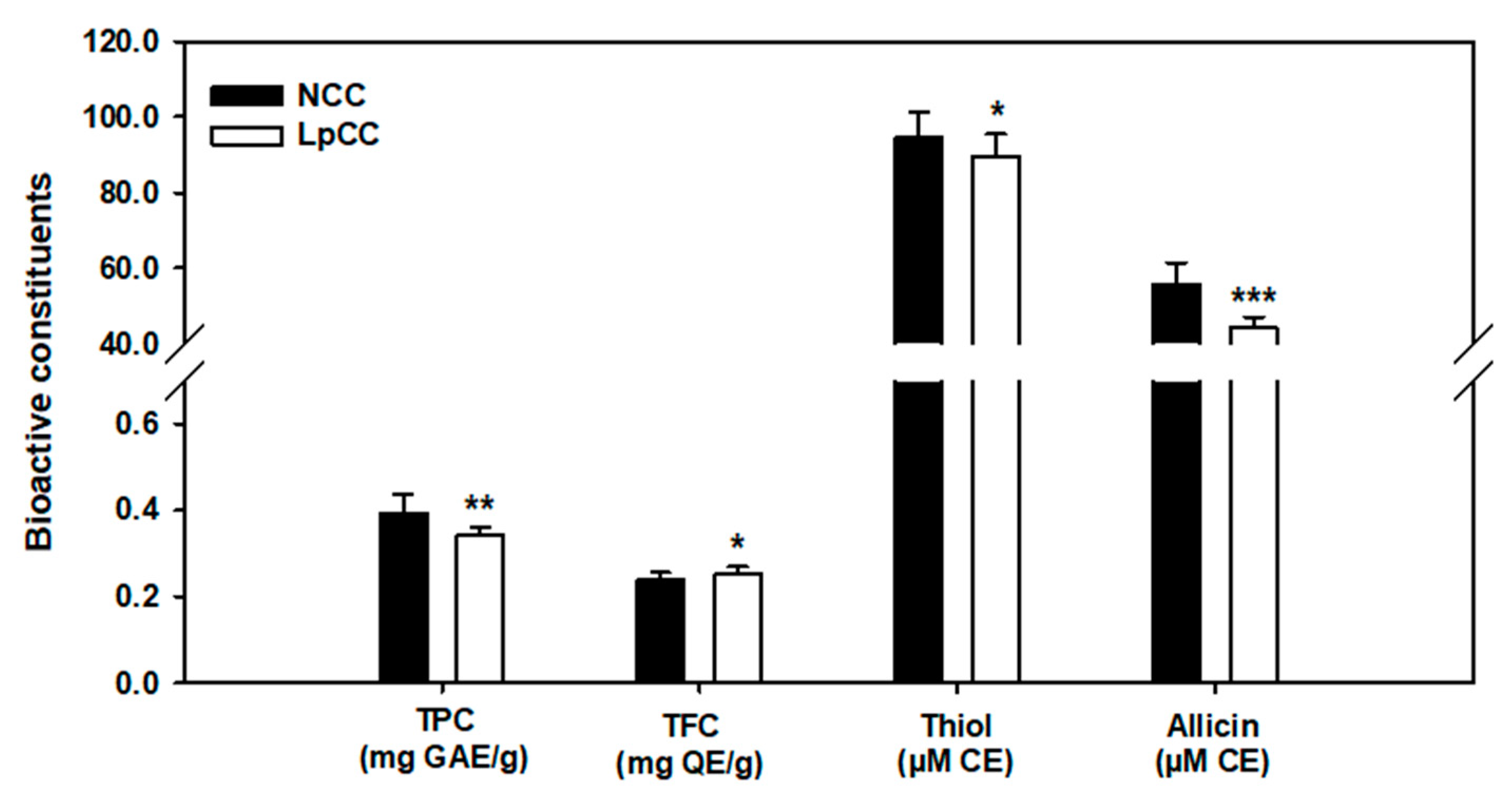
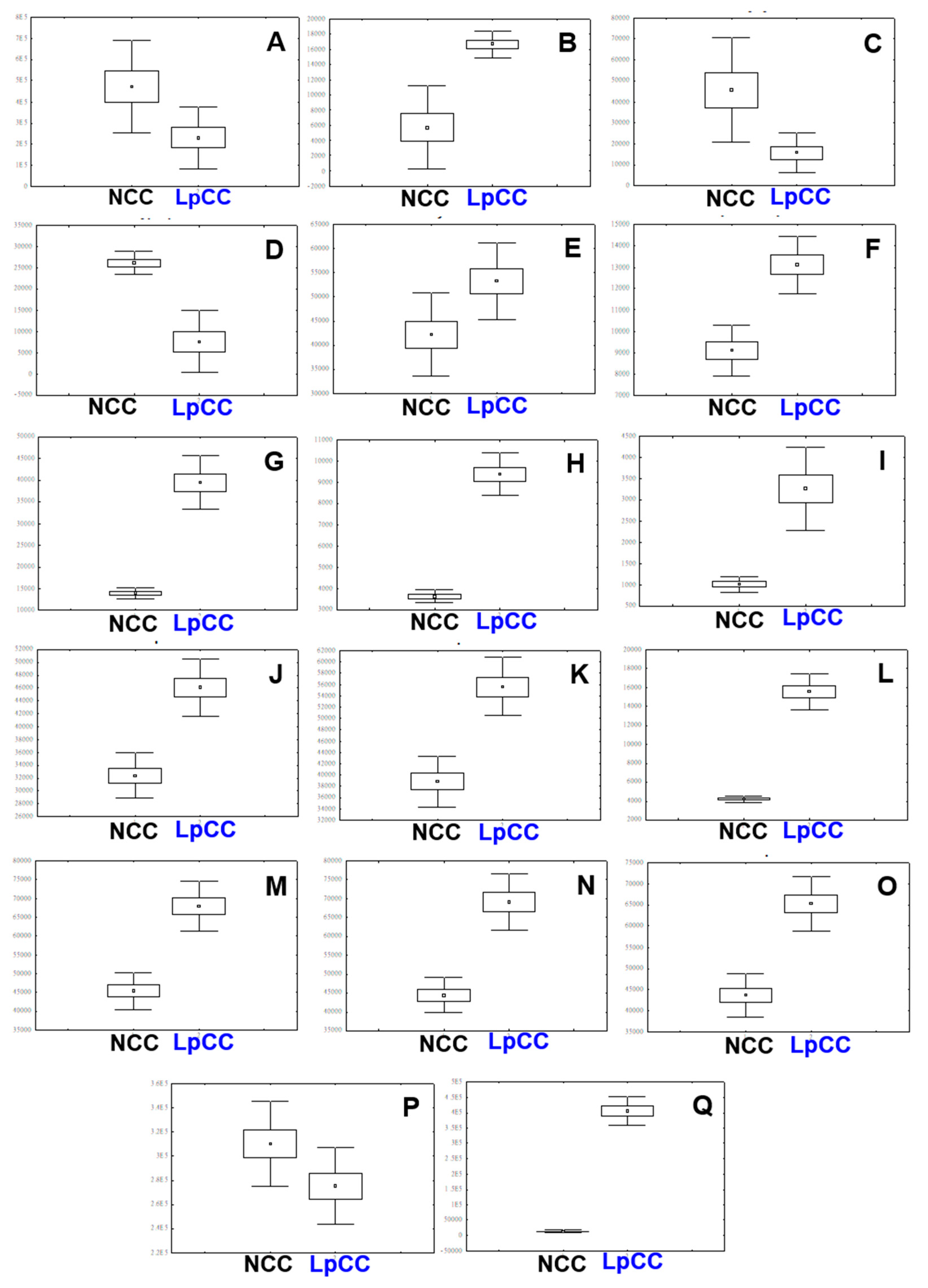
2.4. Biochemical Changes
3. Discussion
4. Materials and Methods
4.1. Isolation, Identification, and Screening of Microorganisms from CC Juice
4.2. Fermentation of CC Juice
4.3. Antibacterial Activity Using Agar Well-Diffusion Assay
4.4. Preparation of Extracts
4.5. Antiviral Activity Using Hemagglutination Assay
4.6. Antioxidant Assays
4.7. Biochemical Constituent Analysis
4.7.1. Total Phenolic Content (TPC)
4.7.2. Total Flavonoid Content (TFC)
4.7.3. Thiol and Allicin Contents
4.7.4. UHPLC-LTQ-Orbitrap-MS/MS Analysis
4.7.5. Data Processing and Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial resistance in bacterial poultry pathogens: A review. Front. Vet. Sci. 2017, 4, 126. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Osman, K.M.; Kappell, A.D.; Elhadidy, M.; ElMougy, F.; El-Ghany, W.A.A.; Orabi, A.; Mubarak, A.S.; Dawoud, T.M.; Hemeg, H.A.; Moussa, I.M. Poultry hatcheries as potential reservoirs for antimicrobial-resistant Escherichia coli: A risk to public health and food safety. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Collignon, P.; Aarestrup, F.M.; Irwin, R.; McEwen, S. Human deaths and third-generation cephalosporin use in poultry, Europe. Emerg. Infect. Dis. 2013, 19, 1339–1340. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Suresh, G.; Das, R.K.; Kaur Brar, S.; Rouissi, T.; Avalos Ramirez, A.; Chorfi, Y.; Godbout, S. Alternatives to antibiotics in poultry feed: Molecular perspectives. Crit. Rev. Microbiol. 2018, 44, 318–335. [Google Scholar] [CrossRef]
- Salem, W.; El-Hamed, D.S.; Sayed, W.; Elamary, R. Alterations in virulence and antibiotic resistant genes of multidrug-resistant Salmonella serovars isolated from poultry: The bactericidal efficacy of Allium sativum. Microb. Pathog. 2017, 108, 91–100. [Google Scholar] [CrossRef]
- Pan, M.; Wu, Q.; Tao, X.; Wan, C.; Shah, N.P.; Wei, H. Fermentation of Allium chinense bulbs with Lactobacillus plantarum ZDY 2013 shows enhanced biofunctionalities, and nutritional and chemical properties. J. Food Sci. 2015, 80, 2272–2278. [Google Scholar] [CrossRef]
- Kyung, K.H. Antimicrobial properties of allium species. Curr. Opin. Biotechnol. 2012, 23, 142–147. [Google Scholar] [CrossRef]
- Yang, E.J.; Kim, S.I.; Park, S.Y.; Bang, H.Y.; Jeong, J.H.; So, J.H.; Rhee, I.K.; Song, K.S. Fermentation enhances the in vitro antioxidative effect of onion (Allium cepa) via an increase in quercetin content. Food Chem. Toxicol. 2012, 50, 2042–2048. [Google Scholar] [CrossRef]
- Elmowalid, G.A.; El-Hamid, M.I.A.; El-Wahab, A.M.A.; Atta, M.; El-Naser, G.A.; Attia, A.M. Garlic and ginger extracts modulated broiler chicks innate immune responses and enhanced multidrug resistant Escherichia coli O78 clearance. Comp. Immunol. Microbiol. Infect. Dis. 2019, 66, 101334. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, S.-L.; Lee, S.; Lee, S.-Y.; Ko, S.; Yoo, M. UPLC/ESI-MS/MS analysis of compositional changes for organosulfur compounds in garlic (Allium sativum L.) during fermentation. Food Chem. 2016, 211, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Millet, A.S.; Lamy, E.; Jonas, D.; Stintzing, F.; Mersch-Sundermann, V.; Merfort, I. Fermentation enhances the biological activity of Allium cepa bulb extracts. J. Agric. Food Chem. 2012, 60, 2148–2156. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Cui, C.; Ruan, Z. Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Lee, J.H.; Jin, Y.H.; Park, Y.K.; Yun, S.J.; Mah, J.H. Formation of biogenic amines in Pa (green onion) kimchi and Gat (mustard leaf) kimchi. Foods 2019, 8, 109. [Google Scholar] [CrossRef]
- Ye, J.H.; Huang, L.Y.; Terefe, N.S.; Augustin, M.A. Fermentation-based biotransformation of glucosinolates, phenolics and sugars in retorted broccoli puree by lactic acid bacteria. Food Chem. 2019, 286, 616–623. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- Torres, S.; Verón, H.; Contreras, L.; Isla, M.I. An overview of plant-autochthonous microorganisms and fermented vegetable foods. Food Sci. Hum. Wellness. 2020, in press. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Dec, M.; Puchalski, A.; Urban-Chmiel, R.; Wernicki, A. Screening of Lactobacillus strains of domestic goose origin against bacterial poultry pathogens for use as probiotics. Poult. Sci. 2014, 93, 2464–2472. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Peng, Q.; Jia, H.; Zeng, X.; Zhu, J.; Hou, C.; Liu, X.; Yang, F.; Qiao, S. Prevention of Escherichia coli infection in broiler chickens with Lactobacillus plantarum B1. Poult. Sci. 2017, 96, 2576–2586. [Google Scholar] [CrossRef]
- Torino, M.I.; Limón, R.I.; Martínez-Villaluenga, C.; Mäkinen, S.; Pihlanto, A.; Vidal-Valverde, C.; Frias, J. Antioxidant and antihypertensive properties of liquid and solid-state fermented lentils. Food Chem. 2013, 136, 1030–1037. [Google Scholar] [CrossRef]
- Wouters, D.; Bernaert, N.; Conjaerts, W.; Van Droogenbroeck, B.; De Loose, M.; De Vuyst, L. Species diversity, community dynamics, and metabolite kinetics of spontaneous leek fermentations. Food Microbiol. 2013, 33, 185–196. [Google Scholar] [CrossRef]
- Xiong, T.; Song, S.; Huang, X.; Feng, C.; Liu, G.; Huang, J.; Xie, M. Screening and identification of functional Lactobacillus specific for vegetable fermentation. J. Food Sci. 2013, 78, 84–89. [Google Scholar] [CrossRef]
- Casella, S.; Leonardi, M.; Melai, B.; Fratini, F.; Pistelli, L. The role of diallyl sulfides and dipropyl sulfides in the in vitro antimicrobial activity of the essential oil of garlic, Allium sativum L., and leek, Allium porrum L. Phytother. Res. 2013, 27, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Gabric, D.; Roohinejad, S.; Barba, F.J.; Granato, D.; Mallikarjunan, K.; Lorenzo, J.M.; Bursac Kovacevic, D. An overview of organosulfur compounds from Allium spp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem. 2019, 276, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Ming, T.; Han, J.; Li, Y.; Lu, C.; Qiu, D.; Li, Y.; Zhou, J.; Su, X. A metabolomics and proteomics study of the Lactobacillus plantarum in the grass carp fermentation. BMC Microbiol. 2018, 18, 216. [Google Scholar] [CrossRef]
- Brizuela, N.; Tymczyszyn, E.E.; Semorile, L.C.; La Hens, D.V.; Delfederico, L.; Hollmann, A.; Bravo-Ferrada, B. Lactobacillus plantarum as a malolactic starter culture in winemaking: A new (old) player? Electron. J. Biotechnol. 2019, 38, 10–18. [Google Scholar] [CrossRef]
- Othman, N.B.; Roblain, D.; Chammen, N.; Thonart, P.; Hamdi, M. Antioxidant phenolic compounds loss during the fermentation of Chétoui olives. Food Chem. 2009, 116, 662–669. [Google Scholar] [CrossRef]
- Park, D.H.; Kothari, D.; Niu, K.-M.; Han, S.G.; Yoon, J.E.; Lee, H.-G.; Kim, S.-K. Effect of fermented medicinal plants as dietary additives on food preference and fecal microbial quality in dogs. Animals 2019, 9, 690. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, Y.; Mukaida, Y.; Saito, Y.; Oshima, K.; Takahashi, T.; Muroi, E.; Hashimoto, K.; Uda, Y. Characterisation of volatile sulphur-containing compounds generated in crushed leaves of Chinese chive (Allium tuberosum Rottler). Food Chem. 2010, 120, 343–348. [Google Scholar] [CrossRef]
- Filocamo, A.; Nueno-Palop, C.; Bisignano, C.; Mandalari, G.; Narbad, A. Effect of garlic powder on the growth of commensal bacteria from the gastrointestinal tract. Phytomedicine 2012, 19, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ji, Y.; Park, H.; Lee, J.; Park, S.; Yeo, S.; Shin, H.; Holzapfel, W.H. Selection of functional lactic acid bacteria as starter cultures for the fermentation of Korean leek (Allium tuberosum Rottler ex Sprengel.). Int. J. Food Microbiol. 2014, 191, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Lee, W.-D.; Niu, K.-M.; Kim, S.-K. The genus Allium as poultry feed additive: A review. Animals 2019, 9, 1032. [Google Scholar] [CrossRef]
- Hossain, M.M.; Lee, S.I.; Kim, I.H. Effect of dietary Korean aged garlic extract by Leukonostoc citreum SK2556 on production, hematological status, meat quality, relative organ weight, targeted Escherichia coli colony and excreta gas emission in broilers. Anim. Feed Sci. Technol. 2014, 198, 333–340. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Dayem, A.A.; Choi, H.Y.; Kim, Y.B.; Cho, S.-G. Antiviral effect of methylated flavonol isorhamnetin against influenza. PLoS ONE 2015, 10, e0121610. [Google Scholar] [CrossRef]
- Al-Yousef, H.M.; Ahmed, A.F.; Al-Shabib, N.A.; Laeeq, S.; Khan, R.A.; Rehman, M.T.; Alsalme, A.; Al-Ajmi, M.F.; Khan, M.S.; Husain, F.M. Onion peel ethylacetate fraction and its derived constituent quercetin 4′-O-β-D glucopyranoside attenuates quorum sensing regulated virulence and biofilm formation. Front. Microbiol. 2017, 8, 1675. [Google Scholar] [CrossRef]
- Yang, W.Y.; Won, T.H.; Ahn, C.H.; Lee, S.H.; Yang, H.C.; Shin, J.; Oh, K.B. Streptococcus mutans sortase A inhibitory metabolites from the flowers of Sophora japonica. Bioorg. Med. Chem. Lett. 2015, 25, 1394–1397. [Google Scholar] [CrossRef]
- Ghoke, S.; Sood, R.; Kumar, N.; Pateriya, A.; Bhatia, S.; Mishra, A.; Dixit, R.; Singh, V.; Desai, D.; Kulkarni, D. Evaluation of antiviral activity of Ocimum sanctum and Acacia arabica leaves extracts against H9N2 virus using embryonated chicken egg model. BMC Complement. Altern. Med. 2018, 18, 174. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, R.; Xiong, X.; Yin, Y.; Cai, Y.; Ma, Z.; Liu, N.; Zhu, Z.-J. Metabolic reaction network-based recursive metabolite annotation for untargeted metabolomics. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.H.; Jung, E.S.; Park, H.M.; Kim, S.H.; Lee, S.; Jo, Y.H.; Lee, M.K.; Jung, G.; Do, S.G.; Lee, C.H. Comparison of metabolites variation and antiobesity effects of fermented versus nonfermented mixtures of Cudrania tricuspidata, Lonicera caerulea, and soybean according to fermentation in vitro and in vivo. PLoS ONE 2016, 11, e0149022. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Oh, D.G.; Singh, D.; Lee, J.S.; Lee, S.; Lee, C.H. Exploring the metabolomic diversity of plant species across spatial (leaf and stem) components and phylogenic groups. BMC Plant Biol. 2020, 20, 39. [Google Scholar] [CrossRef]
- Han, J.; Lawson, L.; Han, G.; Han, P. Spectrophotometric method for quantitative determination of allicin and total garlic thiosulfinates. Anal. Biochem. 1995, 225, 157–160. [Google Scholar] [CrossRef]
| Isolates | Poultry Pathogens | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Salmonella Gallinarum | Salmonella Enteritidis | Salmonella Pullorum | Salmonella Typhimurium | Salmonella Anatum | Salmonella Typhi | Salmonella Paratyphi | Clostridium perfringens | Staphylococcus aureus | Escherichia coli | Enterococcus faecalis | |
| Leuconostoc mesenteroides SK4645 | - | + | - | - | - | - | + | - | - | + | - |
| Lactobacillus sakei SK4688 | - | + | - | + | - | - | - | - | - | - | - |
| Lactobacillus plantarum SK4719 | + | + | + | + | + | + | + | + | + | + | + |
| Weissella cibaria SK4720 | + | - | - | - | + | - | - | - | - | - | - |
| Weissella paramesenteroides SK4721 | + | - | - | - | + | - | - | - | - | + | - |
| Bacillus megaterium SK4723 | - | - | + | - | - | + | - | - | + | - | - |
| Bacillus aryabhattai SK4724 | - | - | - | + | - | - | - | - | - | - | - |
| Bacillus pumilus SK4726 | - | + | - | - | - | - | - | + | - | - | - |
| Bacillus subtilis SK4730 | - | - | - | - | - | - | - | - | - | - | - |
| Staphylococcus sciuri SK4727 | - | - | - | - | - | - | - | + | - | - | - |
| Micrococcus luteus SK4728 | - | - | - | - | - | - | - | - | - | - | - |
| Saccharomyces cerevisiae SK4690 | - | - | - | - | - | + | - | - | + | - | - |
| No. | Tentatively Identified Metabolites | RT (min) | MW | Measured Mass | MS/MS Fragments | Class of Compounds |
|---|---|---|---|---|---|---|
| Negative Mode (m/z) * | ||||||
| 1 | N-(1-Deoxy-1-fructosyl)phenylalanine | 1.09 | 327 | 326.1204 | 326 > 308/278/236/206/164 | Amino acid |
| 2 | 3-(2,3,4-trihydroxy-5-methoxyphenyl)propanoic acid | 1.75 | 228 | 227.1379 | 227 > 183/209 | Organic acid |
| 3 | Glycyrol | 1.83 | 366 | 365.1305 | 365 > 275/347/203/317 | Coumestan |
| 4 | Tryptophan | 1.92 | 204 | 203.0811 | 203 > 159/116/142/186 | Amino acid |
| 5 | Benzoylmesaconine derivative | 3.79 | 559 | 558.2698 | 558 > 540/514/496/470/452/395 | Alkaloid |
| 6 | Feruloyl-galactaric acid | 3.97 | 386 | 385.0720 | 385 > 191/209/367 | Organic acid |
| 7 | Kaempferol-diglucoside | 4.00 | 610 | 609.1408 | 609 > 447/285/489/581 | Flavonol |
| 8 | Isorhamnetin 3,4’-diglucoside | 4.21 | 640 | 639.3306 | 639 > 621/579/549/519/477 | Flavonol |
| 9 | Quercetin-diglucoside | 4.33 | 626 | 625.1360 | 625 > 463/300/445/505/607 | Flavonol |
| 10 | Quercetin-hexoside | 4.76 | 464 | 463.0843 | 463 > 301 | Flavonol |
| 11 | Kaempferol diglucoside-(feruloylglucoside) | 4.77 | 948 | 947.2372 | 94 7> 623/785/447/609/285 | Flavonol |
| 12 | Saponin 1 | 4.92 | 808 | 807.4156 | 807 > 789/763/717/645 | Saponin |
| 13 | Saponin 2 | 4.99 | 852 | 851.4416 | 851 > 833/807/761/689/512 | Saponin |
| 14 | Kaempferol-glucoside | 5.01 | 448 | 447.0897 | 447 > 284/255 | Flavonol |
| 15 | Saponin 3 | 5.05 | 896 | 895.4661 | 895 > 877/859/763/745 | Saponin |
| 16 | Saponin 4 | 5.11 | 940 | 939.4932 | 939 > 921/895/848/776 | Saponin |
| 17 | Saponin 5 | 5.17 | 984 | 983.5189 | 983 > 789/803/771/951/821 | Saponin |
| 18 | Oxo-dihydroxy-octadecenoic acid | 6.25 | 328 | 327.2145 | 327 > 309/291/273/247/239 | Fatty acid |
| 19 | Tianshic acid | 6.50 | 330 | 329.2291 | 329 > 311/293/229/211/171 | Fatty acid |
| 20 | 12-Hydroxystearic acid | 9.66 | 300 | 299.2562 | 299 > 281/253 | Fatty acid |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kothari, D.; Lee, W.-D.; Jung, E.S.; Niu, K.-M.; Lee, C.H.; Kim, S.-K. Controlled Fermentation Using Autochthonous Lactobacillus plantarum Improves Antimicrobial Potential of Chinese Chives against Poultry Pathogens. Antibiotics 2020, 9, 386. https://doi.org/10.3390/antibiotics9070386
Kothari D, Lee W-D, Jung ES, Niu K-M, Lee CH, Kim S-K. Controlled Fermentation Using Autochthonous Lactobacillus plantarum Improves Antimicrobial Potential of Chinese Chives against Poultry Pathogens. Antibiotics. 2020; 9(7):386. https://doi.org/10.3390/antibiotics9070386
Chicago/Turabian StyleKothari, Damini, Woo-Do Lee, Eun Sung Jung, Kai-Min Niu, Choong Hwan Lee, and Soo-Ki Kim. 2020. "Controlled Fermentation Using Autochthonous Lactobacillus plantarum Improves Antimicrobial Potential of Chinese Chives against Poultry Pathogens" Antibiotics 9, no. 7: 386. https://doi.org/10.3390/antibiotics9070386
APA StyleKothari, D., Lee, W.-D., Jung, E. S., Niu, K.-M., Lee, C. H., & Kim, S.-K. (2020). Controlled Fermentation Using Autochthonous Lactobacillus plantarum Improves Antimicrobial Potential of Chinese Chives against Poultry Pathogens. Antibiotics, 9(7), 386. https://doi.org/10.3390/antibiotics9070386





