Candida auris: An Overview of How to Screen, Detect, Test and Control This Emerging Pathogen
Abstract
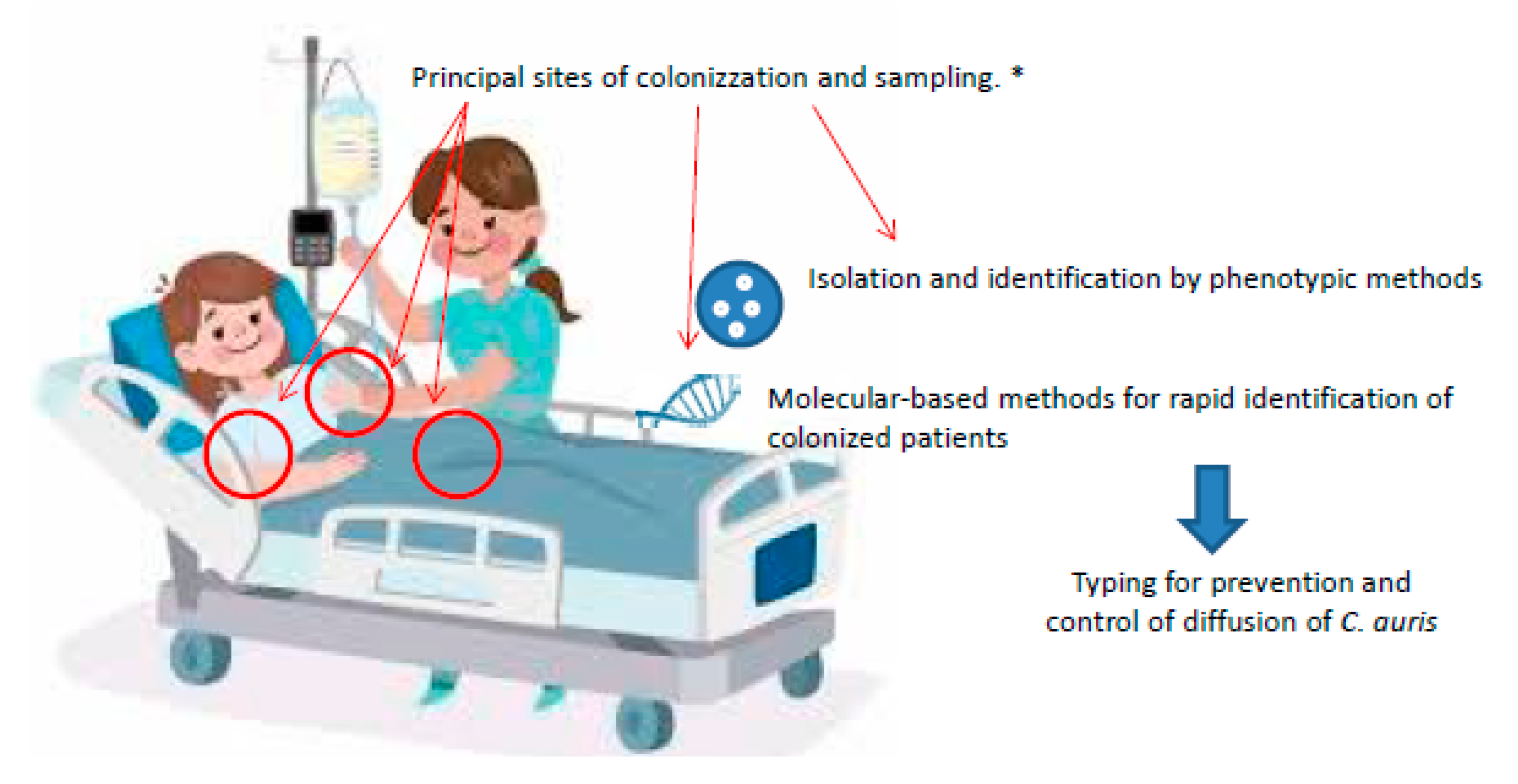
1. Introduction
2. C. auris Screening
3. C. auris Identification
3.1. Phenotypic Methods and Distinctive Characteristics
3.2. Biochemical Methods
3.3. Molecular Methods
3.4. MALDI-TOF
4. Typing
5. C. auris Resistance Profile and Antifungal Susceptibility Testing
5.1. C. auris Resistance Profile
5.2. Antifungal Susceptibility Testing
6. Infection Control Recommendations
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida aurissp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.G.; Shin, J.H.; Uh, Y.; Kang, M.G.; Kim, S.H.; Park, K.H.; Jang, H.-C. First Three Reported Cases of Nosocomial Fungemia Caused by Candida auris. J. Clin. Microbiol. 2011, 49, 3139–3142. [Google Scholar] [CrossRef] [PubMed]
- Magobo, R.E.; Corcoran, C.; Seetharam, S.; Govender, N.P. Candida auris–Associated Candidemia, South Africa. Emerg. Infect. Dis. 2014, 20, 1250–1251. [Google Scholar] [CrossRef] [PubMed]
- Calvo, B.; Melo, A.S.; Perozo-Mena, A.; Hernandez, M.; Francisco, E.C.; Hagen, F.; Meis, J.F.; Colombo, A.L. First report of Candida auris in America: Clinical and microbiological aspects of 18 episodes of candidemia. J. Infect. 2016, 73, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gaitán, A.; Moret, A.M.; Tasias-Pitarch, M.; Aleixandre-Lopez, A.I.; Martínez-Morel, H.; Calabuig, E.; Salavert-Lletí, M.; Ramirez, P.; Hontangas, J.L.L.; Hagen, F.; et al. An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses 2018, 61, 498–505. [Google Scholar] [CrossRef]
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob. Resist. Infect. Control. 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Plachouras, D.; Lötsch, F.; Kohlenberg, A.; Monnet, D.L.; The Candida auris survey collaborative group. Candida auris: Epidemiological situation; laboratory capacity and preparedness in the European Union and European Economic Area, January 2018 to May 2019. Euro Surveill. 2020, 25, 2000240. [Google Scholar] [CrossRef]
- Chaabane, F.; Graf, A.; Jequier, L.; Coste, A.T. Review on antifungal resistance mechanisms in the emerging pathogen Candida auris. Front. Microbiol. 2019, 10, 2788. [Google Scholar] [CrossRef] [PubMed]
- Kohlenberg, A.; Struelens, M.J.; Monnet, D.L.; Plachouras, D.; The Candida auris survey collaborative group. Candida auris: Epidemiological situation, laboratory capacity and preparedness in European Union and European Economic Area countries, 2013 to 2017. Euro. Surveill. 2018, 23, 18-00136. [Google Scholar] [CrossRef]
- Wang, T.Z.; White, K.N.; Scarr, J.V.; Simon, M.S.; Calfee, D.P. Preparing your healthcare facility for the new fungus among us: An infection preventionist’s guide to Candida auris. Am. J. Infect. Control. 2020, 48, 825–827. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Candida auris in Healthcare Settings–Europe–First Update, 23 April 2018. Stockholm: ECDC; 2018. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/RRA-Candida-auris-European-Union-countries-first-update.pdf (accessed on 20 August 2020).
- Centre for Disease Prevention and Control. Identification of Candida auris. Available online: https://www.cdc.gov/fungal/candida-auris/identification.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Ffungal%2Fcandida-auris%2Frecommendations.html (accessed on 20 August 2020).
- Welsh, R.M.; Bentz, M.L.; Shams, A.; Houston, H.; Lyons, A.; Rose, L.J.; Litvintseva, A.P. Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. J. Clin. Microbiol. 2017, 55, 2996–3005. [Google Scholar] [CrossRef] [PubMed]
- Tsay, S.; Kallen, A.; Jackson, B.R.; Chiller, T.M.; Vallabhaneni, S. Approach to the investigation and management of patients with candida auris, an emerging multidrug-resistant yeast. Clin. Infect. Dis. 2018, 66, 306–311. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Candida auris in Healthcare Settings–Europe–19 December 2016. Stockholm: ECDC; 2016. Available online: https://www.ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/Candida-in-healthcare-settings_19-Dec-2016.pdf (accessed on 20 August 2020).
- Cortegiani, A.; Misseri, G.; Fasciana, T.; Giammanco, A.; Giarratano, A.; Chowdhary, A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J. Intensiv. Care 2018, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sachu, A.; Mohan, K.; Vinod, V.; Dinesh, K.; Karim, S. Simple low cost differentiation of Candida auris from Candida haemulonii complex using CHROMagar Candida medium supplemented with Pal’s medium. Revista Iberoamericana de Micología 2017, 34, 109–111. [Google Scholar] [CrossRef]
- Borman, A.M.; Fraser, M.; Johnson, E.M. CHROMagarTM Candida Plus: A novel chromogenic agar that permits the rapid identification of Candida auris. Med. Mycol. 2020. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Afshari, S.A.K.; Gharehbolagh, S.A.; Mirhendi, H.; Makimura, K. Methods for identification of Candida auris, the yeast of global public health concern: A review. Journal de Mycologie Médicale 2019, 29, 174–179. [Google Scholar] [CrossRef]
- Yue, H.; Bing, J.; Zheng, Q.; Zhang, Y.; Hu, T.; Du, H.; Wang, H.; Huang, G. Filamentation in Candida auris, an emerging fungal pathogen of humans: Passage through the mammalian body induces a heritable phenotypic switch. Emerg. Microbes Infect. 2018, 7, 1–13. [Google Scholar] [CrossRef]
- Wang, X.; Bing, J.; Zheng, Q.; Zhang, F.; Liu, J.; Yue, H.; Tao, L.; Du, H.; Wang, Y.; Wang, H.; et al. The first isolate of Candida auris in China: Clinical and biological aspects. Emerg. Microbes Infect. 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Spivak, E.S.; E Hanson, K. Candida auris: An emerging fungal pathogen. J. Clin. Microbiol. 2017, 56. [Google Scholar] [CrossRef]
- Escandón, P.; A Chow, N.; Caceres, D.H.; Gade, L.; Berkow, E.L.; Armstrong, P.; Rivera, S.; Misas, E.; Duarte, C.; Moulton-Meissner, H.; et al. Molecular epidemiology of Candida auris in Colombia reveals a highly related, countrywide colonization with regional patterns in amphotericin B resistance. Clin. Infect. Dis. 2018, 68, 15–21. [Google Scholar] [CrossRef]
- Shapiro, R.S.; Robbins, N.; Cowen, L.E. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol. Mol. Biol. Rev. 2011, 75, 213–267. [Google Scholar] [CrossRef]
- Larkin, E.; Hager, C.; Chandra, J.; Mukherjee, P.K.; Retuerto, M.; Salem, I.; Long, L.; Isham, N.; Kovanda, L.L.; Borroto-Esoda, K.; et al. The Emerging pathogen Candida auris: Growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob. Agents Chemother. 2017, 61, 02396-16. [Google Scholar] [CrossRef]
- Singh, R.; Kaur, M.; Chakrabarti, A.; Shankarnarayan, S.A.; Rudramurthy, S.M. Biofilm formation by Candida auris isolated from colonising sites and candidemia cases. Mycoses 2019, 62, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Alampalli, S.V.; Nageshan, R.K.; Chettiar, S.T.; Joshi, S.; Tatu, U.S. Draft genome of a commonly misdiagnosed multidrug resistant pathogen Candida auris. BMC Genom. 2015, 16, 1–16. [Google Scholar] [CrossRef]
- Ambaraghassi, G.; Dufresne, P.J.; Dufresne, S.F.; Vallières, É.; Muñoz, J.F.; Cuomo, C.A.; Berkow, E.L.; Lockhart, S.R.; Luong, M.-L. Identification of Candida auris by use of the Updated Vitek 2 Yeast Identification System, Version 8.01: A multilaboratory evaluation study. J. Clin. Microbiol. 2019, 57, 00884-19. [Google Scholar] [CrossRef]
- Kordalewska, M.; Perlin, D. Molecular diagnostics in the times of surveillance for Candida auris. J. Fungi 2019, 5, 77. [Google Scholar] [CrossRef]
- Smith, A.J.; Taori, S.K.; Schelenz, S.; Jeffery, K.; Johnson, E.M.; Borman, A. Candida auris Incident Management Team, Manuel, R.; Brown, C.S. Candida auris: A review of the literature. Clin. Microbiol. Rev. 2018, 31, e00029-17. [Google Scholar]
- Kordalewska, M.; Perlin, D.S. Identification of Drug Resistant Candida auris. Front. Microbiol. 2019, 10, 1918. [Google Scholar] [CrossRef]
- Sexton, D.J.; Kordalewska, M.; Bentz, M.L.; Welsh, R.M.; Perlin, D.S.; Litvintseva, A.P. Direct detection of emergent fungal pathogen Candida auris in clinical skin swabs by SYBR Green-Based Quantitative PCR Assay. J. Clin. Microbiol. 2018, 56, e01337-18. [Google Scholar] [CrossRef]
- Leach, L.; Zhu, Y.; Chaturvedi, S. Development and validation of a Real-Time PCR Assay for rapid detection of Candida auris from surveillance samples. J. Clin. Microbiol. 2017, 56. [Google Scholar] [CrossRef]
- Khan, Z.; Ahmad, S.; Al-Sweih, N.; Joseph, L.; Alfouzan, W.; Asadzadeh, M. Increasing prevalence, molecular characterization and antifungal drug susceptibility of serial Candida auris isolates in Kuwait. PLoS ONE 2018, 13, e0195743. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gaitán, A.C.; Fernández-Pereira, J.; Valentin, E.; Tormo-Mas, M.A.; Eraso, E.; Pemán, J.; De Groot, P.W. Molecular identification of Candida auris by PCR amplification of species-specific GPI protein-encoding genes. Int. J. Med Microbiol. 2018, 308, 812–818. [Google Scholar] [CrossRef]
- Arastehfar, A.; Fang, W.; Badali, H.; Vaezi, A.; Jiang, W.; Liao, W.; Pan, W.; Hagen, F.; Boekhout, T. Low-Cost Tetraplex PCR for the global spreading multi-drug resistant fungus, Candida auris and its phylogenetic relatives. Front. Microbiol. 2018, 9, 1119. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Spencer, J.E.; Lockhart, S.R.; Singleton, S.; Petway, D.J.; Bagarozzi, D.A., Jr.; Herzegh, O.T. A high-throughput and rapid method for accurate identification of emerging multidrug-resistant Candida auris. Mycoses 2019, 62, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Theill, L.; Dudiuk, C.; Morales-López, S.E.; Berrio, I.; Rodriguez, J.Y.; Marín, A.; Gamarra, S.; Garcia-Effron, G. Single-tube classical PCR for Candida auris and Candida haemulonii identification. Revista Iberoamericana de Micología 2018, 35, 110–112. [Google Scholar] [CrossRef]
- Arastehfar, A.; Fang, W.; Pan, W.; Lackner, M.; Liao, W.; Badiee, P.; Zomorodian, K.; Badali, H.; Hagen, F.; Lass-Flörl, C.; et al. YEAST PANEL multiplex PCR for identification of clinically important yeast species: Stepwise diagnostic strategy, useful for developing countries. Diagn. Microbiol. Infect. Dis. 2019, 93, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Murcia, A.J.; Navarro, A.; Bru, G.; Chowdhary, A.; Hagen, F.; Meis, J.F. Internal validation of GPS™ MONODOSE CanAur dtec-qPCR kit following the UNE/EN ISO/IEC 17025:2005 for detection of the emerging yeast Candida auris. Mycoses 2018, 61, 877–884. [Google Scholar] [CrossRef]
- Sexton, D.J.; Bentz, M.L.; Welsh, R.M.; Litvintseva, A.P. Evaluation of a new T2 Magnetic Resonance assay for rapid detection of emergent fungal pathogen Candida auris on clinical skin swab samples. Mycoses 2018, 61, 786–790. [Google Scholar] [CrossRef]
- Yamamoto, M.; Alshahni, M.M.; Tamura, T.; Satoh, K.; Iguchi, S.; Kikuchi, K.; Mimaki, M.; Makimura, K. Rapid Detection of Candida auris based on Loop-Mediated Isothermal Amplification (LAMP). J. Clin. Microbiol. 2018, 56, e00591-18. [Google Scholar] [CrossRef]
- Lima, A.; Widen, R.; Vestal, G.; Uy, D.; Silbert, S. A TaqMan Probe-Based Real-Time PCR Assay for the rapid identification of the emerging multidrug-resistant pathogen Candida auris on the BD Max System. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef]
- Tsay, S.; Welsh, R.M.; Adams, E.H.; Chow, N.A.; Gade, L.; Berkow, E.L.; Poirot, E.; Lutterloh, E.; Quinn, M.; Chaturvedi, S.; et al. Notes from the field: Ongoing transmission of Candida auris in health care facilities—United States, June 2016–May 2017. Morb. Mortal. Wkly. Rep. 2017, 66, 514–515. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Berkow, E.L.; Chow, N.; Welsh, R.M. Candida auris for the clinical microbiology laboratory: Not your grandfather’s Candida species. Clin. Microbiol. Newsl. 2017, 39, 99–103. [Google Scholar] [CrossRef]
- Caceres, D.H.; Forsberg, K.; Welsh, R.M.; Sexton, D.J.; Lockhart, S.R.; Jackson, B.R.; Chiller, T. Candida auris: A review of recommendations for detection and control in healthcare settings. J. Fungi 2019, 5, 111. [Google Scholar] [CrossRef]
- Girard, V.; Mailler, S.; Chetry, M.; Vidal, C.; Durand, G.; Van Belkum, A.; Colombo, A.L.; Hagen, F.; Meis, J.F.; Chowdhary, A. Identification and typing of the emerging pathogen Candida auris by matrix-assisted laser desorption ionisation time of flight mass spectrometry. Mycoses 2016, 59, 535–538. [Google Scholar] [CrossRef]
- Grenfell, R.C.; Junior, A.R.D.S.; Del Negro, G.M.B.; Munhoz, R.B.; Gimenes, V.M.F.; Assis, D.M.; Rockstroh, A.C.; Motta, A.L.; Rossi, F.; Juliano, L.; et al. Identification of Candida haemulonii complex species: Use of ClinProToolsTM to overcome limitations of the Bruker BiotyperTM, VITEK MSTM IVD, and VITEK MSTM RUO databases. Front. Microbiol. 2016, 7, 940. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of High-Consequence Pathogens and Pathology (DHCPP). MicrobeNet. Available online: https://www.cdc.gov/microbenet/index.html (accessed on 20 August 2020).
- Sterkel, A.; Bateman, A.; Valley, A.; Warshauer, D. Viability of Candida auris and other Candida species after various Matrix-Assisted Laser Desorption Ionization–Time of Flight (MALDI-TOF) mass spectrometry-based extraction protocols. J. Clin. Microbiol. 2018, 56, e00886-18. [Google Scholar] [CrossRef]
- Desoubeaux, G.; Bailly, E.; Guillaume, C.; De Kyvon, M.-A.; Tellier, A.-C.; Morange, V.; Bernard, L.; Salamé, E.; Quentin, R.; Chandenier, J. Candida auris in contemporary mycology labs: A few practical tricks to identify it reliably according to one recent French experience. J. Mycologie Médicale 2018, 28, 407–410. [Google Scholar] [CrossRef]
- Prakash, A.; Sharma, C.; Singh, A.; Singh, P.K.; Kumar, A.; Hagen, F.; Govender, N.P.; Colombo, A.; Meis, J.; Chowdhary, A. Evidence of genotypic diversity among Candida auris isolates by multilocus sequence typing, matrix-assisted laser desorption ionization time-of-flight mass spectrometry and amplified fragment length polymorphism. Clin. Microbiol. Infect. 2016, 22, 277.e1–277.e9. [Google Scholar] [CrossRef] [PubMed]
- Cendejasbueno, E.; Kolecka, A.; Alastruey-Izquierdo, A.; Theelen, B.; Groenewald, M.; Kostrzewa, M.; Cuencaestrella, M.; Gomezlopez, A.; Boekhout, T. Reclassification of the Candida haemulonii complex as Candida haemulonii (C. haemulonii Group I), C. duobushaemulonii sp. nov. (C. haemulonii Group II), and C. haemulonii var. vulnera var. nov.: Three multiresistant human pathogenic yeasts. J. Clin. Microbiol. 2012, 50, 3641–3651. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Kumar, N.; Pandey, R.; Meis, J.; Chowdhary, A. Whole genome sequencing of emerging multidrug resistant Candida auris isolates in India demonstrates low genetic variation. New Microbes New Infect. 2016, 13, 77–82. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous emergence of multidrug-resistant Candida aurison 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 2016, 64, 134–140. [Google Scholar] [CrossRef]
- De Groot, T.; Puts, Y.; Berrio, I.; Chowdhary, A.; Meis, J.F. Development of Candida auris short tandem repeat typing and Its application to a global collection of isolates. mBio 2020, 11. [Google Scholar] [CrossRef]
- Vatanshenassan, M.; Boekhout, T.; Mauder, N.; Robert, V.; Maier, T.; Meis, J.F.; Berman, J.; Then, E.; Kostrzewa, M.; Hagen, F. Evaluation of microsatellite typing, ITS sequencing, AFLP fingerprinting, MALDI-TOF MS, and Fourier-Transform Infrared Spectroscopy Analysis of Candida auris. J. Fungi 2020, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R. Candida auris and multidrug resistance: Defining the new normal. Fungal Genet. Biol. 2019, 131, 103243. [Google Scholar] [CrossRef]
- Forsberg, K.; Woodworth, K.; Walters, M.; Berkow, E.L.; Jackson, B.; Chiller, T.; Vallabhaneni, S. Candida auris: The recent emergence of a multidrug-resistant fungal pathogen. Med. Mycol. 2018, 57, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bidaud, A.; Chowdhary, A.; Dannaoui, E. Candida auris: An emerging drug resistant yeast—A mini-review. J. Mycol. Médicale 2018, 28, 568–573. [Google Scholar] [CrossRef]
- Sarma, S.; Kumar, N.; Sharma, S.; Govil, D.; Ali, T.; Mehta, Y.; Rattan, A. Candidemia caused by amphotericin B and fluconazole resistant Candida auris. Indian J. Med. Microbiol. 2013, 31, 90–91. [Google Scholar] [CrossRef]
- Sabino, R.; Veríssimo, C.; Pereira Álvaro, A.; Antunes, F. Candida auris, an agent of hospital-associated outbreaks: Which challenging issues do we need to have in mind? Microorganisms 2020, 8, 181. [Google Scholar] [CrossRef]
- Ostrowsky, B.; Greenko, J.; Adams, E.; Quinn, M.; O’Brien, B.; Chaturvedi, V.; Berkow, E.; Vallabhaneni, S.; Forsberg, K.; Chaturvedi, S.; et al. Candida auris isolates resistant to three classes of antifungal medications—New York, 2019. Morb. Mortal. Wkly. Rep. 2020, 69, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Zamith-Miranda, D.; Heyman, H.M.; Cleare, L.G.; Couvillion, S.P.; Clair, G.C.; Bredeweg, E.L.; Gacser, A.; Nimrichter, L.; Nakayasu, E.S.; Nosanchuk, J.D. Multi-omics Signature of Candida auris, an emerging and multidrug-resistant pathogen. mSystems 2019, 4, e00257-19. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Prakash, A.; Meletiadis, J.; Sharma, C.; Chowdhary, A. comparison of EUCAST and CLSI reference microdilution MICs of eight antifungal compounds for Candida auris and associated tentative epidemiological cutoff values. Antimicrob. Agents Chemother. 2017, 61, e00485-17. [Google Scholar] [CrossRef]
- Hata, D.J.; Humphries, R.; Lockhart, S.R. College of American Pathologists Microbiology Committee. Candida auris: An emerging yeast pathogen posing distinct challenges for laboratory diagnostics, treatment, and infection prevention. Arch. Pathol. Lab. Med. 2019, 144, 107–114. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Duggal, S.; Agarwal, K.; Prakash, A.; Singh, P.K.; Jain, S.; Kathuria, S.; Randhawa, H.S.; Hagen, F.; et al. New clonal strain of Candida auris, Delhi, India. Emerg. Infect. Dis. 2013, 19, 1670–1673. [Google Scholar] [CrossRef]
- Crea, F.; Codda, G.; Orsi, A.; Battaglini, A.; Giacobbe, D.R.; Delfino, E.; Ungaro, R.; Marchese, A. Isolation of Candida auris from invasive and non-invasive samples of a patient suffering from vascular disease, Italy, July 2019. Euro Surveill. 2019, 24, 1900549. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Prakash, A.; Sharma, C.; Kordalewska, M.; Kumar, A.; Sarma, S.; Tarai, B.; Singh, A.; Upadhyaya, G.; Upadhyay, S.; et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–2017) in India: Role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J. Antimicrob. Chemother. 2018, 73, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, S.M.; Chakrabarti, A.; Paul, R.A.; Sood, P.; Kaur, H.; Capoor, M.R.; Kindo, A.J.; Marak, R.S.K.; Arora, A.; Sardana, R.; et al. Candida auris candidaemia in Indian ICUs: Analysis of risk factors. J. Antimicrob. Chemother. 2017, 72, 1794–1801. [Google Scholar] [CrossRef]
- Ademe, M.; Girma, F. Candida auris: From multidrug resistance to pan-resistant strains. Infect. Drug Resist. 2020, 2020, 1287–1294. [Google Scholar]
- Szekely, A.; Borman, A.M.; Johnson, E.M. Candida auris isolates of the Southern Asian and South African lineages exhibit different phenotypic and antifungal susceptibility profiles in vitro. J. Clin. Microbiol. 2019, 57, e02055-18. [Google Scholar] [CrossRef]
- Kordalewska, M.; Lee, A.; Park, S.; Berrio, I.; Chowdhary, A.; Zhao, Y.; Perlin, D.S. Understanding echinocandin resistance in the emerging pathogen Candida auris. Antimicrob. Agents Chemother. 2018, 62, e00238-18. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Shin, J.H.; A Byun, S.; Choi, M.J.; Won, E.J.; Lee, D.; Lee, S.Y.; Chun, S.; Lee, J.H.; Choi, H.J.; et al. Candida auris clinical isolates from South Korea: Identification, antifungal susceptibility, and genotyping. J. Clin. Microbiol. 2019, 57, 57–01624. [Google Scholar] [CrossRef]
- Kathuria, S.; Singh, P.K.; Sharma, C.; Prakash, A.; Masih, A.; Kumar, A.; Meis, J.F.; Chowdhary, A. Multidrug-Resistant Candida auris misidentified as Candida haemulonii: Characterization by Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry and DNA sequencing and its antifungal susceptibility profile variability by Vitek 2, CLSI broth microdilution, and e-test method. J. Clin. Microbiol. 2015, 53, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Vella, A.; De Carolis, E.; Vaccaro, L.; Posteraro, P.; Perlin, D.S.; Kostrzewa, M.; Posteraro, B.; Sanguinetti, M. Rapid antifungal susceptibility testing by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry Analysis. J. Clin. Microbiol. 2013, 51, 2964–2969. [Google Scholar] [CrossRef]
- Vella, A.; De Carolis, E.; Mello, E.; Perlin, D.S.; Sanglard, D.; Sanguinetti, M.; Posteraro, B. Potential use of MALDI-ToF Mass Spectrometry for rapid detection of antifungal resistance in the human pathogen Candida glabrata. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Vatanshenassan, M.; Boekhout, T.; Lass-Flörl, C.; Lackner, M.; Schubert, S.; Kostrzewa, M.; Sparbier, K. Proof of concept for MBT ASTRA, a rapid Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS)-based method to detect caspofungin resistance in Candida albicans and Candida glabrata. J. Clin. Microbiol. 2018, 56, e00420-18. [Google Scholar] [CrossRef] [PubMed]
- Vatanshenassan, M.; Boekhout, T.; Meis, J.F.; Berman, J.; Chowdhary, A.; Ben-Ami, R.; Sparbier, K.; Kostrzewa, M. Candida auris identification and rapid antifungal susceptibility testing against echinocandins by MALDI-TOF MS. Front. Cell. Infect. Microbiol. 2019, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Lee, A.; Jiménez-Ortigosa, C.; Kordalewska, M.; Perlin, D.S.; Zhao, Y. Rapid detection of ERG11-associated azole resistance and FKS-associated echinocandin resistance in Candida auris. Antimicrob. Agents Chemother. 2018, 63, e01811-18. [Google Scholar] [CrossRef]
- Ku, T.S.N.; Walraven, C.J.; Lee, S.A. Candida auris: Disinfectants and implications for infection control. Front. Microbiol. 2018, 9, 726. [Google Scholar] [CrossRef]
- Kean, R.; McKloud, E.; Townsend, E.M.; Sherry, L.; Delaney, C.; Jones, B.L.; Williams, C.; Ramage, G. The comparative efficacy of antiseptics against Candida auris biofilms. Int. J. Antimicrob. Agents 2018, 52, 673–677. [Google Scholar] [CrossRef]
- Cortegiani, A.; Misseri, G.; Chowdhary, A. What’s new on emerging resistant Candida species. Intensiv. Care Med. 2018, 45, 512–515. [Google Scholar] [CrossRef]
| Commercial Biochemical Systems | Misidentifies C. auris as |
|---|---|
| Vitek 2YST | C. haemulonii Candida duobushaemulonii Candida spp. |
| API 20C | Rhodotorula glutinis (characteristic red colour not present) Candida sake Saccharomyces kluyveri Saccharomyces cerevisiae Candida spp. |
| BD Phoenix | C. haemulonii Candida catenulata Candida spp. |
| MicroScan, Microscan Walkaway, MicroSan AutoScan | C. famata Candida guilliermondii Candida lusitaniae Candida parapsilos Candida spp. R. rubra |
| RapiID Yeast Plus | C. parapsilosis Candida spp. |
| Assay | Identification From | Target | Reference |
|---|---|---|---|
| PCR and real-time qPCR (SYBR Green) | Colony | 5.8S-ITS2-28S of rDNA | [31] |
| real-time qPCR (SYBR Green) | Swabs | 5.8S-ITS2-28S | [32] |
| real-time qPCR (TaqMan) | Swabs and environmental sample | ITS2-rDNA | [33] |
| PCR | Colony | ITS1-5.8S-ITS2 | [34] |
| Duplex PCR | Colony | GPI protein-encoding genes | [35] |
| Tetraplex PCR | Colony | 26s rDNA | [36] |
| real-time qPCR (TaqMan) | Swabs | ITS2-rDNA | [37] |
| Multiplex end-point PCR | Colony | ITS 1-5.8S-ITS2 | [38] |
| YEAST PANEL multiplex PCR | Colony and spiked serum samples | 26S rDNA | [39] |
| GPS MONODOSE dtec-qPCR kit | Colony | Species-specific primers and probes | [39] |
| T2 Magnetic Resonance (T2MR) system | Swabs | Species-specific primers and probes | [41] |
| Loop-mediated isothermal amplification (LAMP) | Colony, swab and environmental sample | The ferredoxin oxidoreductase encoding gene | [42] |
| Methods | Strengths and Limitations | Reference |
|---|---|---|
| Broth Microdilution Methods/Sensititre YeastOne | Determined ECVs are valuable in the analysis of MICs of isolates from the South Asian clade. MIC distributions can vary substantially for C. auris isolates from different clades. Are easy to perform. | [20] |
| E-test gradient diffusion method | Difficulty of interpretation for presence of aggregate directly adjacent to the zone of growth inhibition. The aggregates are present for evaluation of fluconazole, voriconazole, and anidulafungin but not in experiments performed with flucytosine or amphotericin B. | [20] |
| VITEK 2 | MIC distributions can vary substantially for C. auris isolates from different clades | [28] |
| MBT ASTRA | MBT ASTRA has a potential to detect echinocandin nonsusceptible C. auris isolates within 6 h. | [57] |
| Molecular methods | Echinocandin resistance is mediated through limited mutations S639P or S639F in FKS1, and azole resistance through F126L, Y132F, and K143R in ERG11 * | [2,5] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fasciana, T.; Cortegiani, A.; Ippolito, M.; Giarratano, A.; Di Quattro, O.; Lipari, D.; Graceffa, D.; Giammanco, A. Candida auris: An Overview of How to Screen, Detect, Test and Control This Emerging Pathogen. Antibiotics 2020, 9, 778. https://doi.org/10.3390/antibiotics9110778
Fasciana T, Cortegiani A, Ippolito M, Giarratano A, Di Quattro O, Lipari D, Graceffa D, Giammanco A. Candida auris: An Overview of How to Screen, Detect, Test and Control This Emerging Pathogen. Antibiotics. 2020; 9(11):778. https://doi.org/10.3390/antibiotics9110778
Chicago/Turabian StyleFasciana, Teresa, Andrea Cortegiani, Mariachiara Ippolito, Antonino Giarratano, Orazia Di Quattro, Dario Lipari, Domenico Graceffa, and Anna Giammanco. 2020. "Candida auris: An Overview of How to Screen, Detect, Test and Control This Emerging Pathogen" Antibiotics 9, no. 11: 778. https://doi.org/10.3390/antibiotics9110778
APA StyleFasciana, T., Cortegiani, A., Ippolito, M., Giarratano, A., Di Quattro, O., Lipari, D., Graceffa, D., & Giammanco, A. (2020). Candida auris: An Overview of How to Screen, Detect, Test and Control This Emerging Pathogen. Antibiotics, 9(11), 778. https://doi.org/10.3390/antibiotics9110778






