Essential Oils with High Activity against Stationary Phase Bartonella henselae
Abstract
1. Introduction
2. Results
2.1. Subsection Screening Essential Oil Collection to Identify Drugs Active against Non-Growing Stationary Phase B. henselae
2.2. MIC Determination of Active Hits
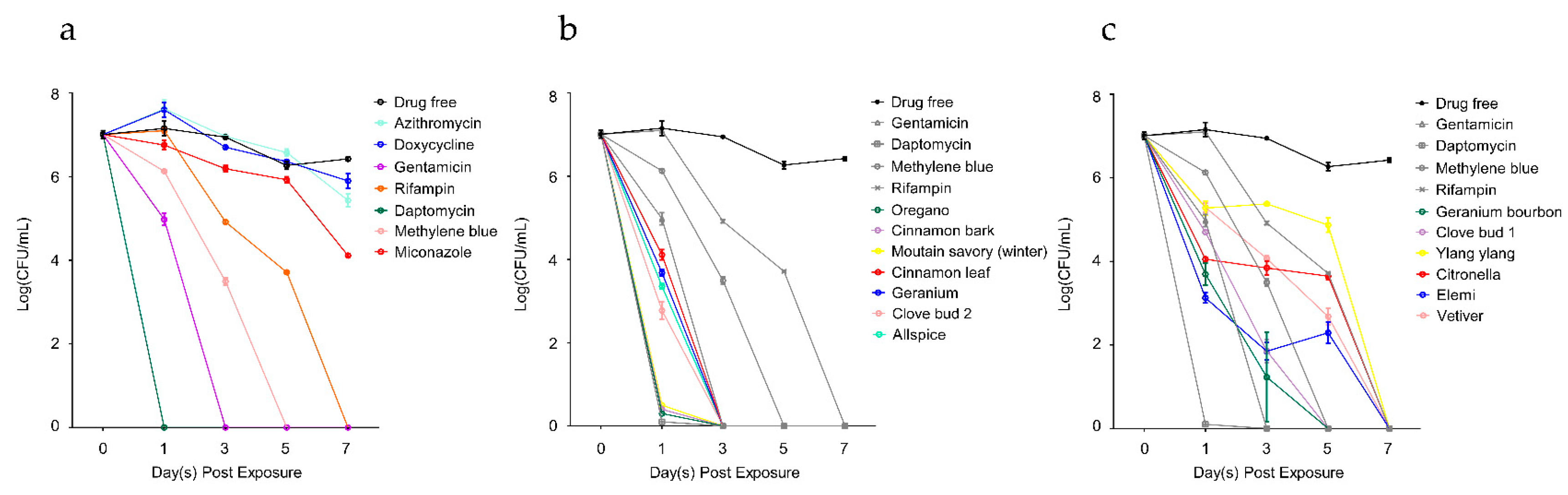
2.3. Time-Kill Curves of Active Hits
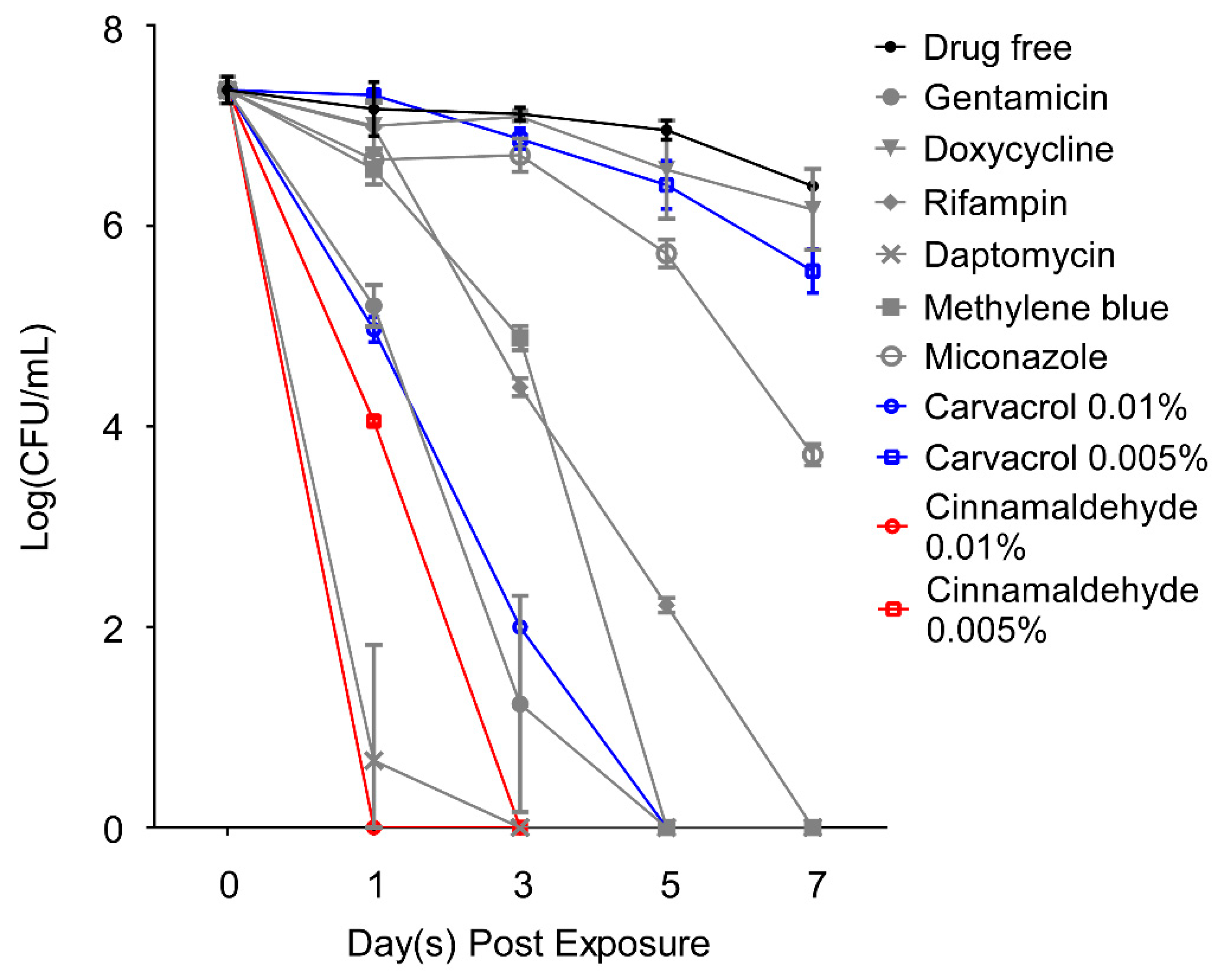
2.4. Carvacrol and Cinnamaldehyde as Highly Potent Active Ingredient of Essential Oils against Stationary Phase B. henselae
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain, Culture Media and Culture Conditions
4.2. Drugs, Essential Oils and their Active Ingredients
4.3. Microscopy Techniques
4.4. Screening of Essential Oil Library against Stationary Phase B. Henselae JK53
4.5. Drug Exposure Assay
4.6. MIC Determination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brenner, D.J.; O’connor, S.P.; Winkler, H.H.; Steigerwalt, A.G. Proposals to unify the genera Bartonella and Rochalimaea, with descriptions of Bartonella quintana comb. nov., Bartonella vinsonii comb. nov., Bartonella henselae comb. nov., and Bartonella elizabethae comb. nov., and to remove the family Bartonellaceae from the order Rickettsiales. Int. J. Syst. Bacteriol. 1993, 43, 777–786. [Google Scholar]
- Peters, D.; Wigand, R. Bartonellaceae. Bacteriol. Rev. 1955, 19, 150–159. [Google Scholar]
- Okaro, U.; Addisu, A.; Casanas, B.; Anderson, B. Bartonella Species, an Emerging Cause of Blood-Culture-Negative Endocarditis. Clin. Microbiol. Rev. 2017, 30, 709–746. [Google Scholar] [CrossRef] [PubMed]
- Breitschwerdt, E.B. Bartonellosis, One Health and all creatures great and small. Vet. Dermatol. 2017, 28, 96. [Google Scholar] [CrossRef] [PubMed]
- Mosepele, M.; Mazo, D.; Cohn, J. Bartonella infection in immunocompromised hosts: Immunology of vascular infection and vasoproliferation. Clin. Dev. Immunol. 2012, 2012, 612809. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Li, Y.; Hua, X.; Bai, Y.; Wang, C.; Zhu, C.; Du, Y.; Yang, Z.; Yuan, C. Lymphatic Circulation Disseminates Bartonella Infection into Bloodstream. J. Infect. Dis. 2017, 215, 303–311. [Google Scholar]
- Rolain, J.M.; La Scola, B.; Liang, Z.; Davoust, B.; Raoult, D. Immunofluorescent detection of intraerythrocytic Bartonella henselae in naturally infected cats. J. Clin. Microbiol. 2001, 39, 2978–2980. [Google Scholar] [CrossRef]
- Jacomo, V.; Kelly, P.J.; Raoult, D. Natural history of Bartonella infections (an exception to Koch’s postulate). Clin. Diagn. Lab. Immunol. 2002, 9, 8–18. [Google Scholar] [CrossRef]
- Chomel, B.B.; Boulouis, H.J.; Maruyama, S.; Breitschwerdt, E.B. Bartonella spp. in pets and effect on human health. Emerg. Infect. Dis. 2006, 12, 389–394. [Google Scholar] [CrossRef]
- Gutierrez, R.; Vayssier-Taussat, M.; Buffet, J.P.; Harrus, S. Guidelines for the Isolation, Molecular Detection, and Characterization of Bartonella Species. Vector Borne Zoonotic Dis. 2017, 17, 42–50. [Google Scholar] [CrossRef]
- Angelakis, E.; Raoult, D. Pathogenicity and treatment of Bartonella infections. Int. J. Antimicrob. Agents 2014, 44, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Rolain, J.M. Bartonella infection: Treatment and drug resistance. Future Microbiol. 2010, 5, 1719–1731. [Google Scholar] [CrossRef] [PubMed]
- Prutsky, G.; Domecq, J.P.; Mori, L.; Bebko, S.; Matzumura, M.; Sabouni, A.; Shahrour, A.; Erwin, P.J.; Boyce, T.G.; Montori, V.M.; et al. Treatment outcomes of human bartonellosis: A systematic review and meta-analysis. Int. J. Infect. Dis. 2013, 17, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Persisters, persistent infections and the Yin-Yang model. Emerg. Microb. Infect. 2014, 3, 3. [Google Scholar] [CrossRef]
- Lee, M.S.; Choi, J.; Posadzki, P.; Ernst, E. Aromatherapy for health care: An overview of systematic reviews. Maturitas 2012, 71, 257–260. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Singh, G.; Kapoor, I.P.; Pandey, S.K.; Singh, U.K.; Singh, R.K. Studies on essential oils: Part 10; antibacterial activity of volatile oils of some spices. Phytother. Res. 2002, 16, 680–682. [Google Scholar] [CrossRef]
- Sakkas, H.; Gousia, P.; Economou, V.; Sakkas, V.; Petsios, S.; Papadopoulou, C. In vitro antimicrobial activity of five essential oils on multidrug resistant Gram-negative clinical isolates. J. Intercult. Ethnopharmacol. 2016, 5, 212–218. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Feng, J.; Wang, T.; Zhang, S.; Shi, W.; Zhang, Y. An optimized SYBR Green I/PI assay for rapid viability assessment and antibiotic susceptibility testing for Borrelia burgdorferi. PLoS ONE 2014, 9, e111809. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, S.; Shi, W.; Zubcevik, N.; Miklossy, J.; Zhang, Y. Selective Essential Oils from Spice or Culinary Herbs Have High Activity against Stationary Phase and Biofilm Borrelia burgdorferi. Front. Med. (Lausanne) 2017, 4, 169. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Shi, W.; Miklossy, J.; Zhang, Y. Additional Essential Oils with High Activity against Stationary Phase Borrelia burgdorferi. bioRxiv 2018, 260091. [Google Scholar] [CrossRef]
- Feng, J.; Shi, W.; Zhang, S.; Sullivan, D.; Auwaerter, P.G.; Zhang, Y. A Drug Combination Screen Identifies Drugs Active against Amoxicillin-Induced Round Bodies of In Vitro Borrelia burgdorferi Persisters from an FDA Drug Library. Front. Microbiol. 2016, 7, 743. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Feng, J.; Xiao, S.; Shi, W.; Sullivan, D.; Zhang, Y. Identification of FDA-Approved Drugs with Activity against Stationary Phase Bartonella henselae. Antibiotics (Basel) 2019, 8, 50. [Google Scholar] [CrossRef]
- Dörbecker, C.; Sander, A.; Oberle, K.; Schülin-Casonato, T. In vitro susceptibility of Bartonella species to 17 antimicrobial compounds: Comparison of Etest and agar dilution. J. Antimicrob. Chemother. 2006, 58, 784–788. [Google Scholar] [CrossRef]
- Rolain, J.M.; Brouqui, P.; Koehler, J.E.; Maguina, C.; Dolan, M.J.; Raoult, D. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob. Agents. Chemother. 2004, 48, 1921–1933. [Google Scholar] [CrossRef]
- Feng, J.; Wang, T.; Shi, W.; Zhang, S.; Sullivan, D.; Auwaerter, P.G.; Zhang, Y. Identification of novel activity against Borrelia burgdorferi persisters using an FDA approved drug library. Emerg. Microb. Infect. 2014, 3, e49. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Ben Halima, N.; Smaoui, S.; Hamdi, N. Citrus lemon essential oil: Chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017, 16, 146. [Google Scholar] [CrossRef]
- Aboelhadid, S.M.; Mahrous, L.N.; Hashem, S.A.; Abdel-Kafy, E.M.; Miller, R.J. In vitro and in vivo effect of Citrus limon essential oil against sarcoptic mange in rabbits. Parasitol. Res. 2016, 115, 3013–3020. [Google Scholar] [CrossRef]
- Maurya, A.K.; Mohanty, S.; Pal, A.; Chanotiya, C.S.; Bawankule, D.U. The essential oil from Citrus limetta Risso peels alleviates skin inflammation: In-vitro and in-vivo study. J. Ethnopharmacol. 2018, 212, 86–94. [Google Scholar] [CrossRef]
- Shen, C.Y.; Jiang, J.G.; Zhu, W.; Ou-Yang, Q. Anti-inflammatory Effect of Essential Oil from Citrus aurantium L. var. amara Engl. J. Agric. Food Chem. 2017, 65, 8586–8594. [Google Scholar] [CrossRef] [PubMed]
- Siti, H.N.; Kamisah, Y.; Nur Iliyani, M.I.; Mohamed, S.; Jaarin, K. Citrus leaf extract reduces blood pressure and vascular damage in repeatedly heated palm oil diet-Induced hypertensive rats. Biomed. Pharmacother. 2017, 87, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, A.; Chaabane, H.; Jemli, M.; Boulila, A.; Boussaid, M. Essential oil composition and antibacterial activity of Origanum vulgare subsp. glandulosum Desf. at different phenological stages. J. Med. Food 2013, 16, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Shelef, L.A.; Naglik, O.A.; Bogen, D.W. Sensitivity of some food-borne to the spices sage, rosemary and allspice. J. Food Sci. 1980, 45, M1042–M1044. [Google Scholar] [CrossRef]
- Eskow, E.; Rao, R.V.; Mordechai, E. Concurrent infection of the central nervous system by Borrelia burgdorferi and Bartonella henselae: Evidence for a novel tick-borne disease complex. Arch. Neurol. 2001, 58, 1357–1363. [Google Scholar] [CrossRef]
- Ren, P.; Ren, X.; Cheng, L.; Xu, L. Frankincense, pine needle and geranium essential oils suppress tumor progression through the regulation of the AMPK/mTOR pathway in breast cancer. Oncol. Rep. 2018, 39, 129–137. [Google Scholar] [CrossRef]
- Lysakowska, M.E.; Sienkiewicz, M.; Banaszek, K.; Sokolowski, J. The Sensitivity of Endodontic Enterococcus spp. Strains to Geranium Essential Oil. Molecules 2015, 20, 22881–22889. [Google Scholar] [CrossRef]
- Al-Yasiry, A.R.; Kiczorowska, B. Frankincense--therapeutic properties. Postep. Hig. Med. Dosw. (Online) 2016, 70, 380–391. [Google Scholar] [CrossRef]
- Ndoti-Nembe, A.; Vu, K.D.; Han, J.; Doucet, N.; Lacroix, M. Antimicrobial Effects of Nisin, Essential Oil, and gamma-Irradiation Treatments against High Load of Salmonella typhimurium on Mini-carrots. J. Food Sci. 2015, 80, M1544–M1548. [Google Scholar] [CrossRef]
- Sue, C.; Gary, Y.; Craig, O.; Karen, N. Inhibition of methicillin-resistant Staphylococcus aureus (MRSA) by essential oils. Flavour Fragr. J. 2008, 23, 444–449. [Google Scholar]
- Ndoti-Nembe, A.; Vu, K.D.; Doucet, N.; Lacroix, M. Antimicrobial effects of essential oils, nisin, and irradiation treatments against Listeria monocytogenes on ready-to-eat carrots. J. Food Sci. 2015, 80, M795–M799. [Google Scholar] [CrossRef] [PubMed]
- Broznic, D.; Ratkaj, I.; Malenica Staver, M.; Kraljevic Pavelic, S.; Zurga, P.; Bubalo, D.; Gobin, I. Evaluation of the Antioxidant Capacity, Antimicrobial and Antiproliferative Potential of Fir (Abies alba Mill.) Honeydew Honey Collected from Gorski kotar (Croatia). Food Technol. Biotechnol. 2018, 56, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Gobin, I.; Crnkovic, G.; Magdalenic, M.; Begic, G.; Babic, A.; Lusic, D.; Vuckovic, D. Antibacterial potential of Croatian honey against antibiotic resistant pathogenic bacteria. Med. Glas. (Zenica) 2018, 15, 139–144. [Google Scholar] [PubMed]
- Whiley, H.; Gaskin, S.; Schroder, T.; Ross, K. Antifungal properties of essential oils for improvement of indoor air quality: A review. Rev. Environ. Health 2018, 33, 63–76. [Google Scholar] [CrossRef]
- Tan, L.T.; Lee, L.H.; Yin, W.F.; Chan, C.K.; Abdul Kadir, H.; Chan, K.G.; Goh, B.H. Traditional Uses, Phytochemistry, and Bioactivities of Cananga odorata (Ylang-Ylang). Evid. Based Complement. Altern. Med. 2015, 2015, 896314. [Google Scholar] [CrossRef]
- Almeida Lde, F.; Paula, J.F.; Almeida, R.V.; Williams, D.W.; Hebling, J.; Cavalcanti, Y.W. Efficacy of citronella and cinnamon essential oils on Candida albicans biofilms. Acta Odontol. Scand. 2016, 74, 393–398. [Google Scholar] [CrossRef]
- Oliveira, J.B.; Teixeira, M.A.; Paiva, L.F.; Oliveira, R.F.; Mendonca, A.; Brito, M.J.A. In Vitro and In Vivo Antimicrobial Activity of Cymbopogon citratus (DC.) Stapf. Against Staphylococcus spp. Isolated from Newborn Babies in an Intensive Care Unit. Microb. Drug. Resist. 2019. [Google Scholar] [CrossRef]
- Mogana, R.; Teng-Jin, K.; Wiart, C. In Vitro Antimicrobial, Antioxidant Activities and Phytochemical Analysis of Canarium patentinervium Miq. from Malaysia. Biotechnol. Res. Int. 2011, 2011, 768673. [Google Scholar] [CrossRef]
- David, A.; Wang, F.; Sun, X.; Li, H.; Lin, J.; Li, P.; Deng, G. Chemical Composition, Antioxidant, and Antimicrobial Activities of Vetiveria zizanioides (L.) Nash Essential Oil Extracted by Carbon Dioxide Expanded Ethanol. Molecules 2019, 24, 1897. [Google Scholar] [CrossRef]
- Powers, C.N.; Osier, J.L.; Mcfeeters, R.L.; Brazell, C.B.; Olsen, E.L.; Moriarity, D.M.; Satyal, P.; Setzer, W.N. Antifungal and Cytotoxic Activities of Sixty Commercially-Available Essential Oils. Molecules 2018, 23, 1549. [Google Scholar] [CrossRef]
- Zouhir, A.; Jridi, T.; Nefzi, A.; Ben Hamida, J.; Sebei, K. Inhibition of methicillin-resistant Staphylococcus aureus (MRSA) by antimicrobial peptides (AMPs) and plant essential oils. Pharm. Biol. 2016, 54, 3136–3150. [Google Scholar] [CrossRef] [PubMed]
- Gadisa, E.; Weldearegay, G.; Desta, K.; Tsegaye, G.; Hailu, S.; Jote, K.; Takele, A. Combined antibacterial effect of essential oils from three most commonly used Ethiopian traditional medicinal plants on multidrug resistant bacteria. BMC Complement. Altern. Med. 2019, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Orchard, A.; Van Vuuren, S.F.; Viljoen, A.M.; Kamatou, G. The in vitro antimicrobial evaluation of commercial essential oils and their combinations against acne. Int. J. Cosmet. Sci. 2018, 40, 226–243. [Google Scholar] [CrossRef] [PubMed]
- Lanzerstorfer, A.; Hackl, M.; Schlomer, M.; Rest, B.; Deutsch-Grasl, E.; Lanzerstorfer, C. The influence of air-dispersed essential oils from lemon (Citrus limon) and silver fir (Abies alba) on airborne bacteria and fungi in hospital rooms. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2019, 54, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Breitschwerdt, E.B.; Kordick, D.L. Bartonella infection in animals: Carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin. Microbiol. Rev. 2000, 13, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Pulliainen, A.T.; Dehio, C. Persistence of Bartonella spp. stealth pathogens: From subclinical infections to vasoproliferative tumor formation. FEMS Microbiol. Rev. 2012, 36, 563–599. [Google Scholar] [CrossRef]
- Riess, T.; Dietrich, F.; Schmidt, K.V.; Kaiser, P.O.; Schwarz, H.; Schäfer, A.; Kempf, V.A. Analysis of a novel insect cell culture medium-based growth medium for Bartonella species. Appl. Environ. Microbiol. 2008, 74, 5224–5227. [Google Scholar] [CrossRef]
- Performance Standards for Antimicrobial Susceptibility Testing. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 13 December 2018).
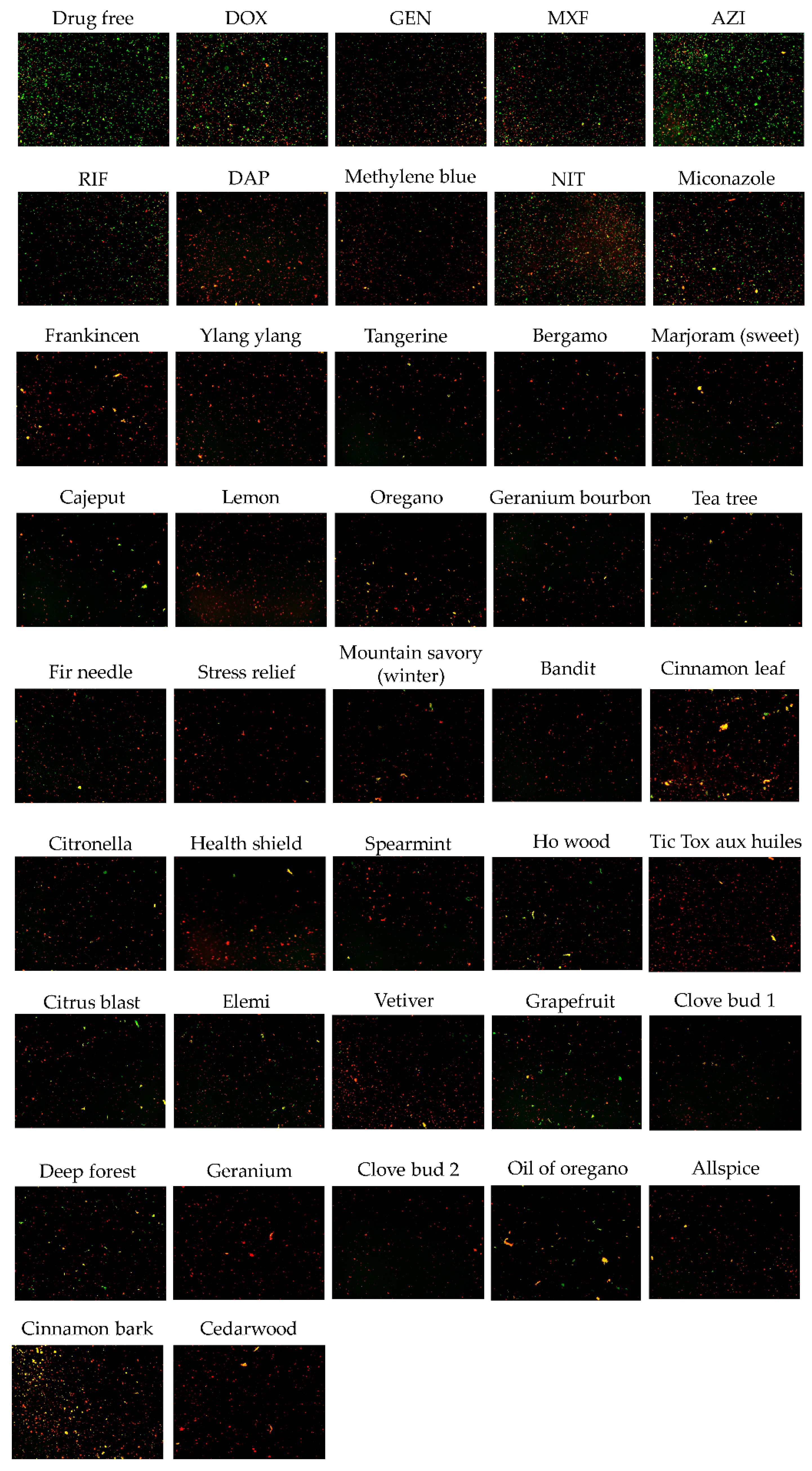
| Essential Oils and Control Drugs | Plant or Ingredients of Essential Oils | Residual Viability (%) after 0.5% EO or 20 μM Antibiotic Treatment | Residual Viability (%) after 0.25% EO Treatment | ||
|---|---|---|---|---|---|
| Plate Reader 2 | Microscope 3 | Plate Reader 2 | Microscope 3 | ||
| Drug free control | 74% | 74% | |||
| Doxycycline | 26% | 57% | |||
| Gentamicin | 9% | 35% | |||
| Moxifloxacin | 22% | 40% | |||
| Azithromycin | 23% | 67% | |||
| Rifampin | 25% | 44% | |||
| Daptomycin | 8% | 18% | |||
| Methylene Blue | 16% | 27% | |||
| Nitrofurantoin | 18% | 50% | |||
| Miconazole | 19% | 44% | |||
| Frankincense | Boswellia serrata | 5% | 11% | 6% | 10% |
| Ylang ylang | Cananga odorata | 5% | 9% | 8% | 10% |
| Tangerine | Citrus reticulata | 6% | 6% | 5% | 12% |
| Bergamot | Citrus bergamia | 6% | 18% | 10% | 15% |
| Marjoram (sweet) | Origanum majorana | 6% | 13% | 5% | 15% |
| Cajeput | Melaleuca cajeputi | 7% | 21% | 9% | 21% |
| Lemon | Citrus limonum | 7% | 10% | 4% | 11% |
| Oregano | Origanum vulgarehirtum | 7% | 7% | 7% | 20% |
| Geranium bourbon | Pelargonium graveolens | 8% | 20% | 11% | 22% |
| Tea tree | Melaleuca alternifolia | 8% | 12% | 5% | 25% |
| Fir needle | Abies siberica | 8% | 25% | 10% | 26% |
| Stress relief | synergy blend of essential oils of bergamot, patchouli, sweet orange, ylang ylang, pink grapefruit, gurjum | 8% | 15% | 6% | 12% |
| Mountain savory (winter) | Satureja montana | 8% | 25% | 21% | 32% |
| Bandit | synergy blend of essential oils of clove, cinnamon, lemon, rosemary, eucalyptus | 8% | 8% | 12% | 20% |
| Cinnamon leaf | Cinnamomum zeylanicum | 8% | 35% | 10% | 25% |
| Citronella | Cymbopogon winterianus | 8% | 15% | 12% | 23% |
| Health shield | blend of cinnamon, clove, eucalyptus, lemon and rosemary oils | 9% | 18% | 17% | 20% |
| Spearmint | Mentha spicata | 9% | 9% | 4% | 20% |
| Ho wood | Cinnamomum camphora | 9% | 20% | 11% | 29% |
| Tic Tox aux huiles essentielles | blend of essential oils of savory, sage officinale, wild chamomile, clove, compact oregano, cinnamon and niaouli | 11% | 21% | 14% | 14% |
| Citrus blast | synergy blend of Citrus sinesis, Citrus limonum, Citrus reticulata blanco var tangerina, Citrus bergamia, Citrus reticulata, Citrus clementina, Vanilla planifolia | 11% | 13% | 11% | 30% |
| Elemi | Canarium luzonicum | 12% | 25% | 14% | 32% |
| Vetiver | Vetiveria zizanoides | 12% | 26% | 8% | 18% |
| Grapefruit | Citrus paradisi | 12% | 35% | 11% | 36% |
| Clove bud 1 | Eugenia caryophyllata | 13% | 36% | 9% | 23% |
| Deep forest | synergy blend of Abies sibirica ledeb, Abies alba, Pinus sylvestris, Cupressus sempervirens, Cedrus deodora | 13% | 20% | 12% | 50% |
| Geranium | Pelargonium asperum | 14% | 23% | 15% | 20% |
| Clove bud 2 | Syzygium aromaticum L | 15% | 15% | 14% | 18% |
| Oil of oregano | Origanum vulgarehirtum | 15% | 52% | 19% | 55% |
| Allspice | Pimenta officinalis | 16% | 35% | 6% | 30% |
| Cedarwood | Cedrus deodora | 17% | 53% | 10% | 23% |
| Cinnamon bark | Cinnamomum zeylanicum | 18% | 40% | 13% | 45% |
| Essential Oils | Plant or Ingredients of Essential Oils | MIC (v/v) |
|---|---|---|
| Cinnamon bark | Cinnamomum zeylanicum | <0.008% |
| Health shield | blend of cinnamon, clove, eucalyptus, lemon and rosemary oils | 0.008–0.016% |
| Bandit | synergy blend of essential oils of clove, cinnamon, lemon, rosemary, eucalyptus | 0.016–0.032% |
| Oregano | Origanum vulgare hirtum | 0.016–0.032% |
| Elemi | Canarium luzonicum | 0.016–0.032% |
| Oil of oregano | Origanum vulgare hirtum | 0.016–0.032% |
| Mountain savory (winter) | Satureja montana | 0.016–0.032% |
| Cedarwood | Cedrus deodora | 0.016–0.032% |
| Ylang ylang | Cananga odorata | 0.032–0.063% |
| Citronella | Cymbopogon winterianus | 0.032–0.063% |
| Clove bud 1 | Eugenia caryophyllata | 0.032–0.063% |
| Clove bud 2 | Syzygium aromaticum L | 0.032–0.063% |
| Geranium bourbon | Pelargonium graveolens | 0.032–0.063% |
| Allspice | Pimenta officinalis | 0.032–0.063% |
| Vetiver | Vetiveria zizanoides | 0.032–0.063% |
| Cinnamon leaf | Cinnamomum zeylanicum | 0.032–0.063% |
| Geranium | Pelargonium asperum | 0.032–0.063% |
| Stress relief | synergy blend of essential oils of bergamot, patchouli, sweet orange, ylang ylang, pink grapefruit, gurjum | 0.063–0.125% |
| Bergamot | Citrus bergamia | 0.063–0.125% |
| Cajeput | Melaleuca cajeputi | 0.063–0.125% |
| Marjoram (sweet) | Origanum majorana | 0.063–0.125% |
| Citrus blast | synergy blend of essential oils of bergamot, patchouli, sweet orange, ylang ylang, pink grapefruit, gurjum | 0.063–0.125% |
| Deep forest | synergy blend of Abies sibirica ledeb, Abies alba, Pinus sylvestris, Cupressus sempervirens, Cedrus deodora | 0.063–0.125% |
| Fir needle | Abies siberica | 0.063–0.125% |
| Grapefruit | Citrus paradisi | 0.063–0.125% |
| Spearmint | Mentha spicata | 0.125–0.25% |
| Tangerine | Citrus reticulata | 0.125–0.25% |
| Tea tree | Melaleuca alternifolia | 0.125–0.25% |
| Lemon | Citrus limonum | 0.125–0.25% |
| Ho wood | Cinnamomum camphora | 0.125–0.25% |
| Frankincense | Boswellia serrata | 0.125–0.25% |
| Tic Tox aux huiles essentielles | blend of essential oils of savory, sage officinale, wild chamomile, clove, compact oregano, cinnamon and niaouli | 0.125–0.25% |
| Essential Oils and Control Drugs 2 | CFU/mL after Drug Exposure | |||
|---|---|---|---|---|
| 1 Day | 3 Day | 5 Day | 7 Day | |
| Drug free control | 1.50 ± 0.53 × 107 | 8.83 ± 0.29 × 106 | 1.88 ± 0.40 × 106 | 2.67 ± 0.29 × 106 |
| Doxycycline | 4.17 ± 1.44 × 107 | 5.07 ± 0.38 × 106 | 2.30 ± 0.10 × 106 | 8.33 ± 2.89 × 105 |
| Azithromycin | 4.50 ± 2.00 × 107 | 9.17 ± 0.29 × 106 | 3.80 ± 0.72 × 106 | 2.83 ± 1.04 × 105 |
| Gentamicin | 9.83 ± 2.93 × 104 | 0 | 0 | 0 |
| Rifampin | 1.27 ± 0.15 × 107 | 8.33 ± 0.76 × 104 | 5.17 ± 0.29 × 103 | 0 |
| Daptomycin | 0 | 0 | 0 | 0 |
| Methylene blue | 1.35 ± 0.13 × 106 | 3.17 ± 0.58 × 103 | 0 | 0 |
| Miconazole | 5.83 ± 1.53 × 106 | 1.57 ± 0.28 × 106 | 8.50 ± 1.32 × 105 | 1.30 ± 0.10 × 104 |
| Oregano | 0 | 0 | 0 | 0 |
| Cinnamon bark | 0 | 0 | 0 | 0 |
| Mountain savory (winter) | 0 | 0 | 0 | 0 |
| Clove bud 2 | 6.50 ± 3.46 × 102 | 0 | 0 | 0 |
| Allspice | 2.27 ± 0.33 × 103 | 0 | 0 | 0 |
| Geranium | 4.83 ± 0.76 × 103 | 0 | 0 | 0 |
| Cinnamon leaf | 1.33 ± 0.35 × 104 | 0 | 0 | 0 |
| Geranium bourbon | 5.50 ± 2.65 × 103 | 5.00 ± 5.00 × 10 | 0 | 0 |
| Clove bud 1 | 5.00 ± 0.00 × 104 | 8.33 ± 5.77 × 10 | 0 | 0 |
| Elemi | 1.38 ± 0.42 × 103 | 5.00 ± 5.00 × 10 | 2.17 ± 1.04 × 102 | 0 |
| Vetiver | 2.00 ± 0.50 × 105 | 1.18 ± 0.19 × 104 | 5.17 ± 2.47 × 102 | 0 |
| Citronella | 1.13 ± 0.12 × 104 | 7.33 ± 2.84 × 103 | 4.50 ± 0.87 × 103 | 0 |
| Ylang ylang | 2.00 ± 0.87 × 105 | 2.38 ± 0.19 × 105 | 7.83 ± 3.01 × 104 | 0 |
| Grapefruit | 1.02 ± 0.19 × 104 | 3.17 ± 1.89 × 104 | 5.33 ± 1.26 × 103 | 6.67 ± 5.77 × 10 |
| Tangerine | 3.17 ± 0.29 × 104 | 2.08 ± 0.58 × 104 | 4.50 ± 2.29 × 103 | 6.67 ± 5.77 × 10 |
| Bergamot | 8.17 ± 2.25 × 103 | 2.62 ± 0.35 × 104 | 6.83 ± 0.76 × 103 | 1.67 ± 0.58 × 102 |
| Fir needle | 4.17 ± 1.61 × 103 | 2.32 ± 0.41 × 104 | 1.10 ± 0.13 × 104 | 1.67 ± 0.58 × 102 |
| Frankincense | 1.35 ± 0.22 × 105 | 8.17 ± 1.53 × 105 | 1.48 ± 0.29 × 106 | 1.83 ± 0.76 × 102 |
| Ho wood | 5.00 ± 0.50 × 106 | 7.50 ± 2.65 × 105 | 1.37 ± 0.28 × 105 | 4.17 ± 1.44 × 102 |
| Lemon | 3.17 ± 1.15 × 104 | 1.03 ± 0.28 × 105 | 8.67 ± 0.76 × 104 | 4.33 ± 2.31 × 103 |
| Marjoram (Sweet) | 2.17 ± 1.53 × 105 | 2.13 ± 0.28 × 106 | 2.22 ± 0.25 × 106 | 7.50 ± 1.32 × 103 |
| Cajeput | 2.50 ± 0.87 × 106 | 9.43 ± 0.40 × 106 | 3.20 ± 0.26 × 106 | 1.62 ± 0.25 × 105 |
| Tea tree | 8.00 ± 2.18 × 105 | 9.33 ± 0.29 × 106 | 3.97 ± 0.45 × 106 | 3.17 ± 0.76 × 106 |
| Cedarwood | 2.33 ± 2.31 × 105 | 2.73 ± 0.33 × 106 | 3.40 ± 0.36 × 106 | 3.52 ± 0.18 × 106 |
| Spearmint | 4.33 ± 1.26 × 105 | 9.17 ± 0.29 × 106 | 3.67 ± 0.58 × 106 | 3.68 ± 0.38 × 106 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Shi, W.; Zhang, Y. Essential Oils with High Activity against Stationary Phase Bartonella henselae. Antibiotics 2019, 8, 246. https://doi.org/10.3390/antibiotics8040246
Ma X, Shi W, Zhang Y. Essential Oils with High Activity against Stationary Phase Bartonella henselae. Antibiotics. 2019; 8(4):246. https://doi.org/10.3390/antibiotics8040246
Chicago/Turabian StyleMa, Xiao, Wanliang Shi, and Ying Zhang. 2019. "Essential Oils with High Activity against Stationary Phase Bartonella henselae" Antibiotics 8, no. 4: 246. https://doi.org/10.3390/antibiotics8040246
APA StyleMa, X., Shi, W., & Zhang, Y. (2019). Essential Oils with High Activity against Stationary Phase Bartonella henselae. Antibiotics, 8(4), 246. https://doi.org/10.3390/antibiotics8040246





