Abstract
The aim of this study was to explore the phytochemical composition, heavy metals analysis and the antibacterial activity of six medicinal plants i.e., Terminalia chebula Retz (fruits), Aegle marmelos L., (fruits), Curcuma longa L., (rhizomes), Syzygium aromaticum L., (flower buds), Piper nigrum L., (seeds), Cinnamomum cassia L., (barks) and its two remedial recipes (recipe 1 and 2) used against diarrhea obtained from the local herbal practitioners (Hakeems). A preliminary phytochemical screening of the above-mentioned plants extract in methanol, chloroform, n-hexane and distilled water revealed the presence of various constituents such as alkaloids, flavonoids, tannins and saponins by using standard procedures. The quantitative phytochemical studies shows that alkaloids, flavonoid and saponins were in maximum amount in Terminalia chebula. The concentration of Cd, Ni, Pb, Fe, Cr, Cu and Zn were investigated by using an atomic absorption spectrometer. The obtained analysis shows that Cr, Fe and Pb were present in the highest concentration in medicinal plants and their recipes. The antibacterial activities of the crude extract found in the recipes of methanol, chloroform, n-hexane and distilled water were analyzed by using agar well disc diffusion assay and minimum inhibitory concentration (MIC) by broth dilution method against four bacterial strains, namely, E. coli, Salmonella, Shigella and Methicillin-resistant Staphylococcus aureus (MRSA), respectively. The maximum zones of inhibition in methanol, water, chloroform and n-hexane extracts were seen in recipe 2 against Shigella (22.16 ± 0.47 mm), recipe 2 against Shigella (20.33 ± 0.24 mm), recipe 1 against Shigella (20.30 ± 0.29 mm) and recipe 2 against E. coli (30.23 ± 0.12 mm), respectively. Furthermore, the recipe extracts are more active against the tested bacterial strains than the extracts from individual plants. Therefore, it is concluded that the use of herbal plants and their recipes are the major source of drugs in a traditional medicinal system to cure different diseases.
1. Introduction
The present study was conducted in district Karak, in the southern region of Khyber Pakhtunkhwa, Pakistan. The total population of district Karak is 706,299, with an area of about 3372 square Kms. Karak is situated between 31°15′ and 36°55′ latitude and between 70°05′ and 74°05′ longitude. Most of the people live in rural areas and depend on medicinal plants for the curing of different diseases. Medicinal plants can act as an indigenous source of new compounds possessing therapeutic value and can also be used in drugs development. According to the World Health Organization (WHO), plants can provide different varieties of drugs for low-income nations to cope with their primary healthcare needs [1]. The plants are medicinally important due to the presence of biologically active secondary metabolites such as alkaloids, flavonoids, steroids, saponins and terpenoids, which exert their effects by interacting with human physiology. The antimicrobial activities of these phytochemicals are due to their chemical nature [2,3] and are a potential source of diarrheal disease [4]. For this reason, the WHO has encouraged the studies for the treatment and prevention of diarrheal diseases using traditional medicinal practices [5]. Presently, a large number of medicinal plants are being used in many countries of the world, including Pakistan, due to their anti-diarrheal properties [6]. In Bangladesh, over 250 floral species are used by the folk medicinal and tribal healers for the treatment of diarrhea [7]. The Indian Himalayan region also support approximately 1700 plant species of known medicinal value [8]. However, there are only few studies on the utility of medicinal plants in the treatment of specific diseases [9]. Various medications are used for diarrheal diseases which possess different adverse effects like nausea, headache, dry mouth and constipation. However, there are many medicinal plants that have anti-diarrheal activities with less, or even no, side-effects than the allopathic drugs. These plants show anti-diarrheal activity by reducing secretions and the gastrointestinal motility [10].
The medicinal plants are destroyed and contaminated by various factors, such as environmental pollution, soil harvesting, microbial growth and introduction of toxic metals. The ingredients of plants include metal ions which are responsible for nutritional as well as medicinal usage [11]. Heavy metals like zinc, manganese, cobalt, iron, copper, chromium and nickel are essential for proper body function and become toxic when they exceed the recommended level and cause various chronic and acute effects in the living organisms [12]; whereas, the metals like lead, mercury and cadmium are non-essential and are toxic in nature even in the trace amount [13].
Thus, due to the hazardous affects as well as antibiotic resistance to the synthetic drugs, researchers are trying to obtain the antimicrobial drugs from medicinal plants due to their non-toxic nature and less side effects [14,15]. In spite of all the progress in the field of allopathic drugs, the traditional medicines, particularly plant-based medicines, also have a key role. Many studies have shown that crude extracts of medicinal plants as well as the pure bioactive components can act as good therapeutic agents [16,17].
Apart from the individual medicinal plants used in this study, we also analyzed the two recipes and their MIC. These recipes were prepared and named according to the traditional Hakeems. Recipe 1 is named Akseer-e-Pechesh and recipe 2 is named Taryaq-e-tabkhir Balghami, respectively. We observed that recipe 1 contains T. chebula (fruits), A. marmelos (fruits) and C. longa (rhizomes), which are mixed in the ratio of 1:1:2, respectively. It is used in the form of powder which is relatively more effective against diarrhea and dysentery. Recipe 2 also contains S. aromaticum (flower buds), P. nigrum (seeds) and C. cassia (barks), which are mixed in the ratio of 1:0.5:1 and ground to powder, which is also quite effective in the treatment of diarrhea and constipation. These plants, as well as their recipes, are substantially used by the local inhabitants and local herbal practitioner (Hakeems) to treat diarrhea caused by the pathogenic microorganisms like E. coli, Salmonella, Shigella and MRSA.
2. Results
Qualitative phytochemical screening shows that the alkaloids were present in chloroform and methanol extracts of T. chebula (fruits), A. marmelos (fruits), S. aromaticum (flower buds), C. longa (rhizomes), C. cassia (barks), P. nigrum (seeds), recipe 1 and recipe 2, while it was not detected in n-hexane extract of A. marmelos (fruits) and in aqueous extracts of C. longa (rhizomes), as shown in Table 1. Whereas, the quantitative amount of alkaloids determined in T. chebula (fruits), A. marmelos (fruits), C. longa (rhizomes), S. aromaticum (flower buds), P. nigrum (seeds), C. cassia (barks), recipe 1 and recipe 2 were 27.84%, 2.54%, 2.66%, 11.88%, 5.06%, 5.28%, 19.66% and 17.78% respectively, as predicted in Table 2. The Flavonoids are present in methanol extract of A. marmelos (fruits), T. chebula (fruits), S. aromaticum (flower buds), recipe 1 and recipe 2, while they were not detected in P. nigrum (seeds), C. longa (rhizomes) and C. cassia (barks). However, in the aqueous solution and chloroform extracts, the flavonoids were found in A. marmelos (fruits) and in recipe 1, while in n-hexane extract the flavonoids were found in S. aromaticum and in recipe 2, as shown in Table 1. The quantity of flavonoids in T. chebula (fruits), A. marmelos (fruits), C. longa (rhizomes), S. aromaticum (flower buds), P. nigrum (seeds), C. cassia (barks), recipe 1 and recipe 2 were found to be 61.21%, 23.81%, 6.82%, 18.6%, 9%, 4.74%, 28.13% and 14.25% respectively, as shown in Table 2. Saponins were found preliminary in methanol, aqueous, chloroform and n-hexane extracts of all the medicinal plants and their recipes, as shown in Table 1. Quantitatively, the amount of saponins were determined in T. chebula (fruits), A. marmelos (fruits), C. longa (rhizomes), S. aromaticum (flower buds), P. nigrum (seeds), C. cassia (barks), recipe 1 and recipe 2 to be 6.32%, 0.24%, 0.36%, 1.10%, 0.19%, 0.23%, 1.19%, and 0.83% respectively, as shown in Table 2. Tannins were detected preliminarily in all the parts of plants as well as their recipes except C. longa (rhizomes) in crude methanol extracts. Tannins were also absent in the aqueous extracts of C. cassia (barks) and C. longa (rhizomes), while in the case of crude chloroform extracts, it was undetected in P. nigrum (seeds), C. longa (rhizomes) and C. cassia (barks). In the n-hexane crude extracts it was found in S. aromaticum (flower buds) and in recipe 2, as shown in Table 1. The results obtained in this study are highly consistent with the previously reported results from the study of medicinal plants of this region [18,19,20,21,22,23].

Table 1.
Qualitative phytochemical screening of alkaloids, flavonoids, saponins and tannins of medicinal plant parts and their recipes.

Table 2.
Quantitative phytochemical screening of alkaloids, flavonoids and saponins of medicinal plant parts and their recipes.
The results of seven different elements shown in Table 3 and Figure 1 indicate that there is no cadmium at all. The cadmium (Cd) is very toxic, non-essential and the accumulation of cadmium may damage the kidneys. According to the WHO, the recommended level of Cd is 0.3 mg/kg in medicinal plants [24]. The results show that in T. chebula, C. cassia, recipe 1 and recipe 2, the Ni is below the detection limit. The S. aromaticum (flower buds) contain 0.825 mg/kg, A. marmelos (fruits) 0.55 mg/kg, P. nigrum (seeds) 0.35 mg/kg and C. longa (rhizomes) 0.05 mg/kg for the mean concentration of Ni respectively, which are below the standard recommended level. According to the WHO, the maximum permissible limit of Nickel in medicinal plants is 1.5 mg/kg, while its recommended level for mankind is 1 mg/day [24]. The results illustrate that the maximum amount of iron present in the S. aromaticum (flower buds) 92.45 mg/kg, T. chebula (fruits) 66.775 mg/kg, C. longa (rhizomes) 55.9 mg/kg, C. cassia (barks) 48.475 mg/kg, A. marmelos (fruits) 48.1 mg/kg, recipe 2 44.475, recipe 1, 43.875 mg/kg and P. nigrum (seeds) 41.975 mg/kg, is beyond the maximum permissible value. According to the WHO, the maximum permissible limit of iron in medicinal plants is 20 mg/kg, while its daily requirement is 10 to 28 mg/day [20]. The results further show that the high concentration of Pb was found in recipe 1, 89.9 mg/kg, recipe 2, 74.45 mg/kg, S. aromaticum (flower buds) 70.675 mg/kg, C. longa (rhizomes) 70.1 mg/kg, C. cassia (barks) 65.875 mg/kg, P. nigrum (seeds) 61.925 mg/kg, T. chebula (fruits) 60.125 mg/kg and A. marmelos (fruits) 58 mg/kg, which are beyond the maximum permissible limit as recommended by the WHO. According to the WHO, the maximum permissible limit of Pb in medicinal plant is 10 mg/kg [24]. The results further predict that the concentration of Zn was found in C. longa (rhizomes) 61.375 mg/kg, recipe 1, 27.925 mg/kg, S. aromaticum (flower buds) 21.75 mg/kg, T. chebula (fruits) 21.075 mg/kg, P. nigrum (seeds) 16.9 mg/kg A. marmelos (fruits) 16.325 mg/kg, C. cassia (barks) 16.025 mg/kg and recipe 2 9.475 mg/kg, which are below the maximum permissible level, except for C. longa (rhizomes), as permitted by the WHO. According to the WHO, the maximum permissible limit of Zn in medicinal plants is 50 mg/kg, while its daily requirement in food is 11 mg/kg [25]. The results in Table 3 reveal that the concentration of Cu was found in P. nigrum (seeds) 10.9 mg/kg, S. aromaticum (flower buds) 8.3 mg/kg, C. longa (rhizomes) 8.225 mg/kg, T. chebula (fruits) 6.8 mg/kg, recipe 2 6.557 mg/kg, C. cassia (barks) 5.875 mg/kg, recipe 1 5.675 mg/kg and A. marmelos (fruits) 5.175 mg/kg, which is below or according to the maximum permissible level. According to the WHO, in medicinal plants, the maximum permissible amount of Cu is 10 mg/kg, while its daily requirement in food is 2–3 mg/day [24]. The results also indicated that the amount of Cr in recipe 1 was 143.9 mg/kg, recipe 2, 140.926 mg/kg, C. cassia (barks), 139.65 mg/kg, A. marmelos (fruits), 127.375 mg/kg, S. aromaticum (flower buds), 125.6 mg/kg, C. longa (rhizomes), 121.05 mg/kg, T. chebula (fruits), 119.475 mg/kg and P. nigrum (seeds), 114.325 mg/kg. The amount of Cr which exists in all these plant parts as well as their recipe is above the maximum permissible value. According to the WHO, the maximum permissible level of chromium in medicinal plants is 1.5 mg/kg and its daily requirement is 0.2 mg [24].

Table 3.
Metals in herbal plants and their recipes e.g., Cd, Ni, Fe, Pb, Zn, Cu and Cr.
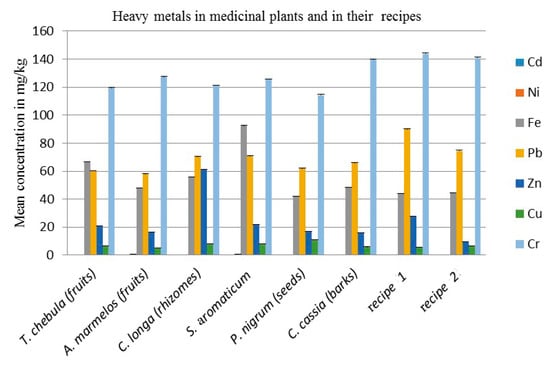
Figure 1.
Mean concentration of heavy metals in mg/kg in medicinal plants and in their recipes. Each column represents the mean value of three independent replicates and the error bars indicate standard deviation.
The comparative antibacterial activities of aqueous, methanol, chloroform and n-hexane extracts of the T. chebula (fruits), A. marmelos (fruits), C. longa (rhizomes) and their recipe 1 are shown in Table 4 and Figure 2. The aqueous extracts of A. marmelos shows very good inhibition effects against all four bacterial strains. The aqueous extracts of T. chebula (fruits) and C. longa (rhizomes) displayed a significant zone of inhibition against Salmonella and MRSA respectively which possess moderate inhibitory effects against the remaining bacterial strains. Recipe 1 presents a very good inhibition zone as compared to its individual plant parts against all bacterial strains except A. marmelos, which produced a large inhibition zone against E-coli only.

Table 4.
Comparative Antibacterial Activities of methanol, Aqueous, Chloroform and n-hexane extracts of the T. chebula (fruits), A. marmelos (fruits), C. longa (rhizomes) and their recipe 1.
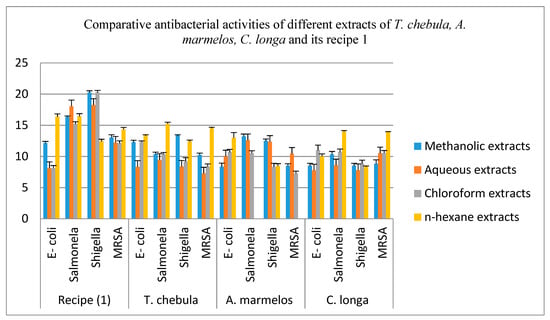
Figure 2.
Comparative Antibacterial Activities of methanol, Aqueous, chloroform and n-hexane extracts of the T. chebula (fruits), A. marmelos (fruits), C. longa (rhizomes) and their recipe 1. Each column represents the mean value of three independent replicates and the error bars indicate standard deviation.
The methanol extracts of T. chebula (fruits) displayed a considerable inhibitory zone against all four bacterial strains. A. marmelos (fruits) and C. longa (rhizomes) also exhibit a strong inhibitory result except for E-coli and MRSA. Besides this, the C. longa also exhibited the moderate inhibitory effect against Shigella. Recipe 1 also revealed a very good inhibition zone as compared to individual plant parts against all four bacterial strains.
The chloroform extracts of T. chebula, A. marmelos and C. longa present good inhibition effects against E-coli and Salmonella. All these extracts exhibited a moderate inhibition zone against Shigella. The extract of C. longa shows a strong inhibitory effect against MRSA, while T. chebula and A. marmelos extracts show a moderate inhibition zone against MRSA. Recipe 1 revealed a significant inhibition effect against three bacterial strains, except for E-coli, as compared to its individual plant extract.
The n-hexane extracts of T. chebula, A. marmelos and C. longa displayed a marked effect against all four bacterial strains except the n-hexane extract of A. marmelos, which shows no inhibitory zone against Salmonella and MRSA. Recipe 1 of n-hexane indicates the better inhibition effect against all four bacterial strains as compared to all of its individual plant extracts.
The comparative antibacterial activities of aqueous, methanol, chloroform and n-hexane extracts of the S. aromaticum (flower buds), P. nigrum (seeds), C. cassia (barks) and their recipe 2 are shown in Table 5 and Figure 3. The aqueous extract of these three plants displayed a strong inhibition zone against E-coli, Salmonella and MRSA. Beside this, the S. aromaticum and C. cassia also show significant antibacterial activity against Shigella but the aqueous extract of P. nigrum indicated a moderate zone of inhibition against Shigella. The aqueous extract of recipe 2 revealed an excellent result, as compared to its individual plant extracts.

Table 5.
Comparative Antibacterial evaluation of methanol, aqueous, chloroform and n-hexane extracts of the S. aromaticum, P. nigrum, C. cassia and recipe 2.
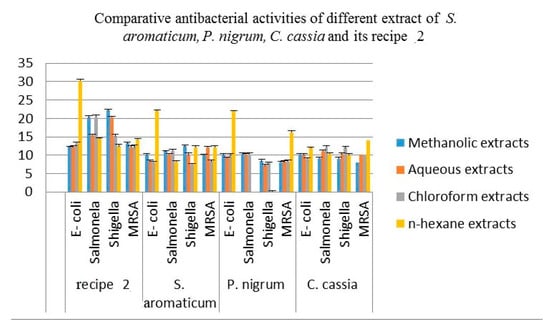
Figure 3.
Antibacterial activities of methanol, aqueous chloroform and n-hexane extracts of the S. aromaticum (flower buds), P. nigrum (seeds), C. cassia and recipe 2. Each column represents the mean value of three independent replicates and the error bars indicate standard deviation.
The methanol extracts of all these plants perceive very influential inhibitory effects against E-coli and Salmonella. The methanol extracts of these medicinal plants indicated a desirable inhibition zone against Shigella and MRSA except for the methanol extract of P. nigrum, which shows a reasonable zone of inhibition against MRSA. The methanol extracts of C. cassia also displayed a moderate zone of inhibition against MRSA. The methanol extract of recipe 2 shows a significant zone of inhibition against all the four bacterial strains as compared to its individual plant extracts.
The chloroform extracts of S. aromaticum displayed a significant inhibition effect against Salmonella, while a moderate inhibition effect against Shigella, E-coli and MRSA. The chloroform extracts of P. nigrum shows strong inhibition effects against Salmonella and E-coli, which demonstrated the moderate inhibition effects against Shigella and MRSA. The chloroform extract of C. cassia has strong inhibition effects against all four bacterial strains. The extracts of recipe 2 showed a very strong inhibitory effect, as compared to the individual plant parts against all four bacterial strains.
The n-hexane extract of S. aromaticum perceived more pronounced effects against Shigella, E-coli and MRSA and exhibited moderate effect against Salmonella. The extracts of P. nigrum demonstrated good inhibitory effects against E-coli, MRSA and show no zone of inhibition against Shigella and Salmonella. The C. cassia had a strong inhibition effect against all four bacterial strains. The n-hexane extracts of recipe 2 exhibited an appreciable inhibition zone as compared to its individual plants against all these four strains.
Minimum inhibitory concentrations (MIC) of recipe 1 and recipe 2 are shown in Table 6 and Table 7, respectively. Both of these recipes displayed minimum inhibitory concentrations (MIC) values against different bacterial strains. Recipe 1 shows MIC in methanol extract against E-coli, Salmonella, Shigella and MRSA at 11,000 mg/L, 12,000 mg/L 11,500 mg/L and 14,000 mg/L respectively, and similarly, in aqueous extracts against E-coli, Salmonella, Shigella and MRSA at 12,500 mg/L, 11500 mg/L, 12,000 mg/L and 14,500 mg/L, respectively. It shows MIC in chloroform extract against E-coli, Salmonella, Shigella and MRSA at 14,000 mg/L, 13,000 mg/L 14,000 mg/L and 15,000 mg/L respectively, and further, in n-hexane extract against E-coli, Salmonella, Shigella and MRSA at 15,000 mg/L, 15,000 mg/L 14,500 mg/L and 15,000 mg/L, respectively.

Table 6.
Minimum inhibitory concentration (MIC) of recipe 1 against different bacterial strains.

Table 7.
Minimum inhibitory concentration (MIC) of recipe 2 against different bacterial strains.
Recipe 2 displayed MIC in methanol extract against E-coli, Salmonella, Shigella and MRSA at 12,000 mg/L, 10,500 mg/L 11,500 mg/L and 15,000 mg/L, respectively. Similarly, it displayed MIC in aqueous extracts against E-coli, Salmonella, Shigella and MRSA at 11,000 mg/L, 12,000 mg/L 12,500 mg/L and 14,000 mg/L, respectively. It displayed MIC in chloroform extract against E-coli, Salmonella, Shigella and MRSA at 13,000 mg/L, 12,500 mg/L 13,000 mg/L and 15,000 mg/L respectively, and in n-hexane extract against E-coli, Salmonella, Shigella and MRSA at 14,000 mg/L, 13,000 mg/L 14,000 mg/L and 15,000 mg/L, respectively.
3. Discussion
Medicinally important plants and their biologically active phytoconstituents are used globally for curing various human diseases including gastrointestinal infections, inflammation, heart disease, cancer and respiratory infection. The use of herbal products and their recipes have fewer side effects as compared to synthetic drugs. All over the world, mostly, people depend upon the herbal products for their healthcare needs. Phytochemicals are the real sources of medicinal value of plants which exert their effects by interacting with human physiology [26]. Preliminary phytochemical screening was performed to find the presence of alkaloids, saponins, flavonoids and tannins. Alkaloids vary greatly in chemical composition and play a vital role in drugs discovery. Typically, the antimicrobial properties of medicinal plants were found due to alkaloids. Typically, herbal species containing flavonoids are known to have therapeutic properties and a declining ratio of cancer has been reported by consuming fruits and vegetables containing flavonoids [27,28]. Saponins are very effective in gastrointestinal infection and have antitumor properties [29]. Tannins have antibacterial activity and are used against diarrhea and dysentery. Tannins play a very important role in the healing of wounds and in bleeding [30]. Many studies have reported the clinical importance of the medicinal plants based on their phytochemical screening, which greatly reinforces the idea of novelty in research in this area [31,32].
Human beings use different medicinal plants from the time immemorial in many aspects, as nutritional values, remedy for different diseases and as essential components in cosmetics. The usefulness of these plants as well as the toxicity is due to their chemical nature, particularly due to the presence of heavy metals like zinc, manganese, cobalt, iron, copper, chromium and nickel. The cadmium (Cd) is very toxic and non-essential and the accumulation of cadmium damages kidneys and liver. Nickel (Ni) is an essential element for all living organisms and is required in a very minute quantity for an individual [33]. Above the permissible level, it is toxic and causes heart failure, loss of vision, loss of body weight and skin irritation [34]. Iron (Fe) is an essential component of hemoglobin. Its deficiency causes nose bleeding, myocardial infractions and gastrointestinal infection [35]. Lead (Pb) is a non-essential trace heavy metal having no functions both in animals as well as in plants. High concentrations of lead causes oxidative stress, brain damage, colic, anemia, headaches and central nervous system disorders [36]. It accumulates in the spleen, kidney and liver through air (20%), food (65%) and water (15%). Zinc (Zn) is an essential trace heavy metal and plays an important role in various processes including bone formation, brain development, wound healing, normal growth and behavioral response. It is essential in protein as well as in DNA synthesis. It regulates structural and catalytic functions of different enzymes [37]. Copper (Cu) is an essential element for normal growth and development as well as for many enzymatic activities. The concentration of copper above the permissible level causes hair and skin discoloration, respiratory and some other lethal effects in human beings [38]. Its deficiency causes anemia and Wilson’s diseases [13]. Chromium (Cr) is very essential for the metabolism of glucose, cholesterol and fat. Its concentration above the permissible level is carcinogenic and toxic in nature. The toxicity of Cr intake may appear in the form of a stomach ulcer, skin rash, kidney damage, lung cancer and nose irritation. Its deficiency may lead to elevated body fat and disturbance in proteins, lipids and glucose metabolism [39].
Due to harmful effects as well as antibiotic resistance to the synthetic drugs, researchers are trying to obtain antimicrobial drugs from medicinal plants due to their non-toxic nature. In this study, the crude extracts of Terminalia chebula (fruits), Aegle marmelos (fruits) and Curcuma longa (rhizomes) Syzygium aromaticum (flower buds), Piper nigrum (seeds) Cinnamomum cassia (barks) recipe 1 and recipe 2 showed a very good zone of inhibition against all four tested bacterial strains.
4. Materials and Methods
4.1. Collection of Medicinal Plants Parts and Their Identification
Medicinally effective parts of the selected plants including Curcuma longa L., (rhizomes), Terminalia chebula Retz., (fruits), Aegle marmelos L., (fruits), Syzygium aromaticum L., (flower buds), Piper nigrum L., (seeds) and Cinnamomum cassia L. (barks) were collected from the local herbal market (pansori shops) of Karak, Khyber Pakhtunkhwa. Plants parts were identified and used for further experimentation.
4.2. Plants Grinding and Recipe Formulation
The collected medicinal plant parts were initially washed using distilled water, dried and then sliced into small pieces, separately. Then, each part was mashed to form powder with the help of mortar and pestle, and these powdered samples were stored in a dirt-free separate closed glass container for further use. Apart from the individual plant parts, two recipes were also formed according to the Hakeem description [40,41,42]. Recipe 1 (Akseer-e-Pechesh) contains T. chebula (fruits), A. marmelos (fruits) and C. longa (rhizomes), which are mixed in the ratio of 1:1:2, respectively. Recipe 2 (Taryaq-e-tabkhir Balghami) contains S. aromaticum (flower buds), P. nigrum (seeds) and C. cassia (barks), which are mixed in the ratio of 1:0.5:1, respectively.
4.3. Preparation of Plant Extracts
Extracts of each plant and its recipe were prepared by soaking 300 g of plant materials in 500 mL of four different solvents like methanol, distill water, chloroform and n-hexane. The mixtures were kept for 48 h stirring at room temperature, followed by the vacuum filtration. After that, the filtrate was rotary evaporated to obtain semi-solid extract. Then, the phytochemicals analysis in each sample were determined qualitatively and quantitatively [43,44].
4.4. Qualitative Phytochemical Screening
Qualitative phytochemical screening of medicinal plant parts and their recipes were carried out by means of some specific methods. Alkaloids, flavonoids, tannins and saponins were detected by the Tyler [45] and Harborne [46] method.
4.5. Quantitative Phytochemical Screening
Quantitative phytochemical analyses were carried by using the Harborne [46] and Obadoni [47] methods for the determination of alkaloids, the Boham [48] method for flavonoids and the Obadoni [47] method for saponins.
4.6. Heavy Metal Analysis of Medicinal Plants and Their Recipes
Heavy metals like Ni, Cd, Fe, Cr, Zn, Cu and Pb in the medicinal plants and its recipes were analyzed by an atomic absorption spectrophotometer (Perkin Elmer analyst 400, UK) using nitrous oxide (N2O)-acetylene flame. For the calibration of equipment, the following sensitivity and detection limits were established, in the amounts of: Ni (0.5, 1 and 2 ppm), Cd (0.5, 1 and 1.5 ppm), Fe (2, 4 and 6 ppm), Cr (2, 4 and 6 ppm), Zn (0.5, 1 and 1.5 ppm), Cu (2, 4 and 6 ppm) and Pb (2, 10 and 20 ppm).
A homogeneous mixture of hydrogen peroxide (H2O2) 30% and nitric acid (HNO3) 65% in 1:2 strength was prepared. One gram of each plant part in the form of dried powder was dissolved in this solution. The sample solutions were heated on a hot plate at 130 °C until the volume of each sample was reduced to 3 mL. Then, the solution was cool down, filtered and the volume was made up to 25 mL [49].
4.7. Antibacterial Activity
Collection of Bacterial strains and antibacterial activity.
Pure cultures of four bacterial strains e.g., Shigella, Escherichia coli, Salmonella and MRSA were obtained and selected for further experimentation. These four bacterial strains were further sub-cultured on nutrient agar. The agar well disc diffusion method was adopted for the evaluation of antibacterial activity. All the equipment was autoclaved and sterilized for 15 min at 120 °C before use. Then, 15 mL of the media was poured in each Petri plate and kept for cooling. The bacterial strains were applied on the Petri dishes by using a sterilized cotton swab. After that, by using the sterilized Cork borer of 6 mm diameter, each Petri plate was punched into five wells for DMSO, distilled water, chloroform, methanol and n-hexane crude extracts, respectively. After that, the stock solution of all crude extract in DMSO each of 30 mg/mL was prepared. Now, each well was filled with 100 µL stock solutions except one, which was filled with DMSO as a negative control. The standard disc of Ceproflaxacene (5 µg) was used as a positive control. All the processes were performed in the laminar flow hood in order to resist contamination. The plates were then incubated in the incubator at 37 °C for 24 h. At last, the zones of inhibition were measured in mm for each crude extract by using Digital Vernier Caliper and the obtained results were noted and recorded [50].
4.8. Determination of Minimum Inhibitory Concentrations (MICs) by Broth Dilution Method
MIC is the lowest concentration (in mg/L) of the antimicrobial agent that prevents visible growth of a microorganism under defined conditions. Broth dilution techniques (micro-dilution) were used to determine the minimal inhibitory concentration (MIC) of antimicrobial agents, including antibiotics that kill (bactericidal activity) or inhibit the growth (bacteriostatic activity) of bacteria. Broth dilution uses liquid growth medium containing geometrically increasing concentrations (typically a two-fold dilution series) of the antimicrobial agent, which is inoculated with a defined number of bacterial cells. The antibacterial agents were dissolved in DMSO. After incubation, the presence of turbidity or sediment indicates growth of the organism [51].
4.9. Statistical Analysis of the Data
The as-recorded data was analyzed and organized by means of Microsoft Excel. The entire experiments were performed three times, consecutively. Standard deviations as well as average zone of inhibitions were calculated by Microsoft Excel 2007. SPSS version 16 was applied to determine the phytoconstituent activities.
5. Conclusions
In summary, the overall results obtained show the medicinal values of the tested plant parts and their recipes, which are used against diarrhea. The qualitative phytochemical screening of these plants and their recipes exhibit the presence of alkaloids, flavonoids, saponins and tannins. The secondary metabolites are more potent in T. chebula, S. aromaticum and in their recipes. The atomic absorption study of the as-prepared plant samples predict the high concentration of Iron (Fe), Lead (Pb) and Chromium (Cr).
Therefore, it has been concluded that the present research work looking at the medicinal plants and their recipes used against diarrhea found that they are therapeutically active substances with enhanced activities. However, most of the crude extracts also show antibacterial activities. Furthermore, the recipes extracts are more active against the tested bacterial strains as compared to the extracts of individual plants. So, for this reason, the present research study is helpful to identify the bioactive compounds obtained from the medicinal plants which are used against different antimicrobial activities.
Author Contributions
N.M. Contributed in methodology, writing original draft preparation, and project administration. R.N. contributed in formal analysis, M.K. in supervision and software work, A.K. in formal analysis, M.A. in investigation, M.U. in Data Curation and H.Y. contributed in Project administration, supervision and writing—review and editing.
Funding
This study was supported by fundamental research funds for the central universities, China 2572017DA07.
Acknowledgments
The author is thankful to Chinese Scholarship Council (CSC), School of International Education and Exchange (SIEE) and Key Laboratory of Saline-Alkali Vegetation Ecology Restoration, Ministry of Education, College of Life Sciences Northeast Forestry University (NEFU), Harbin 150040, China and Kohat University of Science and Technology Kohat, Pakistan for providing research facilities and vibrant research environment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yadav, R.; Agarwala, M. Phytochemical analysis of some medicinal plants. J. Phytol. 2011. Available online: https://updatepublishing.com/journal/index.php/jp/article/view/2737 (accessed on 5 April 2019).
- Babu-Kasimala, M.; Tukue, M.; Ermias, R. Phytochemical screening and antibacterial activity of two common terresterial medicinal plants Rutachalepensis and Rumexnervosus. Available online: https://ojs.unud.ac.id/index.php/bmj/article/view/21605/14295 (accessed on 20 August 2019).
- Ndam, L.; Mih, A.; Fongod, A.; Tening, A.; Tonjock, R.; Enang, J.; Fujii, Y. Phytochemical screening of the bioactive compounds in twenty (20) Cameroonian medicinal plants. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 768–778. [Google Scholar]
- Almeida, C.E.; Karnikowski, M.G.; Foleto, R.; Baldisserotto, B. Analysis of antidiarrhoeic effect of plants used in popular medicine. Rev. Saude Publica 1989, 29, 428. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.H.; Mouneir, S.M. Antidiarrhoeal activity of some Egyptian medicinal plant extracts. J. Ethnopharmacol. 2004, 92, 303. [Google Scholar] [CrossRef] [PubMed]
- Mamoon, S.A.; Azam, M.G. Preliminary phytochemical screening and antidiarrhoeal activity of Derris trifoliata Lour. Int. J. Pharm. Sci. Res. 2012, 3, 97. [Google Scholar]
- Das, P.R.; Akter, S.; Islam, M.T.; Kabir, M.H.; Haque, M.M.; Khatun, Z.; Nurunnabi, M.; Khatun, Z.; Lee, Y.; Jahan, R.; et al. A selection of medicinal plants used for treatment of diarrhea by folk medicinal practitioners of Bangladesh. Am. Eurasian J. Sustain. Agric. 2012, 6, 153. [Google Scholar]
- Samant, S.S.; Dhar, U.; Palni, L.M.S. Medicinal Plants of Indian Himalaya: Diversity, Distribution Potential Values. Nainital Gyanodaya Prakashan 1998, 163. Available online: https://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q))/reference/ReferencesPapers.aspx?ReferenceID=222461 (accessed on 23 October 2019).
- Sharma, V.; Joshi, B.D. Traditional medicines used for dental health Care amongst the local people of Almora district of Central Himalaya in India. Asian J. Tradit. Med. 2010, 5, 117. [Google Scholar]
- Sunil, K.; Rana, A.C. Herbal Approach for Diarrhea. Int. Res. J. Pharm. 2013, 4, 31. [Google Scholar]
- Hussain, I.; Khan, F.; Khan, I.; Khan, L.; Ullah, W. Determination of heavy metals in medicinal plants. J. Chem. Soc. Pak. 2006, 28, 347. [Google Scholar]
- Gawel, J.E.; Ahner, B.A.; Friedland, A.J.; Morel, F.M. Role for heavy metals in forest decline indicated by phytochelatin measurements. Nature 1996, 381, 64. [Google Scholar] [CrossRef]
- Khan, I.; Ali, J.; Tullah, H. Heavy metals determination in medicinal plant Withaniasomnifera growing in various areas of peshawar, NWFP, Pakistan. J. Soc. Pak. 2008, 30, 69. [Google Scholar]
- Abdel-Salam, A. Functional foods: Hopefulness to good health. Am. J. Food Technol. 2010, 2, 86–99. [Google Scholar]
- Thomson, I.F.A.; Ripa, M. Haque and IJ Bulbul. Pak. J. Bio. Sci. 2010, 13, 22–27. [Google Scholar]
- Patsilinakos, A.; Artini, M.; Papa, R.; Saatino, M.; Bozovic, M.; Garzoli, S.; Vrenna, G.; Buzzi, R.; Manfredini, S.; Selan, L.; et al. Machine learning analyses on data including essential oil chemical composition and in vitro experimental antibiofilm activities against staphylococcus species. Molecules 2019, 24, 890. [Google Scholar] [CrossRef]
- Artini, M.; Patsilinakos, A.; Papa, R.; Bozovic, M.; Sabatino, M.; Garzoli, S.; Vrenna, G.; Tilotta, M.; Pepi, F.; Rango, R.; et al. Antimicrobial and antibiofilm activity and machine learning classification analysis of essential oils from different mediterranean plants against pseudomonas aeruginosa. Molecules 2018, 23, 482. [Google Scholar] [CrossRef]
- Vikas, K.; Sharma, N.; Sourirajan, A.; Khosla, P.K.; Dev, K. Comparative evaluation of antimicrobial and antioxidant potential of ethanolic extract and its fractions of bark and leaves of Terminalia arjuna from north-western Himalayas, India. J. Tradit. Complement. Med. 2018, 8, 100–106. [Google Scholar]
- Bikash, M.; Paudel, K.R.; Sharma, B.; Karki, R. Phytochemical profile and pharmacological activity of Aegle marmelos Linn. J. Integr. Med. 2018, 16, 153–163. [Google Scholar]
- Rathinamoorthy, R.; Thilagavathi, G. Terminalia chebula-review on pharmacological and biochemical studies. Int. J. Pharm Technol. Res. 2014, 6, 97–116. [Google Scholar]
- Ahmed, M.; Khan, M.A.; Zafar, M.; Sultana, S. Treatment of common ailments by plant-based remedies among the people of district Attock (Punjab) of Northern Pakistan. Afr. J. Tradit. Altern. Med. 2007, 4, 112–120. [Google Scholar]
- Pfundstein, B.; El Desouky, S.K.; Hull, W.E.; Haubner, R.; Erben, G.; Owen, R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Kakar, S.A.; Tareen, R.B.; Kakar, M.A.; Jabeen, H.; Kakar, S.U.R.; Al-Kahraman, Y.S.M.A.; Shafee, M. Screening of antibacterial activity of four medicinal plants of Balochistan-Pakistan. Pak. J Bot. 2012, 44, 245–250. [Google Scholar]
- World Health Organization. Quality Control Methods for Medicinal Plant Materials. 1998. Available online: https://apps.who.int/iris/handle/10665/44479 (accessed on 15 August 2019).
- Shah, A.; Niaz, A.; Ullah, N.; Rehman, A.; Akhlaq, M.; Zakir, M.; Suleman Khan, M.; Comparative study of heavy metals in soil and selected medicinal plants. J. Chem. 2013. Available online: https://www.hindawi.com/journals/jchem/2013/621265/ (accessed on 15 August 2019).
- Hill, A.F. Economic Botany, A Textbook of Useful Plants and Plant Products, 2nd ed.; McGraw Hill Book Company Inc.: New York, NY, USA, 1952; Volume 205. [Google Scholar]
- Ferguson, P.J.; Kurowska, E.; Freeman, D.J.; Chambers, A.F.; Koropatnick, D.J. A flavonoid fraction from cranberry extract inhibits proliferation of human tumor cell lines. J. Nutr. 2004, 134, 1529–1535. [Google Scholar] [CrossRef]
- Kanadaswami, C.; Lee, L.T.; Lee, P.P.H.; Hwang, J.J.; Ke, F.C.; Huang, Y.T.; Lee, M.T. The antitumor activities of flavonoids. In Vivo 2005, 19, 895–909. [Google Scholar]
- Akinpelu, D.; Onakoya, T. Antimicrobial activities of medicinal plants used in folklore remedies in south-western. Afr. J. Biotechnol. 2006, 5, 11. [Google Scholar]
- Nguyi, A. Tannins of some Nigerian flora. Niger. J. Biotechnol. 1988, 6, 221–226. [Google Scholar]
- Khosit, P.; Hiriote, W.; Soonthornchareonnon, N.; Jongsakul, K.; Sireeratawong, S.; Tor-Udom, S. In vitro and in vivo antiplasmodial activity and cytotoxicity of water extracts of Phyllanthusemblica, Terminalia chebula, and Terminalia bellerica. J. Med. Assoc. Thail. 2011, 93, 120. [Google Scholar]
- Mansour, S.; Mahmoud, M.F.; Hasan, R.A.; Abdelfattah, M.A.O.; Osman, S.; Rashid, H.; El-Shazly, A.M.; Wink, M. Chemical composition, antioxidant and hepatoprotective activities of methanol extracts from leaves of Terminalia bellirica and Terminalia sericea (Combretaceae). PeerJ 2019, 7, e6322. [Google Scholar]
- Hasan, Z.; Anwar, Z.; Khattak, K.U.; Islam, M.; Khan, R.U.; Khattak, J.Z.K. Civic pollution and its effect on water quality of river Toi at district Kohat, NWFP. Res. J. Environ. Earth Sci. 2012, 4, 334–339. [Google Scholar]
- McGrath, S. Chromium and Nickel, Heavy Metals in Soils. 1990; pp. 125–150. Available online: https://trove.nla.gov.au/work/17304135?q&versionId=45617592 (accessed on 23 October 2019).
- Ullah, R.; Khader, J.A.; Hussain, I.; Talha, N.M.A.; Khan, N. Investigation of macro and micro-nutrients in selected medicinal plants. Afr. J. Pharm. Pharmacol. 2012, 6, 1829–1832. [Google Scholar]
- Rehman, A.; Ullah, H.; Khan, R.U.; Ahmad, I. Population based study of heavy metals in medicinal plant Capparis decidua. Int. J. Pharm. Pharmacol. Sci. 2013, 5, 108–113. [Google Scholar]
- Adelekan, B.; Abegunde, K. Heavy metals contamination of soil and groundwater at automobile mechanic villages in Ibadan, Nigeria. Int. J. Phys. Sci. 2011, 5, 1045–1058. [Google Scholar]
- Gupta, U. Copper in the Environment; Nariago, J.O., Ed.; John Wiley and Sons: New York, NY, USA, 1975; Volume 255. [Google Scholar]
- Chishti, K.A.; Khan, F.A.; Shah, S.M.H.; AsifKhan, M.; Khan, J.; Shah, S.M.M.; Hussain, I. Estimation of heavy metals in the seeds of blue and white capitulum’s of silybummarianum grown in various districts of pakistan. J. Basic Appl. Sci. 2011, 7. Available online: https://search.proquest.com/openview/4cc7dfa78ca921cb079d285fe6cf6626/1.pdf?pq-origsite=gscholar&cbl=616537 (accessed on 23 October 2019).
- Adnan, M.; Ullah, I.; Tariq, A.; Murad, W.; Azizullah, A.; Khan, A.L.; Ali, N. Ethnomedicine use in the war affected region of northwest Pakistan. J. Ethnobiol. Ethnomed. 2014, 10, 16. [Google Scholar] [CrossRef]
- Murad, W.; Azizullah, A.; Adnan, M.; Tariq, A.; Khan, K.U.; Waheed, S.; Ahmad, A. Ethnobotanical assessment of plant resources of Banda Daud Shah, District Karak, Pakistan. J. Ethnobiol. Ethnomed. 2013, 9, 77. [Google Scholar] [CrossRef]
- Amjad, M.S.; faisal Qaeem, M.; Ahmad, I.; Khan, S.U.; Chaudhari, S.K.; Malik, N.Z.; Shaheen, H.; Khan, A.M. Descriptive study of plant resources in the context of the ethnomedicinal relevance of indigenous flora: A case study from Toli Peer National Park, Azad Jammu and Kashmir, Pakistan. PLoS ONE 2017, 12, e0171896. [Google Scholar] [CrossRef]
- Mattila, P.; Hellström, J. Phenolic acids in potatoes, vegetables, and some of their products. J. Food. Compos. Anal. 2007, 20, 152–160. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Devi, T.; Bono, A.; Sarbatly, R. Studies on phytochemical constituents of six Malaysian medicinal plants. J. Med. Plants Res. 2009, 3, 67–72. [Google Scholar]
- Tyler, V.E. Phytomedicines in Western Europe: Potential impact on herbal medicine in the United States. ACS Publ. 1993. Available online: https://pubs.acs.org/doi/10.1021/bk-1993-0534.ch003 (accessed on 23 October 2019).
- Harborne, J. Phytochemical methods: London Chapman and hill Ltd. 1973; pp. 49–188. Available online: https://trove.nla.gov.au/version/45450216 (accessed on 23 October 2019).
- Obadoni, B.; Ochuko, P. Phytochemical studies and comparative efficacy of the crude extracts of some haemostatic plants in Edo and Delta States of Nigeria. Glob. J. Pure Appl. Sci. 2002, 8, 203–208. [Google Scholar] [CrossRef]
- Boham, B.; Kocipai-Abuyazan, R. Flavonoids and condensed tannins from the leaves of Vaccinumraticulatum and Vaccinumcalycinium. Pac. Sci. 1994, 48, 458–463. [Google Scholar]
- Khan, S.A.; Khan, L.; Hussain, I.; Marwat, K.B.; Akhtar, N. Profile of heavy metals in selected medicinal plants. Pak. J. Weed Sci. Res. 2008, 14, 101–110. [Google Scholar]
- Kirby, W.; Yoshihara, G.; Sundsted, K.; Warren, J. Clinical usefulness of a single disc method for antibiotic sensitivity testing. Antibiot. Annu. 1956, 892. Available online: https://www.asm.org/getattachment/2594ce26-bd44-47f6-8287-0657aa9185ad/Kirby-Bauer-Disk-Diffusion-Susceptibility-Test-Protocol-pdf.pdf (accessed on 23 October 2019).
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, 1–7. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).