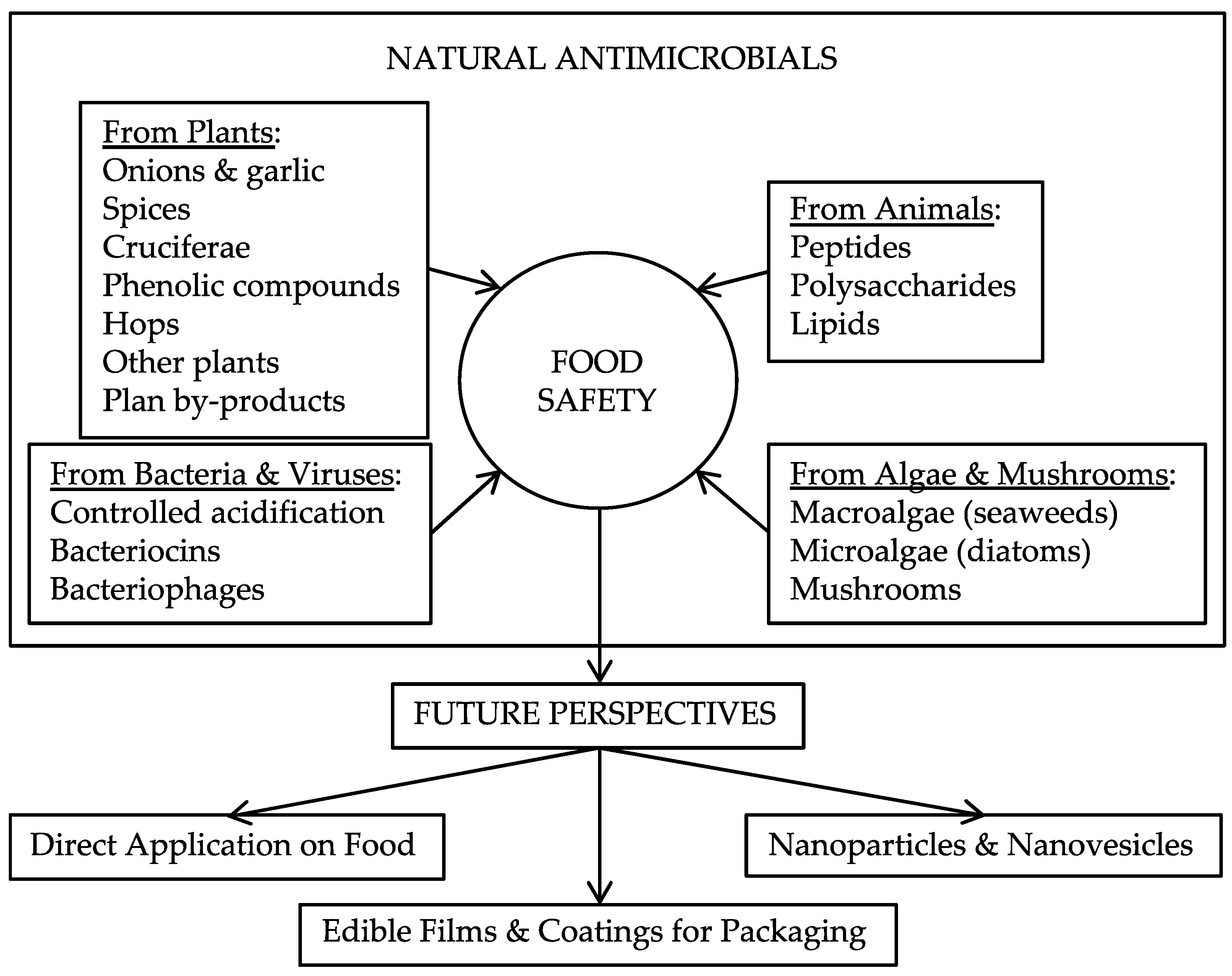
Food Safety through Natural Antimicrobials
Abstract
1. Introduction
2. Natural Antimicrobials from Plants
2.1. Onions and Garlic
2.2. Spices
2.3. Cruciferae
2.4. Phenolic Compounds
2.5. Hops
2.6. Other Plants
2.7. Plant By-Products
3. Natural Antimicrobials from Animals
3.1. Peptides
3.2. Polysaccharides
3.3. Lipids
4. Natural Antimicrobials from Bacteria and Viruses: Biopreservation
4.1. Controlled Acidification
4.2. Bacteriocins
4.3. Bacteriophages
5. Natural Antimicrobials from Algae and Mushrooms
6. Future Perspectives
6.1. Direct Application on Food
6.2. Edible Films and Coatings for Packaging
6.3. Nanoparticles and Nanovesicles
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-K.; Paik, H.-D. Status, antimicrobial mechanism, and regulation of natural preservatives in livestock food systems. Korean J. Food Sci. Anim. Resour. 2016, 36, 547–557. [Google Scholar] [CrossRef]
- Aziz, M.; Karboune, S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 486–511. [Google Scholar] [CrossRef]
- Hygreeva, D.; Pandey, M.C.; Radhakrishna, K. Potential applications of plant based derivatives as fat replacers, antioxidants and antimicrobials in fresh and processed meat products. Meat Sci. 2014, 98, 47–57. [Google Scholar] [CrossRef]
- Burt, S.A.; Reinders, R.D. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Savoia, D. Plant-derived antimicrobial compounds: alternatives to antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, A.; Jackson-Davis, A.; Moutiq, R.; Thomas-Popo, E. Use of natural antimicrobials of plant origin to improve the microbiological safety of foods. In Food and Feed Safety Systems and Analysis; Elsevier: Amsterdam, The Netherlands, 2018; pp. 249–272. ISBN 9780128498880. [Google Scholar]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Proestos, C.; Boziaris, I.S.; Kapsokefalou, M.; Komaitis, M. Natural antioxidant constituents from selected aromatic plants and their antimicrobial activity against selected pathogenic microorganisms. In Proceedings of the Food Technology and Biotechnology, Osijek, Croatia, 17–20 September 2008; Volume 46, pp. 151–156. [Google Scholar]
- Holley, R.A.; Patel, D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005, 22, 273–292. [Google Scholar] [CrossRef]
- Nanasombat, S.; Lohasupthawee, P. Antibacterial activity of crude ethanolic extracts and essential oils of spices against Salmonella and other Enterobacteria. KMITL Sci. Technol. J. 2005, 5, 527–538. [Google Scholar]
- Conner, D.E.; Beuchat, L.R.; Worthington, R.E.; Hitchcock, H.L. Effects of essential oils and oleoresins of plants on ethanol production, respiration and sporulation of yeasts. Int. J. Food Microbiol. 1984, 1, 63–74. [Google Scholar] [CrossRef]
- González-Fandos, E.; García-López, M.L.; Sierra, M.L.; Otero, A. Staphylococcal growth and enterotoxins (A-D) and thermonuclease synthesis in the presence of dehydrated garlic. J. Appl. Bacteriol. 1994, 77, 549–552. [Google Scholar] [CrossRef]
- Cavallito, C.J.; Bailey, J.H. Allicin, the antibacterial principle of Allium sativum. I. Isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 1944, 66, 1950–1951. [Google Scholar] [CrossRef]
- Wills, E.D. Enzyme inhibition by allicin, the active principle of garlic. Biochem. J. 1956, 63, 514–520. [Google Scholar] [CrossRef]
- Barone, F.E.; Tansey, M.R. Isolation, purification, identification, synthesis, and kinetics of activity of the anticandidal component of Allium sativum, and a hypothesis for its mode of action. Mycologia 1977, 69, 793. [Google Scholar] [CrossRef]
- Taylor, T.M.; Davidson, P.M. Chemical preservatives and natural antimicrobial compounds. In Food Microbiology: Fundamentals and Frontiers, 3rd ed.; American Society of Microbiology: Washington, DC, USA, 2014; pp. 713–745. [Google Scholar]
- Kim, J.W.; KIM, Y.S.; Kyung, K.H. Inhibitory activity of essential oils of garlic and onion against bacteria and yeasts. J. Food Prot. 2004, 67, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, E.; Fung, D.Y.C.; Sabah, J.R. Antimicrobial activity and synergistic effect of cinnamon with sodium benzoate or potassium sorbate in controlling Escherichia coli O157:H7 in apple juice. J. Food Sci. 2004, 69, FMS102–FMS106. [Google Scholar] [CrossRef]
- Daferera, D.J.; Ziogas, B.N.; Polissiou, M.G. GC-MS analysis of essential oils from some greek aromatic plants and their fungitoxicity on Penicillium digitatum. J. Agric. Food Chem. 2000, 48, 2576–2581. [Google Scholar] [CrossRef]
- Filoche, S.K.; Soma, K.; Sissons, C.H. Antimicrobial effects of essential oils in combination with chlorhexidine digluconate. Oral Microbiol. Immunol. 2005, 20, 221–225. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002, 65, 1545–1560. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl. Environ. Microbiol. 2004, 70, 5750–5755. [Google Scholar] [CrossRef]
- Nielsen, P.V.; Rios, R. Inhibition of fungal growth on bread by volatile components from spices and herbs, and the possible application in active packaging, with special emphasis on mustard essential oil. Int. J. Food Microbiol. 2000, 60, 219–229. [Google Scholar] [CrossRef]
- Smith-Palmer, A. Influence of subinhibitory concentrations of plant essential oils on the production of enterotoxins A and B and -toxin by Staphylococcus aureus. J. Med. Microbiol. 2004, 53, 1023–1027. [Google Scholar] [CrossRef]
- Moreira, M.R.; Ponce, A.; del Valle, C.E.; Roura, S.I. Inhibitory parameters of essential oils to reduce a foodborne pathogen. LWT Food Sci. Technol. 2005, 38, 565–570. [Google Scholar] [CrossRef]
- Mytle, N.; Anderson, G.L.; Doyle, M.P.; Smith, M.A. Antimicrobial activity of clove (Syzgium aromaticum) oil in inhibiting Listeria monocytogenes on chicken frankfurters. Food Control 2006, 17, 102–107. [Google Scholar] [CrossRef]
- Bagamboula, C.F.; Uyttendaele, M.; Debevere, J. Antimicrobial effect of spices and herbs on Shigella sonnei and Shigella flexneri. J. Food Prot. 2003, 66, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Bennis, S.; Chami, F.; Chami, N.; Bouchikhi, T.; Remmal, A. Surface alteration of Saccharomyces cerevisiae induced by thymol and eugenol. Lett. Appl. Microbiol. 2004, 38, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Gaysinsky, S.; Davidson, P.M.; Bruce, B.D.; Weiss, J. Stability and antimicrobial efficiency of eugenol encapsulated in surfactant micelles as affected by temperature and pH. J. Food Prot. 2005, 68, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, R.G.K.; Zamparini, J. Effects of spices on growth and survival of Escherichia coli 0157 and Salmonella enterica serovar Enteritidis in broth model systems and mayonnaise. Food Control 2002, 13, 399–404. [Google Scholar] [CrossRef]
- López-Malo, A.; Alzamora, S.M.; Palou, E. Aspergillus flavus dose–response curves to selected natural and synthetic antimicrobials. Int. J. Food Microbiol. 2002, 73, 213–218. [Google Scholar] [CrossRef]
- López-Malo, A.; Maris Alzamora, S.; Palou, E. Aspergillus flavus growth in the presence of chemical preservatives and naturally occurring antimicrobial compounds. Int. J. Food Microbiol. 2005, 99, 119–128. [Google Scholar] [CrossRef]
- Rhayour, K.; Bouchikhi, T.; Tantaoui-Elaraki, A.; Sendide, K.; Remmal, A. The mechanism of bactericidal action of oregano and clove essential oils and of their phenolic major components on Escherichia coli and Bacillus subtilis. J. Essent. Oil Res. 2003, 15, 286–292. [Google Scholar] [CrossRef]
- Seaberg, A.C.; Labbe, R.G.; Shetty, K. Inhibition of Listeria monocytogenes by elite clonal extracts of oregano (Origanum vulgare). Food Biotechnol. 2003, 17, 129–149. [Google Scholar] [CrossRef]
- Singh, A.; Singh, R.K.; Bhunia, A.K.; Singh, N. Efficacy of plant essential oils as antimicrobial agents against Listeria monocytogenes in hotdogs. LWT Food Sci. Technol. 2003, 36, 787–794. [Google Scholar] [CrossRef]
- Ultee, A.; Smid, E. Influence of carvacrol on growth and toxin production by Bacillus cereus. Int. J. Food Microbiol. 2001, 64, 373–378. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Burt, S.A.; Vlielander, R.; Haagsman, H.P.; Veldhuizen, E.J.A. Increase in activity of essential oil components carvacrol and thymol against Escherichia coli O157:H7 by addition of food stabilizers. J. Food Prot. 2005, 68, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Di Pasqua, R.; De Feo, V.; Villani, F.; Mauriello, G. In vitro antimicrobial activity of essential oils from Mediterranean Apiaceae, Verbenaceae and Lamiaceae against foodborne pathogens and spoilage bacteria. Ann. Microbiol. 2005, 55, 139–143. [Google Scholar]
- Kiskó, G.; Roller, S. Carvacrol and p-cymene inactivate Escherichia coli O157:H7 in apple juice. BMC Microbiol. 2005, 5, 36. [Google Scholar] [CrossRef]
- Olasupo, N.A.; Fitzgerald, D.J.; Gasson, M.J.; Narbad, A. Activity of natural antimicrobial compounds against Escherichia coli and Salmonella enterica serovar Typhimurium. Lett. Appl. Microbiol. 2003, 37, 448–451. [Google Scholar] [CrossRef]
- Olasupo, N.A.; Fitzgerald, D.J.; Narbad, A.; Gasson, M.J. Inhibition of Bacillus subtilis and Listeria innocua by nisin in combination with some naturally occurring organic compounds. J. Food Prot. 2004, 67, 596–600. [Google Scholar] [CrossRef]
- Özkan, G.; Sağdiç, O.; Özcan, M. Note: Inhibition of pathogenic bacteria by essential oils at different concentrations. Food Sci. Technol. Int. 2003, 9, 85–88. [Google Scholar] [CrossRef]
- Penalver, P.; Huerta, B.; Borge, C.; Astorga, R.; Romero, R.; Perea, A. Antimicrobial activity of five essential oils against origin strains of the Enterobacteriaceae family. APMIS 2005, 113, 1–6. [Google Scholar] [CrossRef]
- Aligiannis, N.; Kalpoutzakis, E.; Mitaku, S.; Chinou, I.B. Composition and antimicrobial activity of the essential oils of two Origanum species. J. Agric. Food Chem. 2001, 49, 4168–4170. [Google Scholar] [CrossRef]
- Chami, N.; Bennis, S.; Chami, F.; Aboussekhra, A.; Remmal, A. Study of anticandidal activity of carvacrol and eugenol in vitro and in vivo. Oral Microbiol. Immunol. 2005, 20, 106–111. [Google Scholar] [CrossRef]
- Elgayyar, M.; Draughon, F.A.; Golden, D.A.; Mount, J.R. Antimicrobial activity of essential oils from plants against selected pathogenic and saprophytic microorganisms. J. Food Prot. 2001, 64, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Falcone, P.; Speranza, B.; Del Nobile, M.A.; Corbo, M.R.; Sinigaglia, M. A study on the antimicrobial activity of thymol intended as a natural preservative. J. Food Prot. 2005, 68, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.-J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Manohar, V.; Ingram, C.; Gray, J.; Talpur, N.A.; Echard, B.W.; Bagchi, D.; Preuss, H.G. Antifungal activities of origanum oil against Candida albicans. Mol. Cell. Biochem. 2001, 228, 111–117. [Google Scholar] [CrossRef]
- Rota, C.; Carramiñana, J.J.; Burillo, J.; Herrera, A. In vitro antimicrobial activity of essential oils from aromatic plants against selected foodborne pathogens. J. Food Prot. 2004, 67, 1252–1256. [Google Scholar] [CrossRef]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Valdramidis, V.P.; O’ Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef]
- Chorianopoulos, N.; Kalpoutzakis, E.; Aligiannis, N.; Mitaku, S.; Nychas, G.J.; Haroutounian, S.A. Essential oils of Satureja, Origanum, and Thymus species: Chemical composition and antibacterial activities against foodborne pathogens. J. Agric. Food Chem. 2004, 52, 8261–8267. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Chaleshtori, A.R.; Tajbakhsh, E.; Momtaz, H.; Rahimi, E.; Shahin, F. Bioactivity of medicinal plant extracts against Listeria monocytogenes isolated from food. J. Food Agric. Environ. 2009, 7, 66–69. [Google Scholar]
- Araújo, C.; Sousa, M.J.; Ferreira, M.F.; Leao, C. Activity of essential oils from mediterranean Lamiaceae species against food spoilage yeasts. J. Food Prot. 2003, 66, 625–632. [Google Scholar] [CrossRef]
- Lachowicz, K.J.; Jones, G.P.; Briggs, D.R.; Bienvenu, F.E.; Wan, J.; Wilcock, A.; Coventry, M.J. The synergistic preservative effects of the essential oils of sweet basil (Ocimum basilicum L.) against acid-tolerant food microflora. Lett. Appl. Microbiol. 1998, 26, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Delaquis, P.; Stanich, K.; Toivonen, P. Effect of pH on the inhibition of Listeria spp. by vanillin and vanillic acid. J. Food Prot. 2005, 68, 1472–1476. [Google Scholar] [CrossRef] [PubMed]
- Delaquis, P. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int. J. Food Microbiol. 2002, 74, 101–109. [Google Scholar] [CrossRef]
- Gill, A.; Delaquis, P.; Russo, P.; Holley, R. Evaluation of antilisterial action of cilantro oil on vacuum packed ham. Int. J. Food Microbiol. 2002, 73, 83–92. [Google Scholar] [CrossRef]
- Thongson, C.; Davidson, P.M.; Mahakarnchanakul, W.; Vibulsresth, P. Antimicrobial effect of Thai spices against Listeria monocytogenes and Salmonella Typhimurium DT104. J. Food Prot. 2005, 68, 2054–2058. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J. Appl. Microbiol. 2003, 95, 853–860. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L. Interactions between components of the essential oil of Melaleuca alternifolia. J. Appl. Microbiol. 2001, 91, 492–497. [Google Scholar] [CrossRef]
- Delaquis, P.J.; Ward, S.M.; Holley, R.A.; Cliff, M.C.; Mazza, G. Microbiological, chemical and sensory properties of pre-cooked roast beef preserved with horseradish essential oil. J. Food Sci. 1999, 64, 519–524. [Google Scholar] [CrossRef]
- Ward, S.M.; Delaquis, P.J.; Holley, R.A.; Mazza, G. Inhibition of spoilage and pathogenic bacteria on agar and pre-cooked roast beef by volatile horseradish distillates. Food Res. Int. 1998, 31, 19–26. [Google Scholar] [CrossRef]
- Nadarajah, D.; Han, J.H.; Holley, R.A. Use of mustard flour to inactivate Escherichia coli O157:H7 in ground beef under nitrogen flushed packaging. Int. J. Food Microbiol. 2005, 99, 257–267. [Google Scholar] [CrossRef]
- Delaquis, P.J.; Sholberg, P.L. Antimicrobial activity of gaseous allyl isothiocyanate. J. Food Prot. 1997, 60, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Wendorff, W.L.; Riha, W.E.; Muehlenkamp, E. Growth of molds on cheese treated with heat or liquid smoke. J. Food Prot. 1993, 56, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.D.; Rico-Munoz, E.; Davidson, P.M. The antimicrobial activity of phenolic compounds against Listeria monocytogenes and their effectiveness in a model milk system. J. Food Prot. 1989, 52, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Herald, P.J.; Davidson, P.M. Antibacterial activity of selected hydroxycinnamic acids. J. Food Sci. 1983, 48, 1378–1379. [Google Scholar] [CrossRef]
- Chipley, J.R.; Uraih, N. Inhibition of Aspergillus growth and aflatoxin release by derivatives of benzoic acid. Appl. Environ. Microbiol. 1980, 40, 352–357. [Google Scholar] [PubMed]
- Ulate-Rodríguez, J.; Schafer, H.W.; Zottola, E.A.; Davidson, P.M. Inhibition of Listeria monocytogenes, Escherichia coli O157:H7, and Micrococcus luteus by linear furanocoumarins in culture media. J. Food Prot. 1997, 60, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Kramer, B.; Thielmann, J.; Hickisch, A.; Muranyi, P.; Wunderlich, J.; Hauser, C. Antimicrobial activity of hop extracts against foodborne pathogens for meat applications. J. Appl. Microbiol. 2015, 118, 648–657. [Google Scholar] [CrossRef]
- Bogdanova, K.; Röderova, M.; Kolar, M.; Langova, K.; Dusek, M.; Jost, P.; Kubelkova, K.; Bostik, P.; Olsovska, J. Antibiofilm activity of bioactive hop compounds humulone, lupulone and xanthohumol toward susceptible and resistant staphylococci. Res. Microbiol. 2018, 169, 127–134. [Google Scholar] [CrossRef]
- Fernandez, J.L.; Simpson, W.J. Aspects of the resistance of lactic acid bacteria to hop bitter acids. J. Appl. Bacteriol. 1993, 75, 315–319. [Google Scholar] [CrossRef]
- Simpson, W.J.; Smith, A.R.W. Factors affecting antibacterial activity of hop compounds and their derivatives. J. Appl. Bacteriol. 1992, 72, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Haas, G.J.; Barsoumian, R. Antimicrobial activity of hop resins. J. Food Prot. 1994, 57, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.E.; Yu, R.R.Y.; Lee, O.A.; Price, S.; Haas, G.J.; Johnson, E.A. Antimicrobial activity of hop extracts against Listeria monocytogenes in media and in food. Int. J. Food Microbiol. 1996, 33, 195–207. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Virani, S.; Zavro, M.; Haas, G.J. Inhibition of Streptococcus mutans and other oral Streptococci by hop (Humulus lupulus L.) constituents. Econ. Bot. 2003, 57, 118–125. [Google Scholar] [CrossRef]
- Shen, C.; Geornaras, I.; Kendall, P.A.; Sofos, J.N. Control of Listeria monocytogenes on frankfurters by dipping in hops beta acids solutions. J. Food Prot. 2009, 72, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Mizobuchi, S.; Sato, Y. A new flavanone with antifungal activity isolated from hops. Agric. Biol. Chem. 1984, 48, 2771–2775. [Google Scholar]
- Mizobuchi, S.; Sato, Y. Antifungal activities of hop bitter resins and related compounds. Agric. Biol. Chem. 1985, 49, 399–403. [Google Scholar]
- Srinivasan, V.; Goldberg, D.; Haas, G.J. Contributions to the antimicrobial spectrum of hop constituents. Econ. Bot. 2004, 58, S230–S238. [Google Scholar] [CrossRef]
- Ahn, J.; Grün, I.U.; Mustapha, A. Antimicrobial and antioxidant activities of natural extracts in vitro and in ground beef. J. Food Prot. 2004, 67, 148–155. [Google Scholar] [CrossRef]
- Markin, D.; Duek, L.; Berdicevsky, I. In vitro antimicrobial activity of olive leaves. Antimikrobielle Wirksamkeit von Olivenblattern in vitro. Mycoses 2003, 46, 132–136. [Google Scholar] [CrossRef]
- Dogasaki, C.; Shindo, T.; Furuhata, K.; Fukuyama, M. Identification of chemical structure of antibacterial components against Legionella pneumophila in a coffee beverage. Yakugaku Zasshi 2002, 122, 487–494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ibrahim, S.; Salameh, M.; Phetsomphou, S.; Yang, H.; Seo, C. Application of caffeine, 1,3,7-trimethylxanthine, to control Escherichia coli O157:H7. Food Chem. 2006, 99, 645–650. [Google Scholar] [CrossRef]
- Kim, S.; Fung, D.Y.C. Antibacterial effect of crude water-soluble arrowroot (Puerariae radix) tea extracts on food-borne pathogens in liquid medium. Lett. Appl. Microbiol. 2004, 39, 319–325. [Google Scholar] [CrossRef]
- Shimamura, T.; Zhao, W.-H.; Hu, Z.-Q. Mechanism of action and potential for use of tea catechin as an antiinfective agent. Antiinfect. Agents Med. Chem. 2007, 6, 57–62. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Cesario, T.; Wang, Y.; Shanbrom, E.; Thrupp, L. Antibacterial activity of vegetables and juices. Nutrition 2003, 19, 994–996. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. Antibacterial properties and major bioactive components of cinnamon stick (Cinnamomum burmannii): Activity against foodborne pathogenic bacteria. J. Agric. Food Chem. 2007, 55, 5484–5490. [Google Scholar] [CrossRef]
- Sagdic, O.; Ozturk, I.; Yilmaz, M.T.; Yetim, H. Effect of grape pomace extracts obtained from different grape varieties on microbial quality of beef patty. J. Food Sci. 2011, 76, M515–M521. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Levin, C.E. Bactericidal activities of health-promoting, food-derived powders against the foodborne pathogens Escherichia coli, Listeria monocytogenes, Salmonella enterica, and Staphylococcus aureus. J. Food Sci. 2013, 78, M270–M275. [Google Scholar] [CrossRef]
- Cicerale, S.; Conlan, X.A.; Barnett, N.W.; Keast, R.S.J. The concentration of oleocanthal in olive oil waste. Nat. Prod. Res. 2011, 25, 542–548. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Sharma, A. Antioxidant and antimicrobial activity of pomegranate peel extract improves the shelf life of chicken products. Int. J. Food Sci. Technol. 2010, 45, 216–222. [Google Scholar] [CrossRef]
- Agourram, A.; Ghirardello, D.; Rantsiou, K.; Zeppa, G.; Belviso, S.; Romane, A.; Oufdou, K.; Giordano, M. Phenolic content, antioxidant potential, and antimicrobial activities of fruit and vegetable by-product extracts. Int. J. Food Prop. 2013, 16, 1092–1104. [Google Scholar] [CrossRef]
- Li, G.; Xu, Y.; Wang, X.; Zhang, B.; Shi, C.; Zhang, W.; Xia, X. Tannin-rich fraction from pomegranate rind damages membrane of Listeria monocytogenes. Foodborne Pathog. Dis. 2014, 11, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, G.; Zhang, B.; Wu, Q.; Wang, X.; Xia, X. Tannin-rich pomegranate rind extracts reduce adhesion to and invasion of Caco-2 cells by Listeria monocytogenes and decrease its expression of virulence genes. J. Food Prot. 2015, 78, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Habibur Rahman, S.M.; Deshmukh, G.; Duganath, N.; Pranitha, C.; Chiranjeevi, A. Antimicrobial screening of various fruit seed extracts. Pharmacogn. J. 2011, 3, 83–86. [Google Scholar] [CrossRef]
- Taveira, M.; Silva, L.R.; Vale-Silva, L.A.; Pinto, E.; Valentão, P.; Ferreres, F.; Guedes de Pinho, P.; Andrade, P.B. Lycopersicon esculentum seeds: An industrial byproduct as an antimicrobial agent. J. Agric. Food Chem. 2010, 58, 9529–9536. [Google Scholar] [CrossRef]
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Recovery of phenolic antioxidants and functional compounds from coffee industry by-products. Food Bioprocess Technol. 2012, 5, 897–903. [Google Scholar] [CrossRef]
- Jenssen, H.; Hamill, P.; Hancock, R.E.W. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef]
- Cole, A.M.; Weis, P.; Diamond, G. Isolation and characterization of pleurocidin, an antimicrobial peptide in the skin secretions of winter flounder. J. Biol. Chem. 1997, 272, 12008–12013. [Google Scholar] [CrossRef]
- Burrowes, O.J.; Hadjicharalambous, C.; Diamond, G.; Lee, T.-C. Evaluation of antimicrobial spectrum and cytotoxic activity of pleurocidin for food applications. J. Food Sci. 2006, 69, FMS66–FMS71. [Google Scholar] [CrossRef]
- Patrzykat, A.; Friedrich, C.L.; Zhang, L.; Mendoza, V.; Hancock, R.E.W. Sublethal concentrations of pleurocidin-derived antimicrobial peptides inhibit macromolecular synthesis in Escherichia coli. Antimicrob. Agents Chemother. 2002, 46, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Potter, R.; Truelstruphansen, L.; Gill, T. Inhibition of foodborne bacteria by native and modified protamine: Importance of electrostatic interactions. Int. J. Food Microbiol. 2005, 103, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Kim, S.M.; Lee, S.Y. Antimicrobial activity of protamine against oral microorganisms. Biocontrol Sci. 2015, 20, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M.; Martin, B.; Chen, H.C. Antimicrobial activity of synthetic magainin peptides and several analogues. Proc. Natl. Acad. Sci. USA 1988, 85, 910–913. [Google Scholar] [CrossRef]
- Zucht, H.-D.; Raida, M.; Adermann, K.; Mägert, H.-J.; Forssmann, W.-G. Casocidin-I: a casein-α s2 derived peptide exhibits antibacterial activity. FEBS Lett. 1995, 372, 185–188. [Google Scholar] [CrossRef]
- Murdock, C.A.; Cleveland, J.; Matthews, K.R.; Chikindas, M.L. The synergistic effect of nisin and lactoferrin on the inhibition of Listeria monocytogenes and Escherichia coli O157:H7. Lett. Appl. Microbiol. 2007, 44, 255–261. [Google Scholar] [CrossRef]
- López-Expósito, I.; Pellegrini, A.; Amigo, L.; Recio, I. Synergistic effect between different milk-derived peptides and proteins. J. Dairy Sci. 2008, 91, 2184–2189. [Google Scholar] [CrossRef]
- Juneja, V.K.; Dwivedi, H.P.; Yan, X. Novel natural food antimicrobials. Annu. Rev. Food Sci. Technol. 2012, 3, 381–403. [Google Scholar] [CrossRef]
- Al-Nabulsi, A.A.; Holley, R.A. Effect of bovine lactoferrin against Carnobacterium viridans. Food Microbiol. 2005, 22, 179–187. [Google Scholar] [CrossRef]
- Seifu, E.; Buys, E.M.; Donkin, E.F. Significance of the lactoperoxidase system in the dairy industry and its potential applications: a review. Trends Food Sci. Technol. 2005, 16, 137–154. [Google Scholar] [CrossRef]
- Bafort, F.; Parisi, O.; Perraudin, J.-P.; Jijakli, M.H. Mode of action of lactoperoxidase as related to its antimicrobial activity: A review. Enzyme Res. 2014, 2014, 517164. [Google Scholar] [CrossRef] [PubMed]
- Gay, M.; Amgar, A. Factors moderating Listeria monocytogenes growth in raw milk and in soft cheese made from raw milk. Lait 2005, 85, 153–170. [Google Scholar] [CrossRef]
- Elliot, R.M.; McLay, J.C.; Kennedy, M.J.; Simmonds, R.S. Inhibition of foodborne bacteria by the lactoperoxidase system in a beef cube system. Int. J. Food Microbiol. 2004, 91, 73–81. [Google Scholar] [CrossRef]
- McLay, J.; Kennedy, M.; O’Rourke, A.-L.; Elliot, R.; Simmonds, R. Inhibition of bacterial foodborne pathogens by the lactoperoxidase system in combination with monolaurin. Int. J. Food Microbiol. 2002, 73, 1–9. [Google Scholar] [CrossRef]
- Kennedy, M.; O’Rourke, A.-L.; McLay, J.; Simmonds, R. Use of a ground beef model to assess the effect of the lactoperoxidase system on the growth of Escherichia coli O157:H7, Listeria monocytogenes and Staphylococcus aureus in red meat. Int. J. Food Microbiol. 2000, 57, 147–158. [Google Scholar] [CrossRef]
- Touch, V.; Hayakawa, S.; Yamada, S.; Kaneko, S. Effects of a lactoperoxidase–thiocyanate–hydrogen peroxide system on Salmonella enteritidis in animal or vegetable foods. Int. J. Food Microbiol. 2004, 93, 175–183. [Google Scholar] [CrossRef]
- Jain, A.; Cheng, K. The principles and applications of avidin-based nanoparticles in drug delivery and diagnosis. J. Control. Release 2017, 245, 27–40. [Google Scholar] [CrossRef]
- Nau, F.; Guérin-Dubiard, C.; Croguennec, T. Avidin. In Bioactive Egg Compounds; Springer: Berlin/Heidelberg, Germany, 2007; pp. 75–80. ISBN 9783540378839. [Google Scholar]
- Diamandis, E.P.; Christopoulos, T.K. The biotin-(strept)avidin system: Principles and applications in biotechnology. Clin. Chem. 1991, 37, 625–636. [Google Scholar]
- Maxwell, P.; Ibrahim, M. Immunocytochemistry. In Advanced Techniques in Diagnostic Cellular Pathology; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 99–134. ISBN 9780470515976. [Google Scholar]
- Kulagina, N.V.; Lassman, M.E.; Ligler, F.S.; Taitt, C.R. Antimicrobial peptides for detection of bacteria in biosensor assays. Anal. Chem. 2005, 77, 6504–6508. [Google Scholar] [CrossRef]
- Juvonen, H.; Oja, T.; Määttänen, A.; Sarfraz, J.; Rosqvist, E.; Riihimäki, T.A.; Toivakka, M.; Kulomaa, M.; Vuorela, P.; Fallarero, A.; et al. Protein and bacterial interactions with nanostructured polymer coatings. Colloids Surf. B Biointerfaces 2015, 136, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Korpela, J.; Salonen, E.-M.; Kuusela, P.; Sarvas, M.; Vaheri, A. Binding of avidin to bacteria and to the outer membrane porin of Escherichia coli. FEMS Microbiol. Lett. 1984, 22, 3–10. [Google Scholar] [CrossRef]
- Wu, J.; Acero-Lopez, A. Ovotransferrin: Structure, bioactivities, and preparation. Food Res. Int. 2012, 46, 480–487. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents—A review. Poult. Sci. 2013, 92, 3292–3299. [Google Scholar] [CrossRef]
- Superti, F.; Ammendolia, M.G.; Berlutti, F.; Valenti, P. Ovotransferrin. In Bioactive Egg Compounds; Springer: Berlin/Heidelberg, Germany, 2007; pp. 43–50. ISBN 9783540378839. [Google Scholar]
- Giansanti, F.; Leboffe, L.; Angelucci, F.; Antonini, G. The nutraceutical properties of ovotransferrin and its potential utilization as a functional food. Nutrients 2015, 7, 9105–9115. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Scott, M.G. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 2000, 97, 8856–8861. [Google Scholar] [CrossRef]
- Mine, Y.; Ma, F.; Lauriau, S. Antimicrobial peptides released by enzymatic hydrolysis of hen egg white lysozyme. J. Agric. Food Chem. 2004, 52, 1088–1094. [Google Scholar] [CrossRef]
- Wang, G. Human antimicrobial peptides and proteins. Pharmaceuticals 2014, 7, 545–594. [Google Scholar] [CrossRef]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLOS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Masschalck, B.; Michiels, C.W. Antimicrobial properties of lysozyme in relation to foodborne vegetative bacteria. Crit. Rev. Microbiol. 2003, 29, 191–214. [Google Scholar] [CrossRef]
- Düring, K.; Porsch, P.; Mahn, A.; Brinkmann, O.; Gieffers, W. The non-enzymatic microbicidal activity of lysozymes. FEBS Lett. 1999, 449, 93–100. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Matsuzaki, T.; Aoki, T. Genetic evidence that antibacterial activity of lysozyme is independent of its catalytic function. FEBS Lett. 2001, 506, 27–32. [Google Scholar] [CrossRef]
- Eby, D.M.; Schaeublin, N.M.; Farrington, K.E.; Hussain, S.M.; Johnson, G.R. Lysozyme catalyzes the formation of antimicrobial silver nanoparticles. ACS Nano 2009, 3, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-I.; Daeschel, M.A.; Zhao, Y. Functional properties of antimicrobial lysozyme-chitosan composite films. J. Food Sci. 2004, 69, M215–M221. [Google Scholar] [CrossRef]
- Herbert, S.; Bera, A.; Nerz, C.; Kraus, D.; Peschel, A.; Goerke, C.; Meehl, M.; Cheung, A.; Götz, F. Molecular basis of resistance to muramidase and cationic antimicrobial peptide activity of lysozyme in Staphylococci. PLoS Pathog. 2007, 3, e102. [Google Scholar] [CrossRef]
- Eby, D.M.; Luckarift, H.R.; Johnson, G.R. Hybrid antimicrobial enzyme and silver nanoparticle coatings for medical instruments. ACS Appl. Mater. Interfaces 2009, 1, 1553–1560. [Google Scholar] [CrossRef]
- Bayarri, M.; Oulahal, N.; Degraeve, P.; Gharsallaoui, A. Properties of lysozyme/low methoxyl (LM) pectin complexes for antimicrobial edible food packaging. J. Food Eng. 2014, 131, 18–25. [Google Scholar] [CrossRef]
- Güçbilmez, Ç.M.; Yemenicioğlu, A.; Arslanoğlu, A. Antimicrobial and antioxidant activity of edible zein films incorporated with lysozyme, albumin proteins and disodium EDTA. Food Res. Int. 2007, 40, 80–91. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Thomas, U.; Pellegrini, A. A helix-loop-helix peptide at the upper lip of the active site cleft of lysozyme confers potent antimicrobial activity with membrane permeabilization action. J. Biol. Chem. 2001, 276, 43767–43774. [Google Scholar] [CrossRef]
- Oh, H.I.; Kim, Y.J.; Chang, E.J.; Kim, J.Y. Antimicrobial characteristics of chitosans against food spoilage microorganisms in liquid media and mayonnaise. Biosci. Biotechnol. Biochem. 2001, 65, 2378–2383. [Google Scholar] [CrossRef]
- Rhoades, J.; Roller, S. Antimicrobial actions of degraded and native chitosan against spoilage organisms in laboratory media and foods. Appl. Environ. Microbiol. 2000, 66, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Roller, S.; Covill, N. The antimicrobial properties of chitosan in mayonnaise and mayonnaise-based shrimp salads. J. Food Prot. 2000, 63, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Sagoo, S.K.; Board, R.; Roller, S. Chitosan potentiates the antimicrobial action of sodium benzoate on spoilage yeasts. Lett. Appl. Microbiol. 2002, 34, 168–172. [Google Scholar] [CrossRef]
- Tsai, G.-J.; Wu, Z.-Y.; Su, W.-H. Antibacterial activity of a chitooligosaccharide mixture prepared by cellulase digestion of shrimp chitosan and its application to milk preservation. J. Food Prot. 2000, 63, 747–752. [Google Scholar] [CrossRef]
- Zivanovic, S.; Basurto, C.C.; Chi, S.; Davidson, P.M.; Weiss, J. Molecular weight of chitosan influences antimicrobial activity in oil-in-water emulsions. J. Food Prot. 2004, 67, 952–959. [Google Scholar] [CrossRef]
- Moreira, M.d.R.; Pereda, M.; Marcovich, N.E.; Roura, S.I. Antimicrobial effectiveness of bioactive packaging materials from edible chitosan and casein polymers: Assessment on carrot, cheese, and salami. J. Food Sci. 2011, 76, M54–M63. [Google Scholar] [CrossRef]
- Ben-Shalom, N.; Ardi, R.; Pinto, R.; Aki, C.; Fallik, E. Controlling gray mould caused by Botrytis cinerea in cucumber plants by means of chitosan. Crop Prot. 2003, 22, 285–290. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Tavaria, F.K.; Soares, J.C.; Ramos, Ó.S.; João Monteiro, M.; Pintado, M.E.; Xavier Malcata, F. Antimicrobial effects of chitosans and chitooligosaccharides, upon Staphylococcus aureus and Escherichia coli, in food model systems. Food Microbiol. 2008, 25, 922–928. [Google Scholar] [CrossRef]
- Park, S.-I.; Stan, S.D.; Daeschel, M.A.; Zhao, Y. Antifungal coatings on fresh strawberries (Fragaria ananassa) to control mold growth during cold storage. J. Food Sci. 2006, 70, M202–M207. [Google Scholar] [CrossRef]
- Pranoto, Y.; Rakshit, S.K.; Salokhe, V.M. Enhancing antimicrobial activity of chitosan films by incorporating garlic oil, potassium sorbate and nisin. LWT Food Sci. Technol. 2005, 38, 859–865. [Google Scholar] [CrossRef]
- Devlieghere, F.; Vermeulen, A.; Debevere, J. Chitosan: antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004, 21, 703–714. [Google Scholar] [CrossRef]
- Sundaram, J.; Pant, J.; Goudie, M.J.; Mani, S.; Handa, H. Antimicrobial and physicochemical characterization of biodegradable, nitric oxide-releasing nanocellulose–chitosan packaging membranes. J. Agric. Food Chem. 2016, 64, 5260–5266. [Google Scholar] [CrossRef] [PubMed]
- Torlak, E.; Sert, D. Antibacterial effectiveness of chitosan–propolis coated polypropylene films against foodborne pathogens. Int. J. Biol. Macromol. 2013, 60, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Torlak, E.; Nizamlioğlu, M. Antimicrobial effectiveness of chitosan-essential oil coated plastic films against foodborne pathogens. J. Plast. Film Sheeting 2011, 27, 235–248. [Google Scholar] [CrossRef]
- Shaaban, H.A.; Ali, H.S.; Bareh, G.F.; Al-khalifa, A.R.S.; Amer, M.M. Antimicrobial activity of two polysaccharide edible films incorporated with essential oils against three pathogenic bacteria. J. Appl. Sci. 2017, 17, 171–183. [Google Scholar] [CrossRef][Green Version]
- Chung, Y.-C.; Yeh, J.-Y.; Tsai, C.-F. Antibacterial characteristics and activity of water-soluble chitosan derivatives prepared by the Maillard reaction. Molecules 2011, 16, 8504–8514. [Google Scholar] [CrossRef]
- Young, D.H.; Köhle, H.; Kauss, H. Effect of chitosan on membrane permeability of suspension-cultured Glycine max and Phaseolus vulgaris cells. Plant Physiol. 1982, 70, 1449–1454. [Google Scholar] [CrossRef]
- Knorr, D. Recovery and utilization of chitin and chitosan in food processing waste management. Food Technol. 1991, 45, 114–122. [Google Scholar]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Raafat, D.; von Bargen, K.; Haas, A.; Sahl, H.-G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Fei Liu, X.; Lin Guan, Y.; Zhi Yang, D.; Li, Z.; De Yao, K. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar] [CrossRef]
- Andres, Y.; Giraud, L.; Gerente, C.; Le Cloirec, P. Antibacterial effects of chitosan powder: Mechanisms of action. Environ. Technol. 2007, 28, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Sprong, R.C.; Hulstein, M.F.E.; Van der Meer, R. Bactericidal activities of milk lipids. Antimicrob. Agents Chemother. 2001, 45, 1298–1301. [Google Scholar] [CrossRef]
- Sprong, R.; Hulstein, M.F.; van der Meer, R. Bovine milk fat components inhibit food-borne pathogens. Int. Dairy J. 2002, 12, 209–215. [Google Scholar] [CrossRef]
- Lock, A.L.; Bauman, D.E. Modifying milk fat composition of dairy cows to enhance fatty acids beneficial to human health. Lipids 2004, 39, 1197–1206. [Google Scholar] [CrossRef]
- Hamosh, M. Protective function of proteins and lipids in human milk. Neonatology 1998, 74, 163–176. [Google Scholar] [CrossRef]
- German, J.B.; Dillard, C.J. Composition, structure and absorption of milk lipids: A source of energy, fat-soluble nutrients and bioactive molecules. Crit. Rev. Food Sci. Nutr. 2006, 46, 57–92. [Google Scholar] [CrossRef]
- El Agamy, E.S.I.; Ruppanner, R.; Ismail, A.; Champagne, C.P.; Assaf, R. Antibacterial and antiviral activity of camel milk protective proteins. J. Dairy Res. 1992, 59, 169–175. [Google Scholar] [CrossRef]
- Shin, S.Y.; Bajpai, V.K.; Kim, H.R.; Kang, S.C. Antibacterial activity of bioconverted eicosapentaenoic (EPA) and docosahexaenoic acid (DHA) against foodborne pathogenic bacteria. Int. J. Food Microbiol. 2007, 113, 233–236. [Google Scholar] [CrossRef]
- Sun, M.; Dong, J.; Xia, Y.; Shu, R. Antibacterial activities of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) against planktonic and biofilm growing Streptococcus mutans. Microb. Pathog. 2017, 107, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Montville, T.J.; Chikindas, M.L. Biopreservation of foods. In Food Microbiology: Fundamentals and Frontiers, 3rd ed.; Doyle, M.P., Beuchat, L.R., Eds.; ASM Press: Washington, DC, USA, 2007; pp. 747–764. ISBN 978-1-55581-407-6. [Google Scholar]
- Saleh, M.A.; Ordal, Z.J. Studies on growth and toxin production of Clostridium botulinum in a precooked frozen food. II. Inhibitioin by lactic acid bacteria. J. Food Sci. 1955, 20, 340–350. [Google Scholar] [CrossRef]
- Ting, P.T.; Freiman, A. The story of Clostridium botulinum: From food poisoning to Botox. Clin. Med. 2004, 4, 258–261. [Google Scholar] [CrossRef]
- Johnson, E.A. CLOSTRIDIUM|Clostridium botulinum. In Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 458–462. ISBN 9780123847331. [Google Scholar]
- Hutton, M.T.; Chehak, P.A.; Hanlin, J.H. Inhibition of botulinum toxin production by Pediococcus acidilactici in temperature abused refrigerated foods. J. Food Saf. 1991, 11, 255–267. [Google Scholar] [CrossRef]
- Cintas, L.M.; Casaus, M.P.; Herranz, C.; Nes, I.F.; Hernández, P.E. Review: Bacteriocins of lactic acid bacteria. Food Sci. Technol. Int. 2001, 7, 281–305. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef]
- Reis, J.A.; Paula, A.T.; Casarotti, S.N.; Penna, A.L.B. Lactic acid bacteria antimicrobial compounds: Characteristics and applications. Food Eng. Rev. 2012, 4, 124–140. [Google Scholar] [CrossRef]
- Klaenhammer, T. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 39–85. [Google Scholar] [CrossRef]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Novel bacteriocins from lactic acid bacteria (LAB): various structures and applications. Microb. Cell Fact. 2014, 13, S3. [Google Scholar] [CrossRef] [PubMed]
- Veskovic-Moracanin, S.; Djukic, D.; Memisi, N. Bacteriocins produced by lactic acid bacteria: A review. Acta Period. Technol. 2014, 2014, 271–283. [Google Scholar] [CrossRef]
- De Vuyst, L.; Leroy, F. Bacteriocins from lactic acid bacteria: Production, purification, and food applications. J. Mol. Microbiol. Biotechnol. 2007, 13, 194–199. [Google Scholar] [CrossRef]
- Woraprayote, W.; Malila, Y.; Sorapukdee, S.; Swetwiwathana, A.; Benjakul, S.; Visessanguan, W. Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Sci. 2016, 120, 118–132. [Google Scholar] [CrossRef]
- Schillinger, U. Bacteriocins of lactic acid bacteria. In Biotechnology and Food Safety; Elsevier: Amsterdam, The Netherlands, 1990; pp. 55–74. [Google Scholar]
- Kalchayanand, N.; Hanlin, M.B.; Ray, B. Sublethal injury makes Gram-negative and resistant Gram-positive bacteria sensitive to the bacteriocins, pediocin AcH and nisin. Lett. Appl. Microbiol. 1992, 15, 239–243. [Google Scholar] [CrossRef]
- Kalchayanand, N.; Sikes, T.; Dunne, C.P.; Ray, B. Hydrostatic pressure and electroporation have increased bactericidal efficiency in combination with bacteriocins. Appl. Environ. Microbiol. 1994, 60, 4174–4177. [Google Scholar]
- Bali, V.; Panesar, P.S.; Bera, M.B. Potential of immobilization technology in bacteriocin production and antimicrobial packaging. Food Rev. Int. 2014, 30, 244–263. [Google Scholar] [CrossRef]
- Mossallam, S.F.; Amer, E.I.; Diab, R.G. Potentiated anti-microsporidial activity of Lactobacillus acidophilus CH1 bacteriocin using gold nanoparticles. Exp. Parasitol. 2014, 144, 14–21. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Synergistic antibacterial efficiency of bacteriocin and silver nanoparticles produced by probiotic Lactobacillus paracasei against multidrug resistant bacteria. Int. J. Pept. Res. Ther. 2019, 25, 1113–1125. [Google Scholar] [CrossRef]
- De Mello, M.B.; da Silva Malheiros, P.; Brandelli, A.; Pesce da Silveira, N.; Jantzen, M.M.; de Souza da Motta, A. Characterization and antilisterial effect of phosphatidylcholine nanovesicles containing the antimicrobial peptide pediocin. Probiotics Antimicrob. Proteins 2013, 5, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Bower, C.K.; McGuire, J.; Daeschel, M.A. Suppression of Listeria monocytogenes colonization following adsorption of nisin onto silica surfaces. Appl. Environ. Microbiol. 1995, 61, 992–997. [Google Scholar] [PubMed]
- Daeschel, M.A.; McGuire, J.; Al-Makhlafi, H. Antimicrobial activity of nisin adsorbed to hydrophilic and hydrophobic silicon surfaces. J. Food Prot. 1992, 55, 731–735. [Google Scholar] [CrossRef]
- Karam, L.; Jama, C.; Nuns, N.; Mamede, A.-S.; Dhulster, P.; Chihib, N.-E. Nisin adsorption on hydrophilic and hydrophobic surfaces: evidence of its interactions and antibacterial activity. J. Pept. Sci. 2013, 19, 377–385. [Google Scholar] [CrossRef]
- Karam, L.; Jama, C.; Mamede, A.-S.; Boukla, S.; Dhulster, P.; Chihib, N.-E. Nisin-activated hydrophobic and hydrophilic surfaces: assessment of peptide adsorption and antibacterial activity against some food pathogens. Appl. Microbiol. Biotechnol. 2013, 97, 10321–10328. [Google Scholar] [CrossRef]
- Karam, L.; Jama, C.; Mamede, A.-S.; Fahs, A.; Louarn, G.; Dhulster, P.; Chihib, N.-E. Study of nisin adsorption on plasma-treated polymer surfaces for setting up materials with antibacterial properties. React. Funct. Polym. 2013, 73, 1473–1479. [Google Scholar] [CrossRef]
- Blay, G.L.; Lacroix, C.; Zihler, A.; Fliss, I. In vitro inhibition activity of nisin A, nisin Z, pediocin PA-1 and antibiotics against common intestinal bacteria. Lett. Appl. Microbiol. 2007, 45, 252–257. [Google Scholar] [CrossRef]
- Todorov, S.D.; Wachsman, M.; Tomé, E.; Dousset, X.; Destro, M.T.; Dicks, L.M.T.; de Melo Franco, B.D.G.; Vaz-Velho, M.; Drider, D. Characterisation of an antiviral pediocin-like bacteriocin produced by Enterococcus faecium. Food Microbiol. 2010, 27, 869–879. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Martínez, M.I.; Kok, J. Pediocin PA-1, a wide-spectrum bacteriocin from lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2002, 42, 91–121. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.d.F.F.; Teófilo, R.F.; Coimbra, J.S.d.R.; Vitor, D.M.; Batista, R.A.; Ferreira, S.O.; de Andrade, N.J.; Medeiros, E.A.A. Physical–mechanical and antimicrobial properties of nanocomposite films with pediocin and ZnO nanoparticles. Carbohydr. Polym. 2013, 94, 199–208. [Google Scholar] [CrossRef]
- Gravesen, A.; Jydegaard Axelsen, A.-M.; Mendes da Silva, J.; Hansen, T.B.; Knochel, S. Frequency of bacteriocin resistance development and associated fitness costs in Listeria monocytogenes. Appl. Environ. Microbiol. 2002, 68, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Silva, P.; Soares, N.F.F.; Nóbrega, J.E.; Júnior, M.A.W.; Barbosa, K.B.F.; Volp, A.C.P.; Zerdas, E.R.M.A.; Würlitzer, N.J. Antimicrobial efficiency of film incorporated with pediocin (ALTA® 2351) on preservation of sliced ham. Food Control 2009, 20, 85–89. [Google Scholar] [CrossRef]
- Rodríguez, E.; Calzada, J.; Arqués, J.L.; Rodríguez, J.M.; Nuñez, M.; Medina, M. Antimicrobial activity of pediocin-producing Lactococcus lactis on Listeria monocytogenes, Staphylococcus aureus and Escherichia coli O157:H7 in cheese. Int. Dairy J. 2005, 15, 51–57. [Google Scholar] [CrossRef]
- Millette, M.; Dupont, C.; Shareck, F.; Ruiz, M.T.; Archambault, D.; Lacroix, M. Purification and identification of the pediocin produced by Pediococcus acidilactici MM33, a new human intestinal strain. J. Appl. Microbiol. 2008, 104, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Lozano, J.C.; Reguera-Useros, J.I.; Peláez-Martínez, M.d.C.; Sacristán-Pérez-Minayo, G.; Gutiérrez-Fernández, Á.J.; de la Torre, A.H. The effect of the pediocin PA-1 produced by Pediococcus acidilactici against Listeria monocytogenes and Clostridium perfringens in Spanish dry-fermented sausages and frankfurters. Food Control 2010, 21, 679–685. [Google Scholar] [CrossRef]
- Renye, J.A.; Somkuti, G.A.; Garabal, J.I.; Du, L. Heterologous production of pediocin for the control of Listeria monocytogenes in dairy foods. Food Control 2011, 22, 1887–1892. [Google Scholar] [CrossRef]
- Langa, S.; Landete, J.M.; Martín-Cabrejas, I.; Rodríguez, E.; Arqués, J.L.; Medina, M. In situ reuterin production by Lactobacillus reuteri in dairy products. Food Control 2013, 33, 200–206. [Google Scholar] [CrossRef]
- Arqués, J.L.; Rodríguez, E.; Nuñez, M.; Medina, M. Combined effect of reuterin and lactic acid bacteria bacteriocins on the inactivation of food-borne pathogens in milk. Food Control 2011, 22, 457–461. [Google Scholar] [CrossRef]
- Elsser-Gravesen, D.; Elsser-Gravesen, A. Biopreservatives. In Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 29–49. [Google Scholar]
- Fontana, C.; Fadda, S.; Cocconcelli, P.S.; Vignolo, G. Lactic acid bacteria in meat fermentations. In Lactic Acid Bacteria: Microbiological and Functional Aspects, Fourth Edition; CRC Press: Boca Raton, FL, USA, 2011; pp. 247–264. ISBN 9781439836781. [Google Scholar]
- Lu, S.; Xu, X.; Zhou, G.; Zhu, Z.; Meng, Y.; Sun, Y. Effect of starter cultures on microbial ecosystem and biogenic amines in fermented sausage. Food Control 2010, 21, 444–449. [Google Scholar] [CrossRef]
- Freire, A.L.; Ramos, C.L.; da Costa Souza, P.N.; Cardoso, M.G.B.; Schwan, R.F. Nondairy beverage produced by controlled fermentation with potential probiotic starter cultures of lactic acid bacteria and yeast. Int. J. Food Microbiol. 2017, 248, 39–46. [Google Scholar] [CrossRef]
- De Melo Pereira, G.V.; Neto, E.; Soccol, V.T.; Medeiros, A.B.P.; Woiciechowski, A.L.; Soccol, C.R. Conducting starter culture-controlled fermentations of coffee beans during on-farm wet processing: Growth, metabolic analyses and sensorial effects. Food Res. Int. 2015, 75, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; El Khoury, M.; Lucas, P.; Bely, M.; Russo, P.; Spano, G.; Capozzi, V. Autochthonous starter cultures and indigenous grape variety for regional wine production. J. Appl. Microbiol. 2015, 118, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Daeschel, M.A. Nisin resistance of foodborne bacteria and the specific resistance responses of Listeria monocytogenes Scott A. J. Food Prot. 1993, 56, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Turovskiy, Y.; Rosenberg, L.; Chikindas, M.L. Autoinducer-2-mediated quorum sensing is not involved in Listeria monocytogenes’ adaptive responses to the food preservatives lactic acid and nisin. J. Food Saf. 2007, 27, 386–399. [Google Scholar] [CrossRef]
- Mazzotta, A.S.; Crandall, A.D.; Montville, T.J. Nisin resistance in Clostridium botulinum spores and vegetative cells. Appl. Environ. Microbiol. 1997, 63, 2654–2659. [Google Scholar]
- Naghmouchi, K.; Kheadr, E.; Lacroix, C.; Fliss, I. Class I/Class IIa bacteriocin cross-resistance phenomenon in Listeria monocytogenes. Food Microbiol. 2007, 24, 718–727. [Google Scholar] [CrossRef]
- Laursen, M.F.; Bahl, M.I.; Licht, T.R.; Gram, L.; Knudsen, G.M. A single exposure to a sublethal pediocin concentration initiates a resistance-associated temporal cell envelope and general stress response in Listeria monocytogenes. Environ. Microbiol. 2015, 17, 1134–1151. [Google Scholar] [CrossRef]
- Hanlin, M.B.; Kalchayanand, N.; Ray, P.; Ray, B. Bacteriocins of lactic acid bacteria in combination have greater antibacterial activity. J. Food Prot. 1993, 56, 252–255. [Google Scholar] [CrossRef]
- Field, D.; Ross, R.P.; Hill, C. Developing bacteriocins of lactic acid bacteria into next generation biopreservatives. Curr. Opin. Food Sci. 2018, 20, 1–6. [Google Scholar] [CrossRef]
- Rekhif, N.; Atrih, A.; Lefebvre, G. Selection and properties of spontaneous mutants of Listeria monocytogenes ATCC 15313 resistant to different bacteriocins produced by lactic acid bacteria strains. Curr. Microbiol. 1994, 28, 237–241. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, T.P.; Malik, R.K. Antibacterial efficacy of nisin, pediocin 34 and enterocin FH99 against Listeria monocytogenes and cross resistance of its bacteriocin resistant variants to common food preservatives. Brazilian J. Microbiol. 2013, 44, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Singh, T.P.; Malik, R.K.; Bhardwaj, A.; De, S. Antibacterial efficacy of nisin, pediocin 34 and enterocin FH99 against L. monocytogenes, E. faecium and E. faecalis and bacteriocin cross resistance and antibiotic susceptibility of their bacteriocin resistant variants. J. Food Sci. Technol. 2014, 51, 233–244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gravesen, A.; Kallipolitis, B.; Holmstrom, K.; Hoiby, P.E.; Ramnath, M.; Knochel, S. pbp2229-mediated nisin resistance mechanism in Listeria monocytogenes confers cross-protection to Class IIa bacteriocins and affects virulence gene expression. Appl. Environ. Microbiol. 2004, 70, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Jończyk, E.; Kłak, M.; Międzybrodzki, R.; Górski, A. The influence of external factors on bacteriophages—review. Folia Microbiol. 2011, 56, 191–200. [Google Scholar] [CrossRef]
- Nobrega, F.L.; Vlot, M.; de Jonge, P.A.; Dreesens, L.L.; Beaumont, H.J.E.; Lavigne, R.; Dutilh, B.E.; Brouns, S.J.J. Targeting mechanisms of tailed bacteriophages. Nat. Rev. Microbiol. 2018, 16, 760–773. [Google Scholar] [CrossRef]
- García, P.; Rodríguez, L.; Rodríguez, A.; Martínez, B. Food biopreservation: promising strategies using bacteriocins, bacteriophages and endolysins. Trends Food Sci. Technol. 2010, 21, 373–382. [Google Scholar] [CrossRef]
- León, M.; Bastías, R. Virulence reduction in bacteriophage resistant bacteria. Front. Microbiol. 2015, 6, 343. [Google Scholar] [CrossRef]
- Brüssow, H. Phage therapy: The Escherichia coli experience. Microbiology 2005, 151, 2133–2140. [Google Scholar] [CrossRef]
- Haq, I.U.; Chaudhry, W.N.; Akhtar, M.N.; Andleeb, S.; Qadri, I. Bacteriophages and their implications on future biotechnology: a review. Virol. J. 2012, 9, 9. [Google Scholar] [CrossRef]
- Golkar, Z.; Bagasra, O.; Pace, D.G. Bacteriophage therapy: a potential solution for the antibiotic resistance crisis. J. Infect. Dev. Ctries. 2014, 8, 129–136. [Google Scholar] [CrossRef]
- García, P.; Martínez, B.; Obeso, J.M.; Rodríguez, A. Bacteriophages and their application in food safety. Lett. Appl. Microbiol. 2008, 47, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Mahony, J.; McAuliffe, O.; Ross, R.P.; van Sinderen, D. Bacteriophages as biocontrol agents of food pathogens. Curr. Opin. Biotechnol. 2011, 22, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Marcó, M.B.; Moineau, S.; Quiberoni, A. Bacteriophages and dairy fermentations. Bacteriophage 2012, 2, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Loessner, M.J. Application of bacteriophages for detection of foodborne pathogens. Bacteriophage 2014, 4, e28137. [Google Scholar] [CrossRef]
- Joerger, R. Alternatives to antibiotics: bacteriocins, antimicrobial peptides and bacteriophages. Poult. Sci. 2003, 82, 640–647. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Singh, S.; Kate, B.N.; Banerjee, U.C. Bioactive compounds from Cyanobacteria and Microalgae: An overview. Crit. Rev. Biotechnol. 2005, 25, 73–95. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Microalgae as sources of pharmaceuticals and other biologically active compounds. J. Appl. Phycol. 1995, 7, 3–15. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Plaza, M.; Ibañez, E. Screening for bioactive compounds from Algae. In Advanced Biofuels and Bioproducts; Springer: New York, NY, USA, 2013; pp. 833–872. ISBN 9781461433484. [Google Scholar]
- Devi, K.P.; Suganthy, N.; Kesika, P.; Pandian, S.K. Bioprotective properties of seaweeds: In vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complement. Altern. Med. 2008, 8, 38. [Google Scholar] [CrossRef]
- Gupta, S.; Rajauria, G.; Abu-Ghannam, N. Study of the microbial diversity and antimicrobial properties of Irish edible brown seaweeds. Int. J. Food Sci. Technol. 2010, 45, 482–489. [Google Scholar] [CrossRef]
- Dussault, D.; Vu, K.D.; Vansach, T.; Horgen, F.D.; Lacroix, M. Antimicrobial effects of marine algal extracts and cyanobacterial pure compounds against five foodborne pathogens. Food Chem. 2016, 199, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Alboofetileh, M.; Rezaei, M.; Hosseini, H.; Abdollahi, M. Antimicrobial activity of alginate/clay nanocomposite films enriched with essential oils against three common foodborne pathogens. Food Control 2014, 36, 1–7. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Fang, Y.; Holley, R.A. Inhibition of Campylobacter jejuni on fresh chicken breasts by κ-carrageenan/chitosan-based coatings containing allyl isothiocyanate or deodorized oriental mustard extract. Int. J. Food Microbiol. 2014, 187, 77–82. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Holley, R.A. Control of Salmonella on fresh chicken breasts by κ-carrageenan/chitosan-based coatings containing allyl isothiocyanate or deodorized Oriental mustard extract plus EDTA. Food Microbiol. 2015, 48, 83–88. [Google Scholar] [CrossRef]
- Ramesh, C.; Pattar, M.G. Antimicrobial properties, antioxidant activity and bioactive compounds from six wild edible mushrooms of western ghats of Karnataka, India. Pharmacognosy Res. 2010, 2, 107–112. [Google Scholar]
- Öztürk, M.; Duru, M.E.; Kivrak, Ş.; Mercan-Doğan, N.; Türkoglu, A.; Özler, M.A. In vitro antioxidant, anticholinesterase and antimicrobial activity studies on three Agaricus species with fatty acid compositions and iron contents: A comparative study on the three most edible mushrooms. Food Chem. Toxicol. 2011, 49, 1353–1360. [Google Scholar] [CrossRef]
- Kalyoncu, F.; Oskay, M.; Sağlam, H.; Erdoğan, T.F.; Tamer, A.Ü. Antimicrobial and antioxidant activities of mycelia of 10 wild mushroom species. J. Med. Food 2010, 13, 415–419. [Google Scholar] [CrossRef]
- Shen, H.-S.; Shao, S.; Chen, J.-C.; Zhou, T. Antimicrobials from mushrooms for assuring food safety. Compr. Rev. Food Sci. Food Saf. 2017, 16, 316–329. [Google Scholar] [CrossRef]
- Alves, M.; Ferreira, I.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef]
- Lallawmsanga; Passari, A.K.; Mishra, V.K.; Leo, V.V.; Singh, B.P.; Valliammai Meyyappan, G.; Gupta, V.K.; Uthandi, S.; Upadhyay, R.C. Antimicrobial potential, identification and phylogenetic affiliation of wild mushrooms from two sub-tropical semi-evergreen indian forest ecosystems. PLoS ONE 2016, 11, e0166368. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Ferreira, I.; Lourenço, I.; Costa, E.; Martins, A.; Pintado, M. Wild mushroom extracts as inhibitors of bacterial biofilm formation. Pathogens 2014, 3, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; van Griensven, L.J. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar]
- Ultee, A.; Slump, R.A.; Steging, G.; Smid, E.J. Antimicrobial activity of carvacrol toward Bacillus cereus on rice. J. Food Prot. 2000, 63, 620–624. [Google Scholar] [CrossRef]
- Ayari, S.; Dussault, D.; Hamdi, M.; Lacroix, M. Growth and toxigenic potential of Bacillus cereus during storage temperature abuse in cooked irradiated chicken rice in combination with nisin and carvacrol. LWT Food Sci. Technol. 2016, 72, 19–25. [Google Scholar] [CrossRef]
- Yuste, J.; Fung, D.Y.C. Inactivation of Listeria monocytogenes Scott A 49594 in apple juice supplemented with cinnamon. J. Food Prot. 2002, 65, 1663–1666. [Google Scholar] [CrossRef]
- Cava, R.; Nowak, E.; Taboada, A.; Marin-Iniesta, F. Antimicrobial activity of clove and cinnamon essential oils against Listeria monocytogenes in pasteurized milk. J. Food Prot. 2007, 70, 2757–2763. [Google Scholar] [CrossRef]
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. The potential application of plant essential oils as natural food preservatives in soft cheese. Food Microbiol. 2001, 18, 463–470. [Google Scholar] [CrossRef]
- Khorshidian, N.; Yousefi, M.; Khanniri, E.; Mortazavian, A.M. Potential application of essential oils as antimicrobial preservatives in cheese. Innov. Food Sci. Emerg. Technol. 2018, 45, 62–72. [Google Scholar] [CrossRef]
- Hyun, J.-E.; Bae, Y.-M.; Yoon, J.-H.; Lee, S.-Y. Preservative effectiveness of essential oils in vapor phase combined with modified atmosphere packaging against spoilage bacteria on fresh cabbage. Food Control 2015, 51, 307–313. [Google Scholar] [CrossRef]
- Karabagias, I.; Badeka, A.; Kontominas, M.G. Shelf life extension of lamb meat using thyme or oregano essential oils and modified atmosphere packaging. Meat Sci. 2011, 88, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Solomakos, N.; Govaris, A.; Koidis, P.; Botsoglou, N. The antimicrobial effect of thyme essential oil, nisin, and their combination against Listeria monocytogenes in minced beef during refrigerated storage. Food Microbiol. 2008, 25, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Solomakos, N.; Govaris, A.; Koidis, P.; Botsoglou, N. The antimicrobial effect of thyme essential oil, nisin and their combination against Escherichia coli O157:H7 in minced beef during refrigerated storage. Meat Sci. 2008, 80, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Piskernik, S.; Klančnik, A.; Riedel, C.T.; Brøndsted, L.; Možina, S.S. Reduction of Campylobacter jejuni by natural antimicrobials in chicken meat-related conditions. Food Control 2011, 22, 718–724. [Google Scholar] [CrossRef]
- DjEnane, D.; Yangüela, J.; Gómez, D.; Roncalés, P. Perspectives on the use of essential oils as antimicrobials against Campylobacter jejuni CECT 7572 in retail chicken meats packaged in microaerobic atmosphere. J. Food Saf. 2012, 32, 37–47. [Google Scholar] [CrossRef]
- Jang, S.-A.; Shin, Y.-J.; Song, K. Bin Effect of rapeseed protein-gelatin film containing grapefruit seed extract on ‘Maehyang’ strawberry quality. Int. J. Food Sci. Technol. 2011, 46, 620–625. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Cerisuelo, J.P.; López-Carballo, G.; Lara, M.; Gavara, R.; Hernández-Muñoz, P. Development of antimicrobial films for microbiological control of packaged salad. Int. J. Food Microbiol. 2012, 157, 195–201. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Cerisuelo, J.P.; López-Carballo, G.; Aucejo, S.; Gavara, R.; Hernández-Muñoz, P. Evaluation of EVOH-coated PP films with oregano essential oil and citral to improve the shelf-life of packaged salad. Food Control 2013, 30, 137–143. [Google Scholar] [CrossRef]
- Cé, N.; Noreña, C.P.Z.; Brandelli, A. Antimicrobial activity of chitosan films containing nisin, peptide P34, and natamycin. CyTA J. Food 2012, 10, 21–26. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y. Effects of chitosan coating on postharvest life and quality of longan fruit. Food Chem. 2001, 73, 139–143. [Google Scholar] [CrossRef]
- Arnon, H.; Zaitsev, Y.; Porat, R.; Poverenov, E. Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest Biol. Technol. 2014, 87, 21–26. [Google Scholar] [CrossRef]
- Hong, K.; Xie, J.; Zhang, L.; Sun, D.; Gong, D. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Sci. Hortic. 2012, 144, 172–178. [Google Scholar] [CrossRef]
- Jianglian, D. Application of chitosan based coating in fruit and vegetable preservation: A review. J. Food Process. Technol. 2013, 4, 227. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Baños, S.B.; Sivakumar, D. Shelf life extension of fresh fruit and vegetables by chitosan treatment. Crit. Rev. Food Sci. Nutr. 2017, 57, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Vodnar, D.C. Inhibition of Listeria monocytogenes ATCC 19115 on ham steak by tea bioactive compounds incorporated into chitosan-coated plastic films. Chem. Cent. J. 2012, 6, 74. [Google Scholar] [CrossRef]
- Park, S.; Marsh, K.S.; Dawson, P. Application of chitosan-incorporated LDPE film to sliced fresh red meats for shelf life extension. Meat Sci. 2010, 85, 493–499. [Google Scholar] [CrossRef]
- Cardoso, G.P.; Dutra, M.P.; Fontes, P.R.; de Lemos Souza Ramos, A.; de Miranda Gomide, L.A.; Ramos, E.M. Selection of a chitosan gelatin-based edible coating for color preservation of beef in retail display. Meat Sci. 2016, 114, 85–94. [Google Scholar] [CrossRef]
- Quesada, J.; Sendra, E.; Navarro, C.; Sayas-Barberá, E. Antimicrobial active packaging including chitosan films with Thymus vulgaris L. essential oil for ready-to-eat meat. Foods 2016, 5, 57. [Google Scholar] [CrossRef]
- Sivarooban, T.; Hettiarachchy, N.S.; Johnson, M.G. Physical and antimicrobial properties of grape seed extract, nisin, and EDTA incorporated soy protein edible films. Food Res. Int. 2008, 41, 781–785. [Google Scholar] [CrossRef]
- Zou, Y.; Lee, H.-Y.; Seo, Y.-C.; Ahn, J. Enhanced antimicrobial activity of nisin-loaded liposomal nanoparticles against foodborne pathogens. J. Food Sci. 2012, 77, M165–M170. [Google Scholar] [CrossRef]
- Field, D.; Begley, M.; O’Connor, P.M.; Daly, K.M.; Hugenholtz, F.; Cotter, P.D.; Hill, C.; Ross, R.P. Bioengineered nisin A derivatives with enhanced activity against both Gram positive and Gram negative pathogens. PLoS ONE 2012, 7, e46884. [Google Scholar] [CrossRef] [PubMed]
- Chatzidaki, M.D.; Balkiza, F.; Gad, E.; Alexandraki, V.; Avramiotis, S.; Georgalaki, M.; Papadimitriou, V.; Tsakalidou, E.; Papadimitriou, K.; Xenakis, A. Reverse micelles as nano-carriers of nisin against foodborne pathogens. Part II: The case of essential oils. Food Chem. 2019, 278, 415–423. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinto, E.J.; Caro, I.; Villalobos-Delgado, L.H.; Mateo, J.; De-Mateo-Silleras, B.; Redondo-Del-Río, M.P. Food Safety through Natural Antimicrobials. Antibiotics 2019, 8, 208. https://doi.org/10.3390/antibiotics8040208
Quinto EJ, Caro I, Villalobos-Delgado LH, Mateo J, De-Mateo-Silleras B, Redondo-Del-Río MP. Food Safety through Natural Antimicrobials. Antibiotics. 2019; 8(4):208. https://doi.org/10.3390/antibiotics8040208
Chicago/Turabian StyleQuinto, Emiliano J., Irma Caro, Luz H. Villalobos-Delgado, Javier Mateo, Beatriz De-Mateo-Silleras, and María P. Redondo-Del-Río. 2019. "Food Safety through Natural Antimicrobials" Antibiotics 8, no. 4: 208. https://doi.org/10.3390/antibiotics8040208
APA StyleQuinto, E. J., Caro, I., Villalobos-Delgado, L. H., Mateo, J., De-Mateo-Silleras, B., & Redondo-Del-Río, M. P. (2019). Food Safety through Natural Antimicrobials. Antibiotics, 8(4), 208. https://doi.org/10.3390/antibiotics8040208






