A Breath of Fresh Air in the Fog of Antimicrobial Resistance: Inhaled Polymyxins for Gram-Negative Pneumonia
Abstract
:1. Introduction
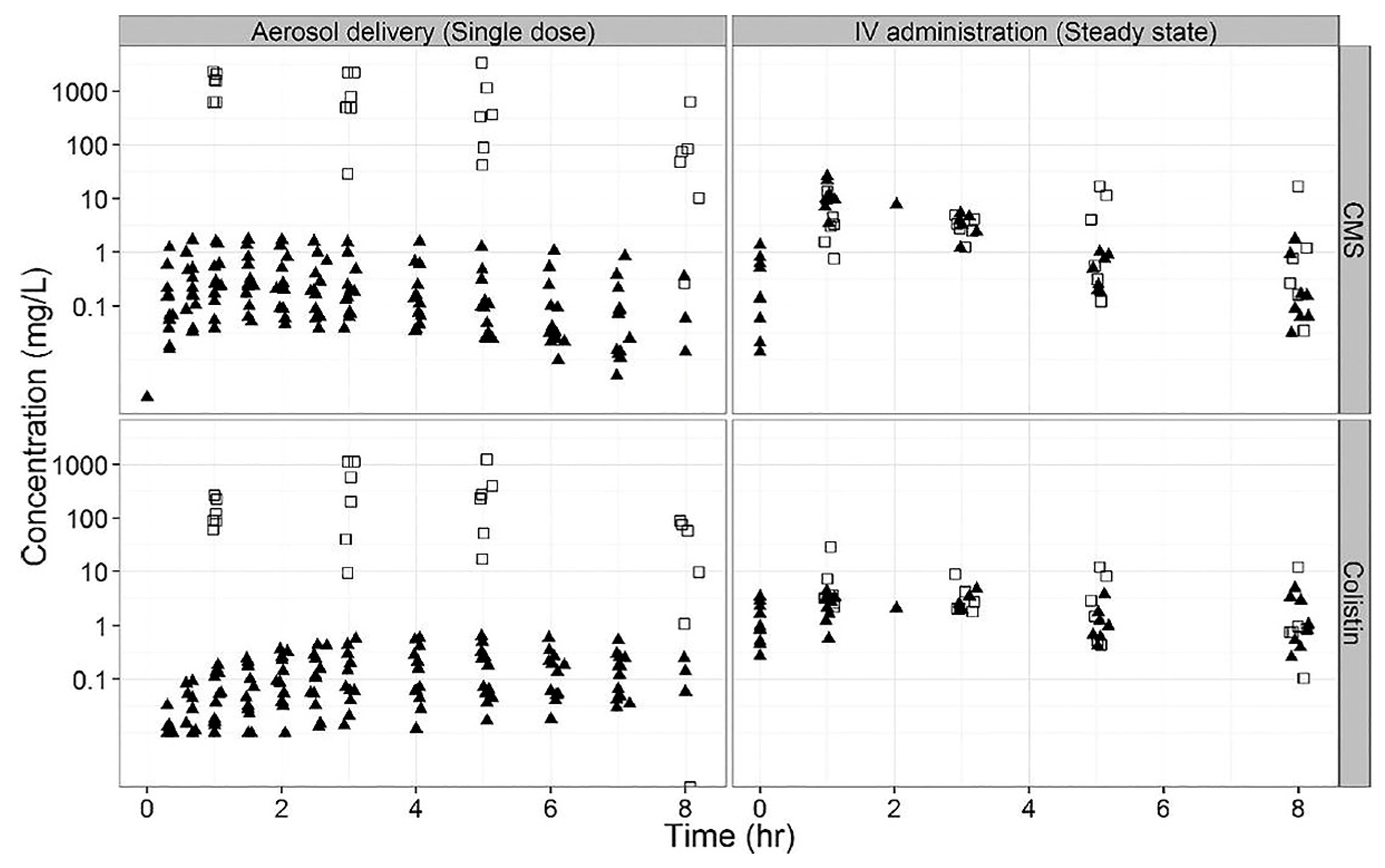
2. Pharmacokinetics and Pharmacodynamics of Inhaled Polymyxins
3. Clinical Outcomes
3.1. Colistin Monotherapy
3.2. Adjunctive Colistin
4. Safety
4.1. Nephrotoxicity
4.2. Bronchoconstriction
4.3. Resistance
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Heron, M. Deaths: Leading Causes for 2016. Natl. Vital Stat. Rep. 2018, 67, 1–77. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2013. Available online: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf (accessed on 1 January 2019).
- Sopena, N.; Heras, E.; Casas, I.; Bechini, J.; Guasch, I.; Pedro-Botet, M.L.; Roure, S.; Sabria, M. Risk factors for hospital-acquired pneumonia outside the intensive care unit: A case-control study. Am. J. Infect. Control 2014, 42, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Micek, S.T.; Chew, B.; Hampton, N.; Kollef, M.H. A Case-Control Study Assessing the Impact of Nonventilated Hospital-Acquired Pneumonia on Patient Outcomes. Chest 2016, 150, 1008–1014. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Giuliano, K.K.; Baker, D.; Quinn, B. The epidemiology of nonventilator hospital-acquired pneumonia in the United States. Am. J. Infect. Control 2018, 46, 322–327. [Google Scholar] [CrossRef]
- Melsen, W.G.; Rovers, M.M.; Koeman, M.; Bonten, M.J. Estimating the attributable mortality of ventilator-associated pneumonia from randomized prevention studies. Crit. Care Med. 2011, 39, 2736–2742. [Google Scholar] [CrossRef] [PubMed]
- Melsen, W.G.; Rovers, M.M.; Groenwold, R.H.; Bergmans, D.C.; Camus, C.; Bauer, T.T.; Hanisch, E.W.; Klarin, B.; Koeman, M.; Krueger, W.A.; et al. Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 2013, 13, 665–671. [Google Scholar] [CrossRef]
- Rodvold, K.A.; Yoo, L.; George, J.M. Penetration of anti-infective agents into pulmonary epithelial lining fluid: Focus on antifungal, antitubercular and miscellaneous anti-infective agents. Clin. Pharmacokinet. 2011, 50, 689–704. [Google Scholar] [CrossRef] [PubMed]
- Mandell, L.A.; Wunderink, R.G. Pneumonia. In Harrison’s Principles of Internal Medicine, 19th ed.; Kasper, D.L., Ed.; McGraw-Hill: New York, NY, USA, 2015. [Google Scholar]
- Baldwin, D.R.; Honeybourne, D.; Wise, R. Pulmonary disposition of antimicrobial agents: Methodological considerations. Antimicrob. Agents Chemother. 1992, 36, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Daugherty, B.; Keise, L.L.; Wei, Z.; Foley, J.P.; Savani, R.C.; Koval, M. Heterogeneity of claudin expression by alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 2003, 29, 62–70. [Google Scholar] [CrossRef]
- Campbell, L.; Abulrob, A.N.; Kandalaft, L.E.; Plummer, S.; Hollins, A.J.; Gibbs, A.; Gumbleton, M. Constitutive expression of p-glycoprotein in normal lung alveolar epithelium and functionality in primary alveolar epithelial cultures. J. Pharmacol. Exp. Ther. 2003, 304, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.M.; Seral, C.; Van Bambeke, F.; Mingeot-Leclercq, M.P.; Tulkens, P.M. Influence of efflux transporters on the accumulation and efflux of four quinolones (ciprofloxacin, levofloxacin, garenoxacin, and moxifloxacin) in J774 macrophages. Antimicrob. Agents Chemother. 2005, 49, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, J.T.; Shimoda, L.A.; Aaronson, P.I.; Ward, J.P. Hypoxic pulmonary vasoconstriction. Physiol. Rev. 2012, 92, 367–520. [Google Scholar] [CrossRef] [PubMed]
- Wenzler, E.; Fraidenburg, D.R.; Scardina, T.; Danziger, L.H. Inhaled Antibiotics for Gram-Negative Respiratory Infections. Clin. Microbiol. Rev. 2016, 29, 581–632. [Google Scholar] [CrossRef]
- Rhodes, N.J.; Cruce, C.E.; O’Donnell, J.N.; Wunderink, R.G.; Hauser, A.R. Resistance Trends and Treatment Options in Gram-Negative Ventilator-Associated Pneumonia. Curr. Infect. Dis. Rep. 2018, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Kidd, J.M.; Kuti, J.L.; Nicolau, D.P. Novel pharmacotherapy for the treatment of hospital-acquired and ventilator-associated pneumonia caused by resistant gram-negative bacteria. Expert Opin. Pharmacother. 2018, 19, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Righi, E.; Russo, A.; Carnelutti, A. New Antibiotics for Pneumonia. Clin. Chest Med. 2018, 39, 853–869. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, K.Z.; Rafailidis, P.I.; Konstantelias, A.A.; Falagas, M.E. Predictors of mortality in patients with infections due to multi-drug resistant Gram negative bacteria: The study, the patient, the bug or the drug? J. Infect. 2013, 66, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Castanheira, M.; Flamm, R.K. Antimicrobial Activity of Ceftazidime-Avibactam against Gram-Negative Bacteria Isolated from Patients Hospitalized with Pneumonia in U.S. Medical Centers, 2011 to 2015. Antimicrob. Agents Chemother. 2017, 61, e02083-16. [Google Scholar]
- Bergen, P.J.; Landersdorfer, C.B.; Lee, H.J.; Li, J.; Nation, R.L. ‘Old’ antibiotics for emerging multidrug-resistant bacteria. Curr. Opin. Infect. Dis. 2012, 25, 626–633. [Google Scholar] [CrossRef]
- Gales, A.C.; Reis, A.O.; Jones, R.N. Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: Review of available interpretative criteria and quality control guidelines. J. Clin. Microbiol. 2001, 39, 183–190. [Google Scholar] [CrossRef]
- Gales, A.C.; Jones, R.N.; Sader, H.S. Contemporary activity of colistin and polymyxin B against a worldwide collection of Gram-negative pathogens: Results from the SENTRY Antimicrobial Surveillance Program (2006–09). J. Antimicrob. Chemother. 2011, 66, 2070–2074. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Hagerman, J.K.; Hancock, K.E.; Klepser, M.E. Aerosolised antibiotics: A critical appraisal of their use. Expert Opin. Drug Deliv. 2006, 3, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Ashley, E.D.; Neuhauser, M.M.; Brown, J.; Gentry, C.; Klepser, M.E.; Marr, A.M.; Schiller, D.; Schwiesow, J.N.; Tice, S.; et al. Consensus summary of aerosolized antimicrobial agents: Application of guideline criteria. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2010, 30, 562–584. [Google Scholar] [CrossRef] [PubMed]
- Bergen, P.J.; Bulman, Z.P.; Saju, S.; Bulitta, J.B.; Landersdorfer, C.; Forrest, A.; Li, J.; Nation, R.L.; Tsuji, B.T. Polymyxin combinations: Pharmacokinetics and pharmacodynamics for rationale use. Pharmacotherapy 2015, 35, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Shortridge, D.; Castanheira, M.; Pfaller, M.A.; Flamm, R.K. Ceftolozane-Tazobactam Activity against Pseudomonas aeruginosa Clinical Isolates from U.S. Hospitals: Report from the PACTS Antimicrobial Surveillance Program, 2012 to 2015. Antimicrob. Agents Chemother. 2017, 61, e00465-17. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.E.; Feola, D.J.; Rapp, R.P. Polymyxin B sulfate and colistin: Old antibiotics for emerging multiresistant gram-negative bacteria. Ann. Pharmacother. 1999, 33, 960–967. [Google Scholar] [CrossRef]
- Bergen, P.J.; Li, J.; Rayner, C.R.; Nation, R.L. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 1953–1958. [Google Scholar] [CrossRef]
- Yapa, S.W.S.; Li, J.; Porter, C.J.; Nation, R.L.; Patel, K.; McIntosh, M.P. Population pharmacokinetics of colistin methanesulfonate in rats: Achieving sustained lung concentrations of colistin for targeting respiratory infections. Antimicrob. Agents Chemother. 2013, 57, 5087–5095. [Google Scholar] [CrossRef] [PubMed]
- Marchand, S.; Gobin, P.; Brillault, J.; Baptista, S.; Adier, C.; Olivier, J.C.; Mimoz, O.; Couet, W. Aerosol therapy with colistin methanesulfonate: A biopharmaceutical issue illustrated in rats. Antimicrob. Agents Chemother. 2010, 54, 3702–3707. [Google Scholar] [CrossRef] [PubMed]
- Imberti, R.; Cusato, M.; Villani, P.; Carnevale, L.; Iotti, G.A.; Langer, M.; Regazzi, M. Steady-state pharmacokinetics and BAL concentration of colistin in critically Ill patients after IV colistin methanesulfonate administration. Chest 2010, 138, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Boisson, M.; Jacobs, M.; Gregoire, N.; Gobin, P.; Marchand, S.; Couet, W.; Mimoz, O. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob. Agents Chemother. 2014, 58, 7331–7339. [Google Scholar] [CrossRef] [PubMed]
- Athanassa, Z.E.; Markantonis, S.L.; Fousteri, M.Z.; Myrianthefs, P.M.; Boutzouka, E.G.; Tsakris, A.; Baltopoulos, G.J. Pharmacokinetics of inhaled colistimethate sodium (CMS) in mechanically ventilated critically ill patients. Intensive Care Med. 2012, 38, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, B.T.; Pogue, J.M.; Zavascki, A.P.; Paul, M.; Daikos, G.L.; Forrest, A.; Giacobbe, D.R.; Viscoli, C.; Giamarellou, H.; Karaiskos, I.; et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 2019, 39, 10–39. [Google Scholar] [PubMed]
- Ari, A.; Atalay, O.T.; Harwood, R.; Sheard, M.M.; Aljamhan, E.A.; Fink, J.B. Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respir. Care 2010, 55, 845–851. [Google Scholar] [PubMed]
- Lu, Q.; Luo, R.; Bodin, L.; Yang, J.; Zahr, N.; Aubry, A.; Golmard, J.L.; Rouby, J.J. Efficacy of high-dose nebulized colistin in ventilator-associated pneumonia caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Anesthesiology 2012, 117, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; De Pascale, G.; Trecarichi, E.M.; De Martino, S.; Bello, G.; Maviglia, R.; Spanu, T.; Antonelli, M. Effect of aerosolized colistin as adjunctive treatment on the outcomes of microbiologically documented ventilator-associated pneumonia caused by colistin-only susceptible gram-negative bacteria. Chest 2013, 144, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Kofteridis, D.P.; Alexopoulou, C.; Valachis, A.; Maraki, S.; Dimopoulou, D.; Georgopoulos, D.; Samonis, G. Aerosolized plus intravenous colistin versus intravenous colistin alone for the treatment of ventilator-associated pneumonia: A matched case-control study. Clin. Infect. Dis. 2010, 51, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Kwa, A.L.; Falagas, M.E.; Michalopoulos, A.; Tam, V.H. Benefits of aerosolized colistin for ventilator-associated pneumonia: Absence of proof versus proof of absence? Clin. Infect. Dis. 2011, 52, 1278–1279, author reply 1279–1280. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, D.H. A closer look at aerosolized colistin. Clin. Infect. Dis. 2011, 52, 1472–1473, author reply 1473–1474. [Google Scholar] [CrossRef]
- Kwa, A.L.; Loh, C.; Low, J.G.; Kurup, A.; Tam, V.H. Nebulized colistin in the treatment of pneumonia due to multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Clin. Infec. Dis. 2005, 41, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Siempos, I.I.; Rafailidis, P.I.; Korbila, I.P.; Ioannidou, E.; Michalopoulos, A. Inhaled colistin as monotherapy for multidrug-resistant gram (-) nosocomial pneumonia: a case series. Respir. Med. 2009, 103, 707–713. [Google Scholar] [CrossRef]
- Choi, H.K.; Kim, Y.K.; Kim, H.Y.; Uh, Y. Inhaled colistin for treatment of pneumonia due to colistin-only-susceptible Acinetobacter baumannii. Yonsei Med. J. 2014, 55, 118–125. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Chen, F.L.; Ou, T.Y.; Jean, S.S.; Lee, W.S. Role of aerosolized colistin methanesulfonate therapy for extensively-drug-resistant Acinetobacter baumannii complex pneumonia and airway colonization. J. Microbiol. Immunol. Infect. 2016, 49, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.H.; Tsai, C.M.; Wu, T.H.; Wu, H.Y.; Chung, M.Y.; Chen, C.C.; Huang, Y.C.; Liu, S.F.; Liao, D.L.; Niu, C.K.; et al. Colistin inhalation monotherapy for ventilator-associated pneumonia of Acinetobacter baumannii in prematurity. Pediatr. Pulmonol. 2014, 49, 381–388. [Google Scholar] [CrossRef]
- Chen, Y.M.; Fang, W.F.; Kao, H.C.; Chen, H.C.; Tsai, Y.C.; Shen, L.S.; Li, C.L.; Chang, H.C.; Huang, K.T.; Lin, M.C.; et al. Influencing factors of successful eradication of multidrug-resistant Acinetobacter baumannii in the respiratory tract with aerosolized colistin. Biomed. J. 2014, 37, 314–320. [Google Scholar] [CrossRef]
- Jean, S.S.; Hsieh, T.C.; Lee, W.S.; Hsueh, P.R.; Hsu, C.W.; Lam, C. Treatment outcomes of patients with non-bacteremic pneumonia caused by extensively drug-resistant Acinetobacter calcoaceticus-Acinetobacter baumannii complex isolates: Is there any benefit of adding tigecycline to aerosolized colistimethate sodium? Medicine (Baltimore) 2018, 97, e12278. [Google Scholar] [CrossRef]
- Korkmaz Ekren, P.; Toreyin, N.; Sayiner, A.; Bacakoglu, F.; Colistin Study, G. The Role of Aerolized Colistin in the Treatment of Hospital-Acquired Pneumonia: Experience of Multicenter From Turkey. Crit. Care Med. 2016, 44, e304. [Google Scholar] [CrossRef] [PubMed]
- Demirdal, T.; Sari, U.S.; Nemli, S.A. Is inhaled colistin beneficial in ventilator associated pneumonia or nosocomial pneumonia caused by Acinetobacter baumannii? Ann. Clin. Microbiol. Antimicrob. 2016, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, S.; Trifi, A.; Daly, F.; Mahjoub, K.; Nasri, R.; Ben Lakhal, S. Efficacy and toxicity of aerosolised colistin in ventilator-associated pneumonia: a prospective, randomised trial. Ann. Intensive Care 2016, 6, 26. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lee, J.H.; Lee, H.K.; Chung, B.C.; Yu, S.J.; Lee, H.Y.; Park, J.H.; Kim, S.; Kim, H.K.; Kiem, S.; et al. Efficacy of nebulized colistin-based therapy without concurrent intravenous colistin for ventilator-associated pneumonia caused by carbapenem-resistant Acinetobacter baumannii. J. Thorac. Dis. 2017, 9, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, A.; Fotakis, D.; Virtzili, S.; Vletsas, C.; Raftopoulou, S.; Mastora, Z.; Falagas, M.E. Aerosolized colistin as adjunctive treatment of ventilator-associated pneumonia due to multidrug-resistant Gram-negative bacteria: a prospective study. Respir. Med. 2008, 102, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Rattanaumpawan, P.; Lorsutthitham, J.; Ungprasert, P.; Angkasekwinai, N.; Thamlikitkul, V. Randomized controlled trial of nebulized colistimethate sodium as adjunctive therapy of ventilator-associated pneumonia caused by Gram-negative bacteria. J. Antimicrob. Chemother. 2010, 65, 2645–2649. [Google Scholar] [CrossRef] [PubMed]
- Kalin, G.; Alp, E.; Coskun, R.; Demiraslan, H.; Gundogan, K.; Doganay, M. Use of high-dose IV and aerosolized colistin for the treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia: do we really need this treatment? J. Infect. Chemother. 2012, 18, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Naesens, R.; Vlieghe, E.; Verbrugghe, W.; Jorens, P.; Ieven, M. A retrospective observational study on the efficacy of colistin by inhalation as compared to parenteral administration for the treatment of nosocomial pneumonia associated with multidrug-resistant Pseudomonas aeruginosa. BMC Infect. Dis. 2011, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Liu, T.C.; Kuo, C.F.; Liu, C.P.; Lee, C.M. Aerosolized colistin for the treatment of multidrug-resistant Acinetobacter baumannii pneumonia: experience in a tertiary care hospital in northern Taiwan. J. Microbiol. Immunol. Infect. 2010, 43, 323–331. [Google Scholar] [CrossRef]
- Doshi, N.M.; Cook, C.H.; Mount, K.L.; Stawicki, S.P.; Frazee, E.N.; Personett, H.A.; Schramm, G.E.; Arnold, H.M.; Murphy, C.V. Adjunctive aerosolized colistin for multi-drug resistant gram-negative pneumonia in the critically ill: a retrospective study. BMC Anesthesiol. 2013, 13, 45. [Google Scholar] [CrossRef]
- Mastoraki, A.; Douka, E.; Kriaras, I.; Stravopodis, G.; Manoli, H.; Geroulanos, S. Pseudomonas aeruginosa susceptible only to colistin in intensive care unit patients. Surg. Infect. (Larchmt) 2008, 9, 153–160. [Google Scholar] [CrossRef]
- Berlana, D.; Llop, J.M.; Fort, E.; Badia, M.B.; Jodar, R. Use of colistin in the treatment of multiple-drug-resistant gram-negative infections. Am. J. Health Syst. Pharm. 2005, 62, 39–47. [Google Scholar] [CrossRef]
- Korbila, I.P.; Michalopoulos, A.; Rafailidis, P.I.; Nikita, D.; Samonis, G.; Falagas, M.E. Inhaled colistin as adjunctive therapy to intravenous colistin for the treatment of microbiologically documented ventilator-associated pneumonia: a comparative cohort study. Clin. Microbiol. Infect. 2010, 16, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, A.; Kasiakou, S.K.; Mastora, Z.; Rellos, K.; Kapaskelis, A.M.; Falagas, M.E. Aerosolized colistin for the treatment of nosocomial pneumonia due to multidrug-resistant Gram-negative bacteria in patients without cystic fibrosis. Crit. Care 2005, 9, R53–R59. [Google Scholar] [CrossRef]
- Ganapathy, H.; Pal, S.K.; Teare, L.; Dziewulski, P. Use of colistin in treating multi-resistant Gram-negative organisms in a specialised burns unit. Burns 2010, 36, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pedrero, M.J.; Sanchez-Casado, M.; Rodriguez-Villar, S. [Nebulized colistin treatment of multi-resistant Acinetobacter baumannii pulmonary infection in critical ill patients]. Med. Intensiva 2011, 35, 226–231. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K.; Kofteridis, D.P.; Roditakis, G.; Samonis, G. Effectiveness and nephrotoxicity of intravenous colistin for treatment of patients with infections due to polymyxin-only-susceptible (POS) gram-negative bacteria. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Rashad, A.; Fouad, A.; Azeem, A. Re-emerging of colistin for treatment of nosocomial pneumonia due to gram negative multi-drug resistant pathogens in critically ill patients. Egypt. J. Chest Dis. Tuberc. 2013, 62, 447–451. [Google Scholar] [CrossRef]
- Bogovic, T.; Budimir, A.; Bosnjak, Z.; Hrabac, P.; Baronica, R.; Tomasevic, B.; Miric, M.; Drvar, Z.; Pavlek, M.; Bratic, V.; et al. Inhalation plus intravenous colistin versus intravenous colistin alone for treatment of ventilator associated pneumonia. Signa. Vitae. 2014, 9, 29–33. [Google Scholar]
- Kuo, S.C.; Lee, Y.T.; Yang, S.P.; Chen, C.P.; Chen, T.L.; Hsieh, S.L.; Siu, L.K.; Fung, C.P. Eradication of multidrug-resistant Acinetobacter baumannii from the respiratory tract with inhaled colistin methanesulfonate: a matched case-control study. Clin. Microbiol. Infect. 2012, 18, 870–876. [Google Scholar] [CrossRef]
- Motaouakkil, S.; Charra, B.; Hachimi, A.; Nejmi, H.; Benslama, A.; Elmdaghri, N.; Belabbes, H.; Benbachir, M. Colistin and rifampicin in the treatment of nosocomial infections from multiresistant Acinetobacter baumannii. J. Infect. 2006, 53, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Nassar, Y.; Saber-Ayad, M.; Shash, R. Combined microbiological and clinical outcomes of short-term inhaled colistin adjunctive therapy in ventilator-associated pneumonia. Egypt. J. Chest Dis. Tuberc. 2018, 67, 376–383. [Google Scholar]
- Park, S.Y.; Park, M.S.; Chung, C.R.; Kim, J.S.; Park, S.J.; Lee, H.B. Clinical Effectiveness and Nephrotoxicity of Aerosolized Colistin Treatment in Multidrug-Resistant Gram-Negative Pneumonia. Korean J. Crit. Care Med. 2016, 31, 208–220. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kwon, H.Y.; Choi, E.H.; Lee, W.Y.; Shim, H.; Bae, K.S. Efficacy and toxicity of high-dose nebulized colistin for critically ill surgical patients with ventilator-associated pneumonia caused by multidrug-resistant Acinetobacter baumannii. J. Crit. Care 2017, 40, 251–256. [Google Scholar] [CrossRef]
- Alikiaie, B.; Mousavi, S.; Yeilaghian, N. Non-inferiority of nebulized colistin compared with conventional systemic antibiotics for the treatment of ventilator-associated pneumonia. Int. J. Pharm. Sci. Res. 2018, 9, 2221–2227. [Google Scholar]
- Elshimy, A.; Shash, R.; Seddik, A. Effectiveness of Adjunctive Inhaled Colistin in Treatment of Ventilator Associated Pneumonia. Egypt. J. Med. Microbiol. 2015, 24, 23–30. [Google Scholar] [CrossRef]
- Valachis, A.; Samonis, G.; Kofteridis, D.P. The role of aerosolized colistin in the treatment of ventilator-associated pneumonia: a systematic review and metaanalysis. Crit. Care Med. 2015, 43, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Koch-Weser, J.; Sidel, V.W.; Federman, E.B.; Kanarek, P.; Finer, D.C.; Eaton, A.E. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann. Int. Med. 1970, 72, 857–868. [Google Scholar] [CrossRef]
- Hartzell, J.D.; Neff, R.; Ake, J.; Howard, R.; Olson, S.; Paolino, K.; Vishnepolsky, M.; Weintrob, A.; Wortmann, G. Nephrotoxicity Associated with Intravenous Colistin (Colistimethate Sodium) Treatment at a Tertiary Care Medical Center. Clin. Infect. Dis. 2009, 48, 1724–1728. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Rafailidis, P.I. Nephrotoxicity of Colistin: New Insight into an Old Antibiotic. Clin. Infect. Dis. 2009, 48, 1729–1731. [Google Scholar] [CrossRef]
- Phe, K.; Lee, Y.; McDaneld, P.M.; Prasad, N.; Yin, T.; Figueroa, D.A.; Musick, W.L.; Cottreau, J.M.; Hu, M.; Tam, V.H. In vitro assessment and multicenter cohort study of comparative nephrotoxicity rates associated with colistimethate versus polymyxin B therapy. Antimicrob. Agents Chemother. 2014, 58, 2740–2746. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Falagas, M.E. The safety of polymyxin antibiotics. Expert Opin. Drug Saf. 2015, 14, 1687–1701. [Google Scholar] [CrossRef]
- Rigatto, M.H.; Oliveira, M.S.; Perdigão-Neto, L.V.; Levin, A.S.; Carrilho, C.M.; Tanita, M.T.; Tuon, F.F.; Cardoso, D.E.; Lopes, N.T.; Falci, D.R.; et al. Multicenter Prospective Cohort Study of Renal Failure in Patients Treated with Colistin versus Polymyxin B. Antimicrob. Agents Chemother. 2016, 60, 2443. [Google Scholar] [CrossRef] [PubMed]
- Zavascki, A.P.; Nation, R.L. Nephrotoxicity of Polymyxins: Is There Any Difference between Colistimethate and Polymyxin B? Antimicrob. Agents Chemother. 2017, 61, e02319-16. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, K.Z.; Falagas, M.E. Colistin versus polymyxin B for the treatment of patients with multidrug-resistant Gram-negative infections: a systematic review and meta-analysis. Int. J. Antimicrob. Agents 2017, 49, 233–238. [Google Scholar] [CrossRef]
- Wenzler, E.; Bunnell, K.L.; Danziger, L.H. Clinical use of the polymyxins: the tale of the fox and the cat. Int. J. Antimicrob. Agents 2018, 51, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kasiakou, S.K. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Couet, W.; Gregoire, N.; Gobin, P.; Saulnier, P.J.; Frasca, D.; Marchand, S.; Mimoz, O. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin. Pharmacol. Ther. 2011, 89, 875–879. [Google Scholar] [CrossRef]
- Sandri, A.M.; Landersdorfer, C.B.; Jacob, J.; Boniatti, M.M.; Dalarosa, M.G.; Falci, D.R.; Behle, T.F.; Bordinhao, R.C.; Wang, J.; Forrest, A.; et al. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin. Infect. Dis. 2013, 57, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Abdelraouf, K.; Braggs, K.H.; Yin, T.; Truong, L.D.; Hu, M.; Tam, V.H. Characterization of polymyxin B-induced nephrotoxicity: implications for dosing regimen design. Antimicrob. Agents Chemother. 2012, 56, 4625–4629. [Google Scholar] [CrossRef]
- Joannidis, M.; Metnitz, B.; Bauer, P.; Schusterschitz, N.; Moreno, R.; Druml, W.; Metnitz, P.G.H. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009, 35, 1692–1702. [Google Scholar] [CrossRef]
- Hoste, E.A.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Voulgaris, G.L.; Samonis, G.; Falagas, M.E. Inhaled colistin monotherapy for respiratory tract infections in adults without cystic fibrosis: a systematic review and meta-analysis. Int. J. Antimicrob. Agents 2018, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, M.I.; Keyt, H.; Reyes, L.F. Aerosolized Antibiotics. Respir. Care 2015, 60, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Pogue, J.M.; Lee, J.; Marchaim, D.; Yee, V.; Zhao, J.J.; Chopra, T.; Lephart, P.; Kaye, K.S. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin. Infect. Dis. 2011, 53, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Phe, K.; Johnson, M.L.; Palmer, H.R.; Tam, V.H. Validation of a model to predict the risk of nephrotoxicity in patients receiving colistin. Antimicrob. Agents Chemother. 2014, 58, 6946–6948. [Google Scholar] [CrossRef]
- Sorli, L.; Luque, S.; Grau, S.; Berenguer, N.; Segura, C.; Montero, M.M.; Alvarez-Lerma, F.; Knobel, H.; Benito, N.; Horcajada, J.P. Trough colistin plasma level is an independent risk factor for nephrotoxicity: a prospective observational cohort study. BMC Infect. Dis. 2013, 13, 380. [Google Scholar] [CrossRef]
- Yapa, S.W.S.; Li, J.; Patel, K.; Wilson, J.W.; Dooley, M.J.; George, J.; Clark, D.; Poole, S.; Williams, E.; Porter, C.J.; et al. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: targeting advantage of inhalational administration. Antimicrob. Agents Chemother. 2014, 58, 2570–2579. [Google Scholar] [CrossRef]
- Marchand, S.; Bouchene, S.; de Monte, M.; Guilleminault, L.; Montharu, J.; Cabrera, M.; Gregoire, N.; Gobin, P.; Diot, P.; Couet, W.; et al. Pharmacokinetics of Colistin Methansulphonate (CMS) and Colistin after CMS Nebulisation in Baboon Monkeys. Pharm. Res. 2015, 32, 3403–3414. [Google Scholar] [CrossRef]
- Landersdorfer, C.B.; Nguyen, T.H.; Lieu, L.T.; Nguyen, G.; Bischof, R.J.; Meeusen, E.N.; Li, J.; Nation, R.L.; McIntosh, M.P. Substantial Targeting Advantage Achieved by Pulmonary Administration of Colistin Methanesulfonate in a Large-Animal Model. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Garonzik, S.M.; Li, J.; Thamlikitkul, V.; Paterson, D.L.; Shoham, S.; Jacob, J.; Silveira, F.P.; Forrest, A.; Nation, R.L. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 2011, 55, 3284–3294. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit. Care 2006, 10, R27. [Google Scholar] [CrossRef] [PubMed]
- Le Brun, P.P.; de Boer, A.H.; Mannes, G.P.; de Fraiture, D.M.; Brimicombe, R.W.; Touw, D.J.; Vinks, A.A.; Frijlink, H.W.; Heijerman, H.G. Dry powder inhalation of antibiotics in cystic fibrosis therapy: Part 2. Inhalation of a novel colistin dry powder formulation: A feasibility study in healthy volunteers and patients. Eur. J. Pharm. 2002, 54, 25–32. [Google Scholar] [CrossRef]
- Alothman, G.A.; Ho, B.; Alsaadi, M.M.; Ho, S.L.; O’Drowsky, L.; Louca, E.; Coates, A.L. Bronchial constriction and inhaled colistin in cystic fibrosis. Chest 2005, 127, 522–529. [Google Scholar] [CrossRef]
- Wilson, F.E. Acute respiratory failure secondary to polymyxin-B inhalation. Chest 1981, 79, 237–239. [Google Scholar] [CrossRef]
- McCoy, K.S. Compounded colistimethate as possible cause of fatal acute respiratory distress syndrome. N Engl. J. Med. 2007, 357, 2310–2311. [Google Scholar] [CrossRef]
- Marschke, G.; Sarauw, A. Polymyxin inhalation therapeutic hazard. Ann. Int. Med. 1971, 74, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Jasani, B.; Kreil, G.; Mackler, B.F.; Stanworth, D.R. Further studies on the structural requirements for polypeptide-mediated histamine release from rat mast cells. Biochem. J. 1979, 181, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.U.; Velkov, T.; Lin, Y.-W.; Yun, B.; Nowell, C.J.; Zhou, F.; Zhou, Q.T.; Chan, K.; Azad, M.A.K.; Li, J. Potential Toxicity of Polymyxins in Human Lung Epithelial Cells. Antimicrob. Agents Chemother. 2017, 61, e02690-16. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-W.; Zhou, Q.T.; Hu, Y.; Onufrak, N.J.; Sun, S.; Wang, J.; Forrest, A.; Chan, H.-K.; Li, J. Pulmonary Pharmacokinetics of Colistin following Administration of Dry Powder Aerosols in Rats. Antimicrob. Agents Chemother. 2017, 61, e00973-17. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-W.; Zhou, Q.; Onufrak, N.J.; Wirth, V.; Chen, K.; Wang, J.; Forrest, A.; Chan, H.-K.; Li, J. Aerosolized Polymyxin B for Treatment of Respiratory Tract Infections: Determination of Pharmacokinetic-Pharmacodynamic Indices for Aerosolized Polymyxin B against Pseudomonas aeruginosa in a Mouse Lung Infection Model. Antimicrob. Agents Chemother. 2017, 61, e00211-17. [Google Scholar] [CrossRef]
- Gontijo, A.V.; Gregoire, N.; Lamarche, I.; Gobin, P.; Couet, W.; Marchand, S. Biopharmaceutical characterization of nebulized antimicrobial agents in rats: 2. Colistin. Antimicrob. Agents Chemother. 2014, 58, 3950–3956. [Google Scholar] [CrossRef]
- Dodd, M.E.; Abbott, J.; Maddison, J.; Moorcroft, A.J.; Webb, A.K. Effect of tonicity of nebulised colistin on chest tightness and pulmonary function in adults with cystic fibrosis. Thorax 1997, 52, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.; Prasad, A.; Collyer, L.; Carr, S.; Lynn, I.B.; Wallis, C. Bronchoconstriction following nebulised colistin in cystic fibrosis. Arch. Dis. Child. 2001, 84, 432–433. [Google Scholar] [CrossRef] [PubMed]
- Westerman, E.M.; Le Brun, P.P.; Touw, D.J.; Frijlink, H.W.; Heijerman, H.G. Effect of nebulized colistin sulphate and colistin sulphomethate on lung function in patients with cystic fibrosis: A pilot study. J. Cyst. Fibros. 2004, 3, 23–28. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Tansarli, G.S.; Rafailidis, P.I.; Falagas, M.E. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum beta-lactamases: a systematic review and meta-analysis. J. Antimicrob. Chemother. 2012, 67, 2793–2803. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.H.; Muller, P.R.; Levin, A.S. Salvage treatment of pneumonia and initial treatment of tracheobronchitis caused by multidrug-resistant Gram-negative bacilli with inhaled polymyxin B. Diagn. Microbiol. Infect. Dis. 2007, 58, 235–240. [Google Scholar] [CrossRef]
- Konkyana, S.K.; Akkenapalli, A.K. Treatment of multidrug resistant gram negative bacilli with inhaled polymyxin-B. Asian Pac. J. Health Sci. 2016, 3, 135–141. [Google Scholar] [CrossRef]
- Berlana, D.; Llop, J.M.; Manresa, F.; Jodar, R. Outpatient treatment of Pseudomonas aeruginosa bronchial colonization with long-term inhaled colistin, tobramycin, or both in adults without cystic fibrosis. Pharmacotherapy 2011, 31, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Milne, R.W.; Nation, R.L.; Turnidge, J.D.; Coulthard, K. Stability of colistin and colistin methanesulfonate in aqueous media and plasma as determined by high-performance liquid chromatography. Antimicrob. Agents Chemother. 2003, 47, 1364–1370. [Google Scholar] [CrossRef]
- Information for Healthcare Professionals: Colistimethate (marketed as Coly-Mycin M and generic products). Available online: http://wayback.archive-it.org/7993/20170112032538/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders (accessed on 21 December 2018).
- Choi, C.-u.; Seo, M.-R.; Lim, Y.-H.; Pai, H. Mutant Prevention Concentration of Polymyxin B for the Clinical Isolates of Pseudomonas aeruginosa. Infect. Chemother. 2006, 38, 344–348. [Google Scholar]
- Choi, M.-J.; Ko, K.S. Mutant prevention concentrations of colistin for Acinetobacter baumannii, Pseudomonas aeruginosa and Klebsiella pneumoniae clinical isolates. J. Antimicrob. Chemother. 2014, 69, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, R.; Liang, B.; Bai, N.; Liu, Y.; Wang, R. In Vitro Antimicrobial Activity and Mutant Prevention Concentration of Colistin against Acinetobacter baumannii. Antimicrob. Agents Chemother. 2010, 54, 3998–3999. [Google Scholar] [CrossRef] [PubMed]
- Nordqvist, H.; Nilsson, L.E.; Claesson, C. Mutant prevention concentration of colistin alone and in combination with rifampicin for multidrug-resistant Acinetobacter baumannii. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Feeley, T.W.; Du Moulin, G.C.; Hedley-Whyte, J.; Bushnell, L.S.; Gilbert, J.P.; Feingold, D.S. Aerosol polymyxin and pneumonia in seriously ill patients. N. Engl. J. Med. 1975, 293, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.B.; Phillips, D.; Barker, M.J.; Pieczarka, R.; Sands, M.; Teres, D. Outbreak of nosocomial Flavobacterium meningosepticum respiratory infections associated with use of aerosolized polymyxin B. Am. J. Infect. Control 1989, 17, 121–125. [Google Scholar] [CrossRef]
- Falagas, M.E.; Siempos, I.I.; Bliziotis, I.A.; Michalopoulos, A. Administration of antibiotics via the respiratory tract for the prevention of ICU-acquired pneumonia: A meta-analysis of comparative trials. Crit. Care 2006, 10, R123. [Google Scholar] [CrossRef]
- Rouby, J.J.; Poete, P.; Martin de Lassale, E.; Nicolas, M.H.; Bodin, L.; Jarlier, V.; Korinek, A.M.; Viars, P. Prevention of gram negative nosocomial bronchopneumonia by intratracheal colistin in critically ill patients. Histologic and bacteriologic study. Intensive Care Med. 1994, 20, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, S.; Teres, D.; Bushnell, L.S.; Hedley-Whyte, J.; Feingold, D.S. Prevention of gram-negative bacillary pneumonia using aerosol polymyxin as prophylaxis. I. Effect on the colonization pattern of the upper respiratory tract of seriously ill patients. J. Clin. Investig. 1973, 52, 2935–2940. [Google Scholar] [CrossRef] [PubMed]
- Klick, J.M.; du Moulin, G.C.; Hedley-Whyte, J.; Teres, D.; Bushnell, L.S.; Feingold, D.S. Prevention of gram-negative bacillary pneumonia using polymyxin aerosol as prophylaxis. II. Effect on the incidence of pneumonia in seriously ill patients. J. Clin. Investig. 1975, 55, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Karvouniaris, M.; Makris, D.; Zygoulis, P.; Triantaris, A.; Xitsas, S.; Mantzarlis, K.; Petinaki, E.; Zakynthinos, E. Nebulised colistin for ventilator-associated pneumonia prevention. Eur. Respir. J. 2015, 46, 1732–1739. [Google Scholar] [CrossRef]
- Craven, D.E.; Lichtenberg, D.A.; Goularte, T.A.; Make, B.J.; McCabe, W.R. Contaminated medication nebulizers in mechanical ventilator circuits. Source of bacterial aerosols. Am. J. Med. 1984, 77, 834–838. [Google Scholar] [CrossRef]
- Prober, C.G.; Walson, P.D.; Jones, J. Technical report: precautions regarding the use of aerosolized antibiotics. Committee on Infectious Diseases and Committee on Drugs. Pediatrics 2000, 106, E89. [Google Scholar] [CrossRef]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratala, J.; et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef] [PubMed]
- Muscedere, J.G.; Day, A.; Heyland, D.K. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin. Infect. Dis. 2010, 51 (Suppl. 1), S120–S125. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Hamilton, C.W.; Ernst, F.R. Economic impact of ventilator-associated pneumonia in a large matched cohort. Infect. Control Hosp. Epidemiol. 2012, 33, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Sopena, N.; Sabria, M. Multicenter study of hospital-acquired pneumonia in non-ICU patients. Chest 2005, 127, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Esperatti, M.; Ferrer, M.; Giunta, V.; Ranzani, O.T.; Saucedo, L.M.; Li Bassi, G.; Blasi, F.; Rello, J.; Niederman, M.S.; Torres, A. Validation of predictors of adverse outcomes in hospital-acquired pneumonia in the ICU. Crit. Care Med. 2013, 41, 2151–2161. [Google Scholar] [CrossRef]
- Jones, R.N. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin. Infect. Dis. 2010, 51 (Suppl. 1), S81–S87. [Google Scholar] [CrossRef]
- Sievert, D.M.; Ricks, P.; Edwards, J.R.; Schneider, A.; Patel, J.; Srinivasan, A.; Kallen, A.; Limbago, B.; Fridkin, S. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect. Control Hosp. Epidemiol. 2013, 34, 1–14. [Google Scholar] [CrossRef] [PubMed]
| Ref | Study Design | Patient Population | Comparator Group(s) | Inhaled Group(s) | Duration (Days) A | Clinical Outcome(s) | Toxicity | Comments |
|---|---|---|---|---|---|---|---|---|
| [39] | Prospective, observational | MDR VAP caused by A. baumannii or P. aeruginosa (n = 165) | MDR VAP: Inhaled colistin + IV aminoglycoside (n = 15)Susceptible VAP: IV β-lactam + IV aminoglycoside or fluoroquinolone (n = 122) | 5 MU q8h (n = 28) | 12 B | No significant differences in clinical outcomes between patients with MDR VAP treated with inhaled colistin with or without IV aminoglycoside and patients with susceptible VAP | Serum creatinine remained stable during treatment in both groups | All patients in MDR VAP group received inappropriate empiric therapy |
| [44] | Retrospective | MDR NP caused by COS A. baumannii or P. aeruginosa (n = 21) | None | 1 MU BID (n = 19) 1 MU TID (n = 1) 1 MU QID (n = 1) | 14 (2–36) | Clinical cure or improvement: 57.1% Microbial eradication:Documented: 52.4% Presumed: 33.3% All-cause mortality: 47.6% Attributable mortality: 14.3% | Bronchoconstriction: (n = 1; 4.8%) | Seven patients cured of pneumonia subsequently died of underlying and unrelated conditions Most patients received concomitant antibiotics for additional extrapulmonary sites of infection |
| [45] | Retrospective | NP/VAP (n = 5) | None | 1 MU q8h (n = 4) 500,000 U q6h (n = 1) | 7.2 | Clinical success: 80% Mortality: 20% | NR | All patients received additional systemic antibiotics that were resistant |
| [46] | Retrospective | COS A. baumannii pneumonia (n = 12) | None | 75 mg BID ± systemic antibiotics | 17 (5–31) | Clinical cure or improvement: 83.3% Microbial eradication: 50% All-cause in-hospital mortality: 33.3% | Hypersensitivity: (n = 1; 8.3%) | Most patients received concomitant systemic antibiotics and all patients received other broad-spectrum antibiotics prior to initiation of colistin Three isolates developed colistin resistance |
| [47] | Retrospective | XDR A. baumannii pneumonia (n = 38) | Inhaled colistin + IV tigecycline (n = 29) | 2 MU BID (n = 9) | 13.5 ± 6.5 C | No significant difference in clinical outcomes, microbiological eradication, or 30-day mortality | Bronchospasm: (n = 2; 1.7%) D | Isolates in the inhaled colistin only group were significantly more likely to be tigecycline-resistant |
| [48] | Retrospective | A. baumannii VAP (n = 31 preterm infants) | Systemic antibiotics (n = 23) | 80 mg q12h (n = 8) | 9.1 (4–22) | All patients in both groups were cured and subsequently discharged | NR | Four pre-term infants in the inhaled colistin received active systemic antibiotics for 12–21 days without improvement prior to inhaled colistin monotherapy |
| [49] | Retrospective | MDR A. baumannii pneumonia or colonization (n = 135) | Tigecycline (n = 40) Ampicillin/sulbactam (n = 12) No therapy (n = 2) | 2 MU BID (n = 54) 2 MU BID + systemic antibiotics (n = 27) | NR | Significantly higher eradication rate in patients receiving inhaled colistin monotherapy compared to IV therapy only (61.1% vs. 29.6%; p = 0.001) No significant difference in 28-day or in-hospital mortality | NR | Patients receiving inhaled colistin were significantly more likely to have colonization 10/37 patients who failed to achieve microbiological eradication at day 14 were eradicated at day 28 with prolonged use of inhaled colistin |
| [50] | Retrospective, case-controlled | XDR A. calcoaceticus-A baumannii complex pneumonia (n = 212) | Inhaled colistin + tigecycline (n = 106) | 2 MU TID (n = 106) | 12.2 ± 6 E | No difference in 30-day mortality | Bronchospasm: (n = 4; 1.9%) | Majority of patients received systemic antibiotics prior to enrollment |
| Ref | Study Design | Patient Population | Comparator Group(s) | Inhaled Group(s) | Duration (days) A | Clinical Outcome(s) | Inhaled Toxicity | Comments |
|---|---|---|---|---|---|---|---|---|
| [40] | Retrospective, matched | COS VAP (n = 208) | IV colistin (n = 104) | 1 MU q8h + IV colistin (n = 104) | 7 (5–14) C | Improved clinical cure in inhaled-antibiotic group; no significant difference in microbiological cure | NR | Concomitant systemic antibiotics not described |
| [41] | Retrospective, matched | MDR VAP (n = 86) | IV colistin (n = 43) | 1 MU q12h + IV colistin (n = 43) | 13 (5–56) F | Improved clinical cure in group administered inhaled antibiotic; no difference in microbiological eradication | NR | No detail provided on concomitant systemic antibiotics |
| [51] | Retrospective | MDR A. baumannii or P. aeruginosa HAP (n = 279) | IV colistin ± systemic antibiotics (n = 210) | Inhaled and IV colistin ± systemic antibiotics (n = 69) E | NR | Significantly higher clinical response and eradication rates with inhaled therapy No difference in mortality with inhaled therapy | No significant difference in rate of nephrotoxicity | No data on dose or duration of inhaled colistin therapy Most patients (82.8%) received concomitant systemic antibiotics |
| [52] | Retrospective, case-controlled | A. baumannii NP or VAP (n = 123) | IV colistin + systemic antibiotics (n = 80) | 75 mg q12h + IV colistin + systemic antibiotics (n = 43) | 11.2 ± 6 C | No differences in clinical success, microbiological eradication, or mortality | No significant difference in rate of nephrotoxicity | No assessment of disease severity |
| [53] | Prospective, randomized | VAP (n = 149) | IV colistin + imipenem (n = 76) | 4 MU TID + imipenem (n = 73) | ≥14 B | No significant difference in clinical cure; decreased time to microbiological eradication with inhaled therapy | Bronchospasm: (n = 2; 2.7%) Neurotoxicity: (n = 9; 12.3%) I | Clinical cure rates in a subset of patients receiving colistin monotherapy were 84% and 58% (p = 0.20) for inhaled and IV monotherapy, respectively |
| [54] | Retrospective | CRAB VAP (n = 219) | IV colistin + systemic antibiotics (n = 57) IV colistin (n = 36) | 75 mg q12h − 150 mg q8h + systemic antibiotics (n = 104) 75 mg q12h to 150 mg q8h (n = 22) | 17 (10–25) | Significantly lower rates of clinical failure, ICU mortality, and AKI with inhaled colistin | NR | Patients receiving inhaled colistin were significantly more likely to receive active concomitant antibiotics and less likely to have septic shock |
| [55] | Prospective, observational | MDR VAP (n = 60) | None | 1 MU TID + systemic antibiotics B | 16.4 ± 10.9 | Clinical improvement: 83.3% All-cause mortality: 25% | NR | No comparator group; microbiological data not provided |
| [56] | Randomized, placebo controlled | VAP (n = 100) | Placebo inhalation + systemic antibiotics (n = 49) | 75 mg q12h + systemic antibiotics (n = 51) | 9.5 ± 4.6 | No difference in clinical outcome; improved microbiological outcome with inhaled colistin | Bronchospasm: (n = 4; 7.8%) | Clinical and microbiological outcomes evaluated at 28 days |
| [57] | Retrospective | MDR A. baumannii VAP (n = 45) | IV colistin (n = 15) | 75 mg BID + IV colistin (n = 29) | 14 | No difference in clinical or microbiological outcome at day 5 or end of therapy | NR | 87% of patients with severe sepsis/septic shock at baseline |
| [58] | Retrospective | MDR P. aeruginosa NP (n = 20) | IV colistin + β-lactam (n = 5) | 2 MU TID + β-lactam (n = 6) 2 MU TID + IV colistin + β-lactam (n = 9) | IV + inhaled: 19.3 (3–46) Inhaled alone: 27.2 (6–96) IV alone: 21.0 (9–28) | 78% favorable response with IV + inhaled vs. 100% for inhaled alone and 40% for IV alone; no patients achieved microbiological eradication | NR | 56% of patients on IV and inhaled colistin also had extrapulmonary infection |
| [59] | Retrospective | MDR A. baumannii VAP (n = 45) | None | Mean daily dose of 4.29 MU + IV colistin + systemic antibiotics | 10.29 | Favorable clinical outcome: 57.8% Microbiological eradication: 37.8% | NR | Only 60% had follow-up cultures available |
| [60] | Retrospective, multicenter | MDR NP (n = 95) | IV colistin + systemic antibiotics (n = 51) | 75 or 150 mg q12h + systemic antibiotics (n = 44) | 11 (7–16.25) | No significant difference in clinical cure, microbiological eradication, or mortality | NR | Clinical cure rate higher in group administered inhaled antibiotic when only patients with high quality respiratory cultures were evaluated |
| [61] | Prospective, observational | MDR P. aeruginosa VAP (n = 8) | None | 500,000 IU q8h + IV colistin | 15.9 D | Clinical cure: 70% E | NR | Six patients had concomitant co-infections |
| [62] | Retrospective | Pneumonia (n = 49) | None | 500,000 IU q6h + systemic antibiotics | 12 ± 8 | Microbiological eradication: 93% | NR | Parenteral formulation used for inhalation Concomitant antibiotics not described |
| [63] | Retrospective | VAP (n = 121) | IV colistin + systemic antibiotics (n = 43) | 2.1 MU per day + IV colistin + systemic antibiotics (n = 78) | 16.9 ± 9.8 G | Significantly improved clinical cure in group administered inhaled antibiotic; no difference in mortality | NR | Significantly more patients in group administered IV antibiotic only with COS organisms; use of inhaled colistin was independent predictor of clinical cure |
| [64] | Retrospective | MDR NP (n = 8) | None | 0.5 MU q6h- 2 MU q8h + IV colistin and/or systemicantibiotics | 8.9 (3–19) | Clinical improvement or cure: 87.5% Bacterial eradication: 80% | NR | No uniform inhaled dosing strategy or duration |
| [65] | Retrospective | MDR pneumonia (n = 29) | IV colistin ± systemic antibiotics (n = 6) Inhaled colistin ± systemic antibiotics (n = 6) | Inhaled and IV colistin ± systemic antibiotics (n = 17) H | NR | Survival rates were 41.1% in patients receiving both inhaled and IV colistin compared to 100% with inhaled colistin only and 66.7% with IV colistin only | NR | Patients in the IV + inhaled colistin group may have had additional sites of infection Most patients were reported to have received additional systemic antibiotics |
| [66] | Retrospective | A. baumannii NP or tracheobronchitis (n = 31) | IV colistin ± systemic antibiotics (n = 14) Inhaled colistin ± systemic antibiotics (n = 7) | 500,000 IU q6h-1 MU q8-12h +IV colistin ± systemic antibiotics (n = 10) | NR | Microbiological eradication: Inhaled only: 100% IV only: 42.9% Inhaled + IV: 66.7% | NR | 16/31 patients were diagnosed with tracheobronchitis Additional systemic antibiotics not fully described |
| [67] | Prospective | COS VAP (n = 9) | None | 1 MU q12h + IV colistin ± systemic antibiotics | 13 ± 6.5 | Clinical cure or improvement: 77.8% All-cause in-hospital mortality: 22.2% | NR | No specific information regarding additional systemic antibiotics administered to patients with VAP |
| [68] | Prospective | MDR NP (n = 40) | IV colistin (n = 12) | 2 MU q12h + IV colistin (n = 28) | 12–15 | Clinical failure and mortality significantly higher in the IV colistin group | NR | Concomitant systemic antibiotics not addressed |
| [69] | Retrospective | MDR VAP (n = 31) | IV colistin ± systemic antibiotics (n = 23) | 2 MU BID + IV colistin (n = 8) | 10.3 ± 5.72 C | Microbiological eradication significantly higher in the inhaled group No difference in ICU mortality | Bronchoconstriction: (n = 1; 12.5%) | Some patients also had extrapulmonary sites of infection Concomitant systemic antibiotics not described |
| [70] | Retrospective, matched case-control | A. baumannii NP or colonization (n = 78) | Systemic antibiotics (n = 39) | 2 MU BID + systemic antibiotics (n = 32) 2 MU BID (n = 7) | 10.9 ± 3.6 | Significantly higher 14-day microbiological eradication with inhaled colistin No difference in mortality | No significant differences in incidence of hemodynamic instability, need for intubation, or nephrotoxicity | All isolates were only susceptible to colistin, tigecycline, or sulbactam |
| [71] | Observational cohort | COS A. baumannii VAP (n = 16) | None | 1 MU TID + IV rifampicin | 15 | All patients had clinical and microbiological success | NR | Three patients had concomitant bacteremia Therapy was initiated based on culture results and lack of response to empiric regimen |
| [72] | Prospective, randomized | VAP (n = 102) | Systemic antibiotics (n = 50) | 1 MU q8h + systemic antibiotics (n = 52) | 5 B | Significantly improved rate of favorable outcomes, 30-day mortality, and clearance of MDR pathogens in inhaled colistin group | NR | Inhaled colistin given only 5 days |
| [73] | Retrospective | MDR VAP (n = 25) | None | 75 mg QID − 150 mg BID ± systemic antibiotics B | 11.7 ± 7.1 | In-hospital mortality: 40% Microbiological eradication: 84.6% (11/13) | Patients receiving >2 concomitant nephrotoxins significantly more likely to develop AKI | Concomitant systemic antibiotics not fully described |
| [74] | Retrospective | MDR A. baumannii VAP (n = 95) | IV colistin ± systemic antibiotics (n = 44) | 4.5 MU q8h ± systemic antibiotics (n = 51) | 12.6 ± 6.1 | No significant differences between in clinical cure, microbiological eradication, or infectious mortality | Nephrotoxicity significantly lower with inhaled colistin: (15.7% vs. 60.5%; p < 0.00001) | Patients receiving inhaled colistin were more likely to be older and have higher APACHE II scores |
| [75] | Prospective, case-controlled | COS VAP (n = 40) | Systemic antibiotics (n = 20) | 1 MU q12h + systemic antibiotics (n = 20) | 5 B | No significant differences in clinical cure or mortality Microbiological eradication significantly higher in patients receiving inhaled colistin | NR | Inhaled colistin only administered for 5 days |
| [76] | Prospective, randomized, controlled | VAP (n = 50) | Systemic antibiotics (n = 25) | 2 MU q8h + systemic antibiotics (n = 25) | 5 | Significantly higher microbiological eradication rate at day 5 with inhaled therapy | NR | Systemic antibiotics not described Causative organisms were unevenly distributed among the treatment groups |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biagi, M.; Butler, D.; Tan, X.; Qasmieh, S.; Wenzler, E. A Breath of Fresh Air in the Fog of Antimicrobial Resistance: Inhaled Polymyxins for Gram-Negative Pneumonia. Antibiotics 2019, 8, 27. https://doi.org/10.3390/antibiotics8010027
Biagi M, Butler D, Tan X, Qasmieh S, Wenzler E. A Breath of Fresh Air in the Fog of Antimicrobial Resistance: Inhaled Polymyxins for Gram-Negative Pneumonia. Antibiotics. 2019; 8(1):27. https://doi.org/10.3390/antibiotics8010027
Chicago/Turabian StyleBiagi, Mark, David Butler, Xing Tan, Samah Qasmieh, and Eric Wenzler. 2019. "A Breath of Fresh Air in the Fog of Antimicrobial Resistance: Inhaled Polymyxins for Gram-Negative Pneumonia" Antibiotics 8, no. 1: 27. https://doi.org/10.3390/antibiotics8010027
APA StyleBiagi, M., Butler, D., Tan, X., Qasmieh, S., & Wenzler, E. (2019). A Breath of Fresh Air in the Fog of Antimicrobial Resistance: Inhaled Polymyxins for Gram-Negative Pneumonia. Antibiotics, 8(1), 27. https://doi.org/10.3390/antibiotics8010027





