Polymyxin Acute Kidney Injury: Dosing and Other Strategies to Reduce Toxicity
Abstract
1. Introduction
2. Mechanisms of Nephrotoxicity
3. Risk Factors for Nephrotoxicity
4. Dosing Strategies to Reduce Nephrotoxicity
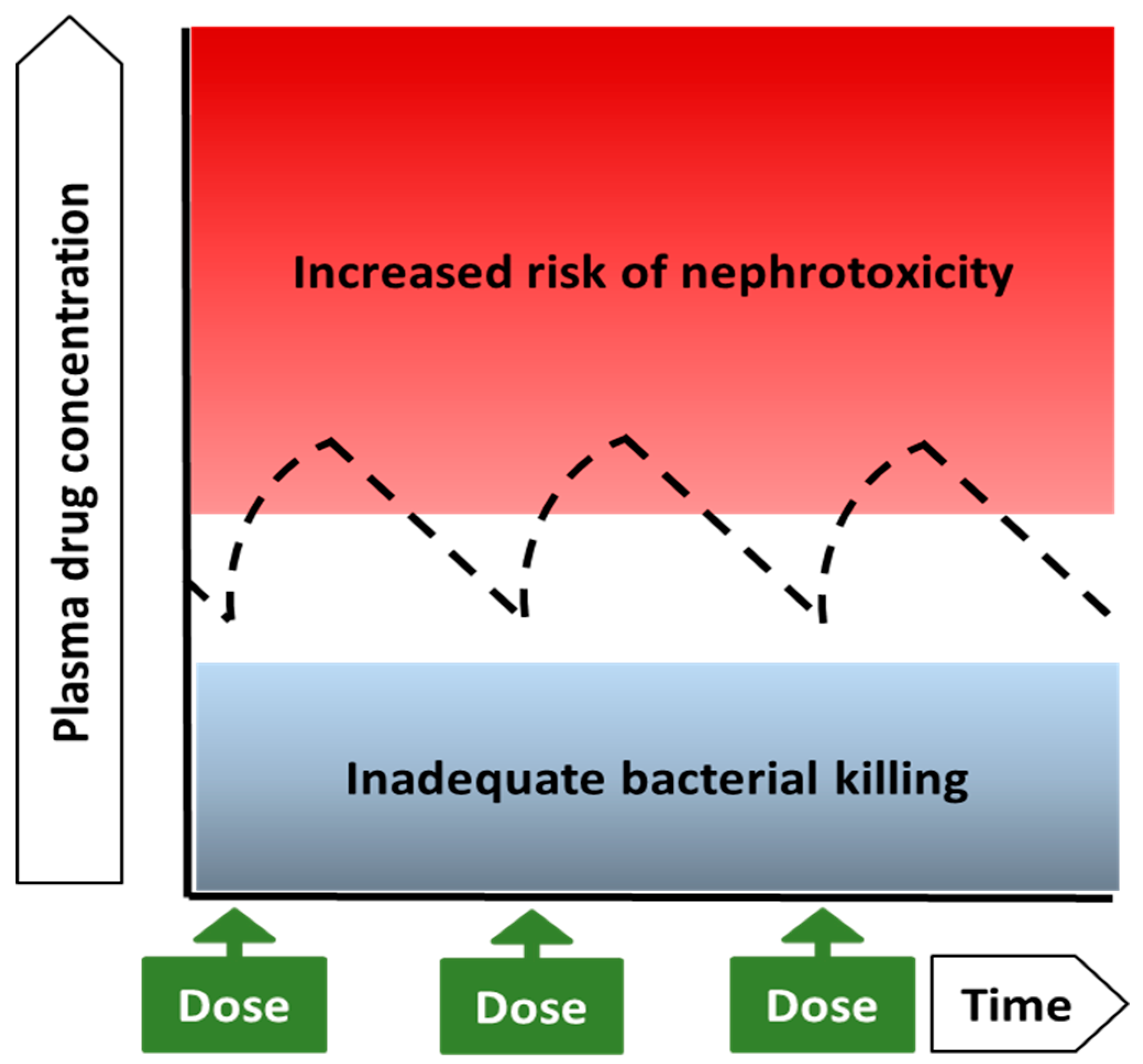
4.1. Colistin and Polymyxin B: Drugs with Very Narrow Therapeutic Windows
4.2. Implications of the Narrow Therapeutic Range for Dosing of Polymyxins
4.2.1. Choice of Polymyxin
4.2.2. To Load or Not to Load?
4.2.3. Selection of the Daily Maintenance Dose at Initiation of Therapy and Ongoing Optimization
Colistin
Polymyxin B
4.2.4. Dosage Interval and Infusion Duration
5. Other Strategies to Reduce Nephrotoxicity
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, J.; Nation, R.L.; Turnidge, J.D.; Milne, R.W.; Coulthard, K.; Rayner, C.R.; Paterson, D.L. Colistin: The re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 2006, 6, 589–601. [Google Scholar] [CrossRef]
- Zavascki, A.P.; Goldani, L.Z.; Li, J.; Nation, R.L. Polymyxin B for the treatment of multidrug-resistant pathogens: A critical review. J. Antimicrob. Chemother. 2007, 60, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kasiakou, S.K. Toxicity of polymyxins: A systematic review of the evidence from old and recent studies. Crit. Care 2006, 10, R27. [Google Scholar] [CrossRef] [PubMed]
- Oliota, A.F.; Penteado, S.T.; Tonin, F.S.; Fernandez-Llimos, F.; Sanches, A.C. Nephrotoxicity prevalence in patients treated with polymyxins: A systematic review with meta-analysis of observational studies. Diagn. Microbiol. Infect. Dis. 2018. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Falagas, M.E. Colistin versus polymyxin B for the treatment of patients with multidrug-resistant Gram-negative infections: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2017, 49, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Milne, R.W.; Nation, R.L.; Turnidge, J.D.; Smeaton, T.C.; Coulthard, K. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob. Agents Chemother. 2003, 47, 1766–1770. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamaguchi, H.; Ogura, J.; Kobayashi, M.; Yamada, T.; Iseki, K. Megalin contributes to kidney accumulation and nephrotoxicity of colistin. Antimicrob. Agents Chemother. 2013, 57, 6319–6324. [Google Scholar] [CrossRef]
- Azad, M.A.; Akter, J.; Rogers, K.L.; Nation, R.L.; Velkov, T.; Li, J. Major pathways of polymyxin-induced apoptosis in rat kidney proximal tubular cells. Antimicrob. Agents Chemother. 2015, 59, 2136–2143. [Google Scholar] [CrossRef]
- Yun, B.; Zhang, T.; Azad, M.A.K.; Wang, J.; Nowell, C.J.; Kalitsis, P.; Velkov, T.; Hudson, D.F.; Li, J. Polymyxin B causes DNA damage in HK-2 cells and mice. Arch. Toxicol. 2018, 92, 2259–2271. [Google Scholar] [CrossRef]
- Dai, C.; Li, J.; Tang, S.; Li, J.; Xiao, X. Colistin-induced nephrotoxicity in mice involves the mitochondrial, death receptor, and endoplasmic reticulum pathways. Antimicrob. Agents Chemother. 2014, 58, 4075–4085. [Google Scholar] [CrossRef]
- Azad, M.A.; Finnin, B.A.; Poudyal, A.; Davis, K.; Li, J.; Hill, P.A.; Nation, R.L.; Velkov, T.; Li, J. Polymyxin B Induces Apoptosis in Kidney Proximal Tubular Cells. Antimicrob. Agents Chemother. 2013, 57, 4329–4335. [Google Scholar] [CrossRef]
- Baradaran, S.; Black, D.J.; Keyloun, K.R.; Hansen, R.N.; Gillard, P.J.; Devine, B. The Impact of Acute Kidney Injury on the Risk of Mortality and Health Care Utilization Among Patients Treated with Polymyxins for Severe Gram-Negative Infections. Open Forum Infect. Dis. 2018, 5, ofy191. [Google Scholar] [CrossRef]
- Miano, T.A.; Lautenbach, E.; Wilson, F.P.; Guo, W.; Borovskiy, Y.; Hennessy, S. Attributable Risk and Time Course of Colistin-Associated Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. Cjasn 2018, 13, 542–550. [Google Scholar] [CrossRef]
- Rigatto, M.H.; Behle, T.F.; Falci, D.R.; Freitas, T.; Lopes, N.T.; Nunes, M.; Costa, L.W.; Zavascki, A.P. Risk factors for acute kidney injury (AKI) in patients treated with polymyxin B and influence of AKI on mortality: A multicentre prospective cohort study. J. Antimicrob. Chemother. 2015, 70, 1552–1557. [Google Scholar] [CrossRef]
- Gomes, E.C.; Falci, D.R.; Bergo, P.; Zavascki, A.P.; Rigatto, M.H. Impact of polymyxin-B-associated acute kidney injury in 1-year mortality and renal function recovery. Int. J. Antimicrob. Agents 2018, 52, 86–89. [Google Scholar] [CrossRef]
- Meraz-Munoz, A.; Gomez-Ruiz, I.; Correa-Rotter, R.; Ramirez-Sandoval, J.C. Chronic kidney disease after acute kidney injury associated with intravenous colistin use in survivors of severe infections: A comparative cohort study. J. Crit. Care 2018, 44, 244–248. [Google Scholar] [CrossRef]
- Akajagbor, D.S.; Wilson, S.L.; Shere-Wolfe, K.D.; Dakum, P.; Charurat, M.E.; Gilliam, B.L. Higher incidence of acute kidney injury with intravenous colistimethate sodium compared with polymyxin B in critically ill patients at a tertiary care medical center. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 57, 1300–1303. [Google Scholar] [CrossRef]
- Crass, R.L.; Rutter, W.C.; Burgess, D.R.; Martin, C.A.; Burgess, D.S. Nephrotoxicity in Patients with or without Cystic Fibrosis Treated with Polymyxin B Compared to Colistin. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Gauthier, T.P.; Wolowich, W.R.; Reddy, A.; Cano, E.; Abbo, L.; Smith, L.B. Incidence and predictors of nephrotoxicity associated with intravenous colistin in overweight and obese patients. Antimicrob. Agents Chemother. 2012, 56, 2392–2396. [Google Scholar] [CrossRef]
- Kwon, J.A.; Lee, J.E.; Huh, W.; Peck, K.R.; Kim, Y.G.; Kim, D.J.; Oh, H.Y. Predictors of acute kidney injury associated with intravenous colistin treatment. Int. J. Antimicrob. Agents 2010, 35, 473–477. [Google Scholar] [CrossRef]
- Min, K.L.; Son, E.S.; Kim, J.S.; Kim, S.H.; Jung, S.M.; Chang, M.J. Risk factors of colistin safety according to administration routes: Intravenous and aerosolized colistin. PLoS ONE 2018, 13, e0207588. [Google Scholar] [CrossRef]
- Balkan, I.I.; Dogan, M.; Durdu, B.; Batirel, A.; Hakyemez, I.N.; Cetin, B.; Karabay, O.; Gonen, I.; Ozkan, A.S.; Uzun, S.; et al. Colistin nephrotoxicity increases with age. Scand. J. Infect. Dis. 2014, 46, 678–685. [Google Scholar] [CrossRef]
- John, J.F.; Falci, D.R.; Rigatto, M.H.; Oliveira, R.D.; Kremer, T.G.; Zavascki, A.P. Severe Infusion-Related Adverse Events and Renal Failure in Patients Receiving High-Dose Intravenous Polymyxin B. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Kwon, K.H.; Oh, J.Y.; Yoon, Y.S.; Jeong, Y.J.; Kim, K.S.; Shin, S.J.; Chung, J.W.; Huh, H.J.; Chae, S.L.; Park, S.Y. Colistin treatment in carbapenem-resistant Acinetobacter baumannii pneumonia patients: Incidence of nephrotoxicity and outcomes. Int. J. Antimicrob. Agents 2015, 45, 605–609. [Google Scholar] [CrossRef]
- Omrani, A.S.; Alfahad, W.A.; Shoukri, M.M.; Baadani, A.M.; Aldalbahi, S.; Almitwazi, A.A.; Albarrak, A.M. High dose intravenous colistin methanesulfonate therapy is associated with high rates of nephrotoxicity; a prospective cohort study from Saudi Arabia. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 3. [Google Scholar] [CrossRef]
- Rocco, M.; Montini, L.; Alessandri, E.; Venditti, M.; Laderchi, A.; De Pascale, G.; Raponi, G.; Vitale, M.; Pietropaoli, P.; Antonelli, M. Risk factors for acute kidney injury in critically ill patients receiving high intravenous doses of colistin methanesulfonate and/or other nephrotoxic antibiotics: A retrospective cohort study. Crit. Care 2013, 17, R174. [Google Scholar] [CrossRef]
- Sorli, L.; Luque, S.; Grau, S.; Berenguer, N.; Segura, C.; Montero, M.M.; Alvarez-Lerma, F.; Knobel, H.; Benito, N.; Horcajada, J.P. Trough colistin plasma level is an independent risk factor for nephrotoxicity: A prospective observational cohort study. Bmc Infect. Dis. 2013, 13, 380. [Google Scholar] [CrossRef]
- Katip, W.; Uitrakul, S.; Oberdorfer, P. Clinical outcomes and nephrotoxicity of colistin loading dose for treatment of extensively drug-resistant Acinetobacter baumannii in cancer patients. Infect. Drug Resist. 2017, 10, 293–298. [Google Scholar] [CrossRef]
- Phe, K.; Lee, Y.; McDaneld, P.M.; Prasad, N.; Yin, T.; Figueroa, D.A.; Musick, W.L.; Cottreau, J.M.; Hu, M.; Tam, V.H. In vitro assessment and multicenter cohort study of comparative nephrotoxicity rates associated with colistimethate versus polymyxin B therapy. Antimicrob. Agents Chemother. 2014, 58, 2740–2746. [Google Scholar] [CrossRef]
- Temocin, F.; Erdinc, S.; Tulek, N.; Demirelli, M.; Bulut, C.; Ertem, G. Incidence and Risk Factors for Colistin-Associated Nephrotoxicity. Jpn. J. Infect. Dis. 2015, 68, 318–320. [Google Scholar] [CrossRef]
- Kubin, C.J.; Ellman, T.M.; Phadke, V.; Haynes, L.J.; Calfee, D.P.; Yin, M.T. Incidence and predictors of acute kidney injury associated with intravenous polymyxin B therapy. J. Infect. 2012, 65, 80–87. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.H.; Yoo, S.; Pai, H. Clinical characteristics and risk factors of colistin-induced nephrotoxicity. Int. J. Antimicrob. Agents 2009, 34, 434–438. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; di Masi, A.; Leboffe, L.; Del Bono, V.; Rossi, M.; Cappiello, D.; Coppo, E.; Marchese, A.; Casulli, A.; Signori, A.; et al. Hypoalbuminemia as a predictor of acute kidney injury during colistin treatment. Sci. Rep. 2018, 8, 11968. [Google Scholar] [CrossRef]
- Nation, R.L.; Garonzik, S.M.; Thamlikitkul, V.; Giamarellos-Bourboulis, E.J.; Forrest, A.; Paterson, D.L.; Li, J.; Silveira, F.P. Reply to Corona and Cattaneo. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 65, 870–871. [Google Scholar] [CrossRef]
- Pogue, J.M.; Lee, J.; Marchaim, D.; Yee, V.; Zhao, J.J.; Chopra, T.; Lephart, P.; Kaye, K.S. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011, 53, 879–884. [Google Scholar] [CrossRef]
- Gul, S.; Kuscu, F.; Aydemir, H.; Ozturk, D.B.; Deveci, O.; Duygu, F.; Kacmaz, B.; Yaman, F.; Aslan, E. Risk Factors for Colistin-Associated Acute Kidney Injury: A Multicenter Study from Turkey. Jpn. J. Infect. Dis. 2016, 69, 109–112. [Google Scholar] [CrossRef]
- Dubrovskaya, Y.; Prasad, N.; Lee, Y.; Esaian, D.; Figueroa, D.A.; Tam, V.H. Risk factors for nephrotoxicity onset associated with polymyxin B therapy. J. Antimicrob. Chemother. 2015, 70, 1903–1907. [Google Scholar] [CrossRef]
- Shields, R.K.; Anand, R.; Clarke, L.G.; Paronish, J.A.; Weirich, M.; Perone, H.; Kieserman, J.; Freedy, H.; Andrzejewski, C.; Bonilla, H. Defining the incidence and risk factors of colistin-induced acute kidney injury by KDIGO criteria. PLoS ONE 2017, 12, e0173286. [Google Scholar] [CrossRef]
- Tuon, F.F.; Rigatto, M.H.; Lopes, C.K.; Kamei, L.K.; Rocha, J.L.; Zavascki, A.P. Risk factors for acute kidney injury in patients treated with polymyxin B or colistin methanesulfonate sodium. Int. J. Antimicrob. Agents 2014, 43, 349–352. [Google Scholar] [CrossRef]
- Aydemir, H.; Akduman, D.; Piskin, N.; Comert, F.; Horuz, E.; Terzi, A.; Kokturk, F.; Ornek, T.; Celebi, G. Colistin vs. the combination of colistin and rifampicin for the treatment of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia. Epidemiol. Infect. 2013, 141, 1214–1222. [Google Scholar] [CrossRef]
- Durante-Mangoni, E.; Signoriello, G.; Andini, R.; Mattei, A.; De Cristoforo, M.; Murino, P.; Bassetti, M.; Malacarne, P.; Petrosillo, N.; Galdieri, N.; et al. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: A multicenter, randomized clinical trial. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 57, 349–358. [Google Scholar] [CrossRef]
- Paul, M.; Daikos, G.L.; Durante-Mangoni, E.; Yahav, D.; Carmeli, Y.; Benattar, Y.D.; Skiada, A.; Andini, R.; Eliakim-Raz, N.; Nutman, A.; et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: An open-label, randomised controlled trial. Lancet. Infect. Dis. 2018, 18, 391–400. [Google Scholar] [CrossRef]
- Wang, J.; Niu, H.; Wang, R.; Cai, Y. Safety and efficacy of colistin alone or in combination in adults with Acinetobacter baumannii infection: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2018. [Google Scholar] [CrossRef]
- Dai, C.; Ciccotosto, G.D.; Cappai, R.; Wang, Y.; Tang, S.; Xiao, X.; Velkov, T. Minocycline attenuates colistin-induced neurotoxicity via suppression of apoptosis, mitochondrial dysfunction and oxidative stress. J. Antimicrob. Chemother. 2017, 72, 1635–1645. [Google Scholar] [CrossRef]
- Lodise, T.P.; Fan, W.; Griffith, D.C.; Dudley, M.N.; Sulham, K.A. A Retrospective Cohort Analysis Shows that Coadministration of Minocycline with Colistin in Critically Ill Patients Is Associated with Reduced Frequency of Acute Renal Failure. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Hartzell, J.D.; Neff, R.; Ake, J.; Howard, R.; Olson, S.; Paolino, K.; Vishnepolsky, M.; Weintrob, A.; Wortmann, G. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2009, 48, 1724–1728. [Google Scholar] [CrossRef] [PubMed]
- Benattar, Y.D.; Omar, M.; Zusman, O.; Yahav, D.; Zak-Doron, Y.; Altunin, S.; Elbaz, M.; Daitch, V.; Granot, M.; Leibovici, L.; et al. The Effectiveness and Safety of High-Dose Colistin: Prospective Cohort Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 63, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Rigatto, M.H.; Oliveira, M.S.; Perdigao-Neto, L.V.; Levin, A.S.; Carrilho, C.M.; Tanita, M.T.; Tuon, F.F.; Cardoso, D.E.; Lopes, N.T.; Falci, D.R.; et al. Multicenter Prospective Cohort Study of Renal Failure in Patients Treated with Colistin versus Polymyxin B. Antimicrob. Agents Chemother. 2016, 60, 2443–2449. [Google Scholar] [CrossRef]
- Elefritz, J.L.; Bauer, K.A.; Jones, C.; Mangino, J.E.; Porter, K.; Murphy, C.V. Efficacy and Safety of a Colistin Loading Dose, High-Dose Maintenance Regimen in Critically Ill Patients with Multidrug-Resistant Gram-Negative Pneumonia. J. Intensive Care Med. 2017, 32, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, J.P.; Sorli, L.; Luque, S.; Benito, N.; Segura, C.; Campillo, N.; Montero, M.; Esteve, E.; Mirelis, B.; Pomar, V.; et al. Validation of a colistin plasma concentration breakpoint as a predictor of nephrotoxicity in patients treated with colistin methanesulfonate. Int. J. Antimicrob. Agents 2016, 48, 725–727. [Google Scholar] [CrossRef]
- Tsuji, B.T.; Pogue, J.M.; Zavascki, A.P.; Paul, M.; Daikos, G.L.; Forrest, A.; Giacobbe, D.R.; Viscoli, C.; Giamarellou, H.; Karaiskos, I.; et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 2019, 39, 10–39. [Google Scholar] [CrossRef] [PubMed]
- Zarjou, A.; Agarwal, A. Sepsis and acute kidney injury. J. Am. Soc. Nephrol. JASN 2011, 22, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Nation, R.L.; Garonzik, S.M.; Li, J.; Thamlikitkul, V.; Giamarellos-Bourboulis, E.J.; Paterson, D.L.; Turnidge, J.D.; Forrest, A.; Silveira, F.P. Updated US and European Dose Recommendations for Intravenous Colistin: How Do They Perform? Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 62, 552–558. [Google Scholar] [CrossRef]
- Nation, R.L.; Garonzik, S.M.; Thamlikitkul, V.; Giamarellos-Bourboulis, E.J.; Forrest, A.; Paterson, D.L.; Li, J.; Silveira, F.P. Dosing guidance for intravenous colistin in critically ill patients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 64, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Mouton, J.W.; Muller, A.E.; Canton, R.; Giske, C.G.; Kahlmeter, G.; Turnidge, J. MIC-based dose adjustment: Facts and fables. J. Antimicrob. Chemother. 2018, 73, 564–568. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing. Clinical Breakpoints—Bacteria (v 9.0). Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 2 January 2019).
- Cheah, S.E.; Wang, J.; Nguyen, V.T.; Turnidge, J.D.; Li, J.; Nation, R.L. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: Smaller response in lung infection. J. Antimicrob. Chemother. 2015, 70, 3291–3297. [Google Scholar] [CrossRef] [PubMed]
- Forrest, A.; Garonzik, S.M.; Thamlikitkul, V.; Giamarellos-Bourboulis, E.J.; Paterson, D.L.; Li, J.; Silveira, F.P.; Nation, R.L. Pharmacokinetic/Toxicodynamic Analysis of Colistin-Associated Acute Kidney Injury in Critically Ill Patients. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Lakota, E.A.; Landersdorfer, C.B.; Nation, R.L.; Li, J.; Kaye, K.S.; Rao, G.G.; Forrest, A. Personalizing Polymyxin B Dosing Using an Adaptive Feedback Control Algorithm. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Landersdorfer, C.B.; Wang, J.; Wirth, V.; Chen, K.; Kaye, K.S.; Tsuji, B.T.; Li, J.; Nation, R.L. Pharmacokinetics/pharmacodynamics of systemically administered polymyxin B against Klebsiella pneumoniae in mouse thigh and lung infection models. J. Antimicrob. Chemother. 2018, 73, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.S.; Prado, G.V.; Costa, S.F.; Grinbaum, R.S.; Levin, A.S. Polymyxin B and colistimethate are comparable as to efficacy and renal toxicity. Diagn. Microbiol. Infect. Dis. 2009, 65, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Dewan, A. Comparison of nephrotoxicity of Colistin with Polymyxin B administered in currently recommended doses: A prospective study. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Zavascki, A.P.; Nation, R.L. Nephrotoxicity of Polymyxins: Is There Any Difference between Colistimethate and Polymyxin B? Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Nation, R.L.; Velkov, T.; Li, J. Colistin and Polymyxin B: Peas in a Pod, or Chalk and Cheese? Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 59, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Nation, R.L.; Li, J.; Cars, O.; Couet, W.; Dudley, M.N.; Kaye, K.S.; Mouton, J.W.; Paterson, D.L.; Tam, V.H.; Theuretzbacher, U.; et al. Framework for optimisation of the clinical use of colistin and polymyxin B: The Prato polymyxin consensus. Lancet. Infect. Dis. 2015, 15, 225–234. [Google Scholar] [CrossRef]
- Barnett, M.; Bushby, S.R.; Wilkinson, S. Sodium sulphomethyl derivatives of polymyxins. Br. J. Pharmacol. Chemother. 1964, 23, 552–574. [Google Scholar] [CrossRef]
- Bergen, P.J.; Li, J.; Rayner, C.R.; Nation, R.L. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 1953–1958. [Google Scholar] [CrossRef]
- Couet, W.; Gregoire, N.; Gobin, P.; Saulnier, P.J.; Frasca, D.; Marchand, S.; Mimoz, O. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin. Pharmacol. Ther. 2011, 89, 875–879. [Google Scholar] [CrossRef]
- Luque, S.; Escano, C.; Sorli, L.; Li, J.; Campillo, N.; Horcajada, J.P.; Salas, E.; Grau, S. Urinary Concentrations of Colistimethate and Formed Colistin after Intravenous Administration in Patients with Multidrug-Resistant Gram-Negative Bacterial Infections. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Bargiacchi, O.; Rossati, A.; Car, P.; Brustia, D.; Brondolo, R.; Rosa, F.; Garavelli, P.L.; De Rosa, F.G. Intrathecal/intraventricular colistin in external ventricular device-related infections by multi-drug resistant Gram negative bacteria: Case reports and review. Infection 2014, 42, 801–809. [Google Scholar] [CrossRef]
- Imberti, R.; Cusato, M.; Accetta, G.; Marino, V.; Procaccio, F.; Del Gaudio, A.; Iotti, G.A.; Regazzi, M. Pharmacokinetics of colistin in cerebrospinal fluid after intraventricular administration of colistin methanesulfonate. Antimicrob. Agents Chemother. 2012, 56, 4416–4421. [Google Scholar] [CrossRef]
- Karaiskos, I.; Galani, L.; Baziaka, F.; Giamarellou, H. Intraventricular and intrathecal colistin as the last therapeutic resort for the treatment of multidrug-resistant and extensively drug-resistant Acinetobacter baumannii ventriculitis and meningitis: A literature review. Int. J. Antimicrob. Agents 2013, 41, 499–508. [Google Scholar] [CrossRef]
- Karaiskos, I.; Galani, L.; Baziaka, F.; Katsouda, E.; Ioannidis, I.; Andreou, A.; Paskalis, H.; Giamarellou, H. Successful treatment of extensively drug-resistant Acinetobacter baumannii ventriculitis and meningitis with intraventricular colistin after application of a loading dose: A case series. Int. J. Antimicrob. Agents 2013, 41, 480–483. [Google Scholar] [CrossRef]
- De Bonis, P.; Lofrese, G.; Scoppettuolo, G.; Spanu, T.; Cultrera, R.; Labonia, M.; Cavallo, M.A.; Mangiola, A.; Anile, C.; Pompucci, A. Intraventricular versus intravenous colistin for the treatment of extensively drug resistant Acinetobacter baumannii meningitis. Eur. J. Neurol. Off. J. Eur. Fed. Neurol. Soc. 2015. [Google Scholar] [CrossRef]
- Fotakopoulos, G.; Makris, D.; Chatzi, M.; Tsimitrea, E.; Zakynthinos, E.; Fountas, K. Outcomes in meningitis/ventriculitis treated with intravenous or intraventricular plus intravenous colistin. Acta Neurochir. 2016, 158, 603–610. [Google Scholar] [CrossRef]
- Inamasu, J.; Ishikawa, K.; Oheda, M.; Nakae, S.; Hirose, Y.; Yoshida, S. Intrathecal administration of colistin for meningitis due to New Delhi metallo-beta-lactamase 1(NDM-1)-producing Klebsiella pneumoniae. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2016, 22, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Bergen, P.J.; Li, J.; Nation, R.L. Dosing of colistin-back to basic PK/PD. Curr. Opin. Pharmacol. 2011, 11, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Couet, W.; Gregoire, N.; Marchand, S.; Mimoz, O. Colistin pharmacokinetics: The fog is lifting. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2012, 18, 30–39. [Google Scholar] [CrossRef]
- Garonzik, S.M.; Li, J.; Thamlikitkul, V.; Paterson, D.L.; Shoham, S.; Jacob, J.; Silveira, F.P.; Forrest, A.; Nation, R.L. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 2011, 55, 3284–3294. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rayner, C.R.; Nation, R.L.; Deans, R.; Boots, R.; Widdecombe, N.; Douglas, A.; Lipman, J. Pharmacokinetics of colistin methanesulfonate and colistin in a critically ill patient receiving continuous venovenous hemodiafiltration. Antimicrob. Agents Chemother. 2005, 49, 4814–4815. [Google Scholar] [CrossRef]
- Spapen, H.D.; Honore, P.M.; Gregoire, N.; Gobin, P.; de Regt, J.; Martens, G.A.; Pierard, D.; Couet, W. Convulsions and apnoea in a patient infected with New Delhi metallo-beta-lactamase-1 Escherichia coli treated with colistin. J. Infect. 2011, 63, 468–470. [Google Scholar] [CrossRef]
- Bode-Boger, S.M.; Schopp, B.; Troger, U.; Martens-Lobenhoffer, J.; Kalousis, K.; Mailander, P. Intravenous colistin in a patient with serious burns and borderline syndrome: The benefits of therapeutic drug monitoring. Int. J. Antimicrob. Agents 2013, 42, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Oh, J.; Lee, K.; Yu, K.S.; Chung, J.Y.; Hwang, J.H.; Nam, E.Y.; Kim, H.S.; Kim, M.; Park, J.S.; et al. A Short Communication: Pharmacokinetic characteristics and limited sampling strategies for therapeutic drug monitoring of colistin in patients with multidrug-resistant Gram-negative bacterial infections. Ther. Drug Monit. 2018. [Google Scholar] [CrossRef]
- Li, J.; Milne, R.W.; Nation, R.L.; Turnidge, J.D.; Coulthard, K. Stability of colistin and colistin methanesulfonate in aqueous media and plasma as determined by high-performance liquid chromatography. Antimicrob. Agents Chemother. 2003, 47, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Jansson, B.; Karvanen, M.; Cars, O.; Plachouras, D.; Friberg, L.E. Quantitative analysis of colistin A and colistin B in plasma and culture medium using a simple precipitation step followed by LC/MS/MS. J. Pharm. Biomed. Anal. 2009, 49, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Dudhani, R.V.; Nation, R.L.; Li, J. Evaluating the stability of colistin and colistin methanesulphonate in human plasma under different conditions of storage. J. Antimicrob. Chemother. 2010, 65, 1412–1415. [Google Scholar] [CrossRef]
- Zavascki, A.P.; Goldani, L.Z.; Cao, G.; Superti, S.V.; Lutz, L.; Barth, A.L.; Ramos, F.; Boniatti, M.M.; Nation, R.L.; Li, J. Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2008, 47, 1298–1304. [Google Scholar] [CrossRef]
- Sandri, A.M.; Landersdorfer, C.B.; Jacob, J.; Boniatti, M.M.; Dalarosa, M.G.; Falci, D.R.; Behle, T.F.; Bordinhao, R.C.; Wang, J.; Forrest, A.; et al. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: Implications for selection of dosage regimens. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 57, 524–531. [Google Scholar] [CrossRef]
- Kwa, A.L.; Abdelraouf, K.; Low, J.G.; Tam, V.H. Pharmacokinetics of polymyxin B in a patient with renal insufficiency: A case report. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011, 52, 1280–1281. [Google Scholar] [CrossRef]
- Thamlikitkul, V.; Dubrovskaya, Y.; Manchandani, P.; Ngamprasertchai, T.; Boonyasiri, A.; Babic, J.T.; Tam, V.H. Dosing and Pharmacokinetics of Polymyxin B in Patients with Renal Insufficiency. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Manchandani, P.; Thamlikitkul, V.; Dubrovskaya, Y.; Babic, J.T.; Lye, D.C.; Lee, L.S.; Tam, V.H. Population Pharmacokinetics of Polymyxin B. Clin. Pharmacol. Ther. 2018, 104, 534–538. [Google Scholar] [CrossRef]
- Miglis, C.; Rhodes, N.J.; Avedissian, S.N.; Kubin, C.J.; Yin, M.T.; Nelson, B.C.; Pai, M.P.; Scheetz, M.H. Population Pharmacokinetics of Polymyxin B in Acutely Ill Adult Patients. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Elias, L.S.; Konzen, D.; Krebs, J.M.; Zavascki, A.P. The impact of polymyxin B dosage on in-hospital mortality of patients treated with this antibiotic. J. Antimicrob. Chemother. 2010, 65, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Jelliffe, R.W.; Schumitzky, A.; Van Guilder, M.; Liu, M.; Hu, L.; Maire, P.; Gomis, P.; Barbaut, X.; Tahani, B. Individualizing drug dosage regimens: Roles of population pharmacokinetic and dynamic models, Bayesian fitting, and adaptive control. Ther. Drug Monit. 1993, 15, 380–393. [Google Scholar] [CrossRef]
- Barza, M.; Ioannidis, J.P.; Cappelleri, J.C.; Lau, J. Single or multiple daily doses of aminoglycosides: A meta-analysis. BMJ 1996, 312, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Pagkalis, S.; Mantadakis, E.; Mavros, M.N.; Ammari, C.; Falagas, M.E. Pharmacological considerations for the proper clinical use of aminoglycosides. Drugs 2011, 71, 2277–2294. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.J.; Li, J.; Nation, R.L.; Rayner, C.R.; Taylor, D.; Middleton, D.; Milne, R.W.; Coulthard, K.; Turnidge, J.D. Subacute toxicity of colistin methanesulfonate in rats: Comparison of various intravenous dosage regimens. Antimicrob. Agents Chemother. 2008, 52, 1159–1161. [Google Scholar] [CrossRef]
- Abdelraouf, K.; Braggs, K.H.; Yin, T.; Truong, L.D.; Hu, M.; Tam, V.H. Characterization of polymyxin B-induced nephrotoxicity: Implications for dosing regimen design. Antimicrob. Agents Chemother. 2012, 56, 4625–4629. [Google Scholar] [CrossRef]
- Okoduwa, A.; Ahmed, N.; Guo, Y.; Scipione, M.R.; Papadopoulos, J.; Eiras, D.P.; Dubrovskaya, Y. Nephrotoxicity Associated with Intravenous Polymyxin B Once- versus Twice-Daily Dosing Regimen. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Tam, V.H.; Schilling, A.N.; Vo, G.; Kabbara, S.; Kwa, A.L.; Wiederhold, N.P.; Lewis, R.E. Pharmacodynamics of polymyxin B against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2005, 49, 3624–3630. [Google Scholar] [CrossRef] [PubMed]
- Bergen, P.J.; Li, J.; Nation, R.L.; Turnidge, J.D.; Coulthard, K.; Milne, R.W. Comparison of once-, twice- and thrice-daily dosing of colistin on antibacterial effect and emergence of resistance: Studies with Pseudomonas aeruginosa in an in vitro pharmacodynamic model. J. Antimicrob. Chemother. 2008, 61, 636–642. [Google Scholar] [CrossRef]
- Kelesidis, T.; Falagas, M.E. The safety of polymyxin antibiotics. Expert Opin. Drug Saf. 2015, 14, 1687–1701. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.K.; Sivanesan, S.; Wang, J.; Chen, K.; Nation, R.L.; Thompson, P.E.; Roberts, K.D.; Velkov, T.; Li, J. Methionine Ameliorates Polymyxin-Induced Nephrotoxicity by Attenuating Cellular Oxidative Stress. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Tang, S.; Deng, S.; Zhang, S.; Zhou, Y.; Velkov, T.; Li, J.; Xiao, X. Lycopene attenuates colistin-induced nephrotoxicity in mice via activation of the Nrf2/HO-1 pathway. Antimicrob. Agents Chemother. 2015, 59, 579–585. [Google Scholar] [CrossRef]
- Dai, C.; Tang, S.; Wang, Y.; Velkov, T.; Xiao, X. Baicalein acts as a nephroprotectant that ameliorates colistin-induced nephrotoxicity by activating the antioxidant defence mechanism of the kidneys and down-regulating the inflammatory response. J. Antimicrob. Chemother. 2017, 72, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Ozyilmaz, E.; Ebinc, F.A.; Derici, U.; Gulbahar, O.; Goktas, G.; Elmas, C.; Oguzulgen, I.K.; Sindel, S. Could nephrotoxicity due to colistin be ameliorated with the use of N-acetylcysteine? Intensive Care Med. 2011, 37, 141–146. [Google Scholar] [CrossRef]
- Arslan, B.Y.; Arslan, F.; Erkalp, K.; Alagol, A.; Sevdi, M.S.; Yildiz, G.; Kucuk, S.H.; Altinay, S. Luteolin ameliorates colistin-induced nephrotoxicity in the rat models. Ren. Fail. 2016, 38, 1735–1740. [Google Scholar] [CrossRef]
- Ceylan, B.; Ozansoy, M.; Kilic, U.; Yozgat, Y.; Ercan, C.; Yildiz, P.; Aslan, T. N-acetylcysteine suppresses colistimethate sodium-induced nephrotoxicity via activation of SOD2, eNOS, and MMP3 protein expressions. Ren. Fail. 2018, 40, 423–434. [Google Scholar] [CrossRef]
- Yousef, J.M.; Chen, G.; Hill, P.A.; Nation, R.L.; Li, J. Melatonin attenuates colistin-induced nephrotoxicity in rats. Antimicrob. Agents Chemother. 2011, 55, 4044–4049. [Google Scholar] [CrossRef]
- Hassan, S.S.; Thomann, C.; Ettarh, R.; Ahmad, Z. Possible protective role of silybin against polymyxin E-induced toxic effect in rat kidneys: A biochemical approach. Neurourol. Urodyn. 2017, 36, 2003–2010. [Google Scholar] [CrossRef]
- Spargias, K.; Alexopoulos, E.; Kyrzopoulos, S.; Iokovis, P.; Greenwood, D.C.; Manginas, A.; Voudris, V.; Pavlides, G.; Buller, C.E.; Kremastinos, D.; et al. Ascorbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation 2004, 110, 2837–2842. [Google Scholar] [CrossRef]
- Dalfino, L.; Puntillo, F.; Ondok, M.J.; Mosca, A.; Monno, R.; Coppolecchia, S.; Spada, M.L.; Bruno, F.; Brienza, N. Colistin-associated Acute Kidney Injury in Severely Ill Patients: A Step Toward a Better Renal Care? A Prospective Cohort Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 61, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Sirijatuphat, R.; Limmahakhun, S.; Sirivatanauksorn, V.; Nation, R.L.; Li, J.; Thamlikitkul, V. Preliminary clinical study of the effect of ascorbic acid on colistin-associated nephrotoxicity. Antimicrob. Agents Chemother. 2015, 59, 3224–3232. [Google Scholar] [CrossRef]
- Li, Z.D.; Luo, J.; Jia, L.H.; Wang, X.Y.; Xun, Z.K.; Liu, M. Cytochrome C suppresses renal accumulation and nephrotoxicity of polymyxin B. Hum. Exp. Toxicol. 2019, 38, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Ulusoy, S.; Orem, A.; Alkanat, M.; Mungan, S.; Yulug, E.; Yucesan, F.B. How does colistin-induced nephropathy develop and can it be treated? Antimicrob. Agents Chemother. 2013, 57, 3463–3469. [Google Scholar] [CrossRef]
- Yousef, J.M.; Chen, G.; Hill, P.A.; Nation, R.L.; Li, J. Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics. J. Antimicrob. Chemother. 2012, 67, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Hrenak, J.; Paulis, L.; Repova, K.; Aziriova, S.; Nagtegaal, E.J.; Reiter, R.J.; Simko, F. Melatonin and renal protection: Novel perspectives from animal experiments and human studies (review). Curr. Pharm. Des. 2015, 21, 936–949. [Google Scholar] [CrossRef]
| Study | Intervention/Exposure Factor | Main Results |
|---|---|---|
| Animal Models | ||
| Li et al., 2019 [114] | Cytochrome C | - Cytochrome C (a megalin ligand) decreased the accumulation of polymyxin B in the kidney and 24-h N-acetyl-β-d-glucosaminidase (NAG) in a dose-dependent manner. - Histological damage was reduced. - No significant differences in serum creatinine, blood urea nitrogen (BUN), and blood β2- microglobulin were seen in the groups that received Cytochrome C compared to the one that received polymyxin B alone. |
| Ceylan et al., 2018 [108] | N-acetylcysteine (NAC) | - Colistin increased the apoptosis index and renal histological damage score significantly and these changes were reduced with NAC co-treatment. - There was no difference between groups regarding total antioxidant and total oxidant status in the kidneys. |
| Dai et al., 2017 [105] | Baicalein | - Baicalein attenuated colistin-induced oxidative and nitrative stress, apoptosis, the infiltration of inflammatory cells, and caused decreases in interleukine-1β and tumor necrosis factor-α levels (all p < 0.05 or 0.01) in kidney tissues. - Baicalein attenuated colistin-induced kidney tissue damage on histopathological analysis, in a dose-dependent manner. |
| Azad et al., 2017 [103] | Methionine | - Histological: polymyxin-induced nephrotoxicity in mice was ameliorated by methionine in a dose-dependent manner. - Attenuation of polymyxin-induced mitochondrial superoxide production in rat kidney cells was observed following pretreatment with methionine. - Pharmacokinetics of polymyxin B in rats were not affected by methionine. |
| Hassan et al., 2017 [110] | Silybin | - Colistin-alone group showed an increase in NAG (p < 0.01) and reduction of renal function compared to other groups (control, vehicle and colistin plus silybin) (p < 0.001), but no difference was found in a direct comparison of colistin plus sybilin group with the colistin-alone group. |
| Arslan et al., 2016 [107] | Luteolin | - Colistin-treated group had statistically higher number of apoptotic cells compared to the other three groups (luteolin, luteolin plus colistin and control) (p = 0.0001) and was the only group to increase serum creatinine values compared to pre-treatment levels. - Renal histological damage was also measured and the score of the colistin-treated group was higher as compared to other groups. |
| Dai et al., 2015 [104] | Lycopene | - Biomarkers of oxidative stress and apoptosis were attenuated in the kidneys of colistin-treated mice by the co-administration of lycopene (5 or 20 mg/kg). |
| Ozkan et al., 2013 [115] | Grape seed proanthocyanidin extract (GSPE) | - Colistin + GSPE group showed significant decreases in BUN levels; creatinine levels; renal histopathological scores; and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling, caspase 1 and 3, calpain 1, iNOS, and eNOS staining when compared to the colistin group alone. |
| Yousef et al., 2012 [116] | Ascorbic acid | - 24-h urinary excretion of NAG was significantly lower in the groups that received ascorbic acid compared to colistin alone (p < 0.01). - The percentage of apoptotic cells decreased in the ascorbic acid group in a dose-dependent manner (p < 0.0001). - Ascorbic acid (200 mg/kg) reduced colistin total body clearance. |
| Yousef et al., 2011 [109] | Melatonin | - The addition of melatonin was associated with lower urinary NAG excretion from day 1 (p < 0.0001). - Significant histological abnormalities (p < 0.0001) were detected only in the kidneys of the colistin group. Melatonin altered colistin pharmacokinetics, reducing total body clearance. |
| Ozyilmaz et al., 2011 [106] | N-acetylcysteine (NAC) | - NAC addition did not change biochemical parameters but reduced the renal tissue superoxide dismutase level, showing a reduction in oxidative stress parameters. |
| Clinical Studies | ||
| Dalfino et al., 2015 [112] | Ascorbic acid | - 70 patients included. Independent predictors of acute kidney injury (AKI) were baseline renal impairment (adjusted Hazar Ratio, 4.15; 95% CI 1.9–9.2; p < 0.001) and age (aHR1.03; 95%CI 1.0–1.05; p = 0.028), whereas ascorbic acid was a protective factor (aHR0.27; 95%CI 12–0.57; p < 0.001). |
| Sirijatuphat et al., 2015 [113] | Ascorbic acid | - Nephrotoxicity incidence was 53.8% (7/13) and 60.0% (9/15) in the colistin-ascorbic acid group and the colistin group, respectively (p = 0.956). - Urinary excretion rates of neutrophil gelatinase-associated lipocalin and NAG increased during colistin treatment compared to baselines in both groups (p < 0.05). |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nation, R.L.; Rigatto, M.H.P.; Falci, D.R.; Zavascki, A.P. Polymyxin Acute Kidney Injury: Dosing and Other Strategies to Reduce Toxicity. Antibiotics 2019, 8, 24. https://doi.org/10.3390/antibiotics8010024
Nation RL, Rigatto MHP, Falci DR, Zavascki AP. Polymyxin Acute Kidney Injury: Dosing and Other Strategies to Reduce Toxicity. Antibiotics. 2019; 8(1):24. https://doi.org/10.3390/antibiotics8010024
Chicago/Turabian StyleNation, Roger L., Maria Helena P. Rigatto, Diego R. Falci, and Alexandre P. Zavascki. 2019. "Polymyxin Acute Kidney Injury: Dosing and Other Strategies to Reduce Toxicity" Antibiotics 8, no. 1: 24. https://doi.org/10.3390/antibiotics8010024
APA StyleNation, R. L., Rigatto, M. H. P., Falci, D. R., & Zavascki, A. P. (2019). Polymyxin Acute Kidney Injury: Dosing and Other Strategies to Reduce Toxicity. Antibiotics, 8(1), 24. https://doi.org/10.3390/antibiotics8010024





