Specificity of Induction of Glycopeptide Antibiotic Resistance in the Producing Actinomycetes
Abstract
1. Introduction
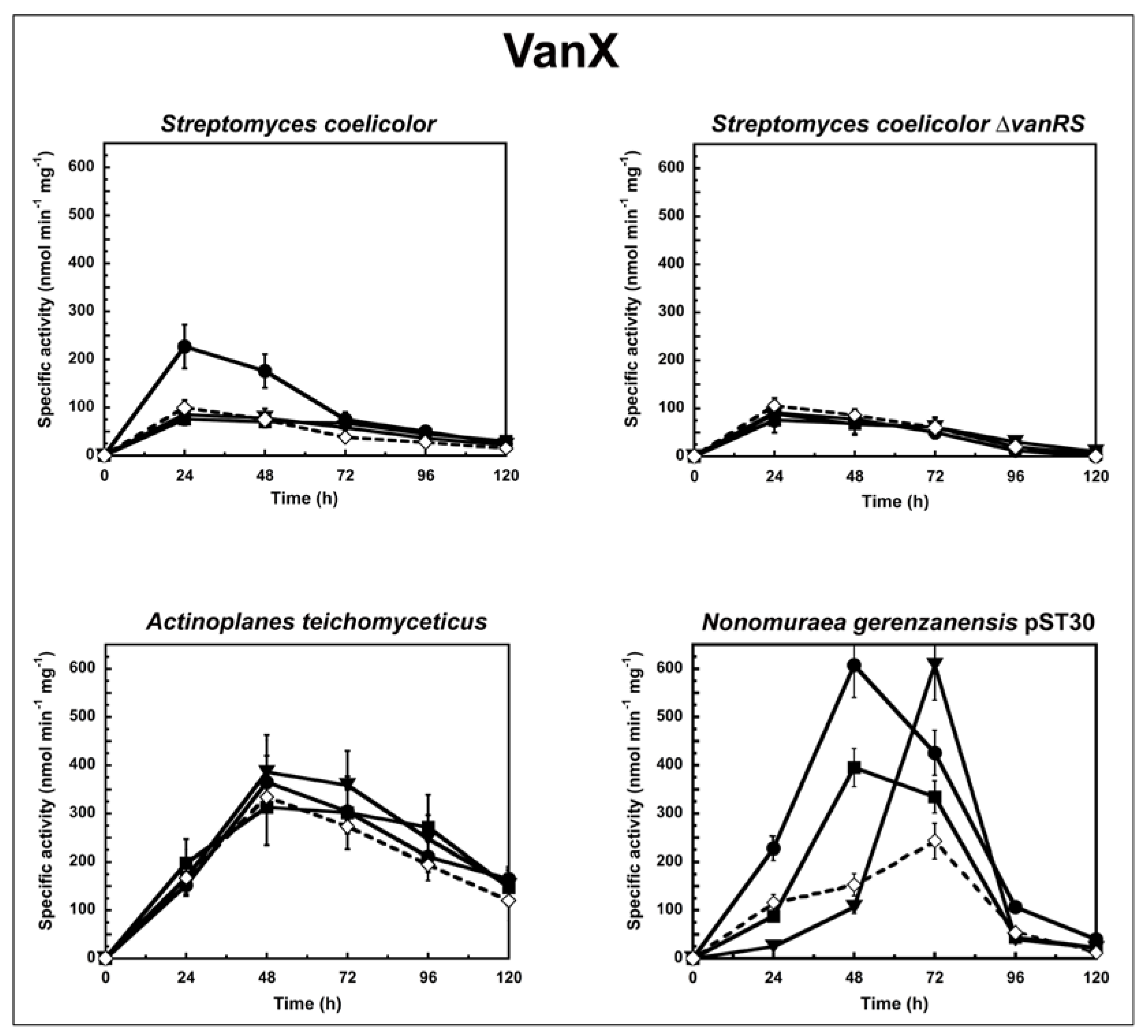
2. Results and Discussion
3. Materials and Methods
3.1. Strains and Media
3.2. Induction Experiments
3.3. MIC Determination
3.4. d,d-dipeptidase and d,d-carboxypeptidase Assays
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- European Centre for Disease Prevention and Control. Available online: https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratoy-networks/ears-net (accessed on 16 January 2018).
- Binda, E.; Marinelli, F.; Marcone, G.L. Old and new glycopeptide antibiotics: Action and resistance. Antibiotics 2014, 3, 572–594. [Google Scholar] [CrossRef] [PubMed]
- Marcone, G.L.; Binda, E.; Berini, F.; Marinelli, F. Old and new glycopeptide antibiotics: From product to gene and back in the post-genomic era. Biotechnol. Adv. 2018, 36, 534–554. [Google Scholar] [CrossRef] [PubMed]
- Van Bambeke, F. Lipoglycopeptide antibacterial agents in Gram-positive infections: A comparative review. Drugs 2015, 18, 2073–2095. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Williams, D.H. Binding of glycopeptide antibiotics to a model of a vancomycin-resistant bacterium. Chem. Biol. 1999, 6, 891–899. [Google Scholar] [CrossRef]
- Perkins, H.R.; Nieto, M. The chemical basis for the action of the vancomycin group of antibiotics. Ann. N. Y. Acad. Sci. 1974, 235, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.E.; Nicas, T.I. Mechanism of action of oritavancin and related glycopeptide antibiotics. FEMS Microbiol. Rev. 2003, 26, 511–532. [Google Scholar] [CrossRef] [PubMed]
- Treviño, J.; Bayón, C.; Ardá, A.; Marinelli, F.; Gandolfi, R.; Molinari, F.; Jimenez-Barbero, J.; Hernáiz, M.J. New insights into glycopeptide antibiotic binding to cell wall precursors using SPR and NMR spectroscopy. Chemistry 2014, 20, 7363–7372. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.D.; Oberthür, M.; Losey, H.C.; Anderson, J.W.; Eggert, U.S.; Peczuh, M.W.; Walsh, C.T.; Kahne, D. The structural basis for induction of VanB resistance. J. Am. Chem. Soc. 2002, 124, 9064–9065. [Google Scholar] [CrossRef] [PubMed]
- Kwun, M.J.; Hong, H.J. The activity of glycopeptide antibiotics against resistant bacteria correlates with their ability to induce the resistance system. Antimicrob. Agents Chemother. 2014, 58, 6306–6310. [Google Scholar] [CrossRef] [PubMed]
- Bugg, T.D.; Wright, G.D.; Dutka-Malen, S.; Arthur, M.; Courvalin, P.; Walsh, C.T. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: Biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry 1991, 30, 10408–10415. [Google Scholar] [CrossRef] [PubMed]
- Arthur, M.; Quintiliani, R. Regulation of VanA- and VanB-type glycopeptide resistance in enterococci. Antimicrob. Agents Chemother. 2001, 45, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Evers, S.; Courvalin, P. Regulation of VanB-type vancomycin resistance gene expression by the VanS(B)-VanR(B) two-component regulatory system in Enterococcus faecalis V583. J. Bacteriol. 1996, 178, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.J.; Hutchings, M.I.; Buttner, M.J. Vancomycin resistance VanS/VanR two-component systems. Adv. Exp. Med. Biol. 2008, 631, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Depardieu, F.; Podglajen, I.; Leclercq, R.; Collatz, E.; Courvalin, P. Modes and modulations of antibiotic resistance gene expression. Clin. Microbiol. Rev. 2007, 20, 79–114. [Google Scholar] [CrossRef] [PubMed]
- Kwun, M.J.; Novotna, G.; Hesketh, A.R.; Hill, L.; Hong, H.J. In vivo studies suggest that induction of VanS-dependent vancomycin resistance requires binding of the drug to d-Ala-d-Ala termini in the peptidoglycan cell wall. Antimicrob. Agents Chemother. 2013, 57, 4470–4480. [Google Scholar] [CrossRef] [PubMed]
- Koteva, K.; Hong, H.J.; Wang, X.D.; Nazi, I.; Hughes, D.; Naldrett, M.J.; Buttner, M.J.; Wright, G.D. A vancomycin photoprobe identifies the histidine kinase VanSsc as a vancomycin receptor. Nat. Chem. Biol. 2010, 6, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.P.; Selva, E.; Gastaldo, L.; Berti, M.; Pallanza, R.; Ripamonti, F.; Ferrari, P.; Denaro, M.; Arioli, V.; Cassani, G. A40926, a new glycopeptide antibiotic with anti-Neisseria activity. Antimicrob. Agents Chemother. 1987, 31, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Dalmastri, C.; Gastaldo, L.; Marcone, G.L.; Binda, E.; Congiu, T.; Marinelli, F. Classification of Nonomuraea sp. ATCC 39727, an actinomycete that produces the glycopeptide antibiotic A40926, as Nonomuraea gerenzanensis sp. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 912–921. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, V.; Petrillo, M.; Pasanisi, D.; Pagliarulo, C.; Colicchio, R.; Talà, A.; de Biase, M.S.; Zanfardino, M.; Scolamiero, E.; Pagliuca, C.; et al. The complete 12 Mb genome and transcriptome of Nonomuraea gerenzanensis with new insights into its duplicated “Magic” RNA polymerase. Sci. Rep. 2016, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.I.; Hong, H.J.; Buttner, M.J. The vancomycin resistance VanRS two-component signal transduction system of Streptomyces coelicolor. Mol. Microbiol. 2006, 59, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Siewert, G.; Strominger, J.L. Bacitracin: An inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in the biosynthesis of the peptidoglycan of bacterial cell walls. Proc. Natl. Acad. Sci. USA 1967, 57, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, J.B.; Hoertz, A.J.; Lee, A.; Senturia, R.J.; McCafferty, D.G.; Loll, P.J. A crystal structure of a dimer of the antibiotic ramoplanin illustrates membrane positioning and a potential lipid II docking interface. Proc. Natl. Acad. Sci. USA 2009, 106, 13759–13764. [Google Scholar] [CrossRef] [PubMed]
- Beltrametti, F.; Consolandi, A.; Carrano, L.; Bagatin, F.; Rossi, R.; Leoni, L.; Zennaro, E.; Selva, E.; Marinelli, F. Resistance to glycopeptide antibiotics in the teicoplanin producer is mediated by van gene homologue expression directing the synthesis of a modified cell wall peptidoglycan. Antimicrob. Agents Chemother. 2007, 51, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Marcone, G.L.; Binda, E.; Carrano, L.; Bibb, M.; Marinelli, F. Relationship between glycopeptide production and resistance in the actinomycete Nonomuraea sp. ATCC 39727. Antimicrob. Agents Chemother. 2014, 58, 5191–5201. [Google Scholar] [CrossRef] [PubMed]
- Marcone, G.L.; Beltrametti, F.; Binda, E.; Carrano, L.; Foulston, L.; Hesketh, A.; Bibb, M.; Marinelli, F. Novel mechanism of glycopeptide resistance in the A40926 producer Nonomuraea sp. ATCC 39727. Antimicrob. Agents Chemother. 2010, 54, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Arthur, M.; Depardieu, F.; Cabanié, L.; Reynolds, P.; Courvalin, P. Requirement of the VanY and VanX d,d-peptidases for glycopeptide resistance in enterococci. Mol. Microbiol. 1998, 30, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Serina, S.; Radice, F.; Maffioli, S.; Donadio, S.; Sosio, M. Glycopeptide resistance determinants from the teicoplanin producer Actinoplanes teichomyceticus. FEMS Microbiol. Lett. 2004, 240, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Marcone, G.L.; Carrano, L.; Marinelli, F.; Beltrametti, F. Protoplast preparation and reversion to the normal filamentous growth in antibiotic-producing uncommon actinomycetes. J. Antibiot. 2010, 63, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Marcone, G.L.; Binda, E.; Reguzzoni, M.; Gastaldo, L.; Dalmastri, C.; Marinelli, F. Classification of Actinoplanes sp. ATCC 33076, an actinomycete that produces the glycolipodepsipeptide antibiotic ramoplanin, as Actinoplanes ramoplaninifer sp. nov. Int. J. Syst. Evol. Microbiol. 2017, 67, 4181–4188. [Google Scholar] [CrossRef] [PubMed]
- Binda, E.; Marcone, G.L.; Pollegioni, L.; Marinelli, F. Characterization of VanYn, a novel d,d-peptidase/d,d-carboxypeptidase involved in glycopeptide antibiotic resistance in Nonomuraea sp. ATCC 39727. FEBS J. 2012, 279, 3203–3213. [Google Scholar] [CrossRef] [PubMed]
- Kilian, R.; Frasch, H.J.; Kulik, A.; Wohlleben, W.; Stegmann, E. The VanRS homologous two-component system VnlRSAb of the glycopeptide producer Amycolatopsis balhimycina activates transcription of the vanHAXSc genes in Streptomyces coelicolor, but not in A. balhimycina. Microb. Drug Resist. 2016, 22, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Sosio, M.; Stinchi, S.; Beltrametti, F.; Lazzarini, A.; Donadio, S. The gene cluster for the biosynthesis of the glycopeptide antibiotic A40926 by Nonomuraea species. Chem. Biol. 2003, 10, 541–549. [Google Scholar] [CrossRef]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics; The John Innes Foundation: Norwich, UK, 2000; ISBN 0-7084-0623-8. [Google Scholar]
- Taurino, C.; Frattini, L.; Marcone, G.L.; Gastaldo, L.; Marinelli, F. Actinoplanes teichomyceticus ATCC 31121 as a cell factory for producing teicoplanin. Microb. Cell Fact. 2011, 10, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Binda, E.; Marcone, G.L.; Berini, F.; Pollegioni, L.; Marinelli, F. Streptomyces spp. as efficient expression system for a d,d-peptidase/d,d-carboxypeptidase involved in glycopeptide antibiotic resistance. BMC Biotechnol. 2013, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Thaker, M.N.; Wang, W.; Spanogiannopoulos, P.; Waglechner, N.; King, A.M.; Medina, R.; Wright, G.D. Identifying producers of antibacterial compounds by screening for antibiotic resistance. Nat. Biotechnol. 2013, 31, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Donadio, S.; Sosio, M.; Stegmann, E.; Weber, T.; Wohlleben, W. Comparative analysis and insights into the evolution of gene clusters for glycopeptide antibiotic biosynthesis. Mol. Genet. Genom. 2005, 274, 40–50. [Google Scholar] [CrossRef] [PubMed]

| Actinomycetes | Vancomycin (µg/mL) | Teicoplanin (µg/mL) | A40926 (µg/mL) | Ramoplanin (µg/mL) | Bacitracin (µg/mL) |
|---|---|---|---|---|---|
| S. coelicolor | >100 | 1.5 ± 0.025 | 1.5 ± 0.075 | 0.9 ± 0.045 | 0.9 ± 0.05 |
| S. coelicolor ∆vanRS | 1.25 ± 0.03 | 1.5 ± 0.02 | 1.5 ± 0.02 | 0.9 ± 0.025 | 0.9 ± 0.035 |
| A. teichomyceticus | 90 ± 4.2 | 20 ± 1.5 | 32.5 ± 1.5 | 20 ± 1 | 20 ± 1.15 |
| N. gerenzanensis | 20 ± 1.6 | 0.9 ± 0.01 | 4 ± 0.2 | 20 ± 1.2 | 20 ± 1.3 |
| N. gerenzanensis pST30 | 20 ± 1.05 | 2.75 ± 0.15 | 17.5 ± 0.875 | 20 ± 1.1 | 20 ± 1.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binda, E.; Cappelletti, P.; Marinelli, F.; Marcone, G.L. Specificity of Induction of Glycopeptide Antibiotic Resistance in the Producing Actinomycetes. Antibiotics 2018, 7, 36. https://doi.org/10.3390/antibiotics7020036
Binda E, Cappelletti P, Marinelli F, Marcone GL. Specificity of Induction of Glycopeptide Antibiotic Resistance in the Producing Actinomycetes. Antibiotics. 2018; 7(2):36. https://doi.org/10.3390/antibiotics7020036
Chicago/Turabian StyleBinda, Elisa, Pamela Cappelletti, Flavia Marinelli, and Giorgia Letizia Marcone. 2018. "Specificity of Induction of Glycopeptide Antibiotic Resistance in the Producing Actinomycetes" Antibiotics 7, no. 2: 36. https://doi.org/10.3390/antibiotics7020036
APA StyleBinda, E., Cappelletti, P., Marinelli, F., & Marcone, G. L. (2018). Specificity of Induction of Glycopeptide Antibiotic Resistance in the Producing Actinomycetes. Antibiotics, 7(2), 36. https://doi.org/10.3390/antibiotics7020036







