Abstract
Fused polyheterocyclic derivatives are available by annulation of a tetramate scaffold, and been shown to have antibacterial activity against a Gram-negative, but not a Gram-positive, bacterial strain. While the activity is not potent, these systems are structurally novel showing, in particular, a high level of polarity, and offer potential for the optimization of antibacterial activity.
1. Introduction
That the emergence of antimicrobial resistance is a challenge that must be faced urgently has been recently recognised [1] and, as a result, there is an ongoing need for new antibacterial drugs [2,3,4]. Unfortunately, this realisation has come at a time when the antibacterial pipeline is poorly populated [5], and key pharmaceutical players are exiting the area. The tetramic acid core occurs in many natural products with antibacterial activity [6,7] and although the core tetramic motif itself tends to have no antibacterial activity [8], tetramates modified with acyl side chains can exhibit high levels of antibacterial (generally Gram-positive) activity [9,10]. Of interest, however, is whether the acyltetramate system is the key pharmacophore responsible for the observed biological activity, and whether the Gram-positive potency can be extended to Gram-negative activity. A probable requirement for success in the latter regard would be the introduction of significantly higher levels of polarity and hydrophilicity to improve membrane permeation, a parameter of crucial importance for antibacterials [11,12], which are generally more polar and different to many other biologically active systems [13]. This requirement does not necessarily intersect with the requirements of Lipinski′s “rule of five” (good absorption predicted if H-bond donors < 5; H-bond acceptors < 10; molecular weight < 500; clogP < 5) which is preferred for good oral absorption in mammalian systems [14]. Some fused-ring quinolone-containing compounds which are structurally similar to tetramates, such as pyrrolo[3,4-c]quinoline-1,3-dione derivatives, exhibit inhibition of Gram-positive and Gram-negative bacteria [15] and of interest to us has been the synthesis of related fused-ring derivatives. We have reported one example of such a system [16], which was found to have a low level of Gram-negative activity, and report here an extension of that work, providing access to a small library of polycyclic heterocyclic derivatives, which also exhibit weak Gram-negative antibacterial activity.
2. Results and Discussion
Our approach began with tetramates 3a–c, which we have reported previously, to be available in enantiopure form from the esters of serine 1a, threonine 1b, and cysteine 1c (Scheme 1) [17,18]; this outcome arises as a result of the selective formation of cis-oxa(thia)zolidines 2a–c, which chemoselectively cyclise to the product tetramates under basic conditions. Reaction of each of these with trimethyl orthoformate and para-bromoaniline (using a protocol which has been recently reported [19]) gave the corresponding enamines 4a–c; these were obtained as E/Z mixtures, and the identity of the major one as Z was established by dihedral-angle-dependent coupling constants [20] from HMBC NMR experiments; thus, the intensity of the coupling between the exocyclic vinylic proton and C-5 is greater than that with C-7 in major product (Z)-4a, and vice versa in the minor product (E)-4a, clearly indicating the double bond geometry in each case (Figure 1).
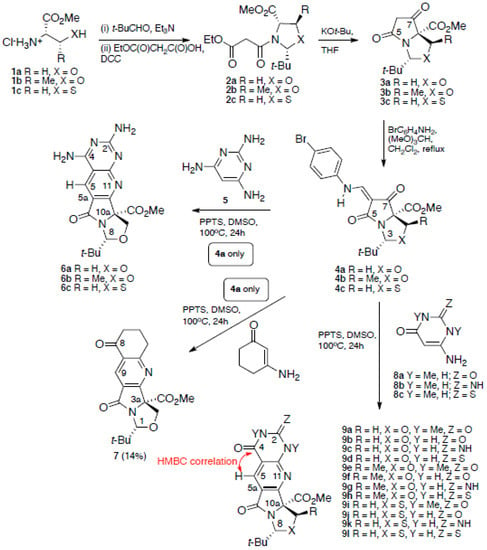
Scheme 1.
Synthetic route for key compounds.
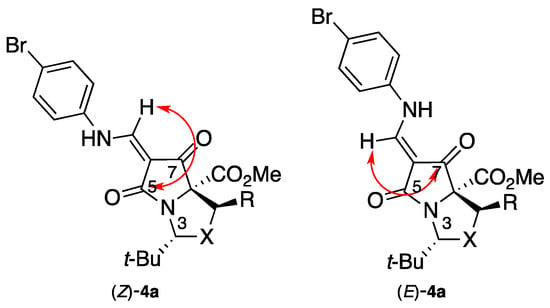
Figure 1.
NMR characterization of key compounds.
Enamine 4a, on heating with 2,4,6-triaminopyrimidine 5, had been previously converted to tetracycle 6a [16]; this reaction was found to give a better yield (68% compared to 47%) when conducted with the addition of pyridinium p-toluenesulfonate (PPTS), giving material which was identical to the previously reported material (NMR data recorded in CD3CN and MeOD respectively). Of interest was whether this approach might be extended, and the other tetramates 4b,c were examined; upon heating them with 2,4,6-triaminopyrimidine 5, products 6b,c were obtained in low yield (Table 1), but their purification was problematic due to their high basicity and polarity. Additional confirmation of the identity of 6b came from its reaction with acetic anhydride [21,22], which indicated the formation of mono and diacetylated products by HRMS analysis. We assumed that orientation of annulation was the same as we had previously reported [16] from the similar values for δH-5 in the compound series; this was confirmed from a later X-ray structure which clearly defined the product structure (vide infra).

Table 1.
Yields and NMR data for key compounds.
When this reaction was applied to 3-aminocyclohex-2-enone with tetramate 4a, the corresponding product 7 was obtained in only 5% yield, which could be improved to a maximum of only 14% yield by using DMSO at 100 °C for 24 h under acid catalysis with PPTS, but of interest is that other acyclic enamine systems (3-aminocyclopent-2-en-1-one, (Z)-3-amino-1,3-diphenylprop-2-en-1-one, 2-aminocyclohexa-2,5-diene-1,4-dione) gave no product. Extension of this approach to other enamine-containing systems, aminopyrimidines 8a-c, which have previously found application in annulation processes with enamines [23], gave the corresponding annulated products in modest to good yield in some cases (Table 1). The serine-derived system 4a gave best yields (9a–d), and interestingly the directly related threonine-derived system reacted poorly or not at all (9e-h), and this presumably reflects the hindrance to reaction imposed by the C-10 methyl group over the endo-face of the tetramate ring. The cysteine-derived systems (9i-j) behaved better, and aminopyrimidine with Z = O gave a higher yield than with Z = NH, while 9l (Z = S) gave a poor yield and could not be obtained in pure form. In the NMR spectra, similar δH-10 values in the compound series were strongly suggestive of similar structure; HMBC experiments also showed strong coupling between H-5 and C-4, supporting the formation of the proposed structure rather than alternative regioisomeric products. In the case of N-Me derivative 9a, the structural assignment was further confirmed from single crystal X-ray analysis (Figure 2).
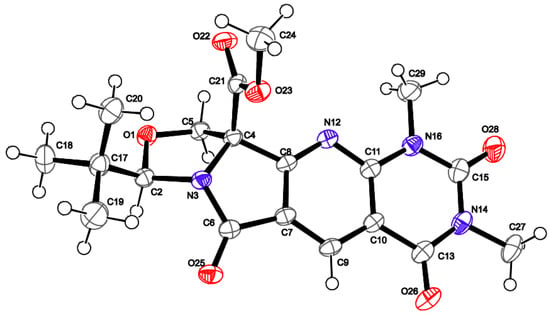
Figure 2.
The crystal structure of compound 9a with the ADPs shown at 50% probability. The second molecule has been omitted for clarity.
The use of enaminones for ring annulation by nucleophilic displacement has been widely reported [24,25] but, of interest, firstly, is the lack of reactivity of the oxazolidine system under these conditions, probably as a result of the unfavourable ring-imine tautomeric equilibrium [26] and, secondly, the regioselectivity of the annulation process, which involves displacement of the p-bromoaniline unit by the β-position of the enaminone and condensation of the remaining amine group with the C-7 carbonyl; this clearly must be the thermodynamic favourable outcome of the reaction process. Moreover, better efficiency of this process for the serine-derived tetramate 4a over the threonine- and cysteine-derived tetramates 4b,c would appear to be due the greater steric hindrance in the two latter systems.
Antimicrobial assays were carried out using the hole-plate method, using both Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus, at a concentration of 4mg/mL (Table 2); a combined solvent system (DMSO and water or MeOH) was required for appropriate solubility depending on the hydrophobicity of each compound. The diameter of the inhibition zone was measured twice, perpendicularly, and the bioactivity of each compound was compared to cephalosporin C; importantly, the solvent blank assays showed no bioactivity, confirming the activity of the test compounds. The selectivity of a number of the test compounds (6a–c, 9b, 9e, 9i, 9j) for Gram-negative (E. coli) activity over Gram-positive (S. aureus) activity is unusual and noteworthy, which we have only seen earlier with a small subset of epoxypyroglutamate systems [27,28]; by comparison, tetramate systems generally exhibit only weak Gram-negative activity but significantly stronger Gram-positive activity. Moreover, the polarity patterns are also different; in the work reported here, the most active Gram-negative systems have clogP and %PSA values in the range 1.64–1.85 and 17.6%–26.6%, respectively (Table 2), as compared to typical values of 4% and 12% observed for Gram-positive active tetramate systems [10,27]. This represents a material difference in polarity and is consistent with a requirement for higher polarity in order to achieve Gram-negative activity, to permit small molecule permeability across the Gram-negative bacterial cell wall. These hole plate bioassay results are, however, weak, and subsequent broth assays indicated MIC values of >32 μg/mL (Antimicrobial screening (broth assay) was performed by CO-ADD (The Community for Antimicrobial Drug Discovery, Brisbane, Australia), funded by the Wellcome Trust (UK) and The University of Queensland (Australia), so although these systems will not find direct therapeutic application and would require substantial optimisation for that purpose, they do indicate that selectivity in favour of Gram-negative systems is possible and not without promise.

Table 2.
Antibacterial assay results for selected compounds a.
3. Materials and Methods
The starting materials 1a–c, 2a–c, and 3a–c were all prepared using reported methods [17,18,30]. The compounds 4a and 6a have been previously reported [16], but were prepared here using slightly different methods (see below).
3.1. General Procedure for Synthesis of Enamines
Tetramic acid 4a–c (1 equiv.), para-bromoaniline (1.05 equiv.), and trimethyl orthoformate (1.05 equiv.) were dissolved in anhydrous dichloromethane (0.1 mmol/mL), and the mixture was heated under reflux for 5 h to 18.5 h. The yellow solution was cooled down to r.t. and concentrated in vacuo to give the product after column chromatography.
Methyl (3R,7aR)-6-{[(4-bromophenyl)amino]methylene}-3-(tert-butyl)-5,7-dioxodihydro-1H,3H-pyrrolo[1,2-c]oxazole-7a(5H)-carboxylate 4a
Bright yellow crystals; 1.8:1 diastereomers (3.87g, quant.); δH (400 MHz, CDCl3) Major: 0.93 (9H, s, C(CH3)3), 3.51 (1H, d, J = 8.8 Hz, C-1HAHB), 3.77 (3H, s, OCH3), 4.82 (1H, d, J = 8.8 Hz, C-1HAHB), 4.86 (1H, s, C-3H), 7.08–7.11 (2H, m, ArH), 7.53–7.56 (2H, m, ArH), 8.19 (1H, d, J = 13.6 Hz, =CH), 11.00 (1H, d, J = 13.6 Hz, NHAr); Minor: 0.92 (9H, s, C(CH3)3), 3.50 (1H, d, J = 8.8 Hz, C-1HAHB), 3.78 (3H, s, OCH3), 4.82 (1H, d, J = 8.8 Hz, C-1HAHB), 4.89 (1H, s, C-3H), 7.08–7.11 (2H, m, ArH), 7.53–7.56 (2H, m, ArH), 8.10 (1H, s, =CH), 10.88 (1H, br s, NHAr); δC (101 MHz, CDCl3) Major: 24.8 (C(CH3)3), 35.4 (C(CH3)3), 53.3 (OCH3), 68.5 (C-1), 78.1 (C-7a), 98.3 (C-3), 99.1 (C-6), 119.3, 120.2, 133.4 (all ArC), 136.8 (ArC), 146.4 (=CH), 168.3 (CO2Me), 177.7 (C-5), 189.1 (C-7); Minor: 24.86 (C(CH3)3), 35.43 (C(CH3)3), 53.33 (OCH3), 68.40 (C-1), 77.39 (C-7a), 98.35 (C-3), 100.28 (C-6), 119.41, 120.38, 133.38, 136.79 (all ArC), 147.23 (=CH), 168.37 (CO2Me), 174.35 (C-5), 192.50 (C-7); m/z (ESI+) 437.1 ([M+H]+); HRMS (ESI+) found 437.0707, 439.0686, 440.0720 ([M+H]+) requires 437.0707, 439.0686, 440.0720; νmax/cm-1 2957 (C–H), 2870 (C–H), 1748 (C=O), 1708 (C=O) 1656, 1620 (C=O); m.p. 111–114 °C; Rf 0.2 (2:8 EtOAc:petroleum ether).
Methyl (1R,3R,7aR)-6-{[(4-bromophenyl)amino]methylene}-3-(tert-butyl)-1-methyl-5,7-dioxodihydro-1H,3H-pyrrolo[1,2-c]oxazole-7a(5H)-carboxylate 4b
Light yellow crystals; 1.8:1 diastereomers (1.66 g, 88%); δH (400 MHz, CDCl3) Major: 0.92 (9H, s, C(CH3)3), 1.07 (3H, d, J = 6.8 Hz, C-1CH3), 3.76 (3H, s, OCH3), 4.97 (1H, s, C-3H), 5.10–5.16 (1H, m, C-1H), 7.07–7.11 (2H, m, ArH), 7.53–7.56 (2H, m, ArH), 8.14 (1H, d, J = 13.4 Hz, =CH), 10.91 (1H, d, J = 13.4 Hz, NHAr); Minor: 0.91 (9H, s, C(CH3)3), 1.04 (3H, d, J = 6.8 Hz, C-1CH3), 3.76 (3H, s, OCH3), 4.99 (1H, s, C-3H), 5.10-5.16 (1H, m, C-1H), 7.07–7.11 (2H, m, ArH), 7.53–7.56 (2H, m, ArH), 8.05 (1H, br s, =CH), 10.86 (1H, br s, NHAr); δC (101 MHz, CDCl3) Major: 14.9 (C-1CH3), 24.9 (C(CH3)3), 35.5 (C(CH3)3), 53.3 (OCH3), 74.9 (C-1), 81.1 (C-7a), 95.7 (C-3), 100.8 (C-6), 119.2, 120.0, 133.4, 136.9 (all ArC), 145.5 (=CH), 168.6 (CO2Me), 177.3 (C-5), 189.4 (C-7); Minor: 15.0 (C-1CH3), 24.9 (C(CH3)3), 35.5 (C(CH3)3), 53.3 (OCH3), 74.7 (C-1), 80.3 (C-7a), 95.8 (C-3), 102.0 (C-6), 119.3 (ArC), 120.2 (ArC), 133.4 (ArC), 136.9 (ArC), 146.1 (=CH), 168.7 (CO2Me), 174.0 (C-5), 192.8 (C-7); m/z (ESI−) 449.0 ([M-H]−); HRMS (ESI−) found 449.0723, 451.0702, 452.0734, 450.0754 ([M+H]−) requires 449.0718, 451.0697, 452.0731, 450.0751; νmax/cm-1 2958 (C–H), 2871 (C–H), 1747 (C=O), 1708 (C=O), 1656, 1620 (C=O); m.p. 90–92 °C; Rf 0.5 (1:5 EtOAc:petroleum ether).
Methyl (3R,7aR)-6-{[(4-bromophenyl)amino]methylene}-3-(tert-butyl)-5,7-dioxodihydro-1H,3H-pyrrolo[1,2-c]thiazole-7a(5H)-carboxylate 4c
Bright yellow crystals; 1.9:1 diastereomers (0.45 g, 30%); δH (400 MHz, CDCl3) Major: 0.98 (9H, s, C(CH3)3), 2.87 (1H, d, J = 11.4 Hz, C-1HAHB), 3.77 (3H, s, OCH3), 3.80 (1H, br s, C-1HAHB), 5.13 (1H, s, C-3H), 7.07–7.10 (2H, m, ArH), 7.52–7.56 (2H, m, ArH), 8.14 (1H, d, J = 13.5 Hz, =CH), 10.94 (1H, d, J = 13.5 Hz, NHAr); Minor: 0.97 (9H, s, C(CH3)3), 2.86 (1H, d, J = 11.4 Hz, C-1HAHB), 3.77 (3H, s, OCH3), 3.79 (1H, br s, C-1HAHB), 5.18 (1H, s, C-2H), 7.07–7.10 (2H, m, ArH), 7.52–7.56 (2H, m, ArH), 8.05 (1H, d, J = 13.5 Hz, =CH), 10.76 (1H, d, J = 13.5 Hz, NHAr); δC (101 MHz, CDCl3) Major: 26.6 (C(CH3)3), 33.7 (C-1), 36.9 (C(CH3)3), 53.4 (OCH3), 73.7 (C-3), 82.7 (C-7a), 97.7 (C-6), 119.2, 120.0, 133.4, 136.9 (all ArC), 145.9 (=CH), 168.3 (CO2Me), 175.3 (C-5), 189.8 (C-7); Minor: 26.6 (C(CH3)3), 33.9 (C-4), 37.0 (C(CH3)3), 53.4 (OCH3), 73.8 (C-2), 81.8 (C-5), 98.9 (C-7), 119.3, 120.2, 133.4, 136.9 (all ArC), 146.8 (C-10), 168.4 (CO2Me), 172.2 (C-5), 193.1 (C-7); m/z (ESI+) 453.0 ([M+H]+); HRMS (ESI+) found 453.0477, 455.0456, 456.0491, 454.0512 ([M+H]+) requires 453.0478, 455.0458, 456.0491, 454.0512; νmax/cm−1 3025(N–H), 2953 (C–H), 1748 (C=O), 1707 (C=O) 1622 (C=O); m.p. 164–167 °C; Rf 0.3 (15:85 EtOAc:petroleum ether).
3.2. General Procedure for Annulation Process
Respective tetramate enamine 4a–c (1 equiv.) was dissolved in anhydrous DMSO, along with corresponding amine and/or catalytic PPTS. The solution was stirred under nitrogen at 100 °C until all starting material was consumed by TLC analysis. After cooling to r.t. the mixture was diluted with EtOAc and washed with saturated brine. The organic layer was separated, dried over MgSO4, filtered, and concentrated in vacuo. The product was purified by flash column chromatography.
Methyl (8R,10aS)-2,4-diamino-8-(tert-butyl)-6-oxo-6H,8H-oxazolo[3′′,4′′:1′,5′] pyrrolo[3′,4′:5,6]pyrido[2,3-d]pyrimidine-10a(10H)-carboxylate 6a
2,4,6-Triaminopyrimidine (1.3 equiv.) and PPTS (0.2 equiv.) were used in DMSO (0.032 mmol/mL), reaction time 24 h. Pale pink solid (0.038 g, 68%); δH (400 MHz, CD3CN) 0.95 (9H, s, C(CH3)3), 3.51 (1H, d, J = 8.8 Hz, C-10HAHB), 3.68 (3H, s, OCH3), 4.89 (1H, s, C-8H), 4.92 (1H, d, J = 8.8 Hz, C-10HAHB), 5.87 (2H, br s, NH2), 6.61 (2H, br s, NH2), 8.53 (1H, s, C-5H); δC (101 MHz, CD3CN) 25.2 (-C(CH3)3), 36.0 (-C(CH3)3), 53.8 (-OCH3), 70.1 (C-10), 76.5 (C-10a), 98.3 (C-8), 106.5, 118.0 (C-5a, C-4a), 131.6 (C-5), 162.0, 163.6, 165.3, 165.5 (C-10b, C-4, C-2, C-11a), 170.1 (CO2Me), 176.6 (C-6); m/z (ESI+) 373.2 ([M+H]+); HRMS (ESI+) found 373.1619 ([M+H]+) requires 373.1619; νmax/cm-1 3338 (N–H), 3179 (N–H), 2958 (C–H), 1716 (C=O), 1610 (C=O), 1549 and 1479 (C=C aromatics); m.p. 215–220 °C [DEC]; Rf 0.31 (1:10 MeOH:CHCl3); [α]D25 +231.1 (c=0.7 in MeOH).
Methyl (8R,10R,10aS)-2,4-diamino-8-(tert-butyl)-10-methyl-6-oxo-6H,8H-oxazolo[3′′,4′′:1′,5′]pyrrolo[3′,4′:5,6]pyrido[2,3-d]pyrimidine-10a(10H)-carboxylate 6b
2,4,6-Triaminopyrimidine (7.5 equiv.) was used in DMSO (0.032 mmol/mL), reaction time 24 h. Pale yellow solid (0.0044 g, 10%); δH (400 MHz, CD3CN) 0.68 (3H, d, J = 6.7 Hz, C-10CH3), 0.94 (9H, s, C(CH3)3), 3.66 (3H, s, OCH3), 4.97 (1H, s, C-8H), 5.29 (1H, q, J = 6.7 Hz, C-10H), 5.75 (2H, br s, NH2), 6.52 (2H, br s, NH2), 8.52 (1H, s, C-5H); δC (101 MHz, CD3CN) 15.2 (C-10CH3), 25.2 (-C(CH3)3), 36.1 (-C(CH3) 3), 53.8(-OCH3), 75.6(C-10), 79.4 (C-10a), 95.6 (C-8), 106.3, 131.1 (C-5a, C-4a), 131.0 (C-5), 165.4, 165.5, 165.6, 168.8 (C-10b, C-4, C-2, C-11a), 170.4 (CO2Me), 173.0 (C-6); m/z (ESI+) 373.2 ([M+H]+); HRMS (ESI+) found 387.1776 ([M+H]+) requires 387.1775; νmax/cm-1 3724 (N–H), 3647 (N–H), 2973 (C-H), 1717 (C=O), 1606 (C=O), 1546 and 1469 (C=C aromatics); m.p. 200–203 °C; Rf: 0.33 (1:10 MeOH:CHCl3); [α]D25 -15.8 (c=0.3in MeOH).
Methyl (8R,10aR)-2,4-diamino-8-(tert-butyl)-6-oxo-6H,8H-thiazolo[3′′,4′′:1′,5′] pyrrolo[3′,4′:5,6]pyrido[2,3-d]pyrimidine-10a(10H)-carboxylate 6c
2,4,6-Triaminopyrimidine (2.0 equiv.) was used in DMSO (0.032 mmol/mL), reaction time 24 h. Pale yellow solid (0.015 g, 13%); δH (400 MHz, CD3CN) 1.01 (9H, s, C(CH3)3), 2.87 (1H, d, J = 11.3 Hz, C-10HAHB), 3.66 (3H, s, OCH₃), 3.90 (1H, d, J = 11.3Hz, C-10HAHB), 5.14 (1H, s, C-8H), 5.71 (2H, br s, NH2), 6.47 (2H, br s, NH2), 8.48 (1H, s, C-5H); m/z (ESI+) 389.1 ([M+H]+); HRMS (ESI+) found 389.1387 ([M+H]+) requires 389.1390; νmax/cm−1: 3348 (N-H), 3191 (N-H), 2955 (C-H), 1740 (C=O), 1620 (C=O), 1542 and 1464 (C=C aromatics); m.p. 230–233 °C [dec.]; Rf 0.27 (1:10 MeOH:CHCl3).
Methyl-(1S,3aS)-1-(tert-butyl)-8,10-dioxo-5,7,8,10-tetrahydro-1H,3H-oxazolo [3′,4′:1,2]pyrrolo[3,4-b]quinoline-3a(6H)-carboxylate 7
3-Amino-2-cyclohexen-1-one (1.3 equiv.) and PPTS (0.2 equiv.) were used in DMSO (0.018 mmol/mL), reaction time 24 h. Colourless oil (0.0058 g, 14%); δH (400 MHz, CDCl3) 1.00 (9H, s, -C(CH3)3), 2.19–2.27 (2H, m, C-6HAHB), 2.74 (2H, dd, J = 7.7 Hz, 5.5 Hz, C-7HAHB), 3.17–3.29 (2H, m, C-5HAHB), 3.40 (1H, d, J = 8.7 Hz, C3HAHB), 3.75 (3H, s, CO2CH3), 4.95 (1H, s, C-1H), 5.11 (1H, d, J = 8.7 Hz, C3HAHB), 8.69 (1H, s, C-9H); δC (101 MHz, CDCl3) 21.5 (C-6), 24.9 (C(CH3)3), 33.3 (C-5), 35.7 (C(CH3)3), 38.5 (C-7), 53.6 (-OCH3), 69.8 (C-3), 97.8 (C-1), 125.4 (C-9a), 129.7 (C-10), 133.0 (C-8a), 166.4 (C-3b), 168.6 (CO2Me), 169.1 (C-4a), 171.6 (C-10), 196.2 (C-9); m/z (ESI+) 359.4 ([M+H]+); HRMS (ESI+) found 359.3603, (MH+) requires 359.3602; νmax/cm−1 2956(C–H), 2928 (C–H), 1751 (C=O), 1720 (C=O), 1694 (C=O); Rf 0.14 (1:2 EtOAc: petroleum ether); [α]D25 +113.2 (c = 0.4 in chloroform).
Methyl (8R,10aS)-8-(tert-butyl)-1,3-dimethyl-2,4,6-trioxo-1,3,4,6-tetrahydro- 2H,8H-oxazolo[3′′,4′′:1′,5′]pyrrolo[3′,4′:5,6]pyrido[2,3-d]pyrimidine-10a(10H)-carboxylate 9a
6-Amino-1,3-dimethyluracil (4.5 equiv.) was used in DMSO (0.018 mmol/mL), reaction time 20 h. White solid (0.041g, 45%); δH (400 MHz, CDCl3) 0.98 (9H, s, C(CH3)3), 3.41 (1H, d, J = 8.7 Hz, C-10HAHB), 3.47 (3H, s, NCH3), 3.70 (3H, s, NCH3), 3.72 (3H, s, OCH₃), 4.94 (1H, s, C-8H), 5.08 (1H, d, J = 8.7 Hz, C-10HAHB), 8.82 (1H, s, C-5H); δC (101 MHz, CDCl3) 24.9 (C(CH3)3), 28.9 (NCH3), 30.5 (NCH3), 35.6 (C(CH3)3), 53.5 (OCH3), 69.6 (C-10), 75.8 (C-10a), 97.8 (C-8), 112.2 (C-4a), 121.4 (C-5a), 135.9 (C-5), 150.9 (C-2), 154.3, 160.5 (C-4, C-11a), 167.8 (C-10b), 168.2 (CO2Me), 171.1 (C-6); HRMS (ESI+) found 403.16201 ([M+H]+) requires 403.16121; νmax/cm-1 1754 (C=O), 1715 (C=O), 1670 (C=O), 1609 (C=O); m.p. 193–194 °C; Rf (1:2 EtOAc:petroleum ether) 0.35; [α]D25 +70.4 (c = 1 in chloroform). Low temperature [31] single crystal X-ray diffraction data were collected using a (Rigaku) Oxford Diffraction SuperNova diffractometer (λ=1.5418 Å). Raw frame data were reduced using CrysAlisPro and the structures were solved using “Superflip” [32] before refinement with CRYSTALS [33,34] as per the SI (CIF). Full refinement details are given in the Supporting Information (CIF). Single crystal X-ray diffraction data for compound 9a: Mr = 402.41; Monoclinic, P21; a = 12.1913(2), b = 13.3572(2), c = 12.7427(3) Å, β = 109.031(2)º, V = 1961.62(7) Å3; T = 150 K; Z = 4; µ = 0.864 mm−1. Reflections collected = 21772; independent reflections = 7313 (Rint = 0.029); R values [I > 2σ(I), 11067 reflections]: R1 = 0.0301, wR2 = 0.0718. The Flack x parameter [35,36] refined to −0.20 (11). Bayesian analysis of the Bijvoet pair [37] gave the Hooft y parameter as −0.059(71) and the probability that the structure was the correct hand was >99.9% given that the crystal is enantiopure. Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication No. 1517235 (www.ccdc.cam.ac.uk/conts/retrieving.html).
Methyl (8R,10aS)-8-(tert-butyl)-1,3-dimethyl-2,4,6-trioxo-1,3,4,6-tetrahydro-2H,8H-oxazolo[3′′,4′′:1′,5′]pyrrolo[3′,4′:5,6]pyrido[2,3-d]pyrimidine-10a(10H)-carboxylate 9b
6-Aminouracil (3.0 equiv.) was used in DMSO (0.032 mmol/mL), reaction time 24 h. White solid (0.0379 g, 82%); δH (400 MHz, MeOD) 0.97 (9H, s, C(CH3)3), 3.57 (1H, d, J = 8.6 Hz, C-10HAHB), 3.71 (3H, s, OCH3), 4.92 (1H, s, C-8H), 4.96 (1H, d, J = 8.6 Hz, C-10HAHB), 8.61 (1H, s, C-5H); δC (101 MHz, MeOD) 23.9 (C(CH3)3), 35.0 (C(CH3)3), 52.5 (OCH3), 56.9 (C-10), 68.8 (C-10a), 75.6 (C-8), 111.8, 121.1 (C-5a, C-4a), 134.1 (C-5), 150.5, 156.1, 161.9, 168.1 (C-10b, C-4, C-2, C-11a), 168.2 (CO2Me), 171.7 (C-6); m/z (ESI−) 373.1 ([M-H]−); HRMS (ESI+) found 375.1299, (MH+) requires 375.1299; νmax/cm-1 3552 (N–H), 3194 (N–H), 1717 (C=O), 1612 (C=O); m.p. 190–192 °C; Rf 0.43 (1:10 MeOH:CHCl3).
Methyl-(8R,10aS)-2-amino-8-(tert-butyl)-4-hydroxy-6-oxo-6H,8H-oxazolo[3′′,4′′:1′,5′]pyrrolo[3′,4′:5,6]pyrido[2,3-d]pyrimidine-10a(10H)-carboxylate 9c
2,4-Diamino-6-hydroxypyrimidine (3.0 equiv.) was used in DMSO (0.032 mmol/mL), reaction time 24 h. White solid (0.023 g, 49%); δH (400 MHz, DMSO) 0.91 (9H, s, C(CH3)3), 3.57 (1H, d, J = 8.6 Hz, C-10HAHB), 3.65 (3H, s, OCH3), 4.82 (1H, d, J = 8.1 Hz, C-10HAHB), 4.84 (1H, s, C-8H), 8.37 (1H, s, C-5H); δC (101 MHz, DMSO): 24.7 (C(CH3)3), 35.0 (C(CH3)3), 53.2 (OCH3), 68.7 (C-10), 75.2 (C-10a), 96.5 (C-8), 112.9, 117.9 (C-5a, C-4a), 132.5 (C-10), 156.4, 156.4, 164.4, 168.0 (C-10b, C-4, C-2, C-11a), 168.8 (CO2Me), 171.8 (C-6); m/z (ESI−) 372.1 ([M-H]−); HRMS (ESI−) found 372.1313, (MH−) requires 372.1313; νmax/cm−1 3267 (N–H), 3132 (N–H), 1727 (C=O), 1705 (C=O), 1529, 1482 (C=C aromatics); m.p. 260–263 °C [dec.]; Rf 0.29 (1:10 MeOH:CHCl3).
Methyl (8R,10aS)-8-(tert-butyl)-4,6-dioxo-2-thioxo-1,3,4,6-tetrahydro-2H,8H-oxazolo[3′′,4′′:1′,5′]pyrrolo[3′,4′:5,6]pyrido[2,3-d]pyrimidine-10a(10H)-carboxylate 9d
6-Amino-2-thiouracil (3.0 equiv.) was used in DMSO (0.032 mmol/mL), reaction time 42 h. White crystal (0.028 g, 45%); δH (400 MHz, MeOD) 0.97 (9H, s, C(CH3)3), 3.60 (1H, d, J = 8.6 Hz, C-10HAHB), 3.72 (3H, s, OCH3), 4.93 (1H, s, C-8H), 4.97 (1H, d, J = 8.6 Hz, C-10HAHB), 8.61 (1H, s, C-5H); δC (101 MHz, MeOD): 23.9 (C(CH3)3), 35.0 (C(CH3)3), 52.6 (OCH3), 68.8 (C-10), 75.7 (C-10a), 97.5 (C-8), 113.4 and 121.9 (C-5a and C-4a), 133.9 (C-5), 154.9, 159.3, 168.2, 168.3 (C-10b, C-4, C-2, C-11a), 171.4 (CO2Me), 176.6 (C-6); m/z (ESI−): 389.1 ([M-H]−); HRMS (ESI−) found 389.0925, (MH−) requires 389.0923; νmax/cm−1 1752 (C=O), 1714 (C=O), 1615 (C=C aromatics); m.p. 193-194 °C; Rf 0.60 (1:10 MeOH:CHCl3).
Methyl (8R,10R,10aS)-8-(tert-butyl)-1,3,10-trimethyl-2,4,6-trioxo-1,3,4,6-tetrahydro-2H,8H-oxazolo[3′′,4′′:1′,5′]pyrrolo[3′,4′:5,6]pyrido[2,3-d]pyrimidine-10a(10H)-carboxylate 9e
6-Amino-1,3-dimethyluracil (7.0 equiv.) was used in DMSO (0.018 mmol/mL), reaction time 24 h. White solid (0.027g, 53%); δH (400 MHz, CDCl3) 0.71 (3H, d, J = 6.7 Hz, C-10CH3) 0.96 (9H, s, C(CH3)3), 3.46 (3H, s, NCH3), 3.69 (3H, s, NCH3), 3.69 (3H, s, OCH₃), 4.99 (1H, s, C-8H), 5.37 (1H, q, J = 6.7 Hz, C-10H), 8.81 (1H, s, C-10H); δC (101 MHz, CDCl3) 13.9 (C-10CH3), 23.8 (C(CH3)3), 27.7 (NCH3), 28.7 (NCH3), 34.5 (C(CH3)3), 52.3 (OCH3), 73.5 (C-10), 77.7 (C-10a), 94.0 (C-8), 110.9 (C-4a), 121.6 (C-5a), 134.3 (C-5), 149.8 (C-2), 153.2 and 159.2 (C-4, C-11a), 166.4 (C-10b), 167.5 (CO2Me), 168.9 (C-6); m/z (ESI+) 417.2 ([M+H]+); HRMS (ESI+) found 417.1768 ([M+H]+) requires 417.1769; νmax/cm−1 2960 (C–H), 1752 (C=O), 1728 (C=O), 1672 (C=O), 1609 (C=O); m.p. 196–199 °C; Rf 0.31 (1:2 EtOAc: petroleum ether).
Methyl (8R,10aR)-8-(tert-butyl)-1,3-dimethyl-2,4,6-trioxo-1,3,4,6-tetrahydro-2H,8H-thiazolo[3′′,4′′:1′,5′]pyrrolo[3′,4′:5,6]pyrido[2,3-d]pyrimidine-10a(10H)-carboxylate 9i
6-Amino-1,3-dimethyluracil (11.0 equiv.) was used in DMSO (0.018 mmol/mL), reaction time 24 h. White solid (0.041 g, 36%); δH (400 MHz, CDCl3) 1.04 (9H, s, C(CH3)3), 2.75 (1H, d, J = 11.3 Hz, C-10HAHB), 3.49 (3H, s, NCH3), 3.71 (3H, s, NCH3), 3.71 (3H, s, OCH₃), 3.99 (1H, d, J = 11.3 Hz, C-10HAHB), 5.19 (1H, s, C-8H), 8.80 (1H, s, C-5H); δC (101 MHz, CDCl3) 26.6 (C(CH3)3), 28.8 (NCH3), 30.4 (NCH3), 34.1 (C-10), 53.5 (OCH3), 68.5 (C(CH3)3), 72.6 (C-8), 80.3 (C-10a), 112.2 (C-4a), 119.8 (C-5a), 135.7 (C-5), 151.0 (C-2), 160.4, 168.1 (C-4, C-11a), 168.1 (C-10b), 168.7 (CO2Me), 168.9 (C-6); m/z (ESI+) 419.1 ([M+H]+); HRMS (ESI+) found 419.1383 ([M+H]+) requires 419.1384; νmax/cm−1 2957 (C-H), 1723 (C=O), 1670 (C=O), 1612 (C=O); m.p. 189-192 °C; Rf: 0.37 (1:2 EtOAc:petroleum ether); [α]D25 +65.3 (c=0.3 in chloroform).
Methyl (8R,10aR)-8-(tert-butyl)-2,4,6-trioxo-1,3,4,6-tetrahydro-2H,8H-thiazolo[3′′,4′′:1′,5′]pyrrolo[3′,4′:5,6] pyrido[2,3-d]pyrimidine-10a(10H)-carboxylate 9j
6-Aminouracil (3.0 equiv.) was used in DMSO (0.032 mmol/mL), reaction time 32 h. δH (400 MHz, CD3OD) 1.02 (9H, s, C(CH3)3), 2.94 (1H, d, J = 11.3 Hz, C-10HAHB), 3.70 (3H, s, -CO2CH3), 3.88 (1H, d, J = 11.3 Hz, C-10HAHB), 5.15 (1H, s, C-8H), 8.57 (1H, s, C-5H); δC (100 MHz, CD3OD) 27.2 (C(CH3)3), 34.2 (C-10), 38.0 (C(CH3)3), 53.9 (CO2CH3), 73.6 (C-8), 81.7 (C-10a), 113.1 (C-4a), 121.0 (C-5a), 135.2 (C-5), 151.9 (C-2), 157.4 (C-11a), 163.3 (C-4), 169.5 (CO2CH3), 170.5 (C-10b), 171.0 (C-6); m/z (ESI-): 389.1 ([M-H]-); HRMS (ESI+) observed 391.1068, C17H19N4O5S requires 391.1071; Rf (5% MeOH in CHCl3) 0.41.
Methyl (8R,10aR)-8-(tert-butyl)-4,6-dioxo-2-thioxo-1,3,4,6-tetrahydro-2H,8H-thiazolo[3′′,4′′:1′,5′]pyrrolo[3′,4′:5,6]pyrido[2,3-d]pyrimidine-10a(10H)-carboxylate 9l
6-Aminouracil (3.0 equiv.) was used in DMSO (0.032 mmol/mL), reaction time 72 h. δH (400 MHz, CD3OD) 1.02 (9H, s, C(CH3)3), 2.97 (1H, d, J = 11.1 Hz, C10- HAHB), 3.60 (3H, s, CO2CH3), 3.8 (1H, d, J = 11.1 Hz, C10- HAHB), 5.15 (1H, s, C-8H), 8.58 (1H, m, C-5H).
4. Conclusions
We have shown that ring annulation on a tetramate template provides ready access to fused ring heterocycles which exhibit weak Gram-negative activity; the structural novelty of these systems is noteworthy, and their high level of polarity gives promise for further optimization of antibacterial activity.
Supplementary Materials
The following are available online at www.mdpi.com/2079-6382/6/1/2/s1, Spectra: Electronic Supporting Information, Figure S1: E. coli calibration, Figure S2: S. aureus calibration, Table S1: Bioassary data for Ceph. C, Table S2: Bioassary data for solvents.
Author Contributions
Mark G. Moloney conceived and designed the experiments; Yiyuan Chen and Jonathan G. Moloney performed the experiments; Yiyuan Chen and Jonathan G. Moloney analyzed the data; Kirsten E. Christensen performed the X-ray analysis; Mark G. Moloney wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Bush, K.; Courvalin, P.; Dantas, G.; Davies, J.; Eisenstein, B.; Huovinen, P.; Jacoby, G.A.; Kishony, R.; Kreiswirth, B.N.; Kutter, E.; et al. Tackling antibiotic resistance. Nat. Rev. Microbiol. 2011, 9, 894–896. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Pollastri, M.P.; Schiffer, C.A.; Peet, N.P. The challenge of developing robust drugs to overcome resistance. Drug Discovery Today 2011, 16, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Hurdle, J.G.; O′Neill, A.J.; Chopra, I.; Lee, R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Molecular mechanisms of antibiotic resistance. Chem. Commun. 2011, 47, 4055–4061. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Benjamin, D.K.; Bradley, J.; Guidos, R.J.; Jones, R.N.; Murray, B.E.; Bonomo, R.A.; Gilbert, D. 10 × ′20 Progress—Development of new drugs active against gram-negative bacilli: An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Royles, B.J.L. Naturally-occurring tetramic acids—Structure, isolation, and synthesis. Chem. Rev. 1995, 95, 1981–2001. [Google Scholar] [CrossRef]
- Schobert, R.; Schlenk, A. Tetramic and tetronic acids: An update on new derivatives and biological aspects. Bioorg. Med. Chem. 2008, 16, 4203–4221. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-C.; Moloney, M.G. Tetramic acids as scaffolds: Synthesis, tautomeric and antibacterial behaviour. Synlett 2009, 15, 2487–2491. [Google Scholar]
- Jeong, Y.-C.; Moloney, M.G.; Bikadi, Z.; Hazai, E. A detailed study of antibacterial 3-acyltetramic acids and 3-acylpiperidine-2,4-diones. ChemMedChem 2014, 9, 1826–1837. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-C.; Anwar, M.; Moloney, M.G.; Bikadi, Z.; Hazai, E. Natural product inspired antibacterial tetramic acid libraries with dual enzyme target activity. Chem. Sci. 2013, 4, 1008–1015. [Google Scholar] [CrossRef]
- Weidenmaier, C.; Peschel, A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat. Rev. Microbiol. 2008, 6, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.W.; Wade, M.M.; Holman, S.C.; Champlin, F.R. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J. Microbiol. Methods 2001, 43, 153–164. [Google Scholar] [CrossRef]
- O’Shea, R.; Moser, H.E. Physicochemical properties of antibacterial compounds: Implications for drug discovery. J. Med. Chem. 2008, 51, 2871–2878. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Xia, L.; Idhayadhulla, A.; Lee, Y.R.; Kim, S.H.; Wee, Y.-J. Microwave-assisted synthesis of diverse pyrrolo[3,4-c]quinoline-1,3- diones and their antibacterial activities. ACS Comb. Sci. 2014, 16, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Moloney, M.G. (8r,10as)-methyl 2,4-diamino-8-(tert-butyl)-6-oxo-6,8,10,10a-tetrahydro-oxazolo-[3′′,4′′:1′,5′]-pyrrolo[3′,4′:5,6]pyrido[2,3-d]pyrimidine-10a-carboxylate. Molbank 2015. [Google Scholar] [CrossRef]
- Josa-Culleré, L.; Moloney, M.G.; Thompson, A.L. Stereoselectivity in the reduction of bicyclic tetramates. Synlett 2016, 27, 1677–1681. [Google Scholar]
- Anwar, M.; Moloney, M.G. Chiral bicyclic tetramates as non-planar templates for chemical library synthesis. Chem. Biol. Drug Des. 2013, 81, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Oshega, J.S.; Paponov, B.V.; Omelchenko, I.V.; Shishkin, O.V. One-pot three-component synthesis of 3-cyano-4-methyl-2,6-dioxopyridine amino enones. Mendeleev Commun. 2015, 25, 133–134. [Google Scholar] [CrossRef]
- Summers, M.F.; Bax, A. H and 13c assignments from sensitivity-enhanced detection of heteronuclear multiple-bond connectivity by 2d multiple quantum nmr. J. Am. Chem. Soc. 1986, 108, 2093–2094. [Google Scholar]
- Liu, H.; Li, X. Synthesis of protected sugar-amino acid hybrid molecules as platform for further derivatization. Tetrahedron Lett. 2012, 53, 6957–6960. [Google Scholar] [CrossRef]
- Taylor, E.C.; Palmer, D.C.; George, T.J.; Fletcher, S.R.; Tseng, C.P.; Harrington, P.J.; Beardsley, G.P.; Dumas, D.J.; Rosowsky, A.; Wick, M. Synthesis and biological activity of L-5-deazafolic acid and L-deazaaminopterin: synthetic strategies to 5-deazapteridines. J. Org. Chem. 1983, 48, 4852–4860. [Google Scholar] [CrossRef]
- Stark, E.; Breitmaier, E. 5-Desazapteridine, synthese und NMR-spektroskopie. Tetrahedron 1973, 29, 2209–2217. [Google Scholar] [CrossRef]
- Hafiz, A.; Saad, I.; Reheim, M.A.; Ahmed, M.; Mohamed Baker, S.; Mahfouz Ramiz, M. Enaminone in heterocyclic synthesis: synthesis of new pyrazolopyrazole, pyrazolothienooxazine and pyrazolothienopyridine derivatives. J. Chem. Soc. Pak. 2014, 36, 1133–1144. [Google Scholar]
- Abu-Shanab, F.A.; Sherif, S.M.; Mousa, S.A.S. Dimethylformamide dimethyl acetal as a building block in heterocyclic synthesis. J. Heterocycl. Chem. 2009, 46, 801–827. [Google Scholar] [CrossRef]
- Singh, K.; Singh, J.; Singh, H. Carbon transfer reactions of functionalized oxazolidines and their open chain enamine tautomers to enamine nucleophiles. A facile synthesis of substituted pyridines and ring annulated derivatives. Tetrahedron 1998, 54, 935–942. [Google Scholar] [CrossRef]
- Jeong, Y.-C.; Moloney, M.G. Equisetin, reutericyclin and streptolodygin as natural product lead structures for novel antibiotic libraries. Future Med. Chem. 2015, 7, 1861–1877. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.S.W.; Chai, C.L.L.; Moloney, M.G.; Thompson, A.L. Synthesis of mimics of pramanicin from pyroglutamic acid and their antibacterial activity. J. Org. Chem. 2015, 80, 2661–2675. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Warren, S.C.; Newton, G.G.F.; Abraham, E.P. Biosynthesis of penicillin N and cephalosporin C—Antibiotic production and other features of metabolism of a cephalosporium species. Biochem. J. 1967, 103, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Holloway, C.A.; Matthews, C.J.; Moloney, M.G.; Roberts, C.F.; Yaqoob, M. Novel chiral skeletons for drug discovery: Antibacterial tetramic acids. Chem. Biol. Drug Des. 2011, 78, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Cosier, J.; Glazer, A.M. A nitrogen-gas-stream cryostat for general X-ray diffraction studies. J. Appl. Cryst. 1986, 19, 105–107. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. Superflip—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Parois, P.; Cooper, R.I.; Thompson, A.L. Crystal structures of increasingly large molecules: Meeting the challenges with CRYSTALS software. Chem. Cent. J. 2015, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.I.; Thompson, A.L.; Watkin, D.J. CRYSTALS enhancements: Dealing with hydrogen atoms in refinement. J. Appl. Cryst. 2010, 43, 1100–1107. [Google Scholar] [CrossRef]
- Flack, H.D. On enantiomorph-polarity estimation. Acta Crystallogr. 1983, A39, 876–881. [Google Scholar] [CrossRef]
- Flack, H.D.; Bernardinelli, G. Reporting and evaluating absolute-structure and absolute-configuration determinations. J. Appl. Cryst. 2000, 33, 1143–1148. [Google Scholar] [CrossRef]
- Hooft, R.W.W.; Straver, L.H.; Spek, A.L. Determination of absolute structure using Bayesian statistics on Bijvoet differences. J. Appl. Cryst. 2008, 41, 96–103. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).