Antimicrobial Action of Essential Oil of Tagetes minuta: Role of the Bacterial Membrane in the Mechanism of Action
Abstract
1. Introduction
2. Results and Discussion
2.1. EO Characterization
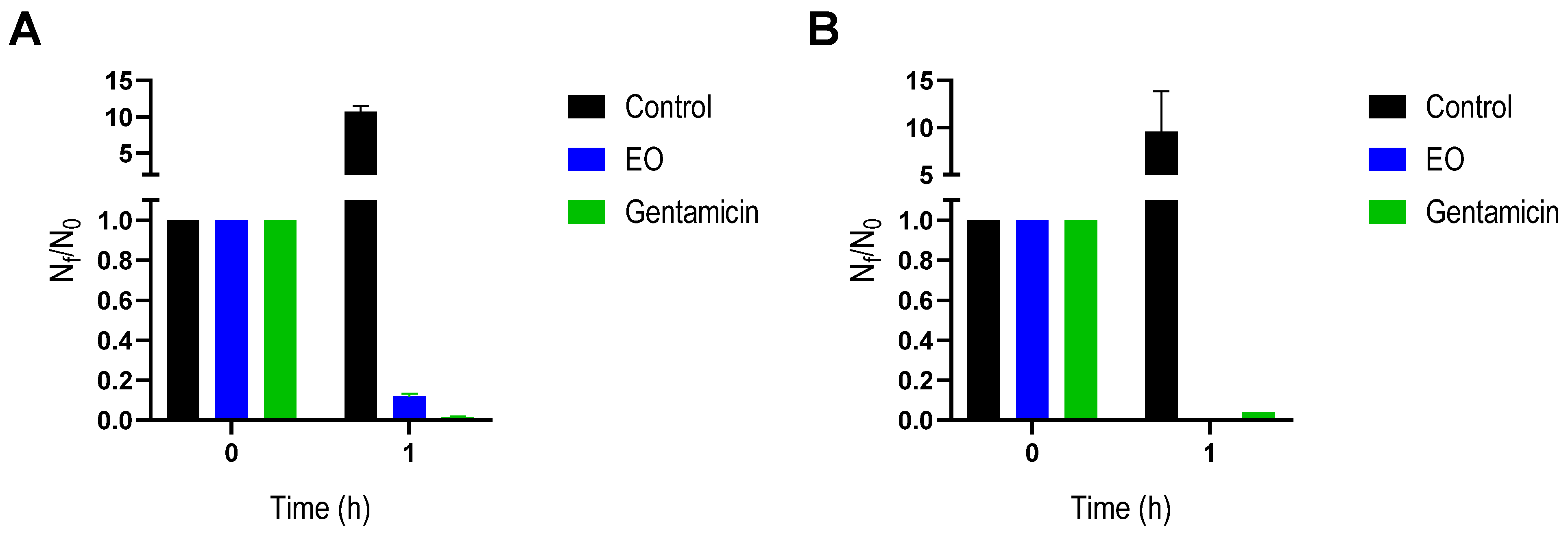
2.2. Antibacterial Activity
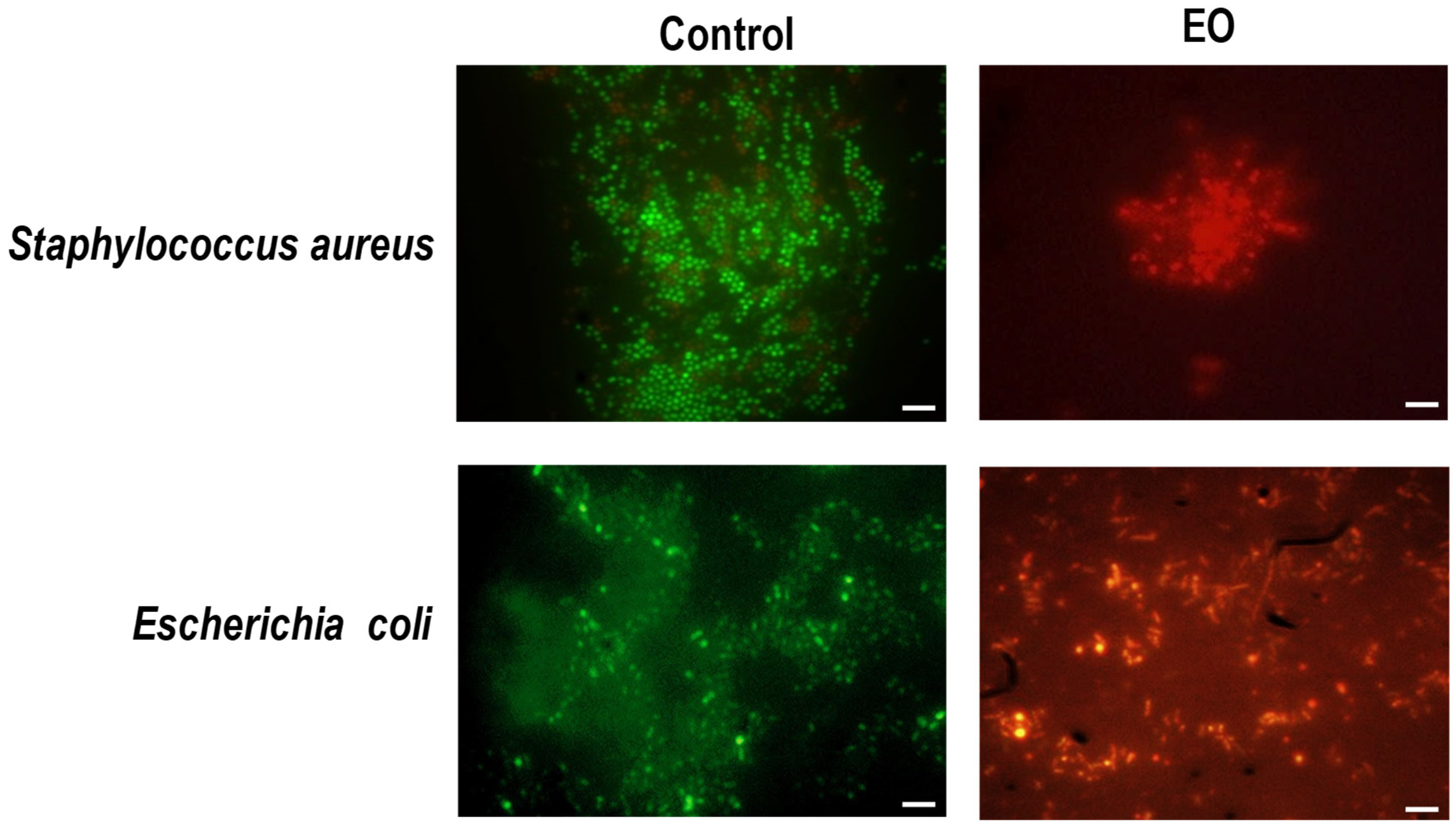
2.3. Interaction of EO with Bacterial Envelope
2.4. Effect of EO on Bacterial Membrane
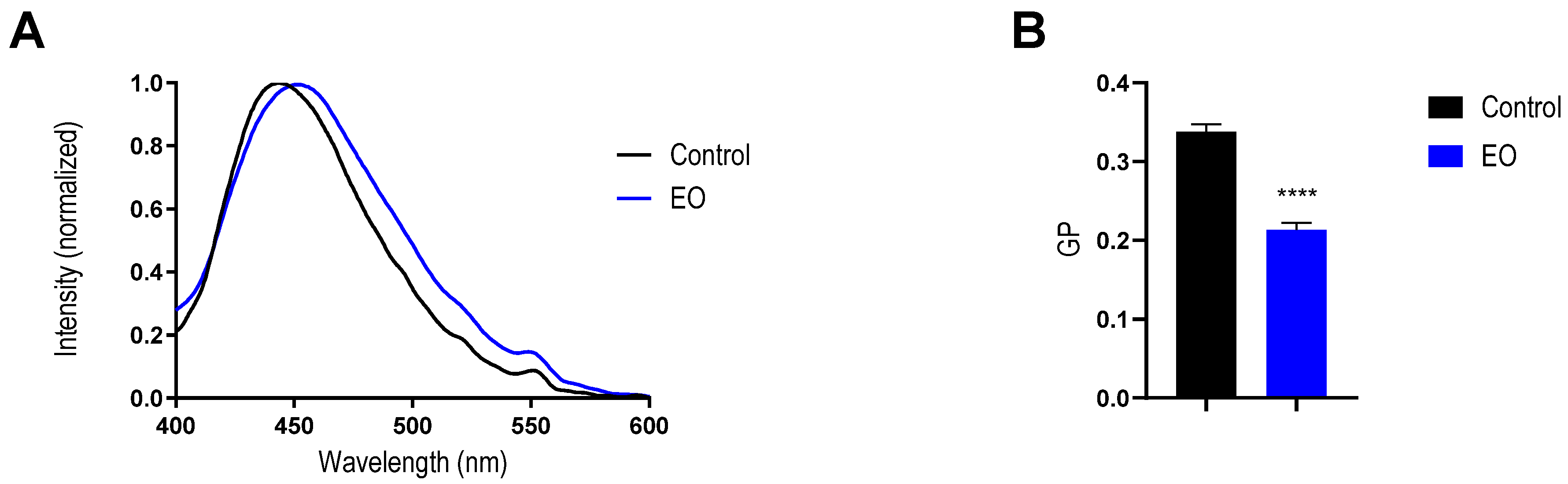
2.5. Effect of EO on Mimetic Lipid Membrane
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Bacterial Strains
4.3. Plant Material, Isolation, and Characterization of Essential Oil
4.4. MIC and MBC Determinations
4.5. Bacterial Viability
4.6. Fluorescence Microscopy
4.7. Fluorescence Spectroscopy
4.8. Zeta Potential
4.9. Liposome Preparations
4.10. Dipole Potential
4.11. Transition Temperature (Tm) by DLS
4.12. Laurdan Fluorescence Spectroscopy in LUVs
4.13. Laurdan Fluorescence Spectroscopy in Bacteria
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DMPC | 1,2-dimyristoyl-sn-glycero-3-phosphocholine |
| DMPG | 1,2-dimyristoyl-sn-glycero-3-phospho-1′-rac-glycerol |
| DMSO | Dimethyl sulfoxide |
| EO | Essential oil |
| GP | Generalized Polarization |
| LUV | Large unilamellar vesicles |
| MBC | Minimal bactericidal concentration |
| MH | Muller Hinton |
| MIC | Minimal inhibitory concentration |
| MLV | multilamellar vesicles |
| PI | Propidium iodide |
| RI | Retention index |
References
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on Plant Antimicrobials: A Mechanistic Viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef]
- Alibi, S.; Crespo, D.; Navas, J. Plant-Derivatives Small Molecules with Antibacterial Activity. Antibiotics 2021, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Ugboko, H.U.; Nwinyi, O.C.; Oranusi, S.U.; Fatoki, T.H.; Omonhinmin, C.A. Antimicrobial Importance of Medicinal Plants in Nigeria. Sci. World J. 2020, 2020, 7059323. [Google Scholar] [CrossRef]
- Assadpour, E.; Can Karaça, A.; Fasamanesh, M.; Mahdavi, S.A.; Shariat-Alavi, M.; Feng, J.; Kharazmi, M.S.; Rehman, A.; Jafari, S.M. Application of Essential Oils as Natural Biopesticides; Recent Advances. Crit. Rev. Food Sci. Nutr. 2023, 64, 6477–6497. [Google Scholar] [CrossRef]
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Al-Sadi, A.M. Biosynthesis of Essential Oils in Aromatic Plants: A Review. Food Rev. Int. 2016, 32, 117–160. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K.; Tan, N.P.; Chong, C.W.; Abushelaibi, A.; Lim, S.H.E.; Lai, K.S. The Missing Piece: Recent Approaches Investigating the Antimicrobial Mode of Action of Essential Oils. Evol. Bioinform. 2021, 17, 1176934320938391. [Google Scholar] [CrossRef]
- Gakuubi, M.; Wanzala, W.; Wagacha, M.; Dossaji, S. Bioactive Properties of Tagetes minuta L. (Asteraceae) Essential Oils: A Review. Am. J. Essent. Oils Nat. Prod. 2016, 4, 27–36. [Google Scholar]
- Abdoul-Latif, F.M.; Elmi, A.; Merito, A.; Nour, M.; Risler, A.; Ainane, A.; Bignon, J.; Ainane, T. Essential Oils of Tagetes minuta and Lavandula coronopifolia from Djibouti: Chemical Composition, Antibacterial Activity and Cytotoxic Activity against Various Human Cancer Cell Lines. Int. J. Plant Biol. 2022, 13, 315–329. [Google Scholar] [CrossRef]
- Alonso, J.D.C. Plantas Medicinales Autóctonas de La Argentina; LOLA (Literature of Latin America): Buenos Aires, Argentina, 2005; ISBN 9789871860258. [Google Scholar]
- Al-Robai, S.A.; Ahmed, A.A.; Ahmed, A.A.E.; Zabin, S.A.; Mohamed, H.A.; Alghamdi, A.A.A. Phenols, Antioxidant and Anticancer Properties of Tagetes minuta, Euphorbia granulata and Galinsoga parviflora: In Vitro and in Silico Evaluation. J. Umm Al-Qura Univ. Appl. Sci. 2023, 9, 15–28. [Google Scholar] [CrossRef]
- Shirazi, M.T.; Gholami, H.; Kavoosi, G.; Rowshan, V.; Tafsiry, A. Chemical Composition, Antioxidant, Antimicrobial and Cytotoxic Activities of Tagetes minuta and Ocimum basilicum Essential Oils. Food Sci. Nutr. 2014, 2, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Walia, S.; Mukhia, S.; Bhatt, V.; Kumar, R.; Kumar, R. Variability in Chemical Composition and Antimicrobial Activity of Tagetes minuta L. Essential Oil Collected from Different Locations of Himalaya. Ind. Crops Prod. 2020, 150, 112449. [Google Scholar] [CrossRef]
- Senatore, F.; Napolitano, F.; Mohamed, M.A.H.; Harris, P.J.C.; Mnkeni, P.N.S.; Henderson, J. Antibacterial Activity of Tagetes minuta L. (Asteraceae) Essential Oil with Different Chemical Composition. Flavour Fragr. J. 2004, 19, 574–578. [Google Scholar] [CrossRef]
- Gakuubi, M.M.; Wagacha, J.M.; Dossaji, S.F.; Wanzala, W. Chemical Composition and Antibacterial Activity of Essential Oils of Tagetes minuta (Asteraceae) against Selected Plant Pathogenic Bacteria. Int. J. Microbiol. 2016, 2016, 7352509. [Google Scholar] [CrossRef]
- Verma, N.; Aggarwal, N.; Sood, P. Exploring the Phytochemistry and Biological Potential of T. minuta (L.): A Comprehensive Review. S. Afr. J. Bot. 2024, 168, 175–195. [Google Scholar] [CrossRef]
- dos Santos, D.C.; Schneider, L.R.; da Silva Barboza, A.; Diniz Campos, Â.; Lund, R.G. Systematic Review and Technological Overview of the Antimicrobial Activity of Tagetes minuta and Future Perspectives. J. Ethnopharmacol. 2017, 208, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.J.; Park, S.M.; Yu, H.; Seo, G.H.; Kim, H.W.; Kim, S.A.; Rhee, M.S. Recent Advances in the Application of Antibacterial Complexes Using Essential Oils. Molecules 2020, 25, 1752. [Google Scholar] [CrossRef]
- Awadh Ali, N.A.; Sharopov, F.S.; Al-kaf, A.G.; Hill, G.M.; Arnold, N.; Al-Sokari, S.S.; Setzer, W.N.; Wessjohann, L. Composition of Essential Oil from Tagetes minuta and Its Cytotoxic, Antioxidant and Antimicrobial Activities. Nat. Prod. Commun. 2014, 9, 265–268. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Guliani, A.; Pooja; Verma, M.; Kumari, A.; Acharya, A. Retaining the ‘Essence’ of Essential Oil: Nanoemulsions of Citral and Carvone Reduced Oil Loss and Enhanced Antibacterial Efficacy via Bacterial Membrane Perturbation: Citral and Carvone Nanoemulsions Were Prepared Using Brij-58 and Lantana Camara Leaf Ex. J. Drug Deliv. Sci. Technol. 2021, 61, 102243. [Google Scholar] [CrossRef]
- Plésiat, P.; Nikaido, H. Outer Membranes of Gram-negative Bacteria Are Permeable to Steroid Probes. Mol. Microbiol. 1992, 6, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In Vitro Antibacterial Activity of Some Plant Essential Oils. BMC Complement. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Van, N.T.B.; Vi, O.T.; Yen, N.T.P.; Nhung, N.T.; Van Cuong, N.; Kiet, B.T.; Van Hoang, N.; Hien, V.B.; Thwaites, G.; Campell, J.; et al. Minimum Inhibitory Concentrations of Commercial Essential Oils against Common Chicken Pathogenic Bacteria and Their Relationship with Antibiotic Resistance. J. Appl. Microbiol. 2022, 132, 1025–1035. [Google Scholar] [CrossRef]
- Gonzalez-Pastor, R.; Carrera-Pacheco, S.E.; Zúñiga-Miranda, J.; Rodríguez-Pólit, C.; Mayorga-Ramos, A.; Guamán, L.P.; Barba-Ostria, C. Current Landscape of Methods to Evaluate Antimicrobial Activity of Natural Extracts. Molecules 2023, 28, 1068. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Ciumac, D.; Gong, H.; Hu, X.; Lu, J.R. Membrane Targeting Cationic Antimicrobial Peptides. J. Colloid Interface Sci. 2019, 537, 163–185. [Google Scholar] [CrossRef]
- Deslouches, B.; Steckbeck, J.D.; Craigo, J.K.; Doi, Y.; Mietzner, T.A.; Montelaro, R.C. Rational Design of Engineered Cationic Antimicrobial Peptides Consisting Exclusively of Arginine and Tryptophan, and Their Activity against Multidrug-Resistant Pathogens. Antimicrob. Agents Chemother. 2013, 57, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra Maillard, A.P.V.; Espeche, J.C.; Maturana, P.; Cutro, A.C.; Hollmann, A. Zeta Potential beyond Materials Science: Applications to Bacterial Systems and to the Development of Novel Antimicrobials. Biochim. Biophys. Acta—Biomembr. 2021, 1863, 183597. [Google Scholar] [CrossRef]
- Domingues, M.M.; Silva, P.M.; Franquelim, H.G.; Carvalho, F.A.; Castanho, M.A.R.B.; Santos, N.C. Antimicrobial Protein RBPI21-Induced Surface Changes on Gram-Negative and Gram-Positive Bacteria. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 543–551. [Google Scholar] [CrossRef]
- Alves, C.S.; Melo, M.N.; Franquelim, H.G.; Ferre, R.; Planas, M.; Feliu, L.; Bardají, E.; Kowalczyk, W.; Andreu, D.; Santos, N.C.; et al. Escherichia coli Cell Surface Perturbation and Disruption Induced by Antimicrobial Peptides BP100 and PepR. J. Biol. Chem. 2010, 285, 27536–27544. [Google Scholar] [CrossRef]
- Ferreyra Maillard, A.; Gonçalves, S.; Santos, N.C.; López de Mishima, B.A.; Dalmasso, P.R.; Hollmann, A. Studies on Interaction of Green Silver Nanoparticles with Whole Bacteria by Surface Characterization Techniques. Biochim. Biophys. Acta—Biomembr. 2019, 1861, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Cutro, A.C.; Castelli, M.V.; López, S.N.; Rosales, M.A.; Hollmann, A.; Rodriguez, S.A. Chemical Composition of Schinus areira Essential Oil and Antimicrobial Action against Staphylococcus aureus. Nat. Prod. Res. 2021, 35, 2931–2936. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K.; Yusoff, K.; Thomas, W.; Akseer, R.; Alhosani, M.S.; Abushelaibi, A.; Lim, S.H.E.; Lai, K.S. Lavender Essential Oil Induces Oxidative Stress Which Modifies the Bacterial Membrane Permeability of Carbapenemase Producing Klebsiella pneumoniae. Sci. Rep. 2020, 10, 819. [Google Scholar] [CrossRef]
- Rao, J.; Chen, B.; McClements, D.J. Improving the Efficacy of Essential Oils as Antimicrobials in Foods: Mechanisms of Action. Annu. Rev. Food Sci. Technol. 2019, 10, 365–387. [Google Scholar] [CrossRef]
- Cutro, A.C.; Coria, M.S.; Bordon, A.; Rodriguez, S.A.; Hollmann, A. Antimicrobial Properties of the Essential Oil of Schinus areira (Aguaribay) against Planktonic Cells and Biofilms of S. aureus. Arch. Biochem. Biophys. 2023, 744, 109670. [Google Scholar] [CrossRef] [PubMed]
- Espina, L.; Gelaw, T.K.; de Lamo-Castellví, S.; Pagán, R.; García-Gonzalo, D. Mechanism of Bacterial Inactivation by (+)-Limonene and Its Potential Use in Food Preservation Combined Processes. PLoS ONE 2013, 8, e56769. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The Antibacterial Mechanism of Carvacrol and Thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Matos, P.M.; Franquelim, H.G.; Castanho, M.A.R.B.; Santos, N.C. Quantitative Assessment of Peptide-Lipid Interactions. Ubiquitous Fluorescence Methodologies. Biochim. Biophys. Acta—Biomembr. 2010, 1798, 1999–2012. [Google Scholar] [CrossRef]
- Gross, E.; Bedlack, R.S.; Loew, L.M. Dual-Wavelength Ratiometric Fluorescence Measurement of the Membrane Dipole Potential. Biophys. J. 1994, 67, 208–216. [Google Scholar] [CrossRef]
- Efimova, S.S.; Ostroumova, O.S. Modulation of the Dipole Potential of Model Lipid Membranes with Phytochemicals: Molecular Mechanisms, Structure–Activity Relationships, and Implications in Reconstituted Ion Channels. Membranes 2023, 13, 453. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. The Biophysical Interaction of Ferulic Acid with Liposomes as Biological Membrane Model: The Effect of the Lipid Bilayer Composition. J. Mol. Liq. 2021, 324, 114689. [Google Scholar] [CrossRef]
- Michel, N.; Fabiano, A.S.; Polidori, A.; Jack, R.; Pucci, B. Determination of Phase Transition Temperatures of Lipids by Light Scattering. Chem. Phys. Lipids 2006, 139, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Gharib, R.; Auezova, L.; Charcosset, C.; Greige-Gerges, H. Effect of a Series of Essential Oil Molecules on DPPC Membrane Fluidity: A Biophysical Study. J. Iran. Chem. Soc. 2018, 15, 75–84. [Google Scholar] [CrossRef]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of Four Monoterpenes Contained in Essential Oils with Model Membranes: Implications for Their Antibacterial Activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef]
- Perez-Lopez, M.I.; Mendez-Reina, R.; Trier, S.; Herrfurth, C.; Feussner, I.; Bernal, A.; Forero-Shelton, M.; Leidy, C. Variations in Carotenoid Content and Acyl Chain Composition in Exponential, Stationary and Biofilm States of Staphylococcus aureus, and Their Influence on Membrane Biophysical Properties. Biochim. Biophys. Acta—Biomembr. 2019, 1861, 978–987. [Google Scholar] [CrossRef]
- Parasassi, T.; Gratton, E. Membrane Lipid Domains and Dynamics as Detected by Laurdan Fluorescence. J. Fluoresc. 1995, 5, 59–69. [Google Scholar] [CrossRef]
- Sherry, M.; Charcosset, C.; Fessi, H.; Greige-Gerges, H. Essential Oils Encapsulated in Liposomes: A Review. J. Liposome Res. 2013, 23, 268–275. [Google Scholar] [CrossRef]
- Kavoosi, G.; Rowshan, V. Chemical Composition, Antioxidant and Antimicrobial Activities of Essential Oil Obtained from Ferula assa-Foetida Oleo-Gum-Resin: Effect of Collection Time. Food Chem. 2013, 138, 2180–2187. [Google Scholar] [CrossRef]
- Blake, M.J.; Page, E.F.; Smith, M.E.; Calhoun, T.R. Miltefosine Impacts Small Molecule Transport in Gram-Positive Bacteria. RSC Chem. Biol. 2024, 5, 981–988. [Google Scholar] [CrossRef]
- Pengfei, S.; Yaqian, L.; Lanlan, X.; Zehao, L.; Yimin, L.; Shasha, L.; Linhui, L.; Yifan, Y.; Linying, Z.; Yong, W. L007-0069 Kills Staphylococcus aureus in High Resistant Phenotypes. Cell. Mol. Life Sci. 2022, 79, 552. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yusoff, K.; Lim, S.H.E.; Chong, C.M.; Lai, K.S. Membrane Disruption Properties of Essential Oils-a Double-Edged Sword? Processes 2021, 9, 595. [Google Scholar] [CrossRef]
- Mattar, V.T.; Borioni, J.L.; Hollmann, A.; Rodriguez, S.A. Insecticidal Activity of the Essential Oil of Schinus areira against Rhipibruchus picturatus (F.) (Coleoptera: Bruchinae), and Its Inhibitory Effects on Acetylcholinesterase. Pestic. Biochem. Physiol. 2022, 185, 105134. [Google Scholar] [CrossRef]
- Adams, R.; Sparkman, O. Review of Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2007, 18, 803–806. [Google Scholar]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Antibacterial Activity and Mechanism of Ginger Essential Oil against Escherichia coli and Staphylococcus aureus. Molecules 2020, 25, 3955. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards, N. M02-A12: Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—Twelfth Edition. Clin. Lab. Stand. Inst. 2015, 35, 73. [Google Scholar]
- Espeche, J.C.; Varas, R.; Maturana, P.; Cutro, A.C.; Maffía, P.C.; Hollmann, A. Membrane Permeability and Antimicrobial Peptides: Much More than Just Making a Hole. Pept. Sci. 2023, 116, e24305. [Google Scholar] [CrossRef]
- Szoka, F.; Olson, F.; Heath, T.; Vail, W.; Mayhew, E.; Papahadjopoulos, D. Preparation of Unilamellar Liposomes of Intermediate Size (0.1-0.2 Μm) by a Combination of Reverse Phase Evaporation and Extrusion through Polycarbonate Membranes. Biochim. Biophys. Acta—Biomembr. 1980, 601, 559–571. [Google Scholar] [CrossRef]
- Maturana, P.; Martinez, M.; Noguera, M.E.; Santos, N.C.; Disalvo, E.A.; Semorile, L.; Maffia, P.C.; Hollmann, A. Lipid Selectivity in Novel Antimicrobial Peptides: Implication on Antimicrobial and Hemolytic Activity. Colloids Surf. B Biointerfaces 2017, 153, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.; Pisco, S.; Silva, A.S.; Nunes, C.; Reis, S. Evaluation of the Effect of Rifampicin on the Biophysical Properties of the Membranes: Significance for Therapeutic and Side Effects. Int. J. Pharm. 2014, 466, 190–197. [Google Scholar] [CrossRef]
- Parasassi, T.; De Stasio, G.; Ravagnan, G.; Rusch, R.M.; Gratton, E. Quantitation of Lipid Phases in Phospholipid Vesicles by the Generalized Polarization of Laurdan Fluorescence. Biophys. J. 1991, 60, 179–189. [Google Scholar] [CrossRef]
- Bessa, L.J.; Ferreira, M.; Gameiro, P. Data on Laurdan Spectroscopic Analyses to Compare Membrane Fluidity between Susceptible and Multidrug-Resistant Bacteria. Data Br. 2018, 21, 128–132. [Google Scholar] [CrossRef] [PubMed]




| Peak | Compound 1 | Area (%) | RI 2 |
|---|---|---|---|
| 1 | β-Pinene | 12.34 | 1004 |
| 2 | Limonene | 7.93 | 1024 |
| 3 | α-Pinene epoxide | 1.19 | 1045 |
| 4 | β-Ocimene | 0.35 | 1054 |
| 5 | Dihydrotagetone | 1.25 | 1063 |
| 6 | γ-Terpinene | 0.85 | 1078 |
| 7 | 1-Octanol | 0.49 | 1086 |
| 8 | α-Terpinolene | 10.75 | 1099 |
| 9 | (E)-Tagetone | 16.60 | 1129 |
| 10 | (Z)-Tagetone | 0.84 | 1153 |
| 11 | Terpinen-4-ol | 0.73 | 1170 |
| 12 | Carvyl acetate | 0.86 | 1186 |
| 13 | Verbenone | 0.41 | 1192 |
| 14 | Thymol | 6.12 | 1210 |
| 15 | (Z)-Ocimenone | 12.57 | 1219 |
| 16 | (E)-Ocimenone | 4.92 | 1257 |
| 17 | Bornyl acetate | 2.27 | 1300 |
| 18 | Isolongifolene | 0.94 | 1332 |
| 19 | β-Resorcylaldehyde | 0.54 | 1346 |
| 20 | β-Elemene | 1.27 | 1356 |
| 21 | (Z)-Jasmone | 5.61 | 1378 |
| 22 | Isocaryophillene | 2.66 | 1395 |
| 23 | (E)-Caryophyllene | 2.12 | 1434 |
| 24 | α-Cadinene | 0.62 | 1446 |
| 25 | α-Humulene | 1.20 | 1459 |
| 26 | Bicyclogermacrene | 1.74 | 1473 |
| 27 | Spathulenol | 2.83 | 1563 |
| - | Total | 100 | - |
| Staphylococcus aureus | Escherichia coli | ||
|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) |
| 8.5 | >17.0 | 17.0 | 17.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordón, A.; Rodríguez, S.A.; Chaves, D.S.d.A.; Cutró, A.C.; Hollmann, A. Antimicrobial Action of Essential Oil of Tagetes minuta: Role of the Bacterial Membrane in the Mechanism of Action. Antibiotics 2025, 14, 632. https://doi.org/10.3390/antibiotics14070632
Bordón A, Rodríguez SA, Chaves DSdA, Cutró AC, Hollmann A. Antimicrobial Action of Essential Oil of Tagetes minuta: Role of the Bacterial Membrane in the Mechanism of Action. Antibiotics. 2025; 14(7):632. https://doi.org/10.3390/antibiotics14070632
Chicago/Turabian StyleBordón, Anahí, Sergio A. Rodríguez, Douglas Siqueira de Almeida Chaves, Andrea C. Cutró, and Axel Hollmann. 2025. "Antimicrobial Action of Essential Oil of Tagetes minuta: Role of the Bacterial Membrane in the Mechanism of Action" Antibiotics 14, no. 7: 632. https://doi.org/10.3390/antibiotics14070632
APA StyleBordón, A., Rodríguez, S. A., Chaves, D. S. d. A., Cutró, A. C., & Hollmann, A. (2025). Antimicrobial Action of Essential Oil of Tagetes minuta: Role of the Bacterial Membrane in the Mechanism of Action. Antibiotics, 14(7), 632. https://doi.org/10.3390/antibiotics14070632









