The Accuracy of Empirical Antibiotic Treatment for Periprosthetic Joint Infections in Total Shoulder and Knee Arthroplasties
Abstract
1. Introduction
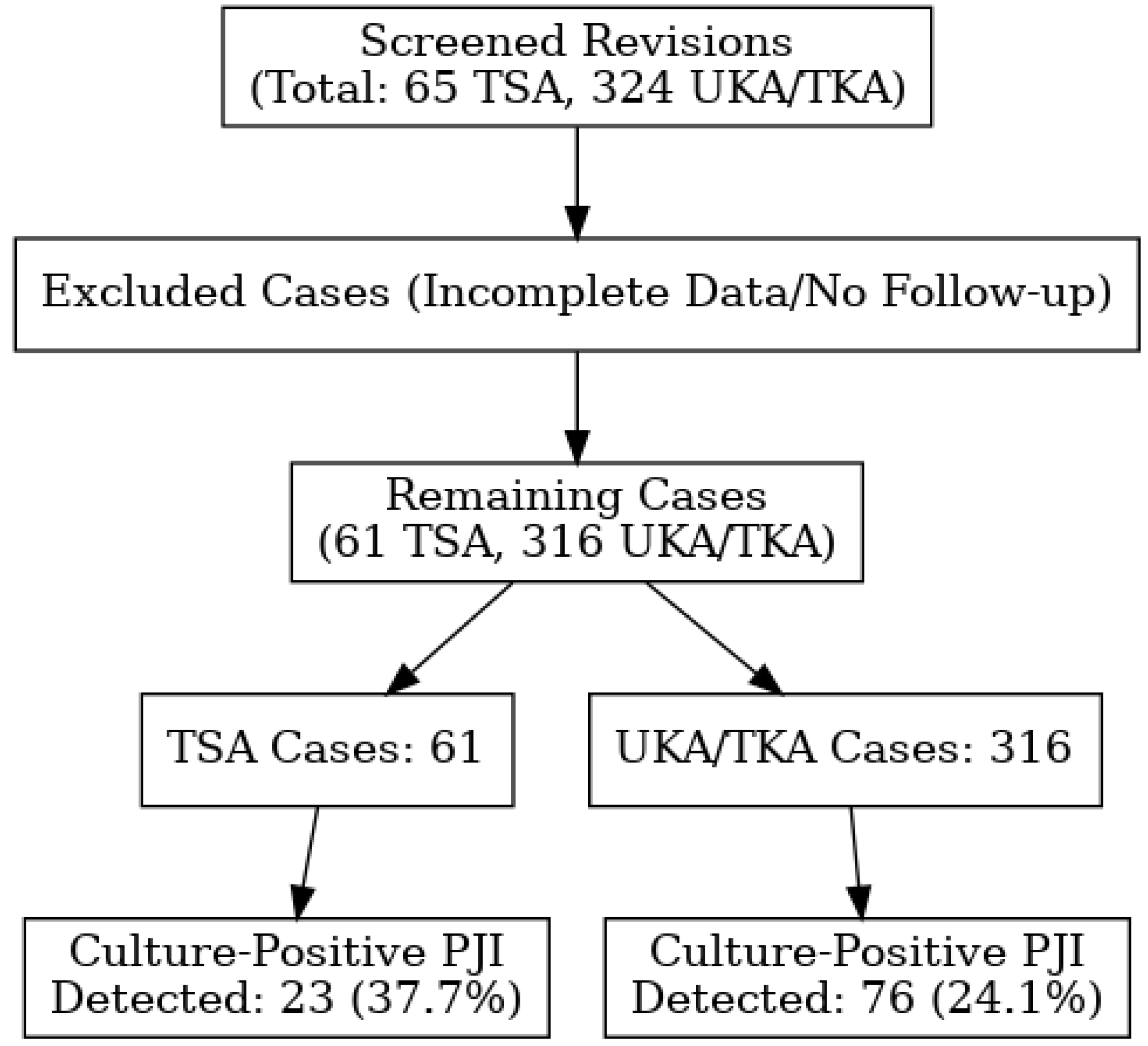
2. Results
2.1. Shoulder Arthroplasty Revision Group
2.2. Knee Arthroplasty Revision Group
2.3. Pathogen Identification
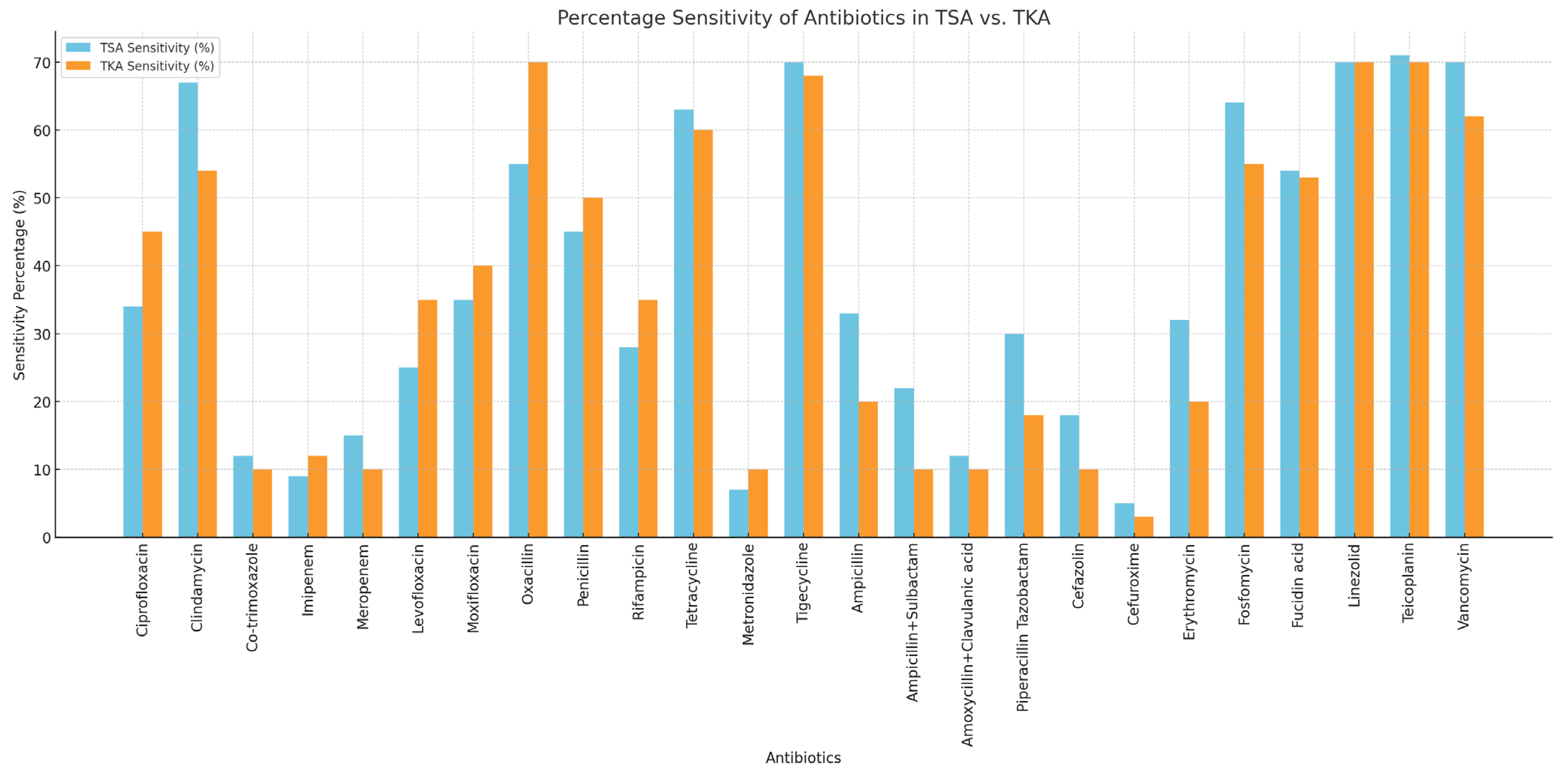
2.4. Empirical Therapy Accuracy
3. Discussion
3.1. Pathogens
3.2. Antibiotic Resistance and Empirical Antibiotics
4. Materials and Methods
4.1. Surgical Treatment Protocol
- Debridement and implant retention (DAIR);
- One-stage arthroplasty revision;
- Two-stage arthroplasty revision.
4.2. Prophylactic Antibiotics
4.3. Empirical Antibiotic Treatment
4.4. Microbiology
4.4.1. Outcome Measures
4.4.2. Statistical Analysis
- Categorical variables were reported as percentages of the total sample for those variables;
- Continuous variables were expressed as means and standard deviations, and follow-up times were expressed as medians and interquartile ranges.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwee, R.M.; Kwee, T.C. (18)F-FDG PET for Diagnosing Infections in Prosthetic Joints. PET Clin. 2020, 15, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, E.J.; Stephens-Shields, A.J.; Newcomb, C.W.; Silibovsky, R.; Nelson, C.L.; O’Donnell, J.A.; Glaser, L.J.; Hsieh, E.; Hanberg, J.S.; Tate, J.P.; et al. Incidence, Microbiological Studies, and Factors Associated With Prosthetic Joint Infection After Total Knee Arthroplasty. JAMA Netw. Open 2023, 6, e2340457. [Google Scholar] [CrossRef] [PubMed]
- Stanborough, R.O.; Bestic, J.M.; Peterson, J.J. Shoulder Osteoarthritis. Radiol. Clin. N. Am. 2022, 60, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Veerman, C.M.; Goosen, J.H.M.; Telgt, D.S.C.; Rijnen, W.H.M.; Nabuurs, M.H.; Wertheim, H.F.L. Assessment of antimicrobial mismatches in empirical treatment in early PJI after aseptic revision arthroplasty. JAC Antimicrob. Resist. 2022, 4, dlac124. [Google Scholar] [CrossRef]
- Nelson, G.N.; Davis, D.E.; Namdari, S. Outcomes in the treatment of periprosthetic joint infection after shoulder arthroplasty: A systematic review. J. Shoulder Elb. Surg. 2016, 25, 1337–1345. [Google Scholar] [CrossRef]
- Bassetti, M.; Castaldo, N.; Cadeo, B.; Carnelutti, A. Prosthetic joint infections: Clinical management, diagnosis, and treatment. Curr. Opin. Infect. Dis. 2019, 32, 102–112. [Google Scholar] [CrossRef]
- Klement, M.R.; Cunningham, D.J.; Wooster, B.M.; Wellman, S.S.; Bolognesi, M.P.; Green, C.L.; Garrigues, G.E. Comparing Standard Versus Extended Culture Duration in Acute Hip and Knee Periprosthetic Joint Infection. J. Am. Acad. Orthop. Surg. 2019, 27, e437–e443. [Google Scholar] [CrossRef]
- Kilgus, D.J.; Howe, D.J.; Strang, A. Results of periprosthetic hip and knee infections caused by resistant bacteria. Clin. Orthop. Relat. Res. 2002, 404, 116–124. [Google Scholar] [CrossRef]
- Tai, D.B.G.; Patel, R.; Abdel, M.P.; Berbari, E.F.; Tande, A.J. Microbiology of hip and knee periprosthetic joint infections: A database study. Clin. Microbiol. Infect. 2022, 28, 255–259. [Google Scholar] [CrossRef]
- Wyles, C.C.; Hevesi, M.; Osmon, D.R.; Park, M.A.; Habermann, E.B.; Lewallen, D.G.; Berry, D.J.; Sierra, R.J. 2019 John Charnley Award: Increased risk of prosthetic joint infection following primary total knee and hip arthroplasty with the use of alternative antibiotics to cefazolin: The value of allergy testing for antibiotic prophylaxis. Bone Jt. J. 2019, 101-b, 9–15. [Google Scholar] [CrossRef]
- Stevoska, S.; Himmelbauer, F.; Stiftinger, J.; Stadler, C.; Gotterbarm, T.; Heyse, T.J.; Klasan, A. Significant Difference in Antimicrobial Resistance of Coagulase Negative Periprosthetic Joint Infection in Septic Revision Total Knee Arthroplasty Between Two Major Orthopedic Centers. J. Arthroplast. 2022, 37, S306–S312. [Google Scholar] [CrossRef]
- Li, C.; Renz, N.; Trampuz, A. Management of Periprosthetic Joint Infection. Hip Pelvis 2018, 30, 138–146. [Google Scholar] [CrossRef]
- Park, C.; Blevins, K.M.; Paul, A.V.; Long, J.S.; Meyer, L.E.; Anakwenze, O.A. Perioperative Management in Shoulder Arthroplasty: A Review of Current Practice. Orthop. Clin. N. Am. 2022, 53, 483–490. [Google Scholar] [CrossRef]
- Bartolotta, R.J.; Ha, A.S. Current Imaging Concepts in Shoulder and Hip Arthroplasty. Radiol. Clin. N. Am. 2022, 60, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Nodzo, S.R.; Boyle, K.K.; Bhimani, S.; Duquin, T.R.; Miller, A.O.; Westrich, G.H. Propionibacterium acnes Host Inflammatory Response During Periprosthetic Infection Is Joint Specific. HSS J. 2017, 13, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Mandell, J.B.; Orr, S.; Koch, J.; Nourie, B.; Ma, D.; Bonar, D.D.; Shah, N.; Urish, K.L. Large variations in clinical antibiotic activity against Staphylococcus aureus biofilms of periprosthetic joint infection isolates. J. Orthop. Res. 2019, 37, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Albavera-Gutierrez, R.R.; Espinosa-Ramos, M.A.; Rebolledo-Bello, E.; Paredes-Herrera, F.J.; Carballo-Lucero, D.; Valencia-Ledezma, O.E.; Castro-Fuentes, C.A. Prevalence of Staphylococcus aureus Infections in the Implantation of Orthopedic Devices in a Third-Level Hospital: An Observational Cohort Study. Pathogens 2024, 13, 630. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef]
- Bdeir, M.; Lerchl, A.; Hetjens, S.; Schilder, A.; Gravius, S.; Baumgartner, T.; Darwich, A. One- vs. Two-Stage Revision for Periprosthetic Shoulder Infections: A Systematic Review and Meta-Analysis. Antibiotics 2024, 13, 440. [Google Scholar] [CrossRef]
- Ernährungssicherheit AÖAfGu. AURES 2023—Austrian Resistance Report: Bericht über Antimikrobielle Resistenzen und Antibiotikaverbrauch in Österreich; Austrian Agency for Health and Food Safety (AGES): Vienna, Austria, 2024. [Google Scholar]
- Zmistowski, B.; Fedorka, C.J.; Sheehan, E.; Deirmengian, G.; Austin, M.S.; Parvizi, J. Prosthetic joint infection caused by gram-negative organisms. J. Arthroplast. 2011, 26 (Suppl. S6), 104–108. [Google Scholar] [CrossRef]
- da Silva, R.B.; Salles, M.J. Outcomes and Risk Factors in Prosthetic Joint Infections by multidrug-resistant Gram-negative Bacteria: A Retrospective Cohort Study. Antibiotics 2021, 10, 340. [Google Scholar] [CrossRef]
- Bdeir, M.; Dally, F.J.; Assaf, E.; Gravius, S.; Mohs, E.; Hetjens, S.; Darwich, A. Periprosthetic Infections of the Shoulder Joint: Characteristics and 5-Year Outcome of a Single-Center Series of 19 Cases. Antibiotics 2021, 10, 1125. [Google Scholar] [CrossRef] [PubMed]
- Rangan, A.; Falworth, M.; Watts, A.C.; Scarborough, M.; Thomas, M.; Kulkarni, R.; Rees, J. Investigation and Management of Periprosthetic Joint Infection in the Shoulder and Elbow: Evidence and consensus based guidelines of the British Elbow and Shoulder Society. Shoulder Elb. 2018, 10, S5–S19. [Google Scholar] [CrossRef]
- Van Erp, J.H.J.; Heineken, A.C.; Van Wensen, R.J.A.; Van Kempen, R.; Hendriks, J.G.E.; Wegdam-Blans, M.; Fonville, J.M.; Van Der Steen, M.C.M. Optimization of the empirical antibiotic choice during the treatment of acute prosthetic joint infections: A retrospective analysis of 91 patients. Acta Orthop. 2019, 90, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.; Pereira, A.; Massada, M.; da Silva, M.V.; Lemos, R.; Costa e Castro, J. Empirical antibiotic therapy in prosthetic joint infections. Acta Orthop. Belg. 2010, 76, 254–259. [Google Scholar]
- Stevoska, S.; Himmelbauer, F.; Stiftinger, J.; Stadler, C.; Pisecky, L.; Gotterbarm, T.; Klasan, A. Significant Difference in Antimicrobial Resistance of Bacteria in Septic Revision between Total Knee Arthroplasty and Total Hip Arthroplasty. Antibiotics 2022, 11, 249. [Google Scholar] [CrossRef]
- Liew-Littorin, C.; Davidsson, S.; Nilsdotter-Augustinsson, A.; Hellmark, B.; Bruggemann, H.; Soderquist, B. Genomic characterization and clinical evaluation of prosthetic joint infections caused by Cutibacterium acnes. Microbiol. Spectr. 2024, 12, e0030324. [Google Scholar] [CrossRef]
- Parvizi, J.; Gehrke, T.; Chen, A.F. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Jt. J. 2013, 95-b, 1450–1452. [Google Scholar] [CrossRef]
- (EUCAST) ECoAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 21 March 2025).
- Hoenig, J.M.; Heisey, D.M. The Abuse of Power. Am. Stat. 2001, 55, 19–24. [Google Scholar] [CrossRef]
| Variable | TSA (n = 23) | TKA/UKA (n = 76) |
|---|---|---|
| Mean age, years (SD) | 67.56 (9.9) | 70.23 (11.0) |
| Gender, n (%): | ||
| Female | 8 (35.0) | 38 (50.0) |
| Male | 15 (65.0) | 38 (50.0) |
| Laterality, n (%): | ||
| Left | 9 (39.1) | 40 (52.6) |
| Right | 14 (60.7) | 36 (47.4) |
| Arthroplasty, n (%) | ||
| Explantation | 10 (43.5) | 8 (11.0) |
| One-stage prosthesis change | 9 (39.1) | 0 (0.0) |
| Inlay change | 4 (17.4) | 68 (89.0) |
| Mean BMI, kg/m2 (SD) | 27.74 (4.3) | 32.63 (7.8) |
| Mean ASA score | 2.4 | 2.4 |
| ASA score, n (%): | ||
| I | 0 (0.0) | 5 (7.0) |
| II | 15 (65.0) | 37 (49.0) |
| III | 8 (35.0) | 33 (43.0) |
| IV | 0 (0.0) | 1 (1.0) |
| Empirical Antibiotic | Shoulder—No. of Patients, n (%) | Knee—No. of Patients, n (%) |
|---|---|---|
| Ampicillin/Sulbactam | 4/23 (17.4) | 40/76 (52.6) |
| Cefuroxime | 8/23 (34.8) | 21/76 (27.6) |
| Ampicillin/Sulbactam + Fosfomycin | 1/76 (1.3) | |
| Clindamycin | 5/23 (21.7) | 5/76 (6.6) |
| Penicillin | 2/23 (8.7) | |
| Amoxicillin/Clavulanic Acid | 2/23 (8.7) | 2/76 (2.6) |
| Cefazolin | 1/76 (1.3) | |
| Daptomycin + Fosfomycin | 1/76 (1.3) | |
| Flucloxacillin | 1/76 (1.3) | |
| Vancomycin | 2/23 (8.7) | 2/76 (2.6) |
| Vancomycin + Fosfomycin | 2/76 (2.6) |
| Bacteria | Occurrence in TSA, n (%) | Occurrence in TKA/UKA, n (%) | p-Value (p < 0.05 Is Significant) |
|---|---|---|---|
| Gram positive | 27 (100.0%) | 78 (94.0%) | p = 0.190 |
| CPS | 3 (11.1%) | 20 (24.1%) | p = 0.150 |
| Staphylococcus aureus | 3 (11.1%) | 19 (22.9%) | |
| Staphylococcus aureus, MRSA | 0 (0.0%) | 1 (1.2%) | |
| CNS | 16 (59.3) | 36 (43.4%) | p = 0.150 |
| Staphylococcus capitis | 1 (3.7%) | 3 (3.6%) | |
| Staphylococcus epidermidis | 12 (44.4%) | 21 (25.3%) | |
| Staphylococcus hemolyticus | 0 (0.0) | 4 (4.8%) | |
| Staphylococcus hominis | 1 (3.7%) | 3 (3.6%) | |
| Staphylococcus lugdunensis | 0 (0.0%) | 3 (3.6%) | |
| Staphylococcus cohnii | 0 (0.0%) | 1 (1.2%) | |
| Staphylococcus warneri | 2 (7.5%) | 1 (1.2%) | |
| Streptococcus spp. | 0 (0.0%) | 13 (15.7%) | p = 0.029 |
| Streptococcus agalactiae | 0 (0.0%) | 6 (7.2%) | |
| Streptococcus dysgalactiae | 0 (0.0%) | 2 (2.4%) | |
| Streptococcus oralis | 0 (0.0%) | 1 (1.2%) | |
| β-hemolytic streptococci group G | 0 (0.0%) | 3 (3.6%) | |
| Streptococcus mitis | 0 (0.0%) | 1 (1.2%) | |
| Anerobes | 7 (25.9%) | 3 (3.6%) | p < 0.001 |
| Cutibacterium acnes | 7 (25.9%) | 0 (0.0%) | |
| Peptoniphilus sp. | 0 (0.0%) | 2 (2.4%) | |
| Actinomyces turicensis | 0 (0.0%) | 1 (1.2%) | |
| Enterococcus spp. | 1 (3.7%) | 6 (7.2%) | p = 0.510 |
| Enterococcus faecalis | 0 (0.0%) | 4 (4.8%) | |
| Enterococcus faecium | 1 (3.7%) | 0 (0.0%) | |
| Corynebacterium | 0 (0.0%) | 2 (2.4%) | |
| Gram negative | 0 (0.0%) | 5 (6.0%) | p = 0.640 |
| Enterobacter cloacae | 0 (0.0%) | 3 (3.6%) | |
| Escherichia coli | 0 (0.0%) | 1 (1.2%) | |
| Serratia marcescens | 0 (0.0%) | 1 (1.2%) | |
| Total number | 27 | 83 |
| Type of Antibiotic | TSA (n = 27) | TKA (n = 83) | Sensitivity p-Value (p < 0.05 Is Significant) | Resistance p-Value (p < 0.05 Is Significant) | ||
|---|---|---|---|---|---|---|
| S | R | S | R | |||
| Ciprofloxacin | 9/14 (64.3) | 5/14 (35.7) | 33/46 (71.7) | 13/46 (28.3) | 0.440 | 0.810 |
| Clindamycin | 18/26 (69.2) | 8/26 (30.8) | 43/66 (65.2) | 23/66 (34.8) | 0.270 | 0.960 |
| Co-trimoxazole | 17/18 (94.4) | 1/18 (5.6) | 41/45 (91.1) | 4/45 (8.9) | 0.320 | 0.770 |
| Imipenem | 2/2 (100.0) | 0/2 (0.0) | 9/13 (69.2) | 4/13 (30.8) | 0.560 | 0.230 |
| Meropenem | 4/4 (100.0) | 0/4 (0.0) | 6/6 (100.0) | 0/6 (0.0) | 0.270 | n/a |
| Levofloxacin | 7/15 (46.7) | 8/15 (53.3) | 28/50 (56.0) | 22/50 (44.0) | 0.360 | 0.860 |
| Moxifloxacin | 15/20 (75.0) | 5/20 (25.0) | 57/70 (81.4) | 13/70 (18.6) | 0.110 | 0.81 |
| Oxacillin | 12/19 (63.2) | 7/19 (36.8) | 37/53 (69.8) | 16/53 (30.2) | 0.830 | 0.540 |
| Penicillin | 7/19 (36.8) | 12/19 (63.2) | 28/60 (46.7) | 32/60 (53.3) | 0.360 | 0.720 |
| Rifampicin | 17/17 (100.0) | 0/17 (0.0) | 47/47 (100.0) | 0/47 (0.0) | 0.750 | n/a |
| Tetracycline | 17/17 (100.0) | 0/17 (0.0) | 49/53 (92.5) | 4/53 (7.5) | 0.930 | 0.230 |
| Metronidazole | 0/6 (0.0) | 6/6 (100.0) | 4/5 (80.0) | 1 (20.0) | 0.320 | <0.001 |
| Tigecycline | 19/19 (100.0) | 0/19 (0.0) | 54/55 (98.2) | 1/55 (1.8) | 0.850 | 0.560 |
| Ampicillin | 0/2 (0.0) | 2/2 (100.0) | 8/16 (50.0) | 8/16 (50.0) | 0.085 | 0.680 |
| Ampicillin + Sulbactam | 9/10 (90.0) | 1/10 (10.0) | 17/30 (56.7) | 13/30 (43.3) | 0.220 | 0.091 |
| Amoxicillin + Clavulanic acid | 3/3 (100.0) | 0/3 (0.0) | 3/5 (60.0) | 2/5 (40.0) | 0.160 | 0.400 |
| Piperacillin Tazobactam | 7/8 (87.5) | 1/8 (12.5) | 12/13 (92.3) | 1/13 (7.7) | 0.210 | 0.420 |
| Cefazolin | 5/7 (71.4) | 2/7 (28.6) | 14/21 (66.7) | 7/21 (33.3) | 0.930 | 0.820 |
| Cefuroxime | 1/1 (100.0) | 0/1 (0.0) | 3/5 (60.0) | 2/5 (40.0) | 0.980 | 0.400 |
| Erythromycin | 7/19 (36.8) | 12/19 (63.2) | 42/66 (63.6) | 22/66 (33.3) | 0.014 | 0.110 |
| Fosfomycin | 18/19 (94.7) | 1/19 (5.3) | 44/56 (78.6) | 12/56 (21.4) | 0.320 | 0.120 |
| Fusidic Acid | 15/20 (75.5) | 5/20 (25.0) | 42/50 (84.0) | 8/50 (16.0) | 0.830 | 0.250 |
| Linezolid | 19/19 (100.0) | 0/19 (0.0) | 56/56 (100.0) | 0/56 (0.0) | 0.960 | n/a |
| Teicoplanin | 19/19 (100.0) | 0/19 (0.0) | 48/48 (100.0) | 0/48 (0.0) | 0.370 | n/a |
| Vancomycin | 19/19 (100.0) | 0/19 (0.0) | 49/49 (100.0) | 0/49 (0.0) | 0.440 | n/a |
| Empirical Antibiotic | Accuracy in TSA Revision Group—No. of Isolated Bacteria, n (%) | Accuracy in TKA/UKA Revision Group—No. of Isolated Bacteria, n (%) | p-Value (p < 0.05 Is Significant) |
|---|---|---|---|
| Ampicillin/Sulbactam | 6/7 (85.7) | 34/49 (69.4) | p = 0.370 |
| Cefuroxime | 6/9 (66.7) | 18/21 (85.7) | p = 0.230 |
| Ampicillin/Sulbactam + Fosfomycin | 1/1 (100.0) | ||
| Clindamycin | 2/6 (33.3) | 3/5 (60.0) | p = 0.080 |
| Penicillin | 3/3 (100.0) | ||
| Cefazolin | 1/1 (100.0) | ||
| Daptomycin + Fosfomycin | 1/1 (100.0) | ||
| Flucloxacillin | 1/1 (100.0) | ||
| Vancomycin | 2/2 (100.0) | 2/2 (100.0) | |
| Vancomycin + Fosfomycin | 2/2 (100.0) | ||
| Total accuracy | 19/27 (70.4) | 63/83 (75.9) | p = 0.200 |
| Preoperative Antibiotic | Shoulder—No. of Patients, n (%) |
|---|---|
| Cefuroxime | 20/23 (87.0) |
| Clindamycin | 3/23 (13.0) |
| Preoperative Antibiotic | Knee—No. of Patients, n (%) |
|---|---|
| Cefuroxime | 67/76 (88.2) |
| Clindamycin | 7/76 (9.2) |
| Ampicillin/Sulbactam | 1/76(1.3) |
| Vancomycin | 1/76 (1.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freller, K.; Punz, H.; Schopper, C.; Gotterbarm, T.; Klasan, A.; Stevoska, S. The Accuracy of Empirical Antibiotic Treatment for Periprosthetic Joint Infections in Total Shoulder and Knee Arthroplasties. Antibiotics 2025, 14, 447. https://doi.org/10.3390/antibiotics14050447
Freller K, Punz H, Schopper C, Gotterbarm T, Klasan A, Stevoska S. The Accuracy of Empirical Antibiotic Treatment for Periprosthetic Joint Infections in Total Shoulder and Knee Arthroplasties. Antibiotics. 2025; 14(5):447. https://doi.org/10.3390/antibiotics14050447
Chicago/Turabian StyleFreller, Katrin, Hannah Punz, Clemens Schopper, Tobias Gotterbarm, Antonio Klasan, and Stella Stevoska. 2025. "The Accuracy of Empirical Antibiotic Treatment for Periprosthetic Joint Infections in Total Shoulder and Knee Arthroplasties" Antibiotics 14, no. 5: 447. https://doi.org/10.3390/antibiotics14050447
APA StyleFreller, K., Punz, H., Schopper, C., Gotterbarm, T., Klasan, A., & Stevoska, S. (2025). The Accuracy of Empirical Antibiotic Treatment for Periprosthetic Joint Infections in Total Shoulder and Knee Arthroplasties. Antibiotics, 14(5), 447. https://doi.org/10.3390/antibiotics14050447







