A One- or Two-Stage Revision of Fungal Prosthetic Joint Infection: A Review of Current Knowledge, Pitfalls and Recommendations
Abstract
1. Introduction
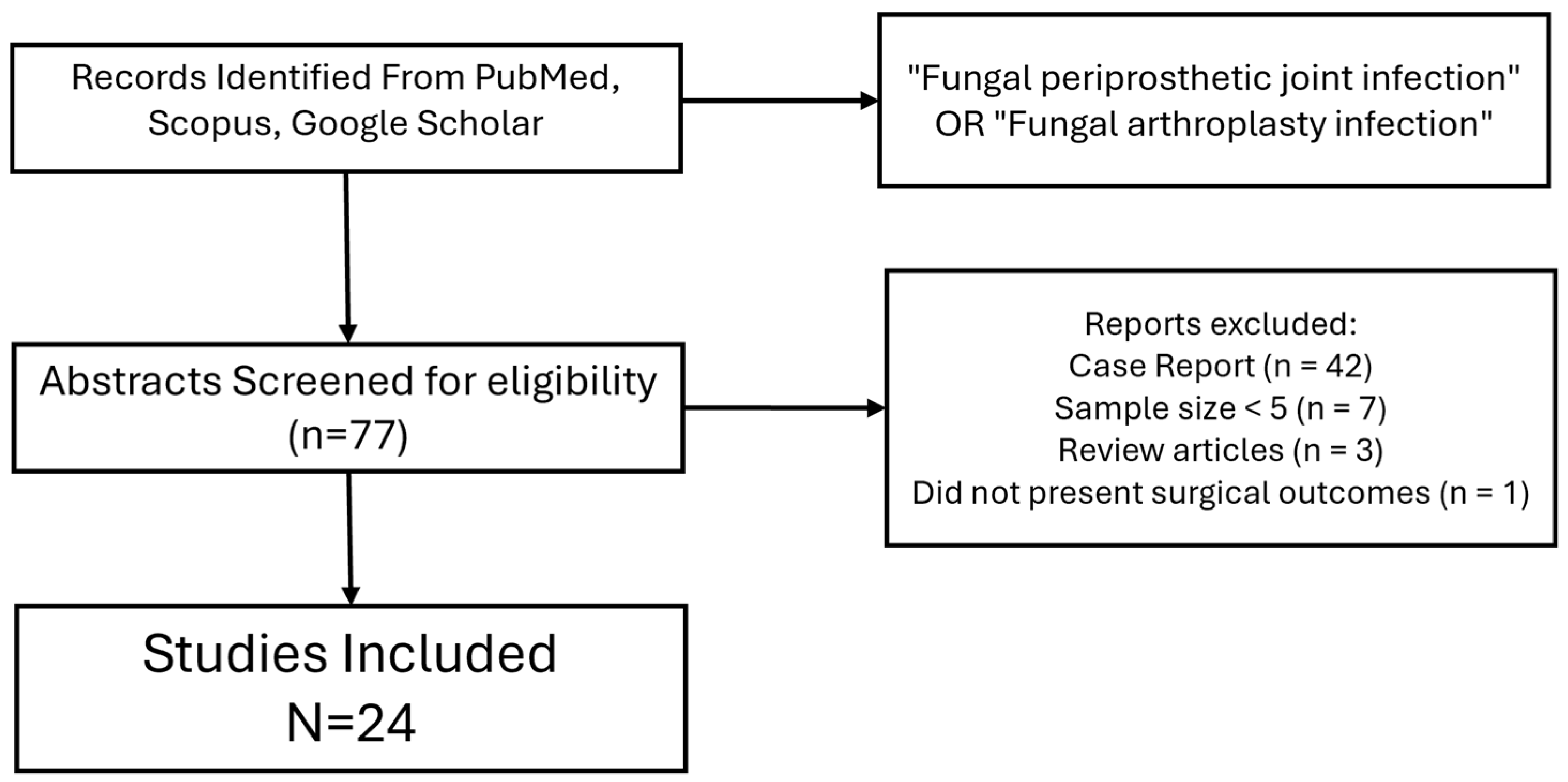
2. Materials and Methods
3. Results
4. Discussion and Literature Review
Limitations of This Study
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobayashi, G.S. Fungi. In Microbiology; Davis, B.D., Dulbecco, R., Eisen, H.N., Ginsberg, H.S., Eds.; JB Lippincott: Philadelphia, PA, USA, 1991; p. 421. [Google Scholar]
- Guan, Y.; Zheng, H.; Zeng, Z.; Tu, Y. Surgical procedures for the treatment of fungal periprosthetic infection following hip arthroplasty: A systematic scoping review. Ann. Med. Surg. 2024, 6, 2786–2793. [Google Scholar] [CrossRef] [PubMed]
- Marta, L.C.; Luis, H.S.; Luis, R.E. Fungal Arthritis. Ann. Rheum. Dis. 1992, 51, 690–697. [Google Scholar]
- Sambri, A.; Zunarelli, R.; Fiore, M.; Bortoli, M.; Paolucci, A.; Filippini, M.; Zamparini, E.; Tedeschi, S.; Viale, P.; De Paolis, M. Epidemiology of Fungal Periprosthetic Joint Infection: A Systematic Review of the Literature. Microorganisms 2022, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Saconi, E.S.; Carvalho, V.C.; Oliveira, P.R.; Lima, A.L. Prosthetic joint infection due to Candida species: Case series and review of literature. Medicine 2020, 99, e19735. [Google Scholar] [CrossRef]
- Bariteau, J.T.; Waryasz, G.R.; McDonnell, M.; Fischer, S.A.; Hayda, C.R.A.; Born, C.T. Fungal osteomyelitis and septic arthritis. J. Am. Acad. Orthop. Surg. 2014, 22, 390–401. [Google Scholar] [CrossRef]
- Koutserimpas, C.; Naoum, S.; Alpantaki, K.; Raptis, K.; Dretakis, K.; Vrioni, G.; Samonis, G. Fungal Prosthetic Joint Infection in Revised Knee Arthroplasty: An Orthopaedic Surgeon’s Nightmare. Diagnostics 2022, 12, 1606. [Google Scholar] [CrossRef]
- Azzam, K.; Parvizi, J.; Jungkind, D.; Hanssen, A.; Fehring, T.; Springer, B.; Bozic, K.; Della Valle, C.; Pulido, L.; Barrack, R. Microbiological, clinical, and surgical features of fungal prosthetic joint infections: A multi-institutional experience. J. Bone Jt. Surg. 2009, 91 (Suppl. S6), 142–149. [Google Scholar] [CrossRef]
- Wang, Q.-J.; Shen, H.; Zhang, X.-L.; Jiang, Y.; Chen, Y.; Shao, J.-J. Staged reimplantation for the treatment of fungal peri-prosthetic joint infection following primary total knee arthroplasty. Orthop. Traumatol. Surg. Res. 2015, 101, 151–156. [Google Scholar] [CrossRef]
- Chisari, E.; Lin, F.; Fei, J.; Parvizi, J. Fungal periprosthetic joint infection: Rare but challenging problem. Chin. J. Traumatol. 2022, 25, 63–66. [Google Scholar] [CrossRef]
- Eric, M.; Ruderman, J.P.F. Fungal Infections of Bones and Joints; 2016. Available online: https://musculoskeletalkey.com/fungal-infections-of-bones-and-joints/ (accessed on 28 October 2024).
- Theil, C.; Schmidt-Braekling, T.; Gosheger, G.; Idelevich, E.A.; Moellenbeck, B.; Dieckmann, R. Fungal prosthetic joint infection in total hip or knee arthroplasty: A retrospective single-centre study of 26 cases. Bone Jt. J. 2019, 101-B, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Riaz, T.; Tande, A.J.; Steed, L.L.; Demos, H.A.; Salgado, C.D.; Osmon, D.R.; Marculescu, C.E. Risk Factors for Fungal Prosthetic Joint Infection. J. Bone Jt. Infect. 2020, 5, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, T.; Parvizi, J. Proceedings of the international consensus meeting on periprosthetic joint infection. Bone Jt. J. 2013, 95-B, 1450–1452. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, M.; Frommelt, L.; Achan, P.; Board, T.N.; Conway, J.; Griffin, W.; Heidari, N.; Kerr, G.; McLaren, A.; Nelson, S.B.; et al. Management of fungal or atypical periprosthetic joint infections. J. Orthop. Res. 2014, 32, S147–S151. [Google Scholar] [CrossRef]
- Herndon, C.L.; Rowe, T.M.; Metcalf, R.W.; Odum, S.M.; Fehring, T.K.; Springer, B.D.; Otero, J.E. Treatment Outcomes of Fungal Periprosthetic Joint Infection. J. Arthroplast. 2023, 38, 2436. [Google Scholar] [CrossRef]
- Yang, H.Y.; Shin, H.H.; Kim, J.W.; Seon, J.K. The fate of fungal periprosthetic joint infection after total knee arthroplasty. Int. Orthop. 2023, 47, 2727–2735. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, Y.; Huang, C.; Huang, Z.; Fang, X.; Bai, G.; Zhang, Z.; Li, W.; Zhang, W. Metagenomic next-generation sequencing assists the diagnosis treatment of fungal osteoarticular infections. Front. Cell. Infect. Microbiol. 2022, 12, 1072539. [Google Scholar] [CrossRef]
- McCulloch, R.A.; Palmer, A.J.; Donaldson, J.; Kendrick, B.J.; Miles, J.; Taylor, A. The Outcomes of Hip and Knee Fungal Periprosthetic Joint Infections: A Retrospective Cohort Study. J. Arthroplast. 2023, 38, 2183–2187.e1. [Google Scholar] [CrossRef]
- Baecker, H.; Frieler, S.; Geßmann, J.; Pauly, S.; Schildhauer, T.A.; Hanusrichter, Y. Three-stage revision arthroplasty for the treatment of fungal periprosthetic joint infection: Outcome analysis of a novel treatment algorithm: A prospective study. Bone Jt. Open 2021, 2, 671–678. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Enz, A.; Mueller, S.C.; Warnke, P.; Ellenrieder, M.; Mittelmeier, W.; Klinder, A. Periprosthetic Fungal Infections in Severe Endoprosthetic Infections of the Hip and Knee Joint—A Retrospective Analysis of a Certified Arthroplasty Centre of Excellence. J. Fungi 2021, 7, 404. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, D.; Ren, Y.; Andronic, O.; Akgün, D.; Perka, C.; Müller, M.; Kienzle, A. Candida periprosthetic joint infections—Risk factors and outcome between albicans and non-albicans strains. Int. Orthop. 2021, 46, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, M.S.; Cooper, G.; Jenkins, N.; Jeys, L.; Parry, M.; Stevenson, J.D. Prosthetic fungal infections: Poor prognosis with bacterial co-infection. Bone Jt. J. 2019, 101-B, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Li, X.; Du, Y.; Peng, Y.; Wu, W.; Zhou, Y. Success Rate of Fungal Peri-Prosthetic Joint Infection Treated by 2-Stage Revision and Potential Risk Factors of Treatment Failure: A Retrospective Study. Med. Sci. Monit. 2018, 24, 5549–5557. [Google Scholar] [CrossRef]
- Kuo, F.-C.; Goswami, K.; Shohat, N.; Blevins, K.; Rondon, A.J.; Parvizi, J. Two-Stage Exchange Arthroplasty Is a Favorable Treatment Option Upon Diagnosis of a Fungal Periprosthetic Joint Infection. J. Arthroplast. 2018, 33, 3555–3560. [Google Scholar] [CrossRef]
- Escolà-Vergé, L.; Rodríguez-Pardo, D.; Lora-Tamayo, J.; Morata, L.; Murillo, O.; Vilchez, H.; Sorli, L.; Carrión, L.G.; Barbero, J.M.; Palomino-Nicás, J.; et al. Candida periprosthetic joint infection: A rare and difficult-to-treat infection. J. Infect. 2018, 77, 151–157. [Google Scholar] [CrossRef]
- Brown, T.S.; Petis, S.M.; Osmon, D.R.; Mabry, T.M.; Berry, D.J.; Hanssen, A.D.; Abdel, M.P. Periprosthetic Joint Infection With Fungal Pathogens. J. Arthroplast. 2018, 33, 2605–2612. [Google Scholar] [CrossRef]
- Ji, B.; Zhang, X.; Xu, B.; Guo, W.; Mu, W.; Cao, L. Single-stage Revision for Chronic Fungal Periprosthetic Joint Infection: An Average of 5 Years of Follow-up. J. Arthroplast. 2017, 32, 2523–2530. [Google Scholar] [CrossRef]
- Kim, J.-K.; Lee, D.-Y.; Kang, D.-W.; Ro, D.-H.; Lee, M.C.; Han, H.-S. Efficacy of antifungal-impregnated cement spacer against chronic fungal periprosthetic joint infections after total knee arthroplasty. Knee 2018, 25, 631–637. [Google Scholar] [CrossRef]
- Klatte, T.O.; Kendoff, D.; Kamath, A.F.; Jonen, V.; Rueger, J.M.; Frommelt, L.; Gebauer, M.; Gehrke, T. Single-stage revision for fungal peri-prosthetic joint infection: A single-centre experience. Bone Jt. J. 2014, 96, 492–496. [Google Scholar] [CrossRef]
- Geng, L.; Xu, M.; Yu, L.; Li, J.; Zhou, Y.; Wang, Y.; Chen, J. Risk factors and the clinical and surgical features of fungal prosthetic joint infections: A retrospective analysis of eight cases. Exp. Ther. Med. 2016, 12, 991–999. [Google Scholar] [CrossRef]
- Ueng, S.W.; Lee, C.Y.; Hu, C.C.; Hsieh, P.H.; Chang, Y. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin. Orthop. Relat. Res. 2013, 471, 3002–3009. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakos, K.; Kelm, J.; Schmitt, E.; Jung, J. Fungal periprosthetic hip and knee joint infections clinical experience with a 2-stage treatment protocol. J. Arthroplast. 2012, 27, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.-H.; Yoon, J.-Y.; Nam, C.-H.; Jung, K.-A.; Lee, S.-C.; Han, C.-D.; Moon, S.-H. Fungal peri-prosthetic joint infection after primary total knee replacement. J. Bone Jt. Surg. Br. Vol. 2012, 94, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Dutronc, H.; Dauchy, F.A.; Cazanave, C.; Rougie, C.; Lafarie-Castet, S.; Couprie, B.; Fabre, T.; Dupon, M. Candida prosthetic infections: Case series and literature review. Scand. J. Infect. Dis. 2010, 42, 890–895. [Google Scholar] [CrossRef]
- Ponde, N.O.; Lortal, L.; Ramage, G.; Naglik, J.R.; Richardson, J.P. Candida albicans biofilms and polymicrobial interactions. Crit. Rev. Microbiol. 2021, 47, 91–111. [Google Scholar] [CrossRef]
- Kuiper, J.W.; Bekerom, M.P.v.D.; van der Stappen, J.; Nolte, P.A.; Colen, S. 2-stage revision recommended for treatment of fungal hip and knee prosthetic joint infections. Acta Orthop. 2013, 84, 517–523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gamaletsou, M.N.; Walsh, T.J.; Sipsas, N.V. Epidemiology of Fungal Osteomyelitis. Curr. Fungal Infect. Rep. 2014, 8, 262–270. [Google Scholar] [CrossRef]
- Koutserimpas, C.; Chamakioti, I.; Zervakis, S.; Raptis, K.; Alpantaki, K.; Kofteridis, D.P.; Vrioni, G.; Samonis, G. Non-Candida Fungal Prosthetic Joint Infections. Diagnostics 2021, 11, 1410. [Google Scholar] [CrossRef]
- Drago, L.; Clerici, P.; Morelli, I.; Ashok, J.; Benzakour, T.; Bozhkova, S.; Alizadeh, C.; del Sel, H.; Sharma, H.K.; Peel, T.; et al. The World Association against Infection in Orthopaedics and Trauma (WAIOT) procedures for Microbiological Sampling and Processing for Periprosthetic Joint Infections (PJIs) and other Implant-Related Infections. J. Clin. Med. 2019, 8, 933. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bracken, C.D.; Berbari, E.F.; Hanssen, A.D.; Mabry, T.M.; Osmon, D.R.; Sierra, R.J. Systemic inflammatory markers and aspiration cell count may not differentiate bacterial from fungal prosthetic infections. Clin. Orthop. Relat. Res. 2014, 472, 3291–3294. [Google Scholar] [CrossRef][Green Version]
- Pérez-Prieto, D.; Portillo, M.E.; Puig-Verdié, L.; Alier, A.; Martínez, S.; Sorlí, L.; Horcajada, J.P.; Monllau, J.C. C-reactive protein may misdiagnose prosthetic joint infections, particularly chronic and low-grade infections. Int. Orthop. 2017, 41, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Beam, E.; Osmon, D. Prosthetic Joint Infection Update. Infect. Dis. Clin. N. Am. 2018, 32, 843–859. [Google Scholar] [CrossRef] [PubMed]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Powderly, W.G.; Opal, S.M. Infectious Diseases E-Book; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Nace, J.; Siddiqi, A.; Talmo, C.T.; Chen, A.F. Diagnosis and Management of Fungal Periprosthetic Joint Infections. J. Am. Acad. Orthop. Surg. 2019, 27, e804–e818. [Google Scholar] [CrossRef]
- Guinea, J.; Zaragoza, Ó.; Escribano, P.; Martín-Mazuelos, E.; Pemán, J.; Sánchez-Reus, F.; Cuenca-Estrella, M. Molecular Identification and Antifungal Susceptibility of Yeast Isolates Causing Fungemia Collected in a Population-Based Study in Spain in 2010 and 2011. Antimicrob. Agents Chemother. 2014, 58, 1529–1537. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Cobo, F.; Rodríguez-Granger, J.; López, E.M.; Jiménez, G.; Sampedro, A.; Aliaga-Martínez, L.; Navarro-Marí, J.M. Candida-induced prosthetic joint infection. A literature review including 72 cases and a case report. Infect. Dis. 2017, 49, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. S1), e1–e38. [Google Scholar] [CrossRef]
- Koutserimpas, C.; Zervakis, S.G.; Maraki, S.; Alpantaki, K.; Ioannidis, A.; Kofteridis, D.P.; Samonis, G. Non-albicans Candida prosthetic joint infections: A systematic review of treatment. World J. Clin. Cases 2019, 7, 1430–1443. [Google Scholar] [CrossRef]
- Haleem, A.A.; Berry, D.J.; Hanssen, A.D. Mid-term to long-term Followup of two-stage reimplantation for infected total knee arthroplasty. Clin. Orthop. Relat. Res. 2004, 428, 35–39. [Google Scholar] [CrossRef]
- Phelan, D.M.; Osmon, D.R.; Keating, M.R.; Hanssen, A.D. Delayed Reimplantation Arthroplasty for Candidal Prosthetic Joint Infection: A Report of 4 Cases and Review of the Literature. Clin. Infect. Dis. 2002, 34, 930–938. [Google Scholar] [CrossRef] [PubMed]

| Study | Year | n | Average Age | Follow Up (Months) | Average ESR (mm/h) | Average CRP (mg/L) | Coinfection |
|---|---|---|---|---|---|---|---|
| Herndon et al. [18] | 2023 | 41 | 64 | 12 | - | - | 35 |
| Yang et al. [19] | 2023 | 41 | 77.6 | 38.9 | 60.5 | 7.9 | 13 |
| Zhang at al. [20] | 2022 | 11 | 59 | 34.5 | 66.1 | 2.04 | 0 |
| McCulloch et al. [21] | 2022 | 69 | 68 | 34 | - | - | 63 |
| Baecker et al. [22] | 2021 | 18 | 72 | 35 | - | - | 6 |
| Enz et al. [23] | 2021 | 18 | 70 | - | - | - | 14 |
| Karczewski et al. [24] | 2021 | 29 | 70.98 | 33.03 | - | 5.17 | 22 |
| Saconi et al. [5] | 2020 | 11 | 65 | 42.5 | 34.8 | 31.2 | 6 |
| Theil et al. [12] | 2019 | 26 | 72 | 27 | - | - | 13 |
| Sidhu et al. [25] | 2019 | 22 | 64 | 46.2 | 47.6 | 6.2 | 22 |
| Gao et al. [26] | 2018 | 18 | 61 | 65 | 57.5 | 4.4 | 9 |
| Kou et al. [27] | 2018 | 29 | 70.7 | 86.4 | 59.2 | 6.6 | 15 |
| Escolà-Vergé et al. [28] | 2018 | 35 | 75 | 16.5 | 66 | 3.8 | 11 |
| Brown et al. [29] | 2017 | 31 | 9 | 48 | 41.0 | 5.3 | 4 |
| Ji et al. [30] | 2017 | 11 | 66 | 12 | - | - | 6 |
| Kim et al. [31] | 2017 | 9 | 76 | 66 | 56 | 2.25 | 3 |
| Klatte et al. [32] | 2014 | 10 | 68 | 96 | - | 22 | 1 |
| Geng et al. [33] | 2014 | 8 | 60 | 55 | 68 | 4.7 | 4 |
| Wang et al. [9] | 2014 | 5 | 67 | 42 | 40 | 2.3 | 0 |
| Ueng et al. [34] | 2012 | 16 | 62 | 41 | - | - | 8 |
| Anagnostakos et al. [35] | 2012 | 7 | 68 | 28 | - | - | 0 |
| Hwang et al. [36] | 2011 | 30 | 69 | 52 | 39 | 4.3 | 6 |
| Dutronc, et al. [37] | 2010 | 7 | 72 | 30 | - | 9.8 | 0 |
| Azzam et al. [8] | 2009 | 31 | 64 | 45 | 54 | 1.75 | 5 |
| Study | Year | n | THA | TKA | DAIR | One-Stage | Two-Stage | Three-Stage | Coinfection | Treatment Success |
|---|---|---|---|---|---|---|---|---|---|---|
| Herndon et al. [18] | 2023 | 41 | 19 | 22 | 14% | 0% | 27% | 0% | 35 | 49 |
| Yang et al. [19] | 2023 | 41 | 0 | 41 | 24% | 0% | 100% | 0% | 13 | 63 |
| Zhang at al. [20] | 2022 | 11 | 1 | 9 | 0% | 9% | 91% | 0% | 0 | 100 |
| McCulloch et al. [21] | 2022 | 69 | 22 | 47 | 23% | 7% | 70% | 0% | 63 | 17 |
| Baecker et al. [22] | 2021 | 18 | 11 | 7 | 0% | 0% | 0% | 100% | 6 | 89 |
| Enz et al. [23] | 2021 | 18 | 14 | 4 | 5% | 0% | 39% | 0% | 14 | 50 |
| Karczewski et al. [24] | 2021 | 29 | 14 | 15 | 7% | 7% | 28% | 41% | 22 | 62 |
| Saconi et al. [5] | 2020 | 11 | 6 | 5 | 9% | 36% | 9% | 0% | 6 | 73 |
| Theil et al. [12] | 2019 | 26 | 18 | 8 | 0% | 8% | 92% | 0% | 13 | 38 |
| Sidhu et al. [25] | 2019 | 22 | 8 | 14 | 32% | 0% | 68% | 0% | 22 | 41 |
| Gao et al. [26] | 2018 | 18 | 5 | 13 | 0% | 0% | 100% | 0% | 9 | 72 |
| Kou et al. [27] | 2018 | 29 | 14 | 15 | 24% | 10% | 65% | 0% | 15 | 41 |
| Escolà-Vergé et al. [28] | 2018 | 35 | 26 | 16 | 43% | 20% | 22% | 0% | 11 | 49 |
| Brown et al. [29] | 2017 | 31 | 13 | 18 | 13% | 3% | 67% | 0% | 4 | 61 |
| Ji et al. [30] | 2017 | 11 | 4 | 7 | 0% | 100% | 0% | 0% | 6 | 64 |
| Kim et al. [31] | 2017 | 9 | 0 | 9 | 78% | 0% | 100% | 0% | 3 | 89 |
| Klatte et al. [32] | 2014 | 10 | 6 | 4 | 0% | 100% | 0% | 0% | 1 | 90 |
| Geng et al. [33] | 2014 | 8 | 4 | 4 | 0% | 0% | 100% | 0% | 4 | 75 |
| Wang et al. [9] | 2014 | 5 | 0 | 5 | 0% | 0% | 100% | 0% | 0 | 100 |
| Ueng et al. [34] | 2012 | 16 | 7 | 9 | 0% | 0% | 56% | 0% | 8 | 50 |
| Anagnostakos et al. [35] | 2012 | 7 | 4 | 3 | 0% | 0% | 100% | 0% | 0 | 100 |
| Hwang et al. [36] | 2011 | 30 | 0 | 30 | 13% | 0% | 87% | 0% | 6 | 93 |
| Dutronc, et al. [37] | 2010 | 7 | 3 | 4 | 14% | 0% | 71% | 0% | 0 | 43 |
| Azzam et al. [8] | 2009 | 31 | 14 | 17 | 23% | 0% | 94% | 0% | 5 | 35 |
| Study | Year | Reinfection | Resection/Arthrodesis | Failed Reimplantation | Amputation | Initial Failure | Salvage | Suppression |
|---|---|---|---|---|---|---|---|---|
| Herndon et al. [18] | 2023 | 51.2 | 17.1 | 22.0 | 4.9 | 0.0 | 17.1 | |
| Yang et al. [19] | 2023 | 36.6 | 34.1 | 29.3 | 0.0 | 36.6 | 0.0 | 31.7 |
| Zhang at al. [20] | 2022 | 0.0 | 0.0 | 27.3 | 0.0 | 0.0 | 0.0 | |
| McCulloch et al. [21] | 2022 | 49.3 | 10.1 | 20.3 | 11.6 | 49.3 | 47.1 | 42.0 |
| Baecker et al. [22] | 2021 | 11.1 | 22.2 | 0.0 | 0.0 | 11.1 | 50.0 | 0.0 |
| Enz et al. [23] | 2021 | 22.2 | 5.6 | 0.0 | 33.3 | 22.2 | 0.0 | 16.7 |
| Karczewski et al. [24] | 2021 | 27.6 | 27.6 | 27.6 | 0.0 | 27.6 | 0.0 | 0.0 |
| Saconi et al. [5] | 2020 | 9.1 | 0.0 | 0.0 | 9.1 | 9.1 | 0.0 | 9.1 |
| Theil et al. [12] | 2019 | 23.1 | 7.7 | 7.7 | 3.8 | 61.5 | 18.8 | 0.0 |
| Sidhu et al. [25] | 2019 | 59.1 | 4.5 | 0.0 | 4.5 | 36.4 | 0.0 | 9.1 |
| Gao et al. [26] | 2018 | 27.8 | 11.1 | 11.1 | 5.6 | 22.2 | 75.0 | 0.0 |
| Kou et al. [27] | 2018 | 58.6 | 37.9 | 41.4 | 3.4 | 69.0 | 5.0 | 0.0 |
| Escolà-Vergé et al. [28] | 2018 | 51.4 | 5.7 | 5.7 | 2.9 | 51.4 | 22.2 | 14.3 |
| Brown et al. [29] | 2017 | 38.7 | 16.1 | 6.5 | 3.2 | 38.7 | 33.3 | 19.4 |
| Ji et al. [30] | 2017 | 27.3 | 0.0 | 0.0 | 9.1 | 18.2 | 100.0 | 0.0 |
| Kim et al. [31] | 2017 | 88.9 | 0.0 | 0.0 | 11.1 | 77.8 | 100.0 | 0.0 |
| Klatte et al. [32] | 2014 | 10.0 | 0.0 | 0.0 | 10.0 | 10.0 | 100.0 | 0.0 |
| Geng et al. [33] | 2014 | 25.0 | 12.5 | 0.0 | 12.5 | 25.0 | 50.0 | 0.0 |
| Wang et al. [9] | 2014 | 0.0 | 0.0 | 0.0 | 20.0 | 0.0 | - | 0.0 |
| Ueng et al. [34] | 2012 | 50.0 | 43.8 | 43.8 | 6.3 | 50.0 | 0.0 | 0.0 |
| Anagnostakos et al. [35] | 2012 | 0.0 | 0.0 | 28.6 | 14.3 | 0.0 | - | 14.3 |
| Hwang et al. [36] | 2011 | 20.0 | 3.3 | 0.0 | 3.3 | 6.7 | 50.0 | 0.0 |
| Dutronc, et al. [37] | 2010 | 42.9 | 0.0 | 0.0 | 14.3 | 42.9 | 0.0 | 0.0 |
| Azzam et al. [8] | 2009 | 32.3 | 19.4 | 0.0 | 3.2 | 38.7 | 33.3 | 9.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhawashki, H.; Benevenia, J.; Drago, L.; Kadkoy, Y. A One- or Two-Stage Revision of Fungal Prosthetic Joint Infection: A Review of Current Knowledge, Pitfalls and Recommendations. Antibiotics 2025, 14, 658. https://doi.org/10.3390/antibiotics14070658
Alkhawashki H, Benevenia J, Drago L, Kadkoy Y. A One- or Two-Stage Revision of Fungal Prosthetic Joint Infection: A Review of Current Knowledge, Pitfalls and Recommendations. Antibiotics. 2025; 14(7):658. https://doi.org/10.3390/antibiotics14070658
Chicago/Turabian StyleAlkhawashki, Hazem, Joseph Benevenia, Lorenzo Drago, and Yazan Kadkoy. 2025. "A One- or Two-Stage Revision of Fungal Prosthetic Joint Infection: A Review of Current Knowledge, Pitfalls and Recommendations" Antibiotics 14, no. 7: 658. https://doi.org/10.3390/antibiotics14070658
APA StyleAlkhawashki, H., Benevenia, J., Drago, L., & Kadkoy, Y. (2025). A One- or Two-Stage Revision of Fungal Prosthetic Joint Infection: A Review of Current Knowledge, Pitfalls and Recommendations. Antibiotics, 14(7), 658. https://doi.org/10.3390/antibiotics14070658








