Frog Skin Peptides Hylin-a1, AR-23, and RV-23: Promising Tools Against Carbapenem-Resistant Escherichia coli and Klebsiella pneumoniae Infections
Abstract
1. Introduction
2. Results
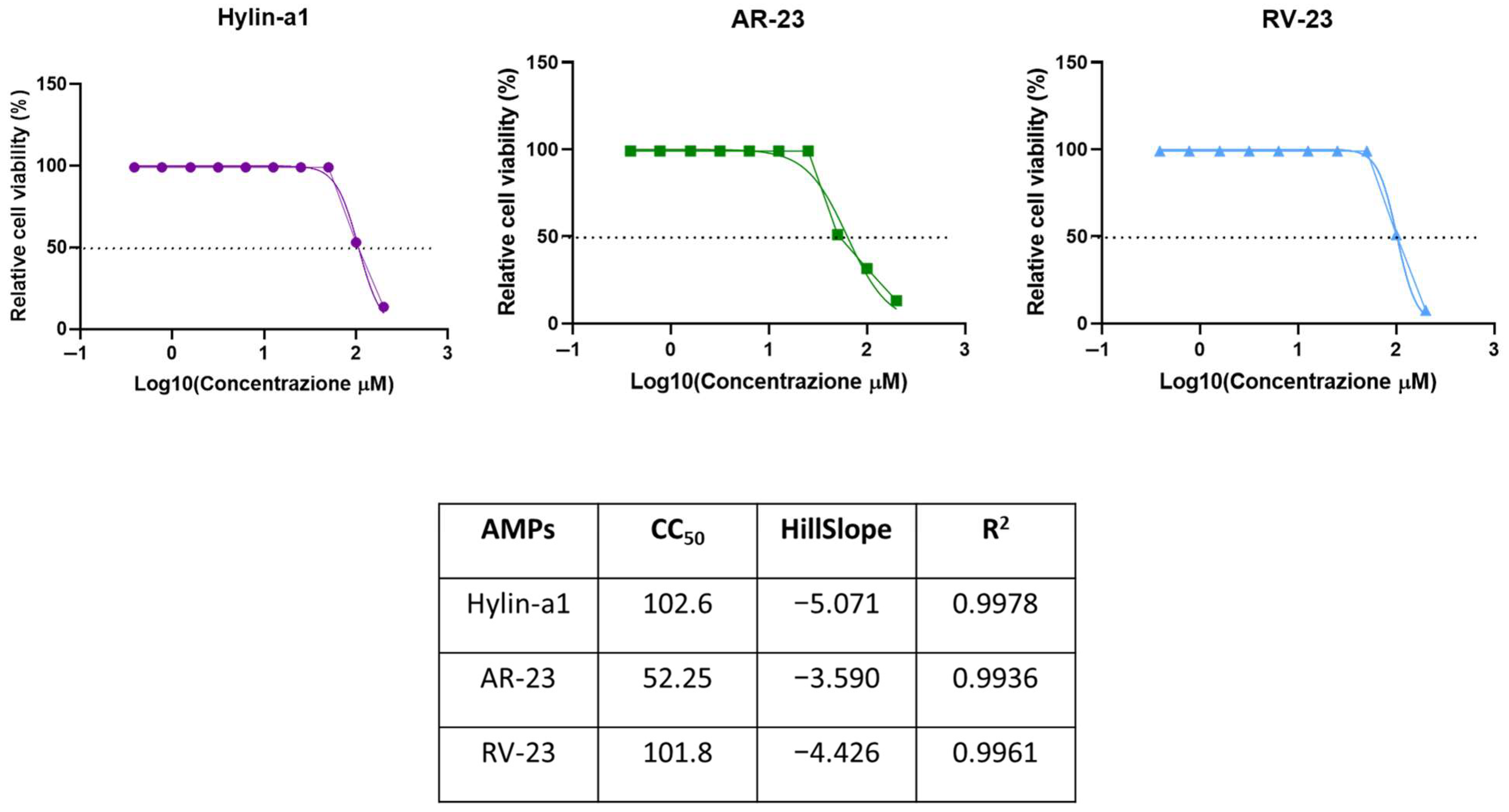
2.1. Cytotoxic Activity
2.2. Antibacterial Activity of Hylin-a1, AR-23, and RV-23
2.3. Time–Kill Assay
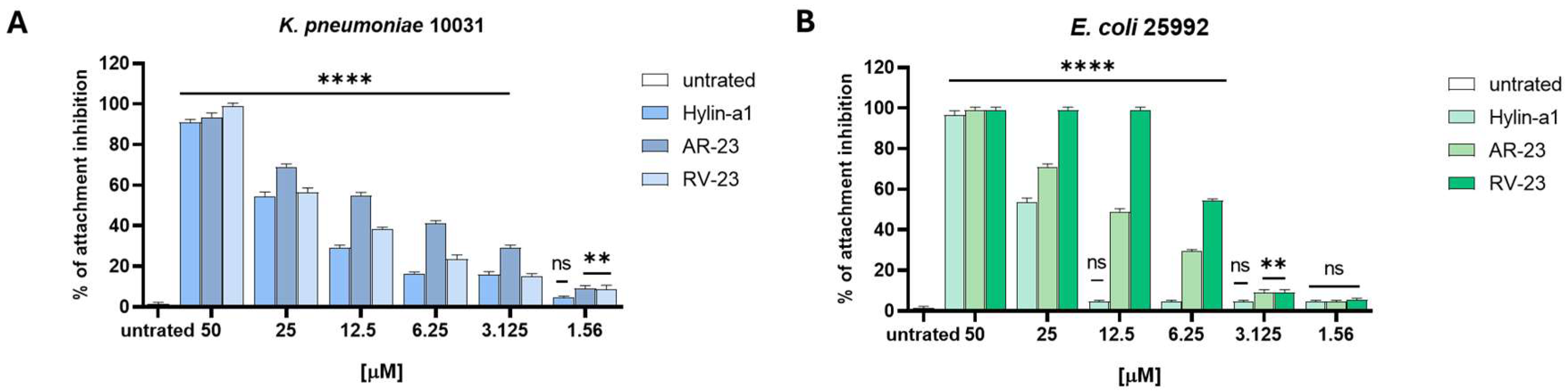
2.4. Antibiofilm Activity
2.5. Synergistic Effect of Hylin-a1, AR-23, and RV-23 with Meropenem
2.6. CD Analysis of Hylin-a1, AR-23, and RV-23 with LPS
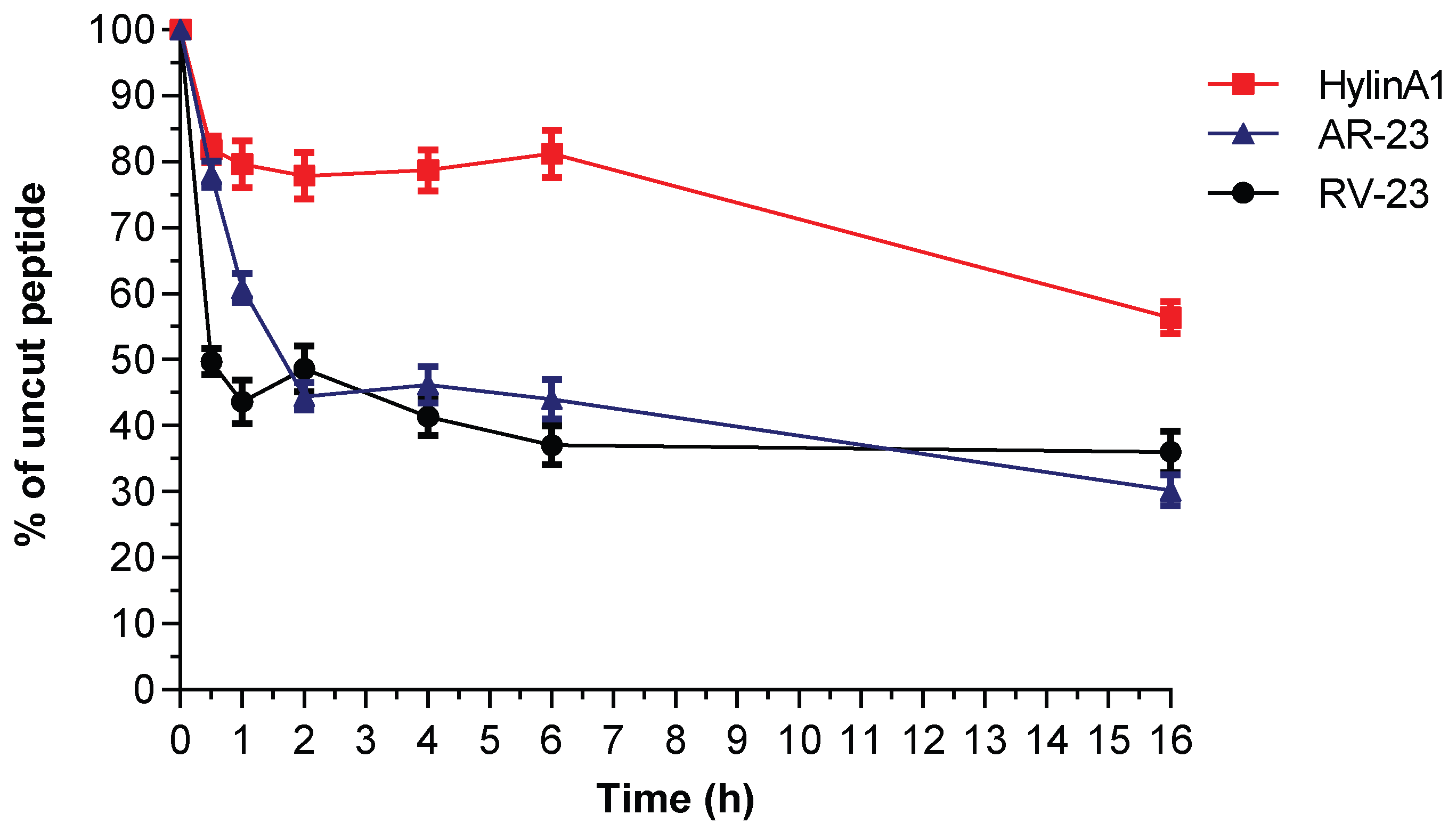
2.7. Serum Stability of Hylin-a1, AR-23, and RV-23
3. Discussion
4. Materials and Methods
4.1. Synthesis and Characterization of Peptides
4.2. Cell Culture and Viability Assay
4.3. Antibacterial Activity
4.3.1. Bacterial Strains
4.3.2. MIC and Minimal Bactericidal Concentration (MBC) Determination
4.3.3. Time-Kill Kinetics Assay
4.4. Antibiofilm Activity
Attachment, Inhibition, and Degradation Assays
4.5. Evaluation of Synergistic Activity of AMPs and Meropenem Against E. coli and K. pneumoniae
4.6. CD Analysis
4.7. Serum Stability Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/europe/health-topics/antimicrobial-resistance#tab=tab_2 (accessed on 9 February 2025).
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 9 February 2025).
- WHO Updates List of Drug-Resistant Bacteria Most Threatening to Human Health. Available online: https://www.who.int/news/item/17-05-2024-who-updates-list-of-drug-resistant-bacteria-most-threatening-to-human-health (accessed on 9 February 2025).
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2023 Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections. Clin. Infect. Dis. 2023, 2024, ciae403. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, Y.; Zong, Z. Reexamining the Association of AmpC Variants with Enterobacter Species in the Context of Updated Taxonomy. Antimicrob. Agents Chemother. 2021, 65, e0159621. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Song, X.; Li, M.; Yu, Z.; Cheng, W.; Yu, Z.; Zhang, W.; Zhang, Y.; Shen, A.; Sun, H.; et al. Global spread of carbapenem-resistant Enterobacteriaceae: Epidemiological features, resistance mechanisms, detection and therapy. Microbiol. Res. 2023, 266, 127249. [Google Scholar] [CrossRef]
- Di Pilato, V.; Pollini, S.; Miriagou, V.; Rossolini, G.M.; D’Andrea, M.M. Carbapenem-resistant Klebsiella pneumoniae: The role of plasmids in emergence, dissemination, and evolution of a major clinical challenge. Expert. Rev. Anti Infect. Ther. 2024, 22, 25–43. [Google Scholar] [CrossRef]
- Budia-Silva, M.; Kostyanev, T.; Ayala-Montano, S.; Bravo-Ferrer Acosta, J.; Garcia-Castillo, M.; Canton, R.; Goossens, H.; Rodriguez-Bano, J.; Grundmann, H.; Reuter, S. International and regional spread of carbapenem-resistant Klebsiella pneumoniae in Europe. Nat. Commun. 2024, 15, 5092. [Google Scholar] [CrossRef]
- Guo, M.Q.; Wang, Y.T.; Wang, S.S.; Chen, L.K.; Xu, Y.H.; Li, G. Genomic epidemiology of hypervirulent carbapenem-resistant Klebsiella pneumoniae at Jinshan local hospital, Shanghai, during 2014–2018. J. Microbiol. Immunol. Infect. 2024, 57, 128–137. [Google Scholar] [CrossRef]
- Li, F.; Ye, K.; Li, X.; Ye, L.; Guo, L.; Wang, L.; Yang, J. Genetic characterization of Carbapenem-Resistant Escherichia coli from China, 2015–2017. BMC Microbiol. 2021, 21, 248. [Google Scholar] [CrossRef]
- Chen, L.; Jian, J.; Xie, Z.; Zhao, P.; Zhang, M. Isolation and Characterization of Carbapenem-Resistant Escherichia coli Carrying bla(NDM) and mcr-1 from Recurrent Urinary Tract Infection Patient. Can. J. Infect. Dis. Med. Microbiol. 2023, 2023, 6640009. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Yin, Y.; Chen, H.; Jin, L.; Gu, B.; Xie, L.; Yang, C.; Ma, X.; Li, H.; et al. Epidemiology of Carbapenem-Resistant Enterobacteriaceae Infections: Report from the China CRE Network. Antimicrob. Agents Chemother. 2018, 62, 10-1128. [Google Scholar] [CrossRef]
- Gajdacs, M.; Abrok, M.; Lazar, A.; Janvari, L.; Toth, A.; Terhes, G.; Burian, K. Detection of VIM, NDM and OXA-48 producing carbapenem resistant Enterobacterales among clinical isolates in Southern Hungary. Acta Microbiol. Immunol. Hung. 2020, 67, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, H.; Glasner, C.; Albiger, B.; Aanensen, D.M.; Tomlinson, C.T.; Andrasevic, A.T.; Canton, R.; Carmeli, Y.; Friedrich, A.W.; Giske, C.G.; et al. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): A prospective, multinational study. Lancet Infect. Dis. 2017, 17, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Canton, R.; Akova, M.; Carmeli, Y.; Giske, C.G.; Glupczynski, Y.; Gniadkowski, M.; Livermore, D.M.; Miriagou, V.; Naas, T.; Rossolini, G.M.; et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 2012, 18, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Girmenia, C.; Serrao, A.; Canichella, M. Epidemiology of Carbapenem Resistant Klebsiella pneumoniae Infections in Mediterranean Countries. Mediterr. J. Hematol. Infect. Dis. 2016, 8, e2016032. [Google Scholar] [CrossRef]
- Pascale, R.; Bussini, L.; Gaibani, P.; Bovo, F.; Fornaro, G.; Lombardo, D.; Ambretti, S.; Pensalfine, G.; Appolloni, L.; Bartoletti, M.; et al. Carbapenem-resistant bacteria in an intensive care unit during the coronavirus disease 2019 (COVID-19) pandemic: A multicenter before-and-after cross-sectional study. Infect. Control Hosp. Epidemiol. 2022, 43, 461–466. [Google Scholar] [CrossRef]
- Nordmann, P. Carbapenemase-producing Enterobacteriaceae: Overview of a major public health challenge. Med. Mal. Infect. 2014, 44, 51–56. [Google Scholar] [CrossRef]
- Aldali, H.J.; Khan, A.; Alshehri, A.A.; Aldali, J.A.; Meo, S.A.; Hindi, A.; Elsokkary, E.M. Hospital-Acquired Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Observational Study. Microorganisms 2023, 11, 1595. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Denissen, J.; Reyneke, B.; Waso-Reyneke, M.; Havenga, B.; Barnard, T.; Khan, S.; Khan, W. Prevalence of ESKAPE pathogens in the environment: Antibiotic resistance status, community-acquired infection and risk to human health. Int. J. Hyg. Environ. Health 2022, 244, 114006. [Google Scholar] [CrossRef]
- Varga, J.F.A.; Bui-Marinos, M.P.; Katzenback, B.A. Frog Skin Innate Immune Defences: Sensing and Surviving Pathogens. Front. Immunol. 2018, 9, 3128. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Shai, Y. Temporins and their synergism against Gram-negative bacteria and in lipopolysaccharide detoxification. Biochim. Biophys. Acta 2009, 1788, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Di Grazia, A.; Cappiello, F.; Imanishi, A.; Mastrofrancesco, A.; Picardo, M.; Paus, R.; Mangoni, M.L. The Frog Skin-Derived Antimicrobial Peptide Esculentin-1a(1-21)NH2 Promotes the Migration of Human HaCaT Keratinocytes in an EGF Receptor-Dependent Manner: A Novel Promoter of Human Skin Wound Healing? PLoS ONE 2015, 10, e0128663. [Google Scholar] [CrossRef] [PubMed]
- Zannella, C.; Chianese, A.; Palomba, L.; Marcocci, M.E.; Bellavita, R.; Merlino, F.; Grieco, P.; Folliero, V.; De Filippis, A.; Mangoni, M.; et al. Broad-Spectrum Antiviral Activity of the Amphibian Antimicrobial Peptide Temporin L and Its Analogs. Int. J. Mol. Sci. 2022, 23, 2060. [Google Scholar] [CrossRef]
- Rizzetto, G.; Gambini, D.; Maurizi, A.; Molinelli, E.; De Simoni, E.; Pallotta, F.; Brescini, L.; Cirioni, O.; Offidani, A.; Simonetti, O.; et al. The sources of antimicrobial peptides against Gram-positives and Gramnegatives: Our research experience. Infez. Med. 2023, 31, 306–322. [Google Scholar]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta 1999, 1462, 55–70. [Google Scholar] [CrossRef]
- Peschel, A.; Sahl, H.G. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 2006, 4, 529–536. [Google Scholar] [CrossRef]
- Schuller, F.; Benz, R.; Sahl, H.G. The peptide antibiotic subtilin acts by formation of voltage-dependent multi-state pores in bacterial and artificial membranes. Eur. J. Biochem. 1989, 182, 181–186. [Google Scholar] [CrossRef]
- Tennessen, J.A. Molecular evolution of animal antimicrobial peptides: Widespread moderate positive selection. J. Evol. Biol. 2005, 18, 1387–1394. [Google Scholar] [CrossRef]
- Yasir, M.; Willcox, M.D.P.; Dutta, D. Action of Antimicrobial Peptides against Bacterial Biofilms. Materials 2018, 11, 2468. [Google Scholar] [CrossRef]
- Mangoni, M.L. Temporins, anti-infective peptides with expanding properties. Cell Mol. Life Sci. 2006, 63, 1060–1069. [Google Scholar] [CrossRef]
- Merlino, F.; Carotenuto, A.; Casciaro, B.; Martora, F.; Loffredo, M.R.; Di Grazia, A.; Yousif, A.M.; Brancaccio, D.; Palomba, L.; Novellino, E.; et al. Glycine-replaced derivatives of [Pro(3),DLeu(9)]TL, a temporin L analogue: Evaluation of antimicrobial, cytotoxic and hemolytic activities. Eur. J. Med. Chem. 2017, 139, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Nacif-Marcal, L.; Pereira, G.R.; Abranches, M.V.; Costa, N.C.; Cardoso, S.A.; Honda, E.R.; de Paula, S.O.; Feio, R.N.; Oliveira, L.L. Identification and characterization of an antimicrobial peptide of Hypsiboas semilineatus (Spix, 1824) (Amphibia, Hylidae). Toxicon 2015, 99, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Attoub, S.; Mechkarska, M.; Sonnevend, A.; Radosavljevic, G.; Jovanovic, I.; Lukic, M.L.; Conlon, J.M. Esculentin-2CHa: A host-defense peptide with differential cytotoxicity against bacteria, erythrocytes and tumor cells. Peptides 2013, 39, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Cappiello, F.; Di Grazia, A.; Segev-Zarko, L.A.; Scali, S.; Ferrera, L.; Galietta, L.; Pini, A.; Shai, Y.; Di, Y.P.; Mangoni, M.L. Esculentin-1a-Derived Peptides Promote Clearance of Pseudomonas aeruginosa Internalized in Bronchial Cells of Cystic Fibrosis Patients and Lung Cell Migration: Biochemical Properties and a Plausible Mode of Action. Antimicrob. Agents Chemother. 2016, 60, 7252–7262. [Google Scholar] [CrossRef]
- Luca, V.; Stringaro, A.; Colone, M.; Pini, A.; Mangoni, M.L. Esculentin(1-21), an amphibian skin membrane-active peptide with potent activity on both planktonic and biofilm cells of the bacterial pathogen Pseudomonas aeruginosa. Cell Mol. Life Sci. 2013, 70, 2773–2786. [Google Scholar] [CrossRef]
- Di Grazia, A.; Cappiello, F.; Cohen, H.; Casciaro, B.; Luca, V.; Pini, A.; Di, Y.P.; Shai, Y.; Mangoni, M.L. D-Amino acids incorporation in the frog skin-derived peptide esculentin-1a(1-21)NH2 is beneficial for its multiple functions. Amino Acids 2015, 47, 2505–2519. [Google Scholar] [CrossRef]
- Chianese, A.; Zannella, C.; Foglia, F.; Nastri, B.M.; Monti, A.; Doti, N.; Franci, G.; De Filippis, A.; Galdiero, M. Hylin-a1: A Host Defense Peptide with Antibacterial Potential against Staphylococcus aureus Multi-Resistant Strains. Pharmaceuticals 2023, 16, 509. [Google Scholar] [CrossRef]
- Chianese, A.; Zannella, C.; Monti, A.; Doti, N.; Sanna, G.; Manzin, A.; De Filippis, A.; Galdiero, M. Hylin-a1: A Pan-Inhibitor against Emerging and Re-Emerging Respiratory Viruses. Int. J. Mol. Sci. 2023, 24, 13888. [Google Scholar] [CrossRef]
- Chianese, A.; Giugliano, R.; Palma, F.; Nastri, B.M.; Monti, A.; Doti, N.; Zannella, C.; Galdiero, M.; De Filippis, A. The antiherpetic and anti-inflammatory activity of the frog-derived peptide Hylin-a1. J. Appl. Microbiol. 2024, 135, lxae165. [Google Scholar] [CrossRef]
- Chianese, A.; Iovane, V.; Zannella, C.; Capasso, C.; Nastri, B.M.; Monti, A.; Doti, N.; Montagnaro, S.; Pagnini, U.; Iovane, G.; et al. Synthetic Frog-Derived-like Peptides: A New Weapon against Emerging and Potential Zoonotic Viruses. Viruses 2023, 15, 1804. [Google Scholar] [CrossRef]
- Zhang, S.K.; Song, J.W.; Gong, F.; Li, S.B.; Chang, H.Y.; Xie, H.M.; Gao, H.W.; Tan, Y.X.; Ji, S.P. Design of an alpha-helical antimicrobial peptide with improved cell-selective and potent anti-biofilm activity. Sci. Rep. 2016, 6, 27394. [Google Scholar] [CrossRef]
- Castro, M.S.; Ferreira, T.C.; Cilli, E.M.; Crusca, E., Jr.; Mendes-Giannini, M.J.; Sebben, A.; Ricart, C.A.; Sousa, M.V.; Fontes, W. Hylin a1, the first cytolytic peptide isolated from the arboreal South American frog Hypsiboas albopunctatus (“spotted treefrog”). Peptides 2009, 30, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Nastri, B.M.; Chianese, A.; Giugliano, R.; Di Clemente, L.; Capasso, C.; Monti, A.; Doti, N.; Iovane, V.; Montagnaro, S.; Pagnini, U.; et al. Oreoch-1: A broad-spectrum virus and host-targeting peptide against animal infections. J. Pept. Sci. 2024, 30, e3593. [Google Scholar] [CrossRef]
- Li, D.; Yang, Y.; Tian, Z.; Lv, J.; Sun, F.; Wang, Q.; Liu, Y.; Xia, P. Synergistic antibiotic effect of looped antimicrobial peptide CLP-19 with bactericidal and bacteriostatic agents. Oncotarget 2017, 8, 55958–55966. [Google Scholar] [CrossRef]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.S.; Yang, H.; Sun, L. Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Updat. 2023, 68, 100954. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Scavello, F.; Amiche, M.; Ghia, J.E. Recent Advances in Multifunctional Antimicrobial Peptides as Immunomodulatory and Anticancer Therapy: Chromogranin A-Derived Peptides and Dermaseptins as Endogenous versus Exogenous Actors. Pharmaceutics 2022, 14, 2014. [Google Scholar] [CrossRef]
- El-Dirany, R.; Shahrour, H.; Dirany, Z.; Abdel-Sater, F.; Gonzalez-Gaitano, G.; Brandenburg, K.; Martinez de Tejada, G.; Nguewa, P.A. Activity of Anti-Microbial Peptides (AMPs) against Leishmania and Other Parasites: An Overview. Biomolecules 2021, 11, 984. [Google Scholar] [CrossRef]
- Zairi, A.; Tangy, F.; Bouassida, K.; Hani, K. Dermaseptins and magainins: Antimicrobial peptides from frogs’ skin-new sources for a promising spermicides microbicides-a mini review. J. Biomed. Biotechnol. 2009, 2009, 452567. [Google Scholar] [CrossRef]
- Casciaro, B.; Cappiello, F.; Cacciafesta, M.; Mangoni, M.L. Promising Approaches to Optimize the Biological Properties of the Antimicrobial Peptide Esculentin-1a(1-21)NH(2): Amino Acids Substitution and Conjugation to Nanoparticles. Front. Chem. 2017, 5, 26. [Google Scholar] [CrossRef]
- Casciaro, B.; Cappiello, F.; Loffredo, M.R.; Ghirga, F.; Mangoni, M.L. The Potential of Frog Skin Peptides for Anti-Infective Therapies: The Case of Esculentin-1a(1-21)NH2. Curr. Med. Chem. 2020, 27, 1405–1419. [Google Scholar] [CrossRef] [PubMed]
- Maloy, W.L.; Kari, U.P. Structure-activity studies on magainins and other host defense peptides. Biopolymers 1995, 37, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, B.; Juhl, D.W.; Glattard, E.; Aisenbrey, C. Revealing the Mechanisms of Synergistic Action of Two Magainin Antimicrobial Peptides. Front. Med. Technol. 2020, 2, 615494. [Google Scholar] [CrossRef]
- D’Andrea, L.D.; Romanelli, A. Temporins: Multifunctional Peptides from Frog Skin. Int. J. Mol. Sci. 2023, 24, 5426. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Grazia, A.D.; Cappiello, F.; Casciaro, B.; Luca, V. Naturally Occurring Peptides from Rana temporaria: Antimicrobial Properties and More. Curr. Top. Med. Chem. 2016, 16, 54–64. [Google Scholar] [CrossRef]
- Marcocci, M.E.; Amatore, D.; Villa, S.; Casciaro, B.; Aimola, P.; Franci, G.; Grieco, P.; Galdiero, M.; Palamara, A.T.; Mangoni, M.L.; et al. The Amphibian Antimicrobial Peptide Temporin B Inhibits In Vitro Herpes Simplex Virus 1 Infection. Antimicrob. Agents Chemother. 2018, 62, 10-1128. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, J.; Chen, Y. Alpha-helical cationic antimicrobial peptides: Relationships of structure and function. Protein Cell 2010, 1, 143–152. [Google Scholar] [CrossRef]
- Huang, Y.; He, L.; Li, G.; Zhai, N.; Jiang, H.; Chen, Y. Role of helicity of alpha-helical antimicrobial peptides to improve specificity. Protein Cell 2014, 5, 631–642. [Google Scholar] [CrossRef]
- Islam, M.M.; Asif, F.; Zaman, S.U.; Arnab, M.K.H.; Rahman, M.M.; Hasan, M. Effect of charge on the antimicrobial activity of alpha-helical amphibian antimicrobial peptide. Curr. Res. Microb. Sci. 2023, 4, 100182. [Google Scholar] [CrossRef]
- Zhang, S.K.; Ma, Q.; Li, S.B.; Gao, H.W.; Tan, Y.X.; Gong, F.; Ji, S.P. RV-23, a Melittin-Related Peptide with Cell-Selective Antibacterial Activity and High Hemocompatibility. J. Microbiol. Biotechnol. 2016, 26, 1046–1056. [Google Scholar] [CrossRef]
- Park, H.J.; Kang, H.K.; Park, E.; Kim, M.K.; Park, Y. Bactericidal activities and action mechanism of the novel antimicrobial peptide Hylin a1 and its analog peptides against Acinetobacter baumannii infection. Eur. J. Pharm. Sci. 2022, 175, 106205. [Google Scholar] [CrossRef] [PubMed]
- Pascale, R.; Giannella, M.; Bartoletti, M.; Viale, P.; Pea, F. Use of meropenem in treating carbapenem-resistant Enterobacteriaceae infections. Expert. Rev. Anti Infect. Ther. 2019, 17, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-review. Res. Rev. J. Eng. Technol. 2017, 6. [Google Scholar]
- Giugliano, R.; Della Sala, G.; Buonocore, C.; Zannella, C.; Tedesco, P.; Palma Esposito, F.; Ragozzino, C.; Chianese, A.; Morone, M.V.; Mazzella, V.; et al. New Imidazolium Alkaloids with Broad Spectrum of Action from the Marine Bacterium Shewanella aquimarina. Pharmaceutics 2023, 15, 2139. [Google Scholar] [CrossRef]



| K. pneumoniae ATCC 10031 | E. coli ATCC 25992 | |||
|---|---|---|---|---|
| MIC (μM) | MBC (μM) | MIC (μM) | MBC (μM) | |
| Hylin-a1 | 6.25 | 6.25 | 25 | 25 |
| AR-23 | 6.25 | 25 | 25 | N/D |
| RV-23 | 6.25 | 12.5 | 12.5 | 12.5 |
| Bacterial Strains | AMPs | |||||
|---|---|---|---|---|---|---|
| Hylin-a1 | AR-23 | RV-23 | ||||
| MIC (μM) | MBC (μM) | MIC (μM) | MBC (μM) | MIC (μM) | MBC (μM) | |
| K. pneumoniae 1711 | 25 | 50 | 50 | N/D | 12.5 | 12.5 |
| K. pneumoniae 311 | N/D | N/D | N/D | N/D | 50 | 50 |
| K. pneumoniae 1745 | 50 | 50 | 50 | N/D | 12.5 | 25 |
| K. pneumoniae 1746 | 25 | N/A | 25 | N/D | 12.5 | N/D |
| E. coli 2267 | 25 | 50 | 25 | N/D | 12.5 | 12.5 |
| E. coli 3140 | 50 | N/D | 50 | N/D | 25 | N/D |
| E. coli 716 | 25 | N/D | 25 | 50 | 25 | 25 |
| E. coli 1376 | 25 | N/D | 25 | 50 | 6.25 | 6.25 |
| E. coli 1441 | 50 | N/D | 25 | N/D | 6.25 | 12.5 |
| Clinical Isolates | Carbapenemase | Source of Isolation |
|---|---|---|
| K. pneumoniae 311 | OXA-48 (class D) | Rectal swab |
| K. pneumoniae 1746 | VIM (class B) | Rectal swab |
| K. pneumoniae 1745 | KPC (class A) | Ulcer |
| K. pneumoniae 1711 | KPC (class A) | Rectal swab |
| E. coli 2267 | NDM (class B) | Rectal swab |
| E. coli 3140 | OXA-48 (class D) | Urine |
| E. coli 716 | IMP1 (class B) | Urine |
| VIM (class B) | ||
| NDM (class B) | ||
| E. coli 1376 | NDM (class B) | Rectal swab |
| E. coli 1441 | KPC (class A) | Rectal swab |
| FICI Values | Effect |
|---|---|
| <0.5 | Synergy |
| 0.5 ≤ FIC < 1 | Partial synergy |
| 1 ≤ FIC < 4 | Additive effect or indifference |
| 4 ≤ FIC | Antagonism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chianese, A.; Ambrosino, A.; Giugliano, R.; Palma, F.; Parimal, P.; Acunzo, M.; Monti, A.; Doti, N.; Zannella, C.; Galdiero, M.; et al. Frog Skin Peptides Hylin-a1, AR-23, and RV-23: Promising Tools Against Carbapenem-Resistant Escherichia coli and Klebsiella pneumoniae Infections. Antibiotics 2025, 14, 374. https://doi.org/10.3390/antibiotics14040374
Chianese A, Ambrosino A, Giugliano R, Palma F, Parimal P, Acunzo M, Monti A, Doti N, Zannella C, Galdiero M, et al. Frog Skin Peptides Hylin-a1, AR-23, and RV-23: Promising Tools Against Carbapenem-Resistant Escherichia coli and Klebsiella pneumoniae Infections. Antibiotics. 2025; 14(4):374. https://doi.org/10.3390/antibiotics14040374
Chicago/Turabian StyleChianese, Annalisa, Annalisa Ambrosino, Rosa Giugliano, Francesca Palma, Preetu Parimal, Marina Acunzo, Alessandra Monti, Nunzianna Doti, Carla Zannella, Massimiliano Galdiero, and et al. 2025. "Frog Skin Peptides Hylin-a1, AR-23, and RV-23: Promising Tools Against Carbapenem-Resistant Escherichia coli and Klebsiella pneumoniae Infections" Antibiotics 14, no. 4: 374. https://doi.org/10.3390/antibiotics14040374
APA StyleChianese, A., Ambrosino, A., Giugliano, R., Palma, F., Parimal, P., Acunzo, M., Monti, A., Doti, N., Zannella, C., Galdiero, M., & De Filippis, A. (2025). Frog Skin Peptides Hylin-a1, AR-23, and RV-23: Promising Tools Against Carbapenem-Resistant Escherichia coli and Klebsiella pneumoniae Infections. Antibiotics, 14(4), 374. https://doi.org/10.3390/antibiotics14040374











