Piloting an Information and Communication Technology Tool to Help Addressing the Challenge of Antimicrobial Resistance in Low-Income Countries
Abstract
1. Introduction
2. Results
2.1. Farmer Pilot Preparation Survey
2.2. Farmer Data Recorded in the ADIS System
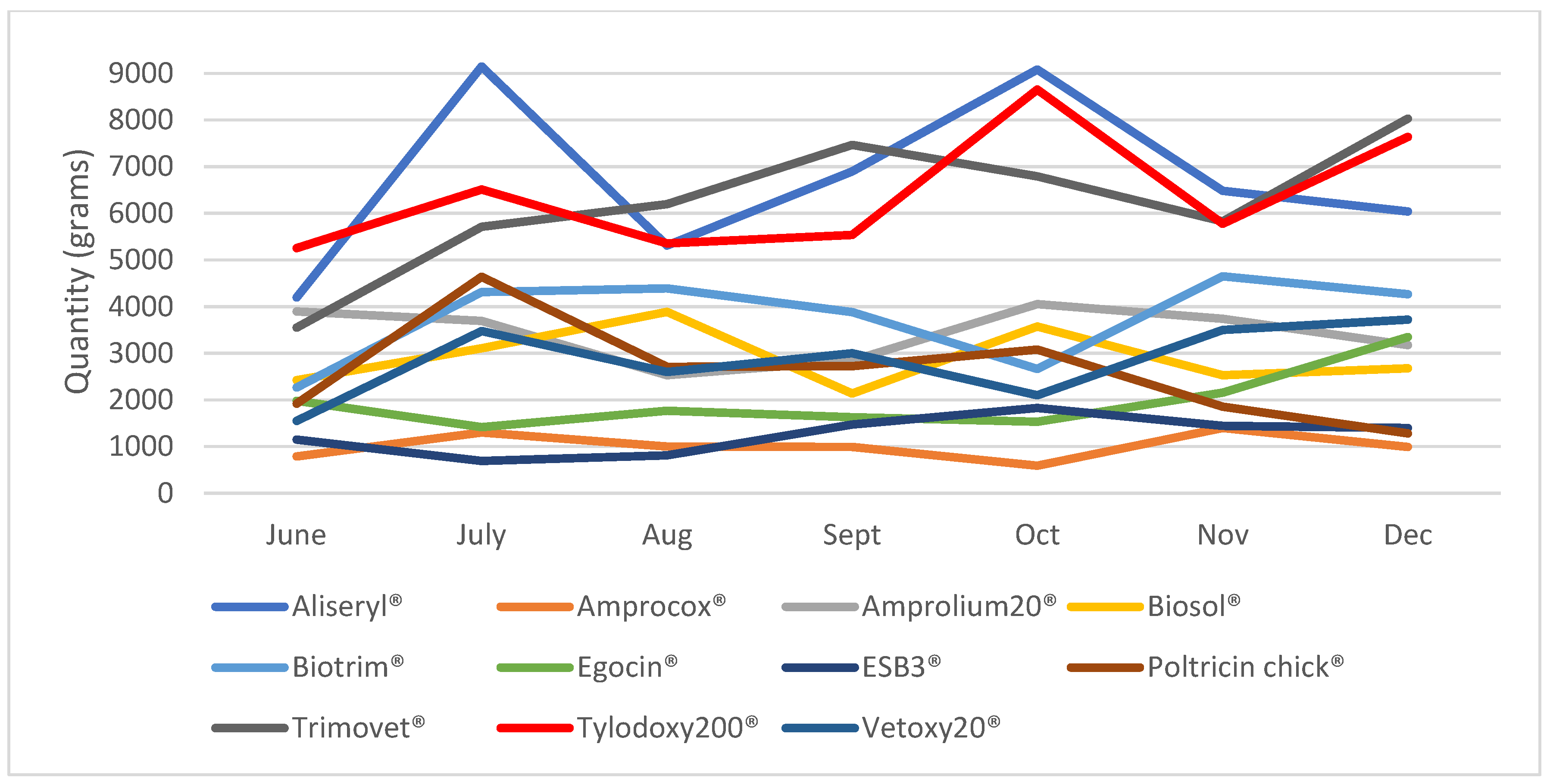
2.3. Agrovet Data Recorded in the ADIS System
2.4. Perceptions and Implementation of ADIS by the Agrovet Attendants
2.5. Perceptions of ADIS and Treatment Practices of the Poultry Farmers
3. Discussion
4. Materials and Methods
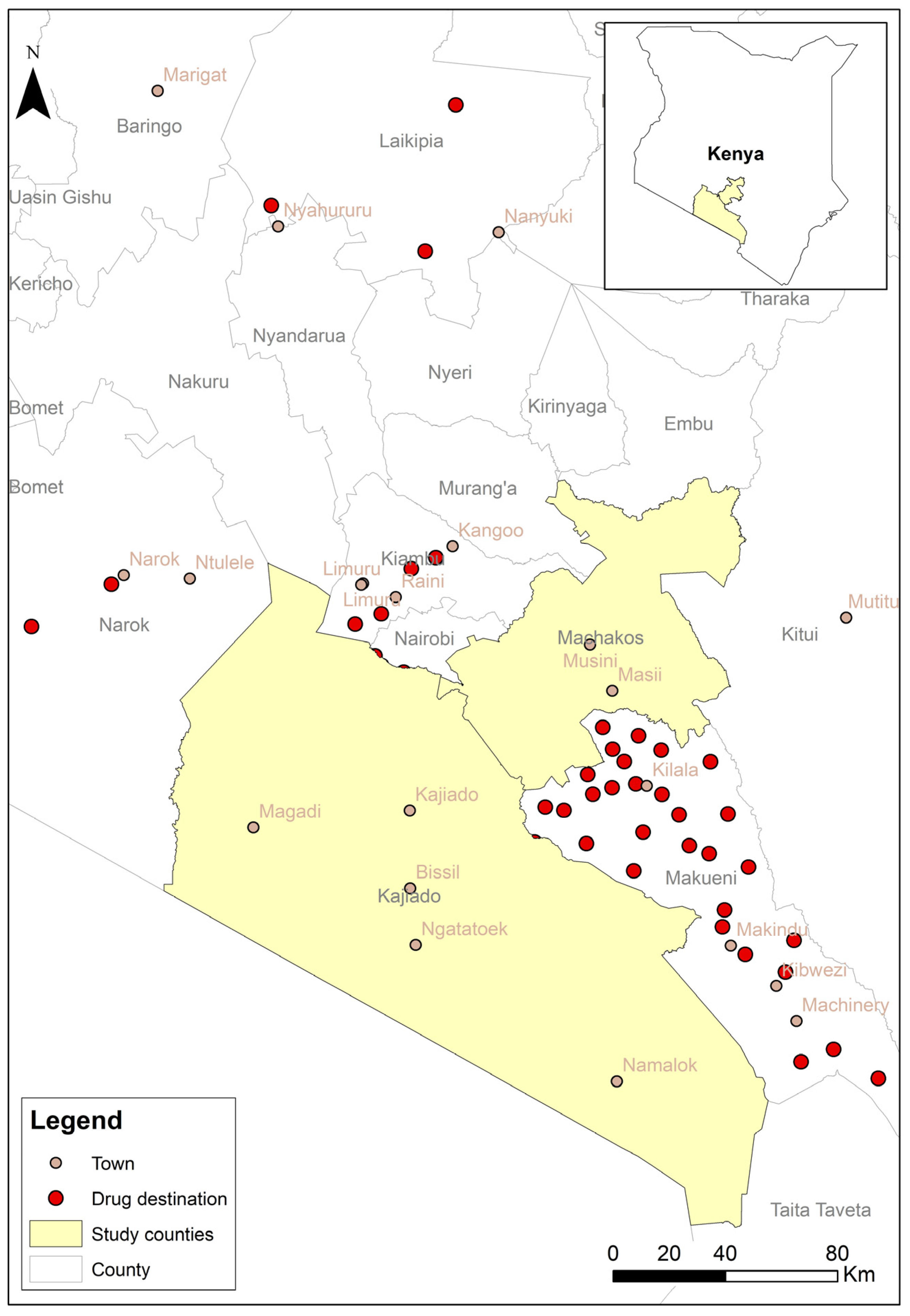
4.1. Study Sites
4.2. Selection of Study Participants
4.3. Piloting the ICT System
4.4. Data Collection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADIS | Animal disease information system |
| ARGs | Antimicrobial resistance genes |
| AMR | Antimicrobial resistance |
| AMU | Antimicrobial use |
| ICT | Information and communication technology |
| JPIAMR | Joint programming initiative on AMR |
| VMD | Veterinary medicines directorate |
| WHO | World health organization |
Appendix A
| Information | Entry Method |
|---|---|
| GPS location of the outlet | Automatically entered |
| Sale ID | Automatically entered |
| Sale Date | Automatically entered but can be modified |
| Customer Location | Manually entered |
| Sale time | Automatically entered |
| Reason for visit | Multiple choice: sick animal; animal not sick but buying drug for disease control; animal not sick but need advice on husbandry; other (specify) |
| Intended animal species | Multiple choice: poultry; cattle; sheep; goats; pigs; fish/aquaculture; Camels; other (specify) |
| Customer requested a specific product | Yes/No choice |
| Drug | Drop-down list with trade names, concentration and application route |
| Package size | Drop-down list with registered packages |
| Quantity | Manually entered |
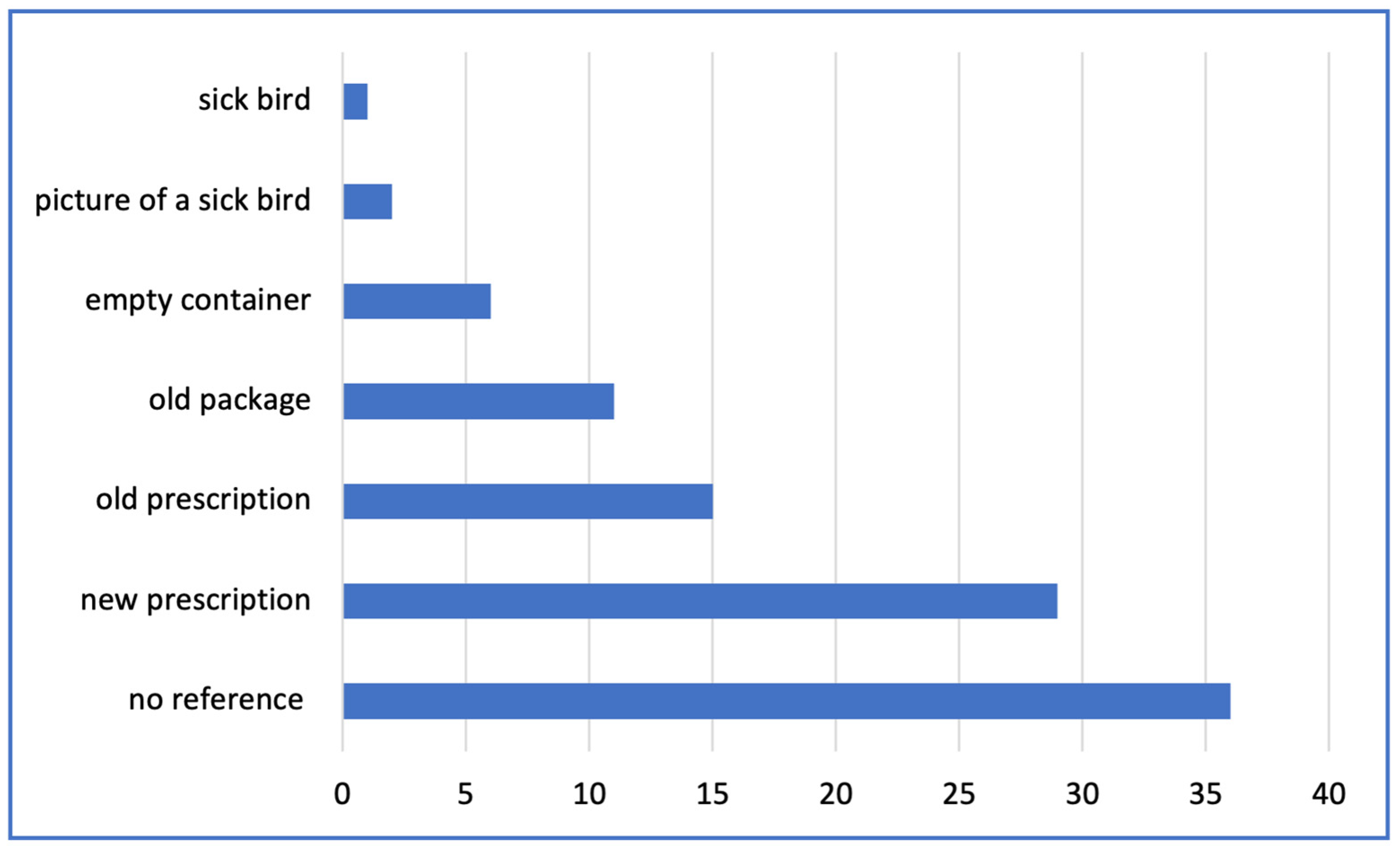
| Reference presented at the time of purchase | Multiple choice: new prescription; old prescription; old package; empty container; sick bird; picture of sick bird |
| User’s IP address | Automatically entered |
Appendix B
- Is everyone in the group still keeping poultry (they should all be poultry farmers, but use the question to open up the discussion)? Are there any major changes that have happened on your farm since the project started (fewer/more birds kept)? Those that do not currently have any poultry, probe to see if the birds were sold, died, etc. and they have plans for restocking.
- What do you think about the MAD-tech-AMR project? What did you like most about the project? [challenges to be probed in detail in later questions]
- The system required farmers to report diseases they encountered on their farms as well as the drugs they used to manage these. How easy was it to do this? Which one(s) did you find difficult to provide? It is important that all enrolled farmers can access the system. An ICT person can run a quick demonstration this time to make sure everyone can log in, and have their inputs in the subsequent discussion.
- The system also provided a way to browse disease information. How often did you browse the disease information page of the system? [probe to see how many did this, and how often] Have you applied this information to your farm, in any way? Please tell us what aspects you found useful. If you did not visit this page, could you tell us why?
- Overall, the number of reports received from farmers was limited. Do you know what could have contributed to this low response? Feel free to explain incidences when this happened to you/what prevented you from using the system and expand on exactly what the problem was [probe using the below as the guide but allow for additional contributions].
- Issues with the phones
- Issues with the Internet
- Time Involvement
- Not able to read on the phone screen.
- Questions are hard to understand? Hard to find medicines etc in the system?
- Problems contacting the project team.
- Needed to be reminded.
- Do you think this system can be of any help to poultry farmers not reached by the project?
- How useful will it be to add contact information for animal health providers in the system, will this help you in any way? Do you see any problem with this?
- What percentage of disease/drug cases did you report in the system?
- What are your thoughts on how unnecessary use of drugs in farms can be reduced?
- How long do you usually wait before considering giving your birds treatment? Do you initiate it directly when observing a sick bird or wait to see if the bird gets better first?
- How do you tell when a treatment/medicine is working? How long do you wait before concluding that the treatment did not work?
- Would you consider continuing to engage with the system after the project? Would you be willing to incur any costs (e.g., internet) to engage with the system?
- Do you have suggestions on how to make the system better?
- Besides using the ICT system as described, has the system/intervention taught you anything that you previously did not know/never used to do?
- Is everyone here still involved in agrovet business? What are some of the challenges agrovet operators face? [ice breaker question]
- Is there any major change that has happened in your business since we started (which you think might have affected the data we collected)—probe for things like staff changes, shop closures, etc.
- What did you think of the MAD-tech-AMR project, overall? Which areas did you like most (ask them if they have looked at all parts, including information about diseases, animal management, and AMR)? [probe for challenges in the next question]
- During the pilot phase, you were issued with a tablet which you used to capture data on drug sales at your agrovet. We visited you several times during the follow-up period.
- Reflecting on the drug recording process, could you tell us the specific areas you found challenging? Facilitator to probe on:
- Using the tablets/navigating through the website
- Internet access
- Time involvement
- Visualizing the form—were you able to see the questions clearly?
- Finding medicines in the system (how easy was it)
- What else was a challenge?
- Did you record the sales in the system instantly or did you enter them all at the end of the day? Were you the only person capturing the data? What did you do to ensure you did not forget to enter any data?
- What would you say was the percentage of sales that got recorded in the system? [probe to establish variations during peak/off-peak periods]
- Do the sales (overall) vary with time, for example in the morning? Which days are busy for the agrovets?
- The sales of antimicrobial substances by agrovets play a crucial role in the overall consumption of antimicrobials among poultry farms. What is your perspective on this role? Do you feel that you have some kind of responsibility? How do you manage this aspect while you still want to improve your business? Would it be useful for you to show the infographic in ADIS to your customers, to provide a service in the form of AMR education?
- Is the problem of AMR something you consider in your daily work and do you adhere to any specific guidelines, to address it? What are the main concerns/problems you face in your daily work when addressing the problem? [it would be good for the facilitator to explain what AMR is at the start, use the infographic from ADIS/let them respond]
- What percentage of customers visiting agrovets request advice on animal health? Do you feel that you are well-capacitated to advise them? If uncertain, who do you consult? Would the information available in ADIS be useful for you in this situation? Were you able to access the additional information on the system (disease control)?
- How useful will it be to add contact information for animal health providers in the system, so farmers can see who to consult easily, do you see any problem with this?
- How do you respond when farmers request a specific medication? Can you easily redirect them if, for example, they describe parasitic symptoms for which antibiotics would be ineffective?
- What are your thoughts on how the use of antimicrobial substances can be optimized and overuse/misuse reduced?
- Besides using the ICT system to record drug sales, has the system/intervention taught you anything new, that you previously did not know/never used to do, and now you do/or are planning to do?
- Would you be interested in using the drug recording system and/or the information available in the system in the future? How do you see yourself using it? What modifications would you like to the done on the system to make it easier for you to use (are there questions that you propose to be removed/or data collected in a different way?)
References
- Pokharel, S.; Priyanka, S.; Bipin, A. Antimicrobial Use in Food Animals and Human Health: Time to Implement ‘One Health’ Approach. Antimicrob. Resist. Infect. Control 2020, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, B.; Gray, A.P.; Davis Weaver, N.; Robles Aguilar, G.; Swetschinski, L.R.; Ikuta, K.S.; Mestrovic, T.; Chung, E.; Wool, E.E.; Han, C.; et al. The Burden of Bacterial Antimicrobial Resistance in the WHO African Region in 2019: A Cross-Country Systematic Analysis. Lancet Glob. Health 2024, 12, e201–e216. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Ungemach, F.R.; Müller-Bahrdt, D.; Abraham, G. Guidelines for Prudent Use of Antimicrobials and Their Implications on Antibiotic Usage in Veterinary Medicine. Int. J. Med. Microbiol. 2006, 296, 33–38. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Caudell, M.A.; Quinlan, M.B.; Subbiah, M.; Call, D.R.; Roulette, C.J.; Roulette, J.W.; Roth, A.; Matthews, L.; Quinla, R.J. Antimicrobial Use and Veterinary Care among Agro-Pastoralists in Northern Tanzania. PLoS ONE 2017, 12, e0170328. [Google Scholar] [CrossRef]
- Baynes, R.E.; Dedonder, K.; Kissell, L.; Mzyk, D.; Marmulak, T.; Smith, G.; Tell, L.; Gehring, R.; Davis, J.; Riviere, J.E. Health concerns and management of select veterinary drug residues. Food Chem. Toxicol. 2016, 88, 112–122. [Google Scholar] [CrossRef]
- Ardakani, Z.; Canali, M.; Aragrande, M.; Tomassone, L.; Simoes, M.; Balzani, A.; Beber, C.L. Evaluating the Contribution of Antimicrobial Use in Farmed Animals to Global Antimicrobial Resistance in Humans. One Health 2023, 17, 100647. [Google Scholar] [CrossRef]
- O’Neill, J. Review on Antimicrobial Resistance: Tackling Drug-Resistant Infections Globally; Wellcome Trust: London, UK, 2016; Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 11 March 2025).
- Chattopadhyay, M.K. Use of Antibiotics as Feed Additives: A Burning Question. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015; Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 11 March 2025).
- Okolie, O.J.; Igwe, U.; Ismail, S.U.; Ighodalo, U.L.; Adukwu, E.C. Systematic Review of Surveillance Systems for AMR in Africa. J. Antimicrob. Chemother. 2023, 78, 31–51. [Google Scholar] [CrossRef]
- Kimera, Z.I.; Mshana, S.E.; Rweyemamu, M.M.; Mboera, L.E.G.; Matee, M.I.N. Antimicrobial Use and Resistance in Food-Producing Animals and the Environment: An African Perspective. Antimicrob. Resist. Infect. Control 2020, 9, e14–e22. [Google Scholar] [CrossRef] [PubMed]
- Musa, K.; Okoliegbe, I.; Abdalaziz, T.; Aboushady, A.T.; Stelling, J.; Gould, I.M. Laboratory Surveillance, Quality Management, and Its Role in Addressing Antimicrobial Resistance in Africa: A Narrative Review. Antibiotics 2023, 12, 1313. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Kenya National Action Plan on Antimicrobial Resistance: Review of Progress in the Human Health Sector; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240062689 (accessed on 11 March 2025).
- Muthuma, E.N.; Gitau, G.K.; Aboge, G.O. Antimicrobial Usage in Broiler Farms in Peri-Urban Nairobi Kenya. Am. J. Res. Commun. 2016, 4, 14–29. [Google Scholar]
- Kariuki, J.W.; Jacobs, J.; Ngogang, M.P.; Howland, O. Antibiotic Use by Poultry Farmers in Kiambu County, Kenya: Exploring Practices and Drivers of Potential Overuse. Antimicrob. Resist. Infect. Control 2023, 12, 3. [Google Scholar] [CrossRef]
- Mutua, F.; Kiarie, G.; Mbatha, M.; Onono, J.; Boqvist, S.; Kilonzi, E.; Mugisha, L.; Moodley, A.; Sternberg-Lewerin, S. Antimicrobial Use by Peri-Urban Poultry Smallholders of Kajiado and Machakos Counties in Kenya. Antibiotics 2023, 12, 905. [Google Scholar] [CrossRef]
- Rware, H.; Kansiime, K.M.; Idah, M.; Makale, F.; Ikiror, D.; Wako, B.; Danielsen, S.; Karanja, D.; Chacha, D.; Byskov, M.; et al. Examining Antibiotic Use in Kenya: Farmers’ Knowledge and Practices in Addressing Antibiotic Resistance. CABI Agric. Biosci. 2024, 5, 21. [Google Scholar] [CrossRef]
- Njenga, M.K.; Kemunto, N.; Kahariri, S.; Holmstrom, L.; Oyas, H.; Biggers, K.; Riddle, A.; Gachohi, J.; Muturi, M.; Mwatondo, A.; et al. High real-time reporting of domestic and wild animal diseases following rollout of mobile phone reporting system in Kenya. PLoS ONE 2021, 16, e0244119. [Google Scholar] [CrossRef]
- Sternberg-Lewerin, S.; Onono, J.O.; Boqvist, S.; Mugisha, L.; Kihara, W.; Lindfors, L.; Strandell, K.; Mutua, F. Development of an Information and Communication Technology (ICT) Tool for Monitoring of Antimicrobial Use, Animal Disease and Treatment Outcome in Low-Income Countries. Antibiotics 2025, 14, 285. [Google Scholar] [CrossRef]
- Lateef, F. Syndromic Surveillance: A Necessary Public Health Tool. J. Acute Dis. 2012, 1, 90–93. [Google Scholar] [CrossRef][Green Version]
- Veterinary Medicines Directorate Kenya. The Veterinary Surgeons and Veterinary Paraprofessionals Act. Available online: https://vmd.go.ke/sites/default/files/2022-10/DRAFT%20VETERINARY%20MEDICINES%20DIRECTORATE%20REGULATIONS%202022.pdf (accessed on 11 March 2025).
- Toghroli, R.; Hassani, L.; Aghamolaei, T.; Sharma, M.; Sharifi, H.; Jajarmi, M. Explaining the barriers faced by veterinarians against preventing antimicrobial resistance: An innovative interdisciplinary qualitative study. BMC Infect. Dis. 2024, 24, 455. [Google Scholar] [CrossRef]
- Moyo, P.; Moyo, E.; Mangoya, D.; Mhango, M.; Mashe, T.; Imran, M.; Dzinamarira, T. Prevention of Antimicrobial Resistance in Sub-Saharan Africa: What Has Worked? What Still Needs to Be Done? J. Infect. Public Health 2023, 16, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Kiambi, S.; Mwanza, R.; Sirma, A.; Czerniak, C.; Kimani, T.; Kabali, E.; Dorado-Garcia, A.; Eckford, S.; Price, C.; Gikonyo, S.; et al. Understanding Antimicrobial Use Contexts in the Poultry Sector: Challenges for Small-Scale Layer Farms in Kenya. Antibiotics 2021, 10, 106. [Google Scholar] [CrossRef]
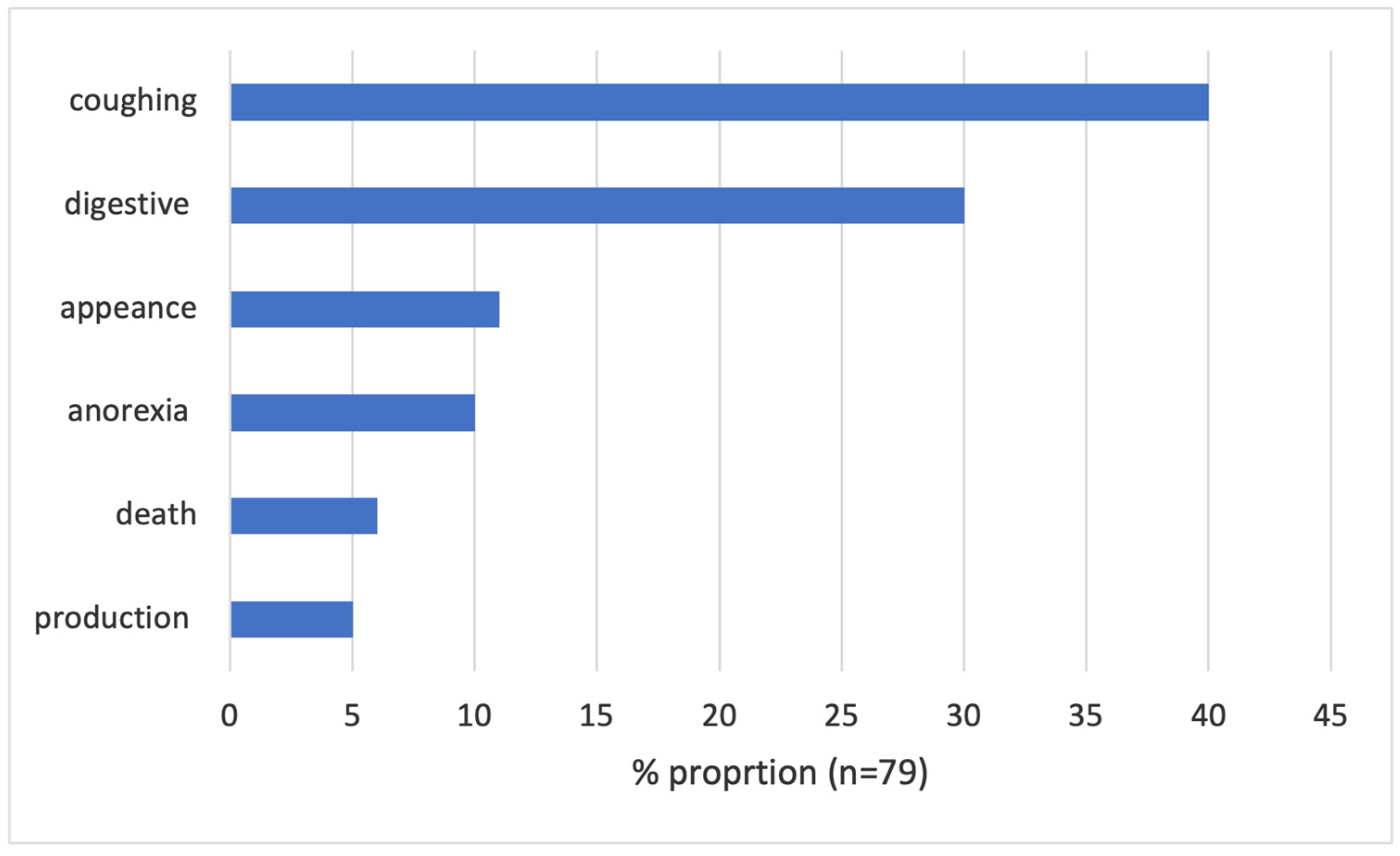

| Drugs Reported in the System | Drug Category | Treatment Cause |
|---|---|---|
| Tylonor 20, KX-Doxytylosin, Gentamox, Ashoxy chick formula 1, Bilosin, Betamox, Collie-AM, Supermed TS 2, Pen & Strep, Tetracycline 25%, Ashtyl, Colivet-4800, Vapcotrim powder, Ashoxy egg formula | Antibiotics | data |
| New-Pacprim | Vitamins | Coughing, diarrhea |
| Alben & Iver Oral, Tectin, Bimectin | Antiparasitics | Lice, wet feces, not eating, worms in feces, gas, something new |
| Animal Species. | Sold by Agrovets in Machakos (n = 4643) | Sold by Agrovets in Kajiado (n = 7832) |
|---|---|---|
| Camel | 1 (<1%) | 2 (<1%) |
| Cat | 25 (<1%) | 48 (<1%) |
| Cattle | 1010 (22%) | 2267 (29%) |
| Dog | 263 (6%) | 470 (6%) |
| Fish/aquaculture | 3 (<1%) | 15 (<1%) |
| Goat | 283 (6%) | 780 (10%) |
| Poultry | 2962 (64%) | 4111 (52%) |
| Rabbit | 96 (2%) | 139 (2%) |
| Key Aspect | Main Points |
|---|---|
| Capacity of agrovet attendants to advise customers on animal health | Attendants are well able to advise their customers, but may sometimes need to consult a colleague (veterinarian or paraveterinarian). Attendants are able to maintain the balance between professionalism and business improvement (by prescribing alternatives to antibiotics). They incur losses if they do not sell drugs. Attendants have a responsibility to advise customers on the right dosage but face challenges when farmers present no prescription. |
| Response to customers requesting specific medical products | Attendants reportedly ask for the case history and redirect them to the appropriate drug. Some farmers refuse to take the advice and are then sold what they have requested. |
| Thoughts on how AMU can be optimized and overuse/misuse reduced | Regulators should enforce implementation of the regulations. Agrovet attendants should be professional and sensitize the public on AMR, including use of alternatives. Ways of tracing animal products to their production source should be explored. Advertising for antibiotics/antimicrobials on social media should be banned. Infographics could be presented in posters for the farmers to read when they visit the shops. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutua, F.; Onono, J.O.; Boqvist, S.; Koech, P.; Abdi, A.M.; Karimi, H.; Sternberg-Lewerin, S. Piloting an Information and Communication Technology Tool to Help Addressing the Challenge of Antimicrobial Resistance in Low-Income Countries. Antibiotics 2025, 14, 373. https://doi.org/10.3390/antibiotics14040373
Mutua F, Onono JO, Boqvist S, Koech P, Abdi AM, Karimi H, Sternberg-Lewerin S. Piloting an Information and Communication Technology Tool to Help Addressing the Challenge of Antimicrobial Resistance in Low-Income Countries. Antibiotics. 2025; 14(4):373. https://doi.org/10.3390/antibiotics14040373
Chicago/Turabian StyleMutua, Florence, Joshua Orungo Onono, Sofia Boqvist, Patricia Koech, Abdullahi M. Abdi, Hildah Karimi, and Susanna Sternberg-Lewerin. 2025. "Piloting an Information and Communication Technology Tool to Help Addressing the Challenge of Antimicrobial Resistance in Low-Income Countries" Antibiotics 14, no. 4: 373. https://doi.org/10.3390/antibiotics14040373
APA StyleMutua, F., Onono, J. O., Boqvist, S., Koech, P., Abdi, A. M., Karimi, H., & Sternberg-Lewerin, S. (2025). Piloting an Information and Communication Technology Tool to Help Addressing the Challenge of Antimicrobial Resistance in Low-Income Countries. Antibiotics, 14(4), 373. https://doi.org/10.3390/antibiotics14040373







