Repurposed Drugs and Efflux Pump Inhibitors Against Gram-Negative Urinary Tract Pathogenic Bacteria
Abstract
1. Introduction
2. Results
2.1. Antibacterial Activity
2.2. Anti-Biofilm Activity
2.3. MIC Reduction Assay
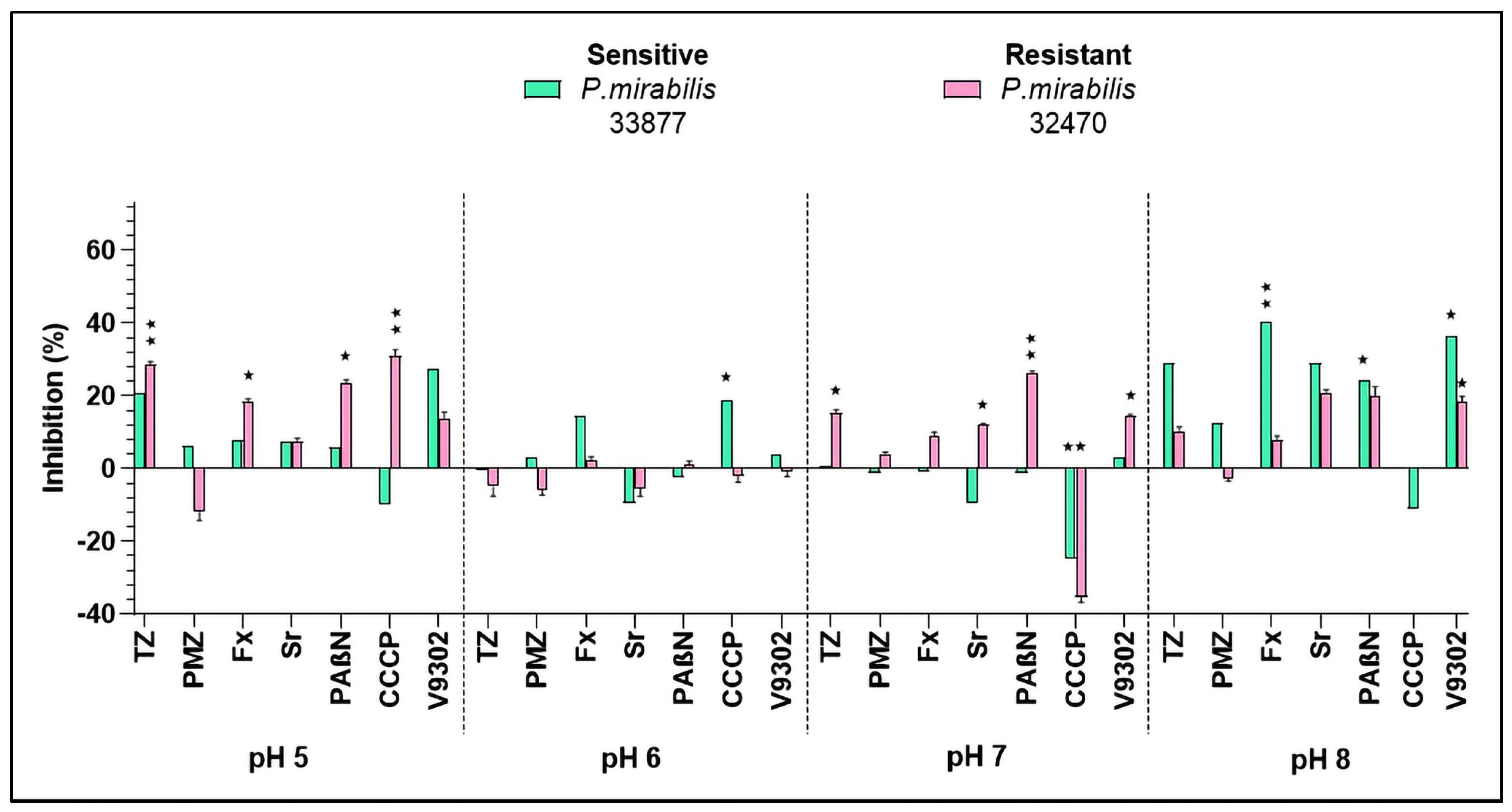
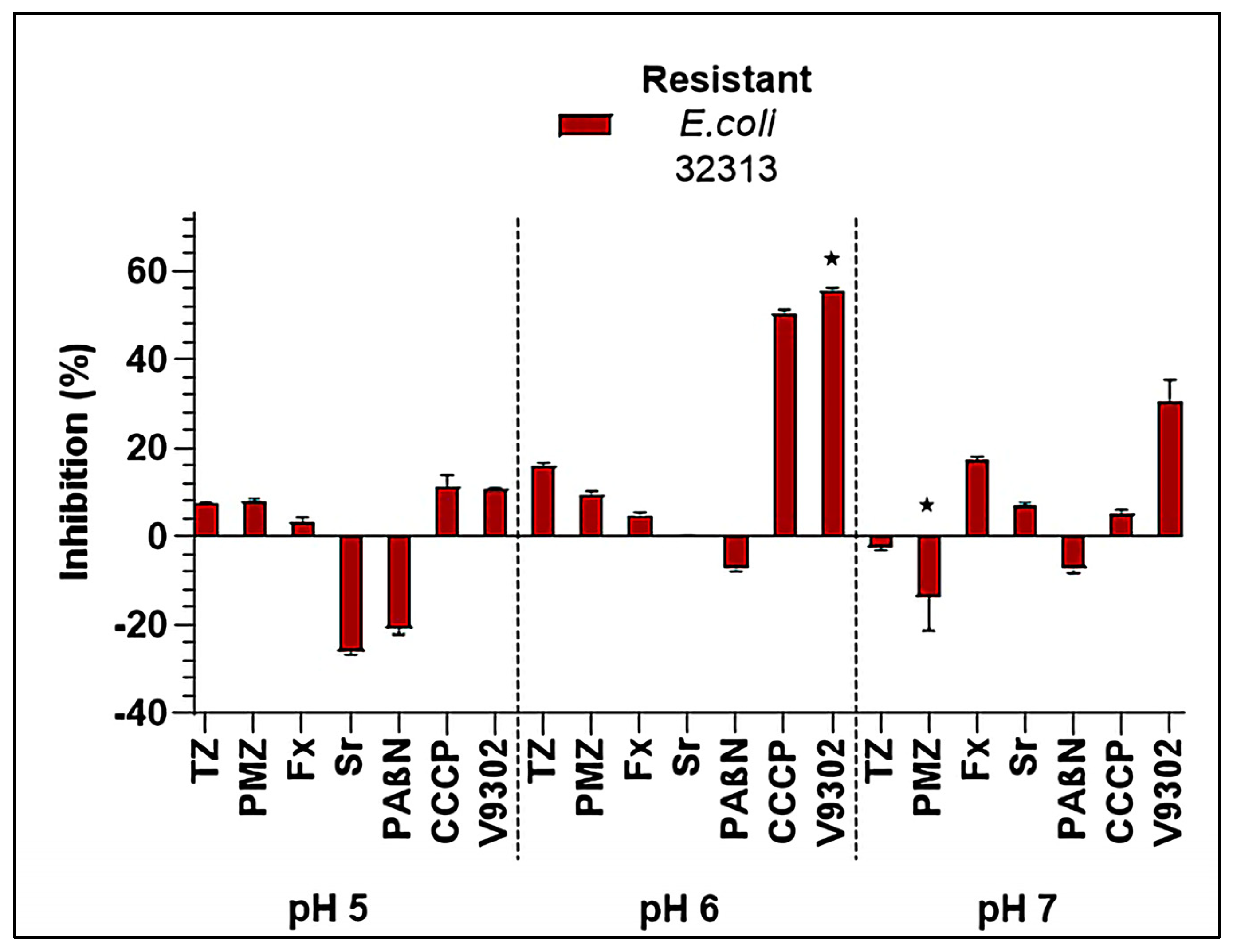
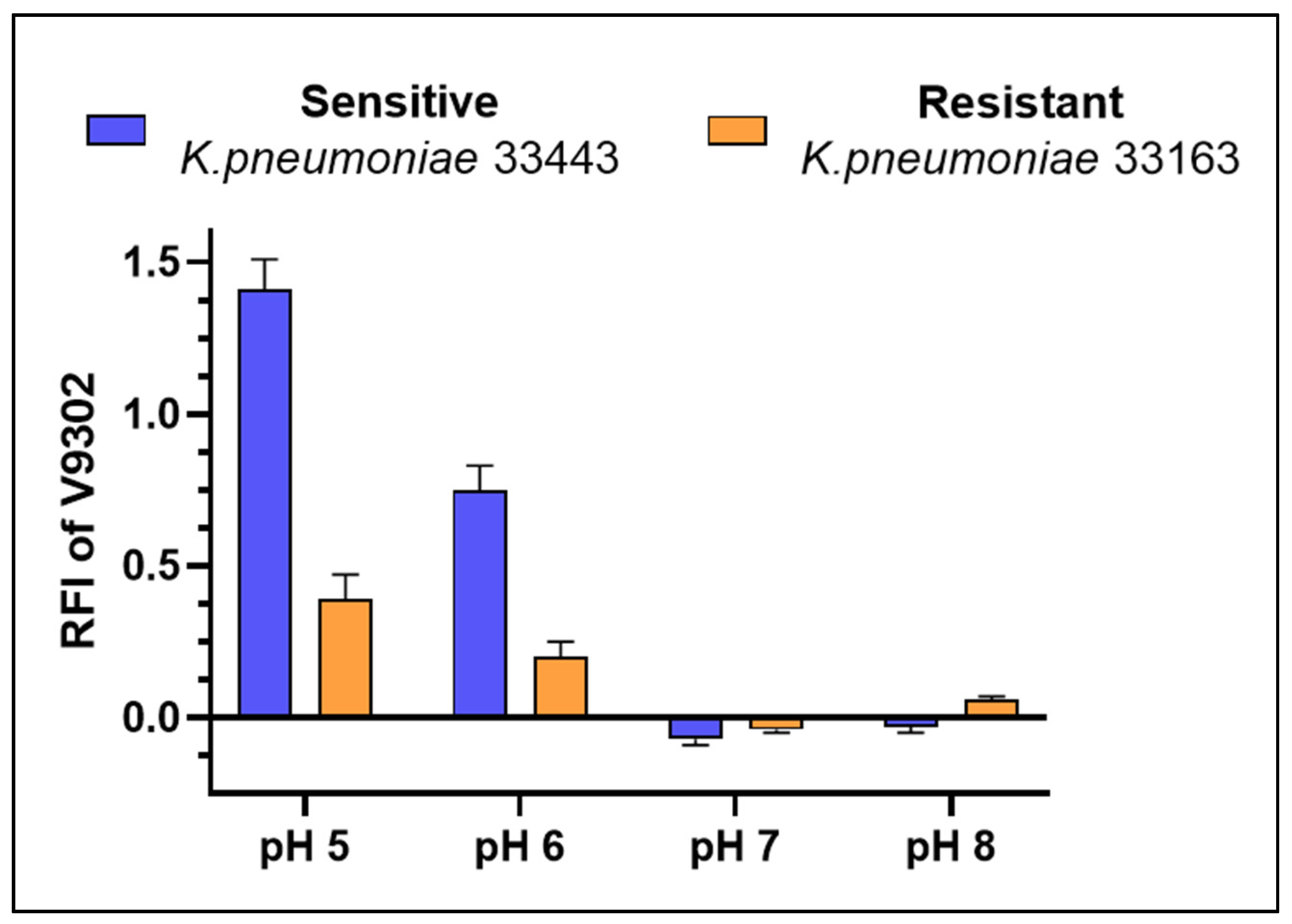
2.4. Inhibition of Bacterial Efflux Pumps
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Determination of Minimum Inhibitory Concentrations by Microdilution Method
4.3. Measuring Biofilm Formation Using Crystal Violet
4.4. Resistance Modulation Assay
4.5. Real-Time Ethidium Bromide Accumulation Assay
4.6. Statystical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alghamdi, A.; Hafiz, T.; Almaghrabi, R.; Al-Qahtani, A.; Hammad, E.; Obeid, D.; Alaidan, A.; Alhamlan, F. Multidrug Resistance Efflux Pump Expression in Uropathogenic Gram-Negative Bacteria in Organ Transplant Recipients. J. Infect. Dev. Ctries. 2025, 19, 641–648. [Google Scholar] [CrossRef]
- Abdelhamid, S.M.; Abozahra, R.R. Expression of the Fluoroquinolones Efflux Pump Genes Acr A and Mdf A in Urinary Escherichia coli Isolates. Pol. J. Microbiol. 2017, 66, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Keeney, D.; Ruzin, A.; McAleese, F.; Murphy, E.; Bradford, P.A. MarA-Mediated Overexpression of the AcrAB Efflux Pump Results in Decreased Susceptibility to Tigecycline in Escherichia coli. J. Antimicrob. Chemother. 2007, 61, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Bhattacharyya, A.; Mandal, S. YnfA, a SMR Family Efflux Pump Is Abundant in Escherichia coli Isolates from Urinary Infection. Indian J. Med. Microbiol. 2015, 33, 139–142. [Google Scholar] [CrossRef]
- Al-Dahmoshi, H.; Ali, S.A.; Al-Khafaji, N. Efflux Pumps among Urinary E. coli and K. pneumoniae Local Isolates in Hilla City, Iraq. In The Global Antimicrobial Resistance Epidemic—Innovative Approaches and Cutting-Edge Solutions; Tellez-Isaias, G., Ed.; IntechOpen: London, UK, 2022; ISBN 978-1-80356-041-0. [Google Scholar]
- Szabo, O.; Kocsis, B.; Szabo, N.; Kristof, K.; Szabo, D. Contribution of OqxAB Efflux Pump in Selection of Fluoroquinolone-Resistant Klebsiella pneumoniae. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 1–5. [Google Scholar] [CrossRef]
- Periyasami, G.; Karuppiah, P.; Karthikeyan, P.; Palaniappan, S. Anti-Infective Efficacy of Duloxetine against Catheter-Associated Urinary Tract Infections Caused by Gram-Positive Bacteria. ACS Omega 2023, 8, 48317–48325. [Google Scholar] [CrossRef]
- Cerna-Vargas, J.P.; Marcos-Torres, F.J. Antidepressants: A New Front in the War against Antibiotics Resistance. Environ. Microbiol. 2022, 24, 4984–4986. [Google Scholar] [CrossRef]
- Bohnert, J.A.; Szymaniak-Vits, M.; Schuster, S.; Kern, W.V. Efflux Inhibition by Selective Serotonin Reuptake Inhibitors in Escherichia coli. J. Antimicrob. Chemother. 2011, 66, 2057–2060. [Google Scholar] [CrossRef]
- Poyil, M.M.; Khan, M.S.; Gowri, M. Repurposing the Anti-Depression Drug Sertraline against Catheter-Associated Urinary Tract Infections. Adv. Hum. Biol. 2023, 13, 271–276. [Google Scholar] [CrossRef]
- Endo, T.H.; Santos, M.H.D.M.; Scandorieiro, S.; Gonçalves, B.C.; Vespero, E.C.; Perugini, M.R.E.; Pavanelli, W.R.; Nakazato, G.; Kobayashi, R.K.T. Selective Serotonin Reuptake Inhibitors: Antimicrobial Activity Against ESKAPEE Bacteria and Mechanisms of Action. Antibiotics 2025, 14, 51. [Google Scholar] [CrossRef]
- Hussein, M.; Schneider-Futschik, E.K.; Paulin, O.K.A.; Allobawi, R.; Crawford, S.; Zhou, Q.T.; Hanif, A.; Baker, M.; Zhu, Y.; Li, J.; et al. Effective Strategy Targeting Polymyxin-Resistant Gram-Negative Pathogens: Polymyxin B in Combination with the Selective Serotonin Reuptake Inhibitor Sertraline. ACS Infect. Dis. 2020, 6, 1436–1450. [Google Scholar] [CrossRef] [PubMed]
- Nzakizwanayo, J.; Scavone, P.; Jamshidi, S.; Hawthorne, J.A.; Pelling, H.; Dedi, C.; Salvage, J.P.; Hind, C.K.; Guppy, F.M.; Barnes, L.M.; et al. Fluoxetine and Thioridazine Inhibit Efflux and Attenuate Crystalline Biofilm Formation by Proteus mirabilis. Sci. Rep. 2017, 7, 12222. [Google Scholar] [CrossRef] [PubMed]
- Rácz, B.; Spengler, G. Repurposing Antidepressants and Phenothiazine Antipsychotics as Efflux Pump Inhibitors in Cancer and Infectious Diseases. Antibiotics 2023, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, K.; Li, H.; Denstedt, J.D.; Cadieux, P.A. The Influence of Urinary pH on Antibiotic Efficacy Against Bacterial Uropathogens. Urology 2014, 84, 731.e1–731.e7. [Google Scholar] [CrossRef]
- Herrera-Espejo, S.; Carretero-Ledesma, M.; Bahamonde-García, M.A.; Cordero, E.; Pachón, J.; Pachón-Ibáñez, M.E. Assessing the Influence of Urine pH on the Efficacy of Ciprofloxacin and Fosfomycin in Immunocompetent and Immunocompromised Murine Models of Escherichia coli and Klebsiella pneumoniae Infection in the Lower Urinary Tract. Antibiotics 2024, 13, 827. [Google Scholar] [CrossRef]
- Kincses, A.; Rácz, B.; Baaity, Z.; Vásárhelyi, O.; Kristóf, E.; Somogyvári, F.; Spengler, G. The Relationship between Antibiotic Susceptibility and pH in the Case of Uropathogenic Bacteria. Antibiotics 2021, 10, 1431. [Google Scholar] [CrossRef]
- Tozar, T.; Santos Costa, S.; Udrea, A.-M.; Nastasa, V.; Couto, I.; Viveiros, M.; Pascu, M.L.; Romanitan, M.O. Anti-Staphylococcal Activity and Mode of Action of Thioridazine Photoproducts. Sci. Rep. 2020, 10, 18043. [Google Scholar] [CrossRef]
- Dastidar, S.; Kristiansen, J.; Molnar, J.; Amaral, L. Role of Phenothiazines and Structurally Similar Compounds of Plant Origin in the Fight against Infections by Drug Resistant Bacteria. Antibiotics 2013, 2, 58–72. [Google Scholar] [CrossRef]
- Kvist, M.; Hancock, V.; Klemm, P. Inactivation of Efflux Pumps Abolishes Bacterial Biofilm Formation. Appl. Environ. Microbiol. 2008, 74, 7376–7382. [Google Scholar] [CrossRef]
- Amaral, L.; Martins, A.; Spengler, G.; Molnar, J. Efflux Pumps of Gram-Negative Bacteria: What They Do, How They Do It, with What and How to Deal with Them. Front. Pharmacol. 2014, 4, 168. [Google Scholar] [CrossRef]
- El Banna, T.E.S.; Ibrahim Sonbol, F. Modulation of Antibiotic Efficacy against Klebsiella pneumoniae by Antihistaminic Drugs. J. Med. Microb. Diagn. 2016, 5, 225. [Google Scholar] [CrossRef]
- Nové, M.; Kincses, A.; Molnár, J.; Amaral, L.; Spengler, G. The Role of Efflux Pumps and Environmental pH in Bacterial Multidrug Resistance. In Vivo 2020, 34, 65–71. [Google Scholar] [CrossRef] [PubMed]
- McGovern, A.S.; Hamlin, A.S.; Winter, G. A Review of the Antimicrobial Side of Antidepressants and Its Putative Implications on the Gut Microbiome. Aust. N. Z. J. Psychiatry 2019, 53, 1151–1166. [Google Scholar] [CrossRef]
- Basil, M.; Khalid, T.W.; Kassim Ghaima, K. Effect of Berberine as Efflux Pump Inhibitor in Multidrug-resistant Klebsiella pneumoniae Isolated from Urinary Tract Infections. Rev. Bionatura 2023, 8, 1–13. [Google Scholar] [CrossRef]
- Reza, A.; Sutton, J.M.; Rahman, K.M. Effectiveness of Efflux Pump Inhibitors as Biofilm Disruptors and Resistance Breakers in Gram-Negative (ESKAPEE) Bacteria. Antibiotics 2019, 8, 229. [Google Scholar] [CrossRef]
- Baugh, S.; Phillips, C.R.; Ekanayaka, A.S.; Piddock, L.J.V.; Webber, M.A. Inhibition of Multidrug Efflux as a Strategy to Prevent Biofilm Formation. J. Antimicrob. Chemother. 2014, 69, 673–681. [Google Scholar] [CrossRef]
- Le, D.; Krasnopeeva, E.; Sinjab, F.; Pilizota, T.; Kim, M. Active Efflux Leads to Heterogeneous Dissipation of Proton Motive Force by Protonophores in Bacteria. mBio 2021, 12, e00676-21. [Google Scholar] [CrossRef]
- Osei Sekyere, J.; Amoako, D.G. Carbonyl Cyanide M-Chlorophenylhydrazine (CCCP) Reverses Resistance to Colistin, but Not to Carbapenems and Tigecycline in Multidrug-Resistant Enterobacteriaceae. Front. Microbiol. 2017, 8, 228. [Google Scholar] [CrossRef]
- Ren, J.; Wang, M.; Zhou, W.; Liu, Z. Efflux Pumps as Potential Targets for Biofilm Inhibition. Front. Microbiol. 2024, 15, 1315238. [Google Scholar] [CrossRef]
- Schulte, M.L.; Fu, A.; Zhao, P.; Li, J.; Geng, L.; Smith, S.T.; Kondo, J.; Coffey, R.J.; Johnson, M.O.; Rathmell, J.C.; et al. Pharmacological Blockade of ASCT2-Dependent Glutamine Transport Leads to Antitumor Efficacy in Preclinical Models. Nat. Med. 2018, 24, 194–202. [Google Scholar] [CrossRef]
- Szemerédi, N.; Schelz, Z.; Horvath, D.A.; Rácz, B.; Szatmári, A.G.; Muddather, H.F.; Bózsity, N.; Zupkó, I.; Spengler, G. Impact of V9302, a Competitive Antagonist of Transmembrane Glutamine Flux on Reversal of Resistance in Breast Cancer Cell Lines. Pharmaceutics 2024, 16, 877. [Google Scholar] [CrossRef]
- Forchhammer, K. Glutamine Signalling in Bacteria. Front. Biosci. 2007, 12, 358. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Z.; Yang, T.; Jiang, M.; Wang, J.; Cheng, Z.; Yang, M.; Zhu, J.; Zhang, T.; Li, H.; et al. Glutamine Promotes Antibiotic Uptake to Kill Multidrug-Resistant Uropathogenic Bacteria. Sci. Transl. Med. 2021, 13, eabj0716. [Google Scholar] [CrossRef]
- CLSI Supplement M100; Performance Standards for Antimicrobial Susceptibility Testing. CLSI: Wayne, PA, USA, 2025.

| MIC (µM) | Thioridazine | Promethazine | Fluoxetine | Sertraline | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | pH | pH | pH | |||||||||||||
| 5 | 6 | 7 | 8 | 5 | 6 | 7 | 8 | 5 | 6 | 7 | 8 | 5 | 6 | 7 | 8 | |
| E. coli 33504 | 100 | >100 | >100 | 100 | 50 | |||||||||||
| E. coli 32313 | >100 | 100 | >100 | >100 | >100 | 50 | ||||||||||
| K. pneumoniae 33443 | >100 | >100 | >100 | >100 | ||||||||||||
| K. pneumoniae 33163 | >100 | >100 | >100 | >100 | ||||||||||||
| P. mirabilis 33877 | >100 | >100 | >100 | >100 | ||||||||||||
| P. mirabilis 32470 | >100 | >100 | >100 | >100 | ||||||||||||
| MIC (µM) | PAβN | CCCP | V9302 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | pH | pH | ||||||||||
| 5 | 6 | 7 | 8 | 5 | 6 | 7 | 8 | 5 | 6 | 7 | 8 | |
| E. coli 33504 | >100 | 12.5 | 6.25 | 25 | 100 | 100 | 50 | >100 | ||||
| E. coli 32313 | >100 | 12.5 | 25 | 100 | 100 | >100 | ||||||
| K. pneumoniae 33443 | >100 | 25 | >100 | >100 | ||||||||
| K. pneumoniae 33163 | 100 | >100 | 100 | >100 | >100 | |||||||
| P. mirabilis 33877 | >100 | 6.25 | 6.25 | 12.5 | 25 | >100 | 50 | >100 | ||||
| P. mirabilis 32470 | >100 | 25 | >100 | 100 | >100 | |||||||
| MIC (µg/mL) | K. pneumoniae 33443 | |||
|---|---|---|---|---|
| pH | ||||
| 5 | 6 | 7 | 8 | |
| CIP | 100 | 100 | 25 | 6.25 |
| CIP + TZ | ND | ND | 12.5 | ND |
| CIP + PMZ | ND | ND | 12.5 | ND |
| CIP + PAβN | 100 | 25 | 1.56 | 0.39 |
| FO | 50 | 100 | 100 | 100 |
| FO + PAβN | 50 | 100 | 100 | 50 |
| MIC (µg/mL) | K. pneumoniae 33163 | |||
| pH | ||||
| 5 | 6 | 7 | 8 | |
| CIP | >100 | >100 | >100 | 100 |
| CIP + PAβN | >100 | >100 | >100 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kincses, A.; Nové, M.; Asefi, J.; Spengler, G. Repurposed Drugs and Efflux Pump Inhibitors Against Gram-Negative Urinary Tract Pathogenic Bacteria. Antibiotics 2025, 14, 988. https://doi.org/10.3390/antibiotics14100988
Kincses A, Nové M, Asefi J, Spengler G. Repurposed Drugs and Efflux Pump Inhibitors Against Gram-Negative Urinary Tract Pathogenic Bacteria. Antibiotics. 2025; 14(10):988. https://doi.org/10.3390/antibiotics14100988
Chicago/Turabian StyleKincses, Annamária, Márta Nové, Jina Asefi, and Gabriella Spengler. 2025. "Repurposed Drugs and Efflux Pump Inhibitors Against Gram-Negative Urinary Tract Pathogenic Bacteria" Antibiotics 14, no. 10: 988. https://doi.org/10.3390/antibiotics14100988
APA StyleKincses, A., Nové, M., Asefi, J., & Spengler, G. (2025). Repurposed Drugs and Efflux Pump Inhibitors Against Gram-Negative Urinary Tract Pathogenic Bacteria. Antibiotics, 14(10), 988. https://doi.org/10.3390/antibiotics14100988








