Co-Producing an Intervention to Reduce Inappropriate Antibiotic Prescribing Among Dental Practitioners in India
Abstract
1. Background
2. Aims and Objectives
- To engage key stakeholders in India and identify priority topics related to antibiotic prescribing in dental practice.
- To collaboratively design a computer-based educational intervention package that addresses inappropriate antibiotic use, ensuring it is evidence-based, contextually relevant, and feasible for use in Indian primary dental care settings.
- To refine the intervention components through iterative feedback and pre-testing.
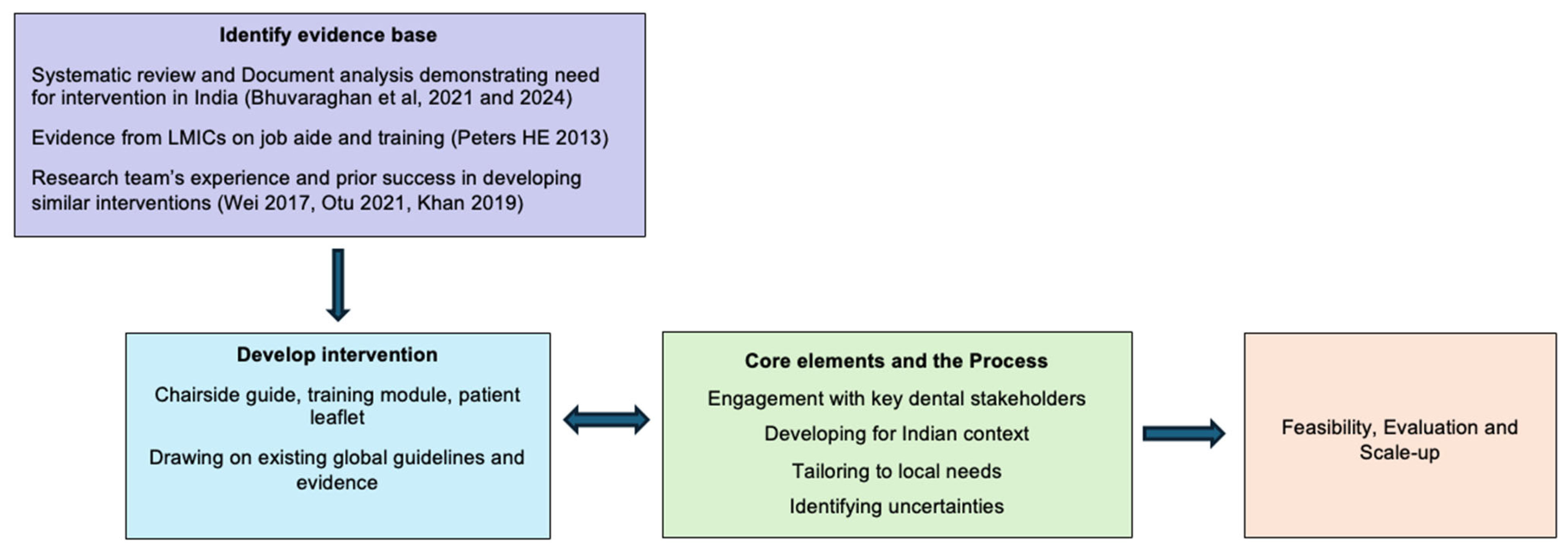
3. Considering the Core Elements of the Medical Research Council (MRC) Framework
3.1. Engaging with Stakeholders
- Helping in identifying/recruiting potential participants;
- Development of resources;
- Endorsing the developed materials;
- Disseminating any developed stewardship resources on their websites;
- Organising stewardship activities, if needed, such as CPD programmes;
- Assistance with future scale-up;
- Bringing the issue to the attention of the dental regulatory authority.
3.2. The Context
3.3. The Programme Theory
3.4. Key Uncertainties
4. Methods
4.1. Ethical Approval
4.2. Setting
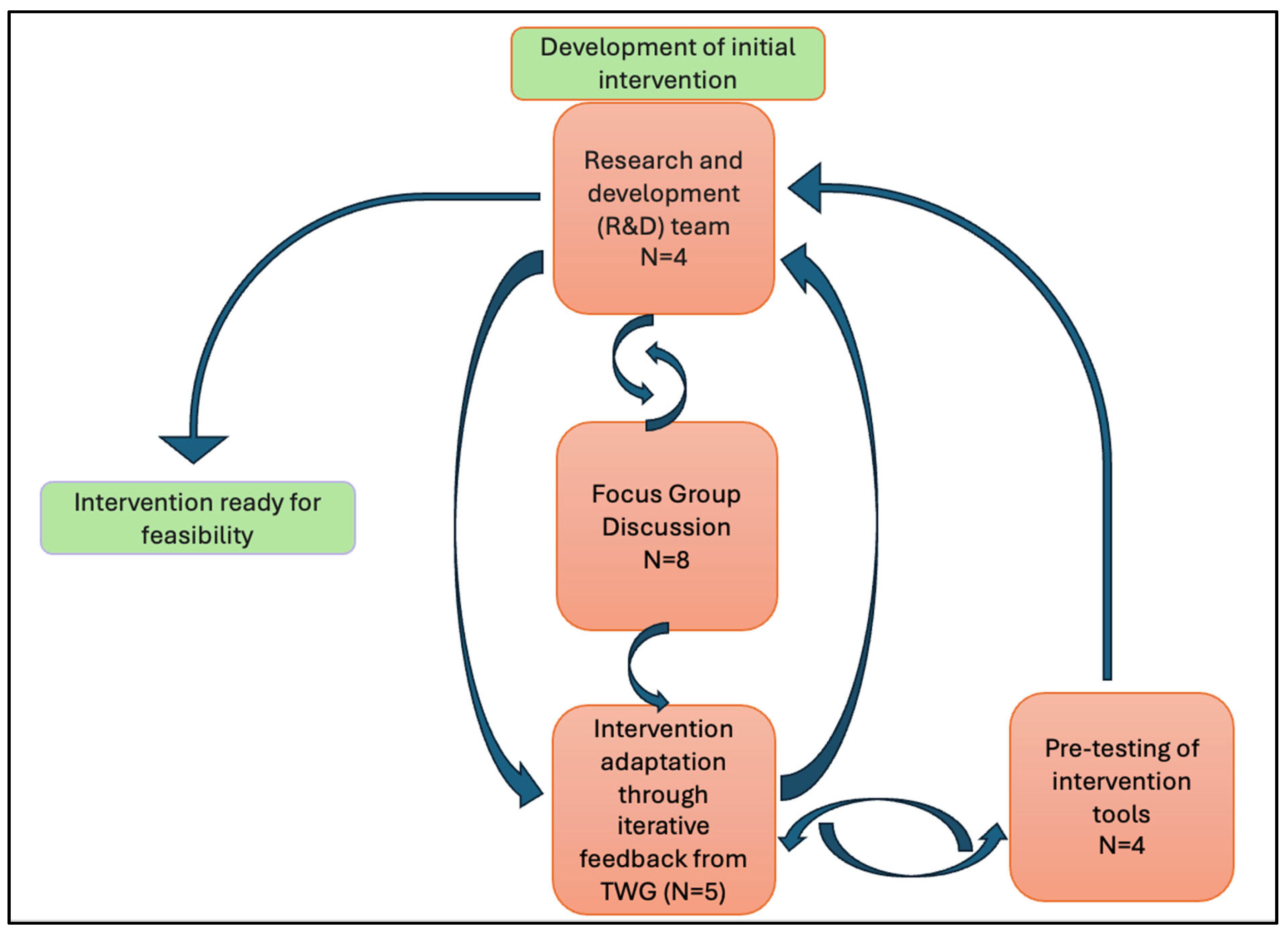
4.3. The Intervention Development Process
- Development of the initial draft intervention by the Research and Development team.
- Focus group discussions (FGDs).
- Enhancing the training module (intervention) based on focus group discussions.
- The Technical Working Group (TWG) and intervention adaptation through iterative feedback.
- Pre-testing of the intervention package with dental practitioners.
- Final refinements with the TWG.
4.3.1. Step 1: Development of the Initial Draft Intervention by the Research and Development Team
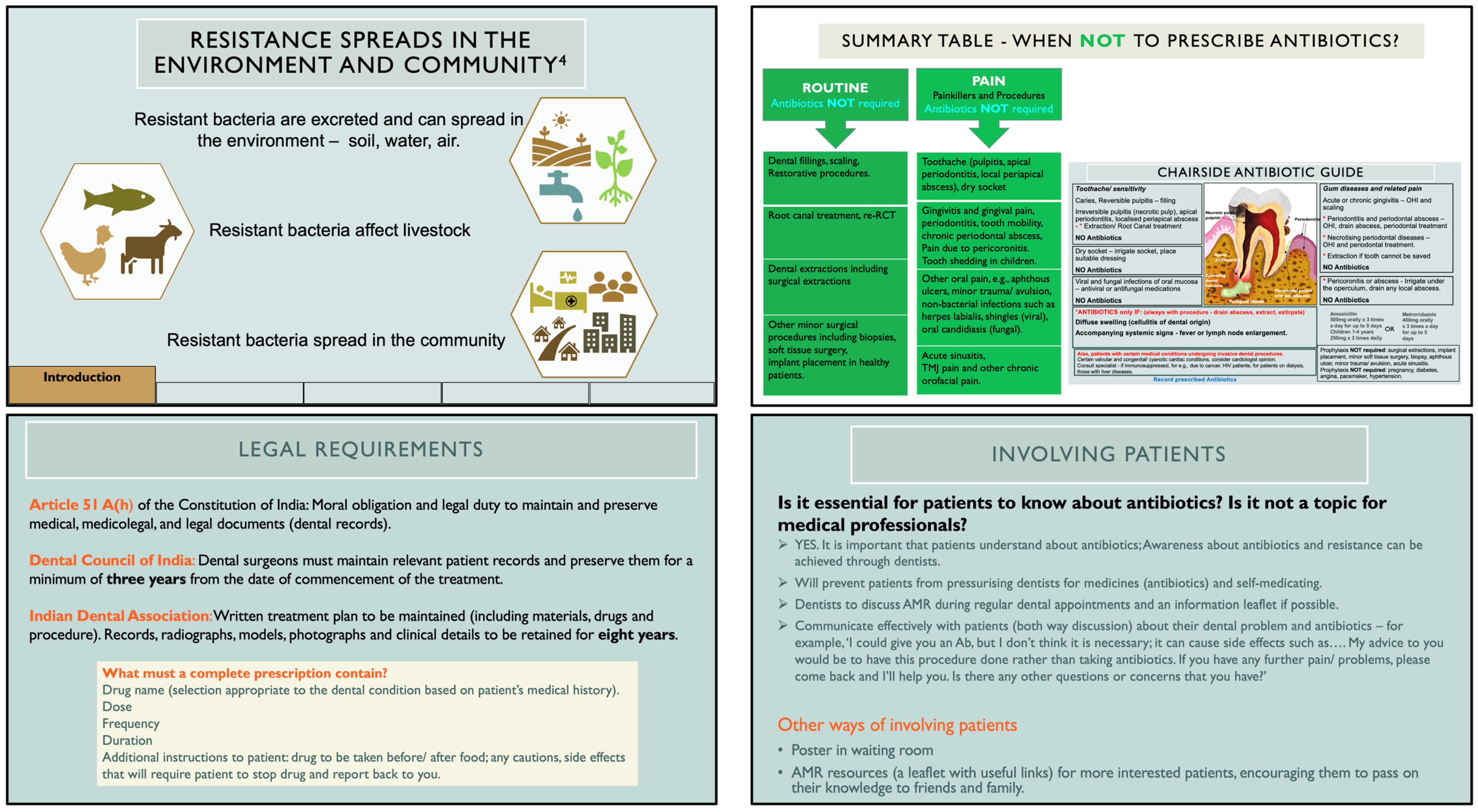
Choice of the Intervention and Content
- (i)
- A chairside antibiotic guide—a one-page illustrated job aid containing dental conditions and procedures encountered in Indian primary care dental settings, for which inappropriate antibiotic prescribing is common, and
- (ii)
- A training module in the form of PowerPoint slides based on this guide.
Format and Delivery
Critical Knowledge Gap to Be Addressed
4.3.2. Step 2: The Focus Group Discussions (FGDs)
Purpose
Sampling
Methods
Analysis
Results
“There is no big use to me recording that(prescription)data now… If I was affected… in the sense sometwoof my patients sued me and I had to go to the court of law, then I will automatically start recording even without anyone’s advice.” FG 5
“If the patient is allergic to a certain medication, I mention that in the case sheet. But I do not routinely record what I prescribe.” FG 8
“If we want them to keep a record, we need to tell first them what will happen if they do not do it. It can be a moral or legal reason.” FG 2
“We were also at that time not convinced about software data safety and cloud storage. But in future, things may change.” FG 3
“I think this is enough, nothing more is required. This will work.” FG 1
“No antibiotics for extractions?!” FG 3
“You cannot skip antibiotics for abscesses.” FG 4
“Instruments we use are not 100% sterile. We only use normal gloves that are not sterile for surgical procedures.” FG 7
“Whatever may happen, antibiotics will take care of it.” FG 2
“My personal opinion is that AMR is never going to be caused by dentists in this lifetime… We use very limited number of antibiotics, mostly amoxy (amoxicillin). How can just amoxy lead to AMR?” FG 5
“I don’t think amoxy (amoxicillin) works. In case I must give antibiotics, I give only Amoxiclav (amoxicillin with clavulanic acid).” FG 1
“After COVID, azithromycin has become resistant. We are using more of Clindamycin.” FG 4
“Ideally, we need to go to every clinic and talk to dentists.” FG 1
“The first reason why dentists have poor practice is because of prescribing antibiotics. Patients don’t go for treatment at all.” FG 2
“There is a tendency from the patient side to ask for a prescription because they have been treated that way for generations. It is our bounded duty to tell them.” (Senior clinician FG 1)
“Many patients do NOT know what antibiotics are.” FG 8
“These dentists (pointing to senior clinicians) are already established. The problem is… patients listen to doctors because you are already an established practice. That’s not the case with us.” FG 5
“If you have earned enough to sustain, you can take a stand… But it takes a bit of time.” FG 3
“Many young dentists… they see lot of (sales) representatives. These ‘rep’ meetings should be banned.” FG 1
“During COVID time, they were able to successfully implement ‘No OTC sale’. That means it is possible, isn’t it?” FG 5
4.3.3. Step 3: Enhancing the Training Module (Intervention) Based on Focus Group Discussions
4.3.4. Step 4: The Technical Working Group (TWG) and Intervention Adaptation Through Iterative Feedback
Purpose
Selection and Composition of the TWG
Methods
Results
4.3.5. Step 5: Pre-Testing of Intervention Package with Dental Practitioners
Purpose
Sample
Methods
Results
4.3.6. Step 6: Final Refinements with the TWG
5. Discussion
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial Resistance |
| AMS | Antimicrobial Stewardship |
| MRC | Medical Research Council |
| CPD | Continuing Professional Development |
| EHR | Electronic Health Records |
| FCG | Focus Group |
| FGD | Focus Group Discussions |
| HIC | High-Income Countries |
| IDA | Indian Dental Association |
| LMICs | Low- and Middle-Income Countries |
| R&D | Research and Development Team |
| TWG | Technical Working Group |
References
- WHO. Antimicrobial Resistance; World Health Organisation: Geneva, Switzerland, 2023. [Google Scholar]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resitant Infections Globally: Final Report and Recommendations; Wellcome Trust: London, UK, 2016. [Google Scholar]
- Holmes, A.H.; Moore, L.S.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- The Burden of Antimicrobial Resistance (AMR) in India; Institute for Health Metrics and Evaluation: Seattle, WA, USA, 2019.
- Sriram, A.; Kalanxhi, E.; Kapoor, G.; Craig, J.; Balasubramanian, R.; Brar, S.; Criscuolo, N.; Hamilton, A.; Klein, E.; Tseng, K. The State of the World’s Antibiotics in 2021: A Global Analysis of Antimicrobial Resistance and Its Drivers; Center for Disease Dynamics, Economics & Policy: Washington, DC, USA, 2021. [Google Scholar]
- Sulis, G.; Daniels, B.; Kwan, A.; Gandra, S.; Daftary, A.; Das, J.; Pai, M. Antibiotic overuse in the primary health care setting: A secondary data analysis of standardised patient studies from India, China and Kenya. BMJ Glob. Health 2020, 5, e003393. [Google Scholar] [CrossRef] [PubMed]
- Innovate UK. Antimicrobial Resistance in India 2023; Global Expert Mission Report; UKRI: Swindon, UK, 2023. [Google Scholar]
- Hicks, L.A.; Bartoces, M.G.; Roberts, R.M.; Suda, K.J.; Hunkler, R.J.; Taylor, T.H., Jr.; Schrag, S.J. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin. Infect. Dis. 2015, 60, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Al-Haroni, M.; Skaug, N. Incidence of antibiotic prescribing in dental practice in Norway and its contribution to national consumption. J. Antimicrob. Chemother. 2007, 59, 1161–1166. [Google Scholar] [CrossRef]
- Thornhill, M.H.; Dayer, M.J.; Durkin, M.J.; Lockhart, P.B.; Baddour, L.M. Oral antibiotic prescribing by NHS dentists in England 2010–2017. Br. Dent. J. 2019, 227, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Cope, A.L.; Francis, N.A.; Wood, F.; Chestnutt, I.G. Antibiotic prescribing in UK general dental practice: A cross-sectional study. Community Dent. Oral Epidemiol. 2016, 44, 145–153. [Google Scholar] [CrossRef]
- Suda, K.J.; Calip, G.S.; Zhou, J.; Rowan, S.; Gross, A.E.; Hershow, R.C.; Perez, R.I.; McGregor, J.C.; Evans, C.T. Assessment of the Appropriateness of Antibiotic Prescriptions for Infection Prophylaxis Before Dental Procedures, 2011 to 2015. JAMA Netw. Open 2019, 2, e193909. [Google Scholar] [CrossRef]
- Bhuvaraghan, A.; King, R.; Larvin, H.; Aggarwal, V.R. Antibiotic Use and Misuse in Dentistry in India—A Systematic Review. Antibiotics 2021, 10, 1459. [Google Scholar] [CrossRef]
- Gross, A.E.; Hanna, D.; Rowan, S.A.; Bleasdale, S.C.; Suda, K.J. Successful Implementation of an Antibiotic Stewardship Program in an Academic Dental Practice. Open Forum Infect. Dis. 2019, 6, ofz067. [Google Scholar] [CrossRef]
- Seager, J.M.; Howell-Jones, R.S.; Dunstan, F.D.; Lewis, M.A.; Richmond, S.; Thomas, D.W. A randomised controlled trial of clinical outreach education to rationalise antibiotic prescribing for acute dental pain in the primary care setting. Br. Dent. J. 2006, 201, 217–222; discussion 216. [Google Scholar] [CrossRef]
- Elouafkaoui, P.; Young, L.; Newlands, R.; Duncan, E.M.; Elders, A.; Clarkson, J.E.; Ramsay, C.R.; Translation Research in a Dental Setting (TRiaDS) Research Methodology Group. An Audit and Feedback Intervention for Reducing Antibiotic Prescribing in General Dental Practice: The RAPiD Cluster Randomised Controlled Trial. PLoS Med. 2016, 13, e1002115. [Google Scholar] [CrossRef] [PubMed]
- Loffler, C.; Bohmer, F. The effect of interventions aiming to optimise the prescription of antibiotics in dental care—A systematic review. PLoS ONE 2017, 12, e0188061. [Google Scholar] [CrossRef] [PubMed]
- Teoh, L.; Stewart, K.; Marino, R.J.; McCullough, M.J. Improvement of dental prescribing practices using education and a prescribing tool: A pilot intervention study. Br. J. Clin. Pharmacol. 2021, 87, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.A.; Mangino, J.E.; Trolli, E.; Scheetz, R.; Goff, D. Private Practice Dentists Improve Antibiotic Use After Dental Antibiotic Stewardship Education from Infectious Diseases Experts. Open Forum Infect Dis. 2022, 9, ofac361. [Google Scholar] [CrossRef]
- Chehabeddine, N.; Lahoud, N.; Noujeim, Z.E.F.; Zeidan, R.K.; Hleyhel, M.; Saleh, N. Effect of an educational intervention among Lebanese dentists on antibiotic prescribing: A randomized controlled study. Clin. Oral Investig. 2022, 26, 4857–4869. [Google Scholar] [CrossRef]
- Angarita-Diaz, M.D.P.; Bernal-Cepeda, L.; Bastidas-Legarda, L.; Forero-Escobar, D.; Ricaurte-Avendano, A.; Mora-Reina, J.; Vergara-Mercado, M.; Herrera-Herrera, A.; Rodriguez-Paz, M.; Caceres-Matta, S.; et al. Impact of a virtual learning environment on the conscious prescription of antibiotics among Colombian dentists. PLoS ONE 2022, 17, e0262731. [Google Scholar] [CrossRef]
- Cooper, L.; Sneddon, J.; Thompson, W.; Guise, T.; Robertson, D.; Smith, A. Tackling antimicrobial resistance in practice: Dental students’ evaluation of university teaching supplemented by an online course. JAC Antimicrob. Resist. 2022, 4, dlac039. [Google Scholar] [CrossRef]
- Bhuvaraghan, A.; King, R.; Walley, J.; Thiruvenkatachari, B.; Aggarwal, V.R. Dental antibiotic policies, stewardship, and implementation in India: A policy document analysis. Community Dent. Oral Epidemiol. 2024, 52, 844–860. [Google Scholar] [CrossRef]
- Ewald, D.R.; Orsini, M.M.; Strack, R.W. The path to good health: Shifting the dialogue and promoting social ecological thinking. SSM Popul. Health 2023, 22, 101378. [Google Scholar] [CrossRef]
- Wight, D.; Wimbush, E.; Jepson, R.; Doi, L. Six steps in quality intervention development (6SQuID). J. Epidemiol. Community Health 2016, 70, 520–525. [Google Scholar] [CrossRef]
- Chaudhuri, C.; Datta, P. Analysis of private healthcare providers. Econ. Political Wkly. 2020, 55, 59–64. [Google Scholar]
- Hunter, B.M.; Murray, S.F.; Marathe, S.; Chakravarthi, I. Decentred regulation: The case of private healthcare in India. World Dev. 2022, 155, 105889. [Google Scholar] [CrossRef]
- Walley, J.; Khan, M.A.; Witter, S.; Haque, R.; Newell, J.; Wei, X. Embedded health service development and research: Why and how to do it (a ten-stage guide). Health Res. Policy Syst. 2018, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Agyepong, I.A.; Godt, S.; Sombie, I.; Binka, C.; Okine, V.; Ingabire, M.G. Strengthening capacities and resource allocation for co-production of health research in low- and middle-income countries. BMJ 2021, 372, n166. [Google Scholar] [CrossRef] [PubMed]
- Skivington, K.; Matthews, L.; Simpson, S.A.; Craig, P.; Baird, J.; Blazeby, J.M.; Boyd, K.A.; Craig, N.; French, D.P.; McIntosh, E.; et al. A new framework for developing and evaluating complex interventions: Update of Medical Research Council guidance. BMJ 2021, 374, n2061. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.H.; Tran, N.T. Implementation Research in Health: A Practical Guide; Alliance for Health Policy and Systems Research; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Wei, X.; Zhang, Z.; Walley, J.D.; Hicks, J.P.; Zeng, J.; Deng, S.; Zhou, Y.; Yin, J.; Newell, J.N.; Sun, Q.; et al. Effect of a training and educational intervention for physicians and caregivers on antibiotic prescribing for upper respiratory tract infections in children at primary care facilities in rural China: A cluster-randomised controlled trial. Lancet Glob. Health 2017, 5, e1258–e1267. [Google Scholar] [CrossRef]
- Das, J.; Holla, A.; Das, V.; Mohanan, M.; Tabak, D.; Chan, B. In urban and rural India, a standardized patient study showed low levels of provider training and huge quality gaps. Health Aff. 2012, 31, 2774–2784. [Google Scholar] [CrossRef]
- Mehndiratta, A.; Sharma, S.; Gupta, N.P.; Sankar, M.J.; Cluzeau, F. Adapting clinical guidelines in India-a pragmatic approach. BMJ 2017, 359, j5147. [Google Scholar] [CrossRef][Green Version]
- Agarwal, R.; Kalita, J.; Misra, U. Barriers to evidence-based medicine practice in South Asia and possible solutions. Neurol. Asia 2008, 13, 87–94. [Google Scholar][Green Version]
- Sasirekha, K.; Sankar, P. A study on the Challenges and Outlooks of Medical Tourism in India with reference to Chennai region. J. Posit. Sch. Psychol. 2022, 6, 7141–7153. [Google Scholar][Green Version]
- Dental Council of India. RESULT. Available online: https://dciindia.gov.in/ (accessed on 20 June 2025).[Green Version]
- About Implementation Science; National Cancer Institute at the National Institutes of Health: Bethesda, MD, USA, 2025.[Green Version]
- Glasgow, R.E.; Eckstein, E.T.; Elzarrad, M.K. Implementation science perspectives and opportunities for HIV/AIDS research: Integrating science, practice, and policy. JAIDS J. Acquir. Immune Defic. Syndr. 2013, 63, S26–S31. [Google Scholar] [CrossRef]
- Peters, D.H. Improving Health Services in Developing Countries: From Evidence to Action; World Bank: Washington, DC, USA, 2009. [Google Scholar]
- Drug Prescribing for Dentistry: Dental Clinical Guidance; Scottish Dental Clinical Effectiveness Programme: Dundee, UK, 2016.
- Prophylaxis Against Infective Endocarditis: Antimicrobial Prophylaxis Against Infective Endocarditis in Adults and Children Undergoing Interventional Procedures; National Institute for Health and Care Excellence: London, UK, 2016.
- Summary of Antimicrobial Prescribing Guidance—Managing Common Infections; National Institute for Health and Care Excellence: London, UK, 2022.
- Antibiotic Stewardship: Antibiotics for Therapeutic Use; American Dental Association: Chicago, IL, USA, 2023.
- Lockhart, P.B.; Tampi, M.P.; Abt, E.; Aminoshariae, A.; Durkin, M.J.; Fouad, A.F.; Gopal, P.; Hatten, B.W.; Kennedy, E.; Lang, M.S.; et al. Evidence-based clinical practice guideline on antibiotic use for the urgent management of pulpal- and periapical-related dental pain and intraoral swelling: A report from the American Dental Association. J. Am. Dent. Assoc. 2019, 150, 906–921.e12. [Google Scholar] [CrossRef] [PubMed]
- Palmer, N. Antimicrobial Prescribing in Dentistry: Good Practice Guidelines; Faculty of General Dental Practice (UK) and Faculty of Dental Surgery: London, UK, 2020. [Google Scholar]
- Khan, M.A.; Khan, N.; Walley, J.D.; Khan, S.E.; Hicks, J.; Sheikh, F.I.; Khan, M.A.; Ali, M.; Ahmed, M.; Khan, H.J.; et al. Enhanced hypertension care through private clinics in Pakistan: A cluster randomised trial. BJGP Open 2019, 3, bjgpopen18X101617. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Otu, A.A.; Effa, E.E.; Onwusaka, O.; Omoyele, C.; Arakelyan, S.; Okuzu, O.; Walley, J. mHealth guideline training for non-communicable diseases in primary care facilities in Nigeria: A mixed methods pilot study. BMJ Open 2022, 12, e060304. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, T.; Mondal, H.; Mondal, S.; Dutta, R.; Saha, K.; Das, D. Prescription digitization, online preservation, and retrieval on a smartphone. J. Family Med. Prim. Care 2020, 9, 5295–5302. [Google Scholar]
- Duncan, E.; O’Cathain, A.; Rousseau, N.; Croot, L.; Sworn, K.; Turner, K.M.; Yardley, L.; Hoddinott, P. Guidance for reporting intervention development studies in health research (GUIDED): An evidence-based consensus study. BMJ Open 2020, 10, e033516. [Google Scholar] [CrossRef]
- National Action Plan on Antimicrobial Resistance (NAP-AMR) 2017–2021; National Centre for Disease Control: Delhi, India, 2017.
- Revised Dentists (Code of Ethics) Regulations; Dental Council of India: New Delhi, India, 2014.
- Selvaraj, S.; Karan, K.A.; Srivastava, S.; Bhan, N.; Mukhopadhyay, I. India Health System Review; World Health Organization, Regional Office for South-East Asia: New Delhi, India, 2022. [Google Scholar]
- Electronic Health Record (EHR) Standards for India v2.0; Ministry of Health & Family Welfare Government of India: New Delhi, India, 2016.
- Ramanarayanan, V.; Prabhu, A.S.; Sundareswaran, A.; Boban, D. Effectiveness of a training program on the knowledge and awareness of antimicrobial resistance and stewardship among dental house-surgeons. Public Health Toxicol. 2024, 4, 1–7. [Google Scholar] [CrossRef]
- McLeroy, K.R.; Bibeau, D.; Steckler, A.; Glanz, K. An ecological perspective on health promotion programs. Health Educ. Q. 1988, 15, 351–377. [Google Scholar] [CrossRef]
- Watt, R.G. From victim blaming to upstream action: Tackling the social determinants of oral health inequalities. Community Dent. Oral Epidemiol. 2007, 35, 1–11. [Google Scholar] [CrossRef]
- Thompson, W.; Sandoe, J.; Pavitt, S.; Walsh, T.; Byrne-Davis, L. Co-Developing an Antibiotic Stewardship Tool for Dentistry: Shared Decision-Making for Adults with Toothache or Infection. Antibiotics 2021, 10, 1345. [Google Scholar] [CrossRef]
- Laks, M.; Guerra, C.M.; Miraglia, J.L.; Medeiros, E.A. Distance learning in antimicrobial stewardship: Innovation in medical education. BMC Med. Educ. 2019, 19, 191. [Google Scholar] [CrossRef]
- Rocha-Pereira, N.; Lafferty, N.; Nathwani, D. Educating healthcare professionals in antimicrobial stewardship: Can online-learning solutions help? J. Antimicrob. Chemother. 2015, 70, 3175–3177. [Google Scholar] [CrossRef]
| Key Actors/ Stakeholder Groups Involved in Development | Composition Number (n)= Male (m)/Female (f) | Background and Expertise Brought to This Research | Contribution/Role in Development |
|---|---|---|---|
| The Indian Dental Association | n/a | n/a | Recruitment of participants—FCG, pre-testing and subsequent piloting, resource development, endorsement, dissemination, lobbying with the dental regulatory body (Dental Council/NDC) |
| Researchers (Research and Development Team) (AB, RK, JW, VA) | n = 4 (m = 2; f = 2) | AB—Dental practitioner and specialist in oral medicine. Lived and has work experience in India and the UK. Understands the Indian context and speaks the local language (Tamil). RK—Social scientist and anthropologist. Expertise in qualitative research, intervention development, and participatory approaches in LMIC contexts, and AMR research. JW—Medical practitioner and specialist in public health. Expertise in AMR research, intervention development in LMIC contexts, primary care health service delivery. Prior success in developing and evaluating online educational resources to improve health behaviours. VA—Dental practitioner and specialist in dental public health. Expertise in epidemiology, quantitative research, management of acute and chronic orofacial pain. | Conducted a needs assessment prior to intervention development to determine the need for and the type of intervention. Developed the initial intervention and refined it based on iterative feedback from the TWG, FCG, and pre-testing. |
| Focus Group | n = 8 (m = 6; f = 2) | Four Academic dental practitioners Four Full-time dental clinicians All members had their own private practices in urban or peri-urban settings within Chennai and had varying levels of clinical experience, ranging from 3 years to 20 years. | Provided inputs on the chairside antibiotic guide (job aid) Provided information on record-keeping and various ways of prescription recording/retrieval in primary care. Helped triangulate findings (from systematic review and document analysis) regarding antibiotic prescribing practices and AMR awareness. |
| Technical Working Group (TWG) | n = 5 (including lead researcher AB) (m = 3; f = 2) | Dental practitioners from India with over 15 years of clinical experience. AMK—Academic dental practitioner and researcher familiar with global antibiotic guidelines; involved in dental curricular development. KGS—Academic dental practitioner, holds an executive post in the Indian Dental Association. BJK—Full-time clinician, antibiotic champion, holds international (JCI) accreditation in the clinic for quality and patient safety. SJ—Experienced full-time clinician and antibiotic champion. AB—Dental practitioner and academic researcher, co-ordination between R&D team and TWG. | Critically reviewed the accuracy of the content and provided multiple iterative refinements. Provided inputs on the format of intervention. Made final refinements and agreed on the intervention after pre-testing. |
| Pre-testing | n = 4 (m = 2; f = 2) | Two academic dental practitioners and two full-time practitioners, with varying clinical experience, practising in urban or peri-urban settings within Chennai. | Critically reviewed the content for readability, understandability, and duration of content of the module and the questionnaire for regular dental practitioners. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhuvaraghan, A.; Walley, J.; King, R.; Aggarwal, V.R. Co-Producing an Intervention to Reduce Inappropriate Antibiotic Prescribing Among Dental Practitioners in India. Antibiotics 2025, 14, 984. https://doi.org/10.3390/antibiotics14100984
Bhuvaraghan A, Walley J, King R, Aggarwal VR. Co-Producing an Intervention to Reduce Inappropriate Antibiotic Prescribing Among Dental Practitioners in India. Antibiotics. 2025; 14(10):984. https://doi.org/10.3390/antibiotics14100984
Chicago/Turabian StyleBhuvaraghan, Aarthi, John Walley, Rebecca King, and Vishal R. Aggarwal. 2025. "Co-Producing an Intervention to Reduce Inappropriate Antibiotic Prescribing Among Dental Practitioners in India" Antibiotics 14, no. 10: 984. https://doi.org/10.3390/antibiotics14100984
APA StyleBhuvaraghan, A., Walley, J., King, R., & Aggarwal, V. R. (2025). Co-Producing an Intervention to Reduce Inappropriate Antibiotic Prescribing Among Dental Practitioners in India. Antibiotics, 14(10), 984. https://doi.org/10.3390/antibiotics14100984








