Microbiological and Clinical Evaluation of the Efficacy of a Chemical Desiccant Agent in Non-Surgical Periodontal Treatment: A Randomized Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design and Setting
2.2. Primary Objectives
2.3. Study Population
2.3.1. Eligibility Criteria
2.3.2. Inclusion and Exclusion Criteria
- -
- diagnosis of periodontitis, stage II–III, grade A/B/C, generalized form;
- -
- at least 4 teeth per quadrant (excluding third molars);
- -
- at least 8 teeth with pocket depth (PPD) ≥ 5 mm and radiographic evidence of bone loss; among these, at least 3 teeth with PPD ≥ 5 mm;
- -
- male and female patients aged ≥ 18 years;
- -
- general systemic good health as determined by medical history and clinical evaluation (ASA status I and II).
- -
- Allergy to sulfonated compounds;
- -
- Presence of decompensated systemic diseases that could compromise study outcomes or patient safety (ASA status III and IV);
- -
- Regular use of antibiotics;
- -
- Regular use of anti-inflammatory drugs (NSAIDs, corticosteroids, aspirin);
- -
- Use of anticoagulant medications;
- -
- Severe cognitive or psychiatric disorders;
- -
- Systemic antibiotic therapy within 1 month prior to enrolment;
- -
- Periodontal therapy within 6 months prior to enrolment.
2.4. Periodontal Treatment
2.5. Harms
2.6. Sample Size
2.7. Randomisation
- -
- HBX application only in the first session + UD-SRP (HBX 1T);
- -
- Repeated HybenX application over three sessions + UD-SRP (HBX 3T);
- -
- SRP only (control group).
2.8. Blinding
2.9. Outcomes
- -
- Bleeding on probing (BOP): This parameter was used to assess gingival inflammation. A periodontal probe was gently inserted into the gingival sulcus, and if bleeding occurred within 10–15 s, the site was considered positive. If no bleeding occurred, it was considered negative. The absence of BOP during maintenance visits has been suggested as a negative predictor for clinical attachment loss [24].
- -
- Probing pocket depth (PPD): Measured from the gingival margin to the base of the periodontal pocket [25].
- -
- Plaque index (PI): Measured with the probe passed along the gingival margin. PI scores ranged from 0 to 3 (0 = no plaque in the gingival area; 1 = no visible plaque, but plaque is detected on the probe tip; 2 = gingival area visibly covered with plaque; 3 = heavy plaque accumulation or calculus in the gingival area [26].
- -
- Gingival recession (REC): Measured as the distance between the gingival margin and the cementoenamel junction (CEJ) [27].
- -
- Clinical Attachment Level (CAL): Measured as the distance from the CEJ to the base of the sulcus or periodontal pocket [28].
2.10. Microbiological Analysis
- Aggregatibacter actinomycetemcomitans (Aa);
- Porphyromonas gingivalis (Pg);
- Prevotella intermedia (Pi);
- Tannerella forsythia (Tf);
- Treponema denticola (Td);
- Actinomyces naeslundii (An).
2.11. Statistical Analysis
3. Results
3.1. Clinical Outcomes
3.2. Microbiological Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nocini, R.; Lippi, G. Periodontal disease: The portrait of an epidemic. J. Public Health Emerg. 2020, 4, 10. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, G. The effects of a desiccant agent in the treatment of chronic periodontitis: A randomized, controlled clinical trial. Clin. Oral Investig. 2018, 22, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.; Eaton, K.A. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J. Clin. Periodontol. 2009, 36, 458–467. [Google Scholar] [CrossRef]
- Reddy, M.S. Reaching a better understanding of non-oral disease and the implication of periodontal infections. Periodontology 2000 2007, 43, 199–206. [Google Scholar] [CrossRef]
- Tonetti, M.S. Periodontitis and risk for atherosclerosis: An update on intervention trials. J. Clin. Periodontol. 2009, 36 (Suppl. S10), 15–19. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. Dental biofilms: Difficult therapeutic targets. Periodontology 2000 2002, 28, 12–55. [Google Scholar] [CrossRef]
- Sağlam, M.; Arslan, U. Boric acid irrigation as an adjunct to mechanical periodontal therapy in patients with chronic periodontitis: A randomized clinical trial. J. Periodontol. 2013, 84, 1297–1308. [Google Scholar] [CrossRef]
- Oda, S.; Nitta, H. Current concepts and advances in manual and power-driven instrumentation. Periodontology 2000 2004, 36, 45–58. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque: Biological significance of a biofilm and community lifestyle. J. Clin. Periodontol. 2005, 32 (Suppl. S6), 7–15. [Google Scholar] [CrossRef]
- Mariotti, A.J.; Rumpf, D.A.H. Chlorhexidine-induced changes to human gingival fibroblast collagen and non-collagen protein production. J. Periodontol. 1999, 70, 1443–1448. [Google Scholar] [CrossRef]
- Bracke, J.; Basara, M. Pilot evaluation of a simple adjunctive method for improved removal of oral biofilm during conventional scaling and root planing therapy. J. Biol. Regul. Homeost. Agents 2015, 29 (Suppl. S1), 6–9. [Google Scholar] [PubMed]
- Rhodus, N.L.; Bereuter, J. An evaluation of a chemical cautery agent and an anti-inflammatory ointment for the treatment of recurrent aphthous stomatitis: A pilot study. Quintessence Int. 1988, 19, 769–770. [Google Scholar]
- Lupșe, I.; Baldea, I. Cytotoxic effects on gingival mesenchymal stromal cells and root surface modifications induced by some local antimicrobial products used in periodontitis treatment. Materials 2021, 14, 5049. [Google Scholar] [CrossRef]
- Porter, S.; Al-Johani, K. Randomised controlled trial of the efficacy of HybenX in the symptomatic treatment of recurrent aphthous stomatitis. Oral Dis. 2009, 15, 155–161. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A. New frontiers on adjuvants drug strategies and treatments in periodontitis. Sci. Pharm. 2021, 89, 46. [Google Scholar] [CrossRef]
- Pardo, A.; Fiorini, V. Topical Agents in Biofilm Disaggregation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 2179. [Google Scholar] [CrossRef]
- Rajagopal, A.; Varghese, J. Anti-infective Efficacy of Mechanical Debridement with Adjunctive Modalities on Clinical and Cytokine Parameters in Treatment of Chronic Periodontitis: Randomized Controlled Clinical Trial. Eur. J. Dent. 2024, 18, 526–533. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomized trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M. Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S173–S182. [Google Scholar] [CrossRef]
- García, L.; Tercero, J.C. Rapid detection of Actinobacillus actinomycetemcomitans, Prevotella intermedia and Porphyromona gingivalis by multiplex PCR. J. Periodontal Res. 1998, 33, 59–64. [Google Scholar] [CrossRef]
- Frequently Asked Questions about HYBENX® Oral Tissue Decontaminant (HOTD). Available online: http://hybenx.it/wp-content/uploads/2015/07/FAQs_HOTD15Nov17-.pdf (accessed on 10 February 2025).
- Khalil, B.; Alqahtani, F. The effects of adjunctive use of a desiccant agent in the treatment of stage III periodontitis: Randomized controlled clinical trial. Saudi Dent. J. 2023, 35, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, G.; Singoretto, C. A topical desiccant agent in association with ultrasonic debridement in the initial treatment of chronic periodontitis: A clinical and microbiological study. New Microbiol. 2015, 38, 393–407. [Google Scholar] [PubMed]
- Gerber, J.A.; Tan, W.C. Bleeding on probing and pocket probing depth in relation to probing pressure and mucosal health around oral implants. Clin. Oral Implant. Res. 2009, 20, 75–78. [Google Scholar] [CrossRef]
- Yoneyama, T.; Okamoto, H. Probing depth, attachment loss and gingival recession. Findings from a clinical examination in Ushiku, Japan. J. Clin. Periodontol. 1988, 15, 581–591. [Google Scholar] [CrossRef]
- Silness, J.; Löe, H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef]
- Tugnait, A.; Clerehugh, V. Gingival recession: Its significance and management. J. Dent. 2001, 29, 381–394. [Google Scholar] [CrossRef]
- Kim, G.Y.; Kim, S. Advancements in methods of classification and measurement used to assess tooth mobility: A narrative review. J. Clin. Med. 2024, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Carelli, M.; Maguolo, A. Oral microbiota in children and adolescents with type 1 diabetes mellitus: Novel insights into the pathogenesis of dental and periodontal disease. Microorganisms 2023, 11, 668. [Google Scholar] [CrossRef]
- Lombardo, G.; Signoriello, A. A Topical Desiccant Agent in Association with Manual Debridement in the Initial Treatment of Peri-Implant Mucositis: A Clinical and Microbiological Pilot Study. Antibiotics 2019, 8, 82. [Google Scholar] [CrossRef]
- Mombelli, A.; Schmid, B. Persistence patterns of Porphyromonas gingivalis, Prevotella intermedia/nigrescens, and Actinobacillus actinomycetemcomitans after mechanical therapy of periodontal disease. J. Periodontol. 2000, 71, 14–21. [Google Scholar] [CrossRef]
- Quirynen, M.; Teughels, W. Topical antiseptics and antibiotics in the initial therapy of chronic adult periodontitis: Microbiological aspects. Periodontology 2000 2002, 28, 72–90. [Google Scholar] [CrossRef]
- Pardo, A.; Butera, A. Photodynamic Therapy in Non-Surgical Treatment of Periodontitis: A Systematic Review and Meta-Analysis. Appl. Sci. 2023, 13, 1086. [Google Scholar] [CrossRef]
- Scribante, A.; Gallo, S. Ozonized gels vs chlorhexidine in non-surgical periodontal treatment: A randomized clinical trial. Oral Dis. 2024, 30, 3993–4000. [Google Scholar] [CrossRef] [PubMed]
- Sun, G. The impact of systemic and topical antimicrobial therapy combined with non-surgical periodontal therapy: A meta-analysis. Adv. Clin. Exp. Med. 2025, 34, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Baccini, F.; De Manzoni, R.; Viviani, M.; Brentaro, S.; Zangani, A.; Faccioni, P.; Luciano, U.; Zuffellato, N.; Signoriello, A.; et al. Air polishing therapy in supportive periodontal treatment: A systematic review. J. Appl. Cosmetol. 2023, 41 (Suppl. 1), 13–24. [Google Scholar] [CrossRef]
- Roussa-Tsoulou, E.; Schug, W. The limits of root planing in the furcation area: A mathematical comparison of the radii of curvature on the root surfaces and on the curettes. Dtsch. Zahn Mund Kieferheilkd. Zentralbl. 1992, 80, 295–298. [Google Scholar]
- Johnson, R.H.; Rozanis, J. A review of chemotherapeutic plaque control. Oral Surg. Oral Med. Oral Pathol. 1979, 47, 136–141. [Google Scholar] [CrossRef]
- Iorio-Siciliano, V.; Ramaglia, L. Changes in clinical parameters following adjunctive local sodium hypochlorite gel in minimally invasive nonsurgical therapy (MINST) of periodontal pockets: A 6-month randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 5331–5340. [Google Scholar] [CrossRef]
- Ramanauskaite, E.; Machiulskiene, V.A. Clinical evaluation of sodium hypochlorite/amino acids and cross-linked hyaluronic acid adjunctive to nonsurgical periodontal treatment: A randomized controlled clinical trial. Clin. Oral Investig. 2023, 27, 6645–6656. [Google Scholar] [CrossRef]
- Soancă, A.; Ilea, A. The treatment of severe periodontitis using a local antiseptic desiccant and subgingival mechanical instrumentation: A pilot study. J. Clin. Med. 2023, 12, 4286. [Google Scholar] [CrossRef]
- Reddy, S.; Kumar, M. Treatment of acute periodontal abscess using a biofilm decontaminant gel (Hybenx®) in patients on periodontal maintenance therapy: A case series. Int. J. Appl. Dent. Sci. 2018, 4, 240–243. [Google Scholar]
- Radulescu, V.; Ilea, A. Clinical and microbiological effects of a single application of sodium hypochlorite gel during subgingival re-instrumentation: A triple-blind randomized placebo-controlled clinical trial. Clin. Oral Investig. 2022, 26, 6639–6652. [Google Scholar] [CrossRef]
- Pini-Prato, G.P.; Magnani, C. Treatment of acute periodontal abscesses using the biofilm decontamination approach: A case-report study. Int. J. Periodontics Restor. Dent. 2016, 36, 55–63. [Google Scholar] [CrossRef]
- Zafar, F.; Ahmad, S. Chemical cleansing as an adjunct to subgingival instrumentation with ultrasonic and hand devices in deep periodontal pockets: A randomized controlled study. J. Periodontal Implant Sci. 2021, 51, 276–284. [Google Scholar] [CrossRef]
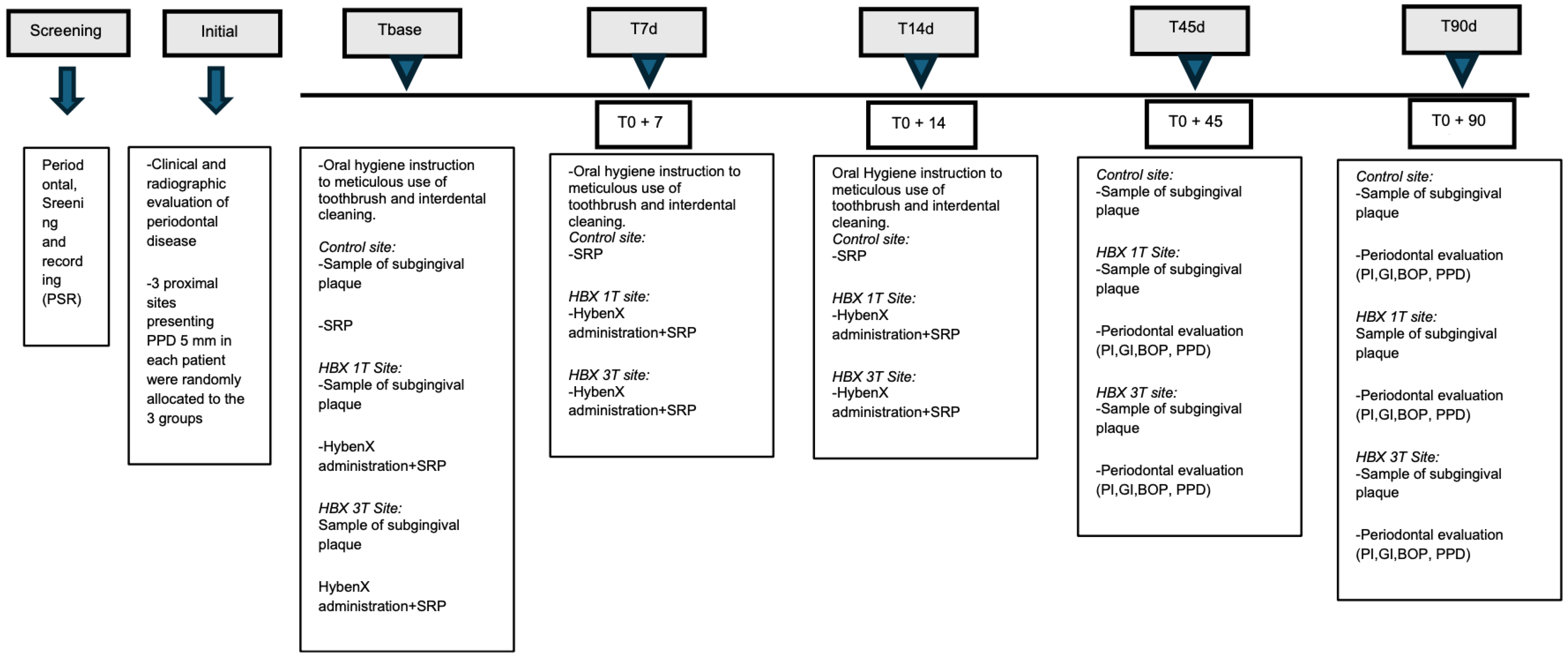
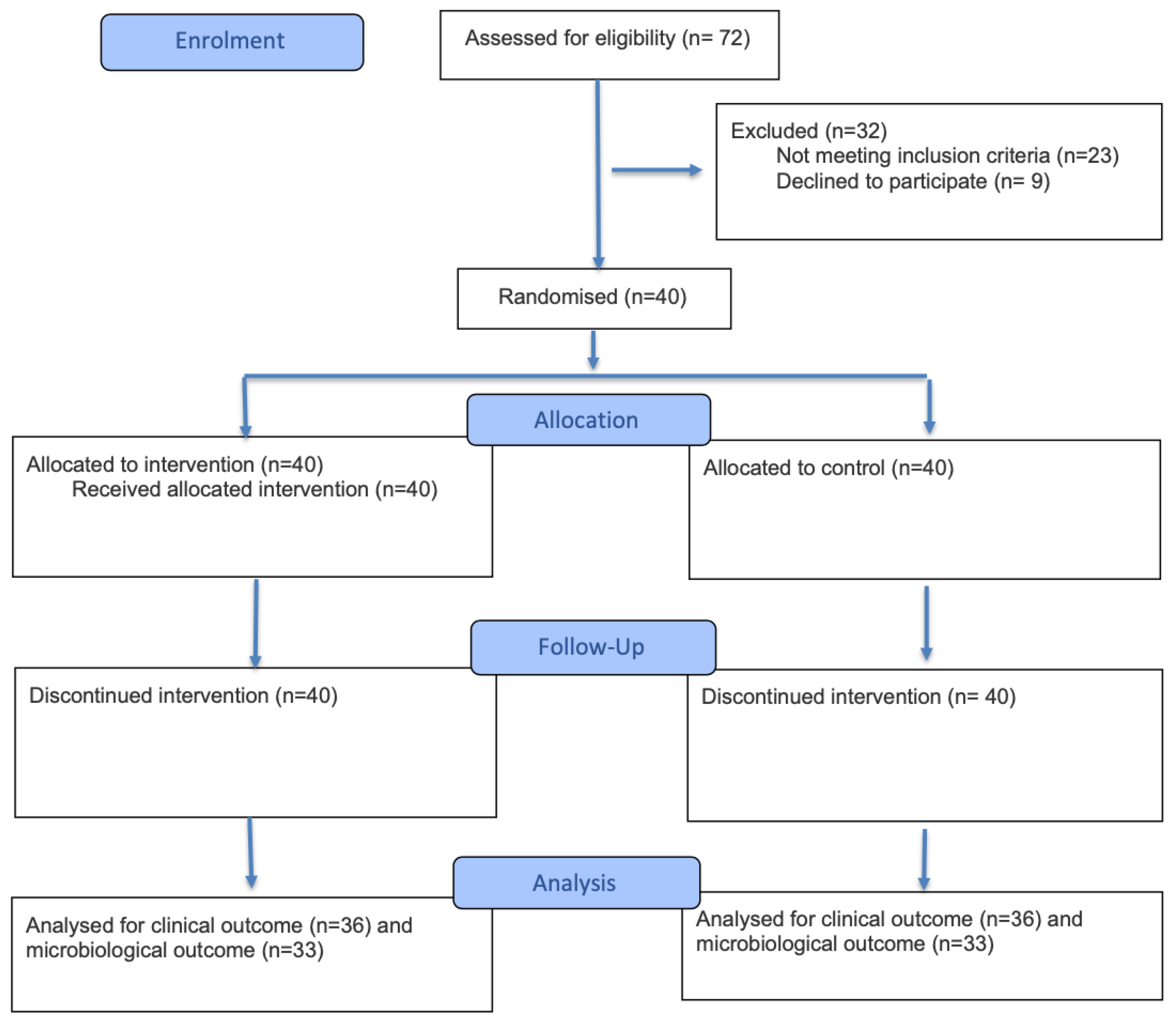
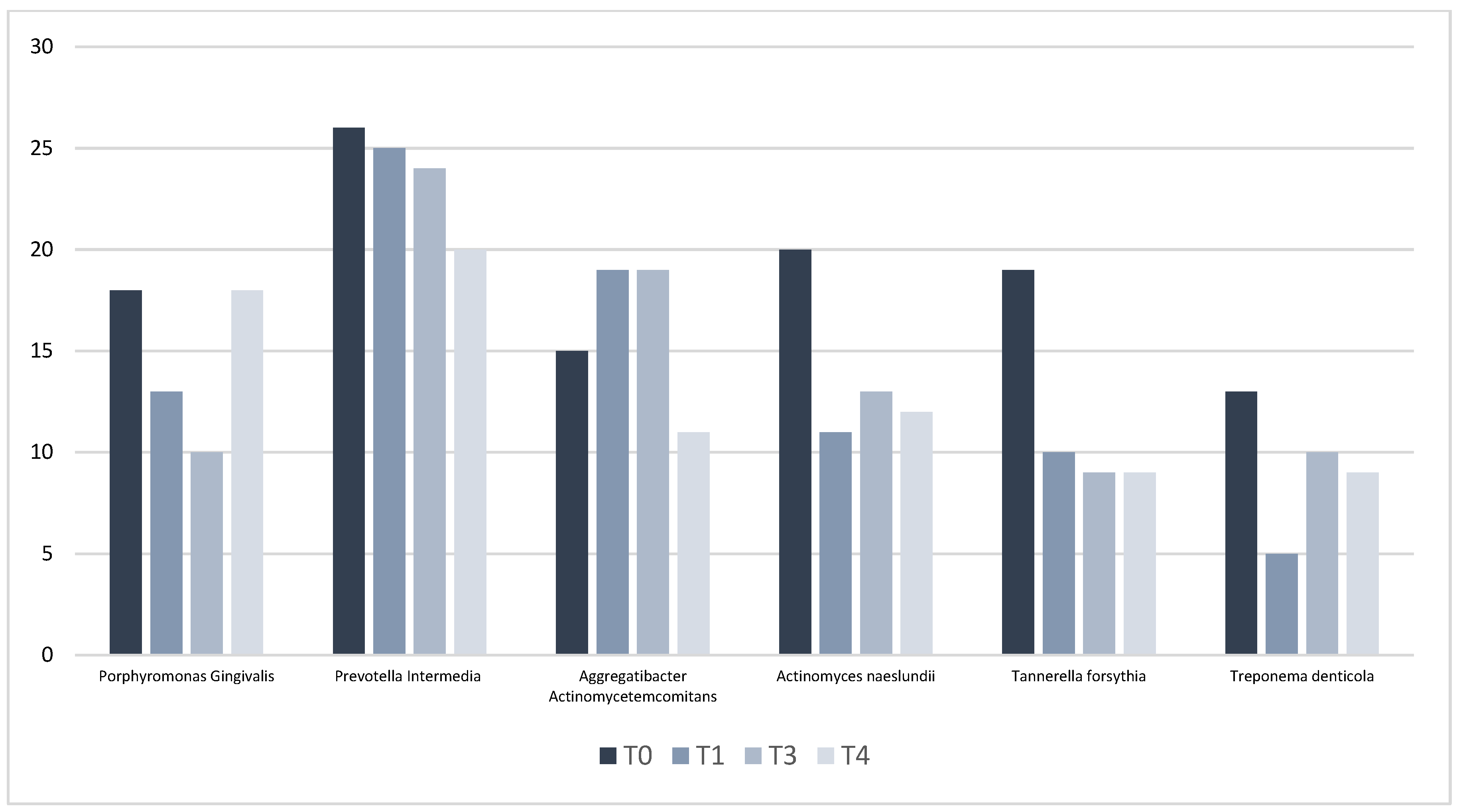
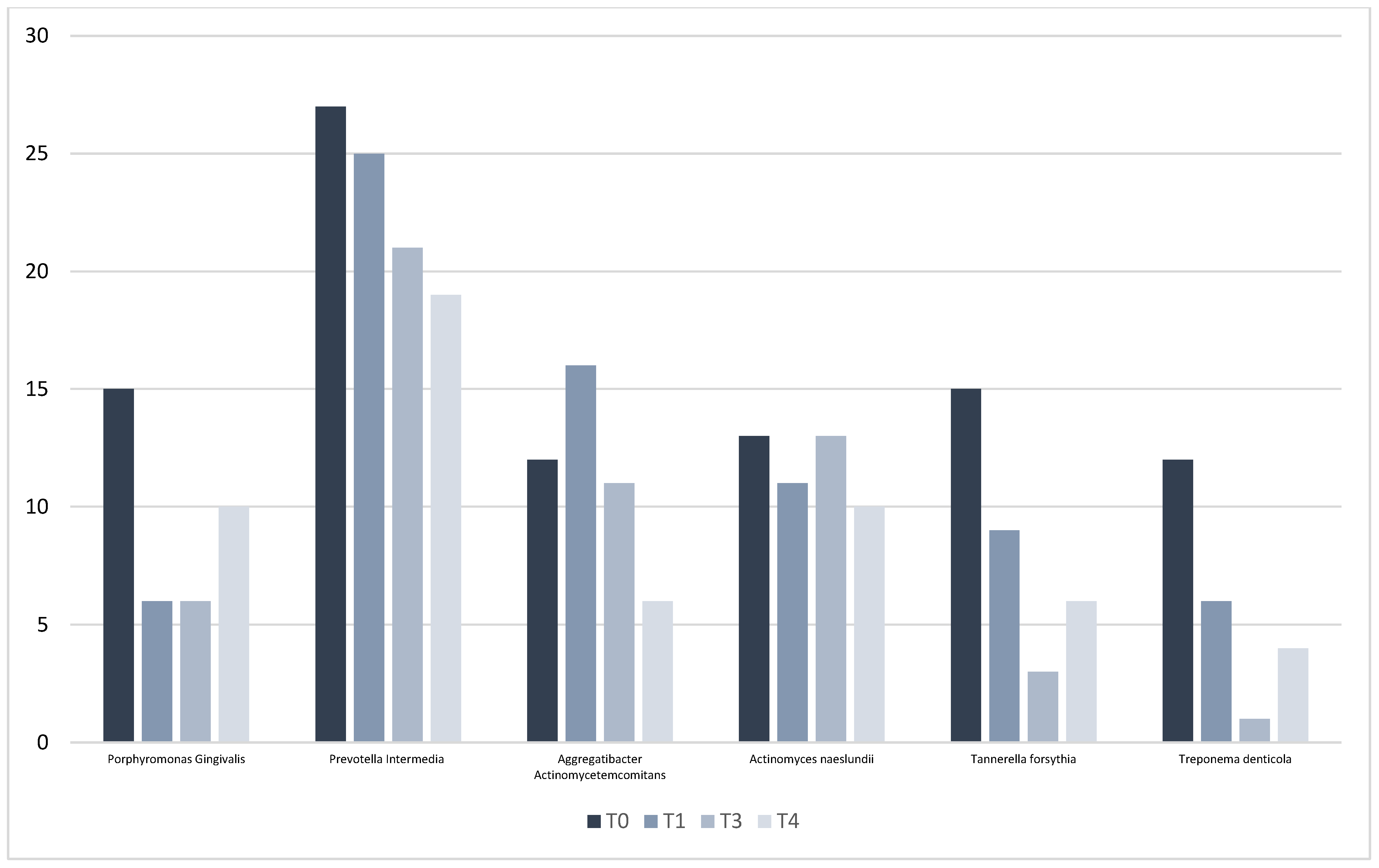
| PPD (mm) | T0 | T3 | T4 | Δ(T0–T3) | p Value | Δ(T3-T4) | p Value | Δ(T0–T4) | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Total | 5.2 ± 0.43 | 3.7 ± 0.90 | 3.8 ± 0.94 | 1.5 ± 0.85 | 0.001 * | 0.1 ± 0.58 | 0.24 | 1.4 ± 0.90 | 0.001 * |
| Control | 5.2 ± 0.42 | 3.9 ± 0.91 | 4.1 ± 1.0 | 1.4 ± 0.82 | 0.001 * | 0.2 ± 0.54 | 0.02 * | 1.1 ± 0.88 | 0.001 * |
| HBX1T | 5.2 ± 0.36 | 3.7 ± 0.85 | 3.7 ± 0.9 | 1.5 ± 0.83 | 0.001 * | 0.1 ± 0.57 | 0.3 | 1.6 ± 0.93 | 0.001 * |
| HBX3T | 5.3 ± 0.49 | 3.6 ± 0.92 | 3.7 ± 0.8 | 1.7 ± 0.88 | 0.001 * | 0.1 ± 0.58 | 0.7 | 1.6 ± 0.81 | 0.001 * |
| p Value | 0.61 | 0.36 | 0.04 * | 0.28 | 0.04 * | 0.04 * |
| BOP (%) | T0 | T3 | T4 | Δ(T0–T3) | p Value | Δ(T3-T4) | p Value | Δ(T0–T4) | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Total | 73 ± 0.44 | 38 ± 0.51 | 43 ± 0.50 | 34 ± 0.61 | 0.002 * | 4 ± 0.56 | 0.7 | 30 ± 0.63 | 0.001 * |
| Control | 69 ± 0.46 | 39 ± 0.49 | 43 ± 0.5 | 28 ± 0.61 | 0.002 * | 3 ± 0.51 | 0.7 | 26 ± 0.61 | 0.001 * |
| HBX1T | 77 ± 0.42 | 33 ± 0.47 | 46 ± 0.5 | 42 ± 0.55 | 0.002 * | 11 ± 0.62 | 0.28 | 29 ± 0.62 | 0.007 * |
| HBX3T | 72 ± 0.45 | 44 ± 0.55 | 43 ± 0.5 | 30 ± 0.66 | 0.01 * | 3 ± 0.55 | 0.7 | 29 ± 0.66 | 0.01 * |
| p Value | 0.74 | 0.72 | 0.96 | 0.61 | 0.56 | 0.96 |
| PI | T0 | T3 | T4 | Δ(T0–T3) | p Value | Δ(T3-T4) | p Value | Δ(T0–T4) | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Total | 1.3 ± 1.17 | 0.7 ± 0.88 | 0.6 ± 0.91 | 0.6 ± 1.27 | 0.002 * | 0.0 ± 1.03 | 0.9 | 0.7 ± 1.36 | 0.001 * |
| Control | 1.5 ± 1.2 | 0.7 ± 0.97 | 0.6 ± 0.96 | 0.8 ± 1.41 | 0.002 * | 0.1 ± 1.31 | 0.9 | 0.8 ± 1.62 | 0.001 * |
| HBX1T | 1 ± 1.13 | 0.5 ± 0.77 | 0.5 ± 0.85 | 0.5 ± 1.06 | 0.006 * | 0.03 ± 0.73 | 0.78 | 0.5 ± 1.14 | 0.003 * |
| HBX3T | 1.4 ± 1.15 | 0.8 ± 0.87 | 0.8 ± 0.93 | 0.6 ± 1.32 | 0.007 * | 0.1 ± 0.93 | 0.55 | 0.7 ± 1.26 | 0.002 * |
| p Value | 0.09 | 0.29 | 0.38 | 0.65 | 0.96 | 0.53 |
| REC (mm) | T0 | T3 | T4 | Δ(T0–T3) | p Value | Δ(T3-T4) | p Value | Δ(T0–T4) | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Total | 0.8 ± 1.04 | 1.4 ± 1.17 | 1.4 ± 1.18 | 0.6 ± 0.52 | 0.001 * | 0.3 ± 0.87 | 0.2 | 0.5 ± 0.63 | 0.001 * |
| Control | 0.8 ± 1.01 | 1.5 ± 1.13 | 1.5 ± 1.17 | 0.6 ± 0.56 | 0.001 * | 0.0 ± 0.41 | 0.2 | 0.6 ± 0.48 | 0.001 * |
| HBX1T | 0.8 ± 0.97 | 1.3 ± 1.12 | 1.2 ± 1.02 | 0.5 ± 0.52 | 0.006 * | 0.1 ± 0.32 | 0.8 | 0.4 ± 0.55 | 0.003 * |
| HBX3T | 0.8 ± 1.14 | 1.4 ± 1.25 | 1.4 ± 1.32 | 0.5 ± 1.32 | 0.001 * | 0.1 ± 0.37 | 0.08 | 0.5 ± 0.58 | 0.001 * |
| p Value | 0.72 | 0.69 | 0.78 | 0.32 | 0.56 | 0.62 |
| CAL (mm) | T0 | T3 | T4 | Δ(T0–T3) | p Value | Δ(T3-T4) | p Value | Δ(T0–T4) | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Total | 6.0 ± 1.18 | 5.1 ± 1.77 | 5.2 ± 1.72 | 0.9 ± 0.93 | 0.001 * | 0.0 ± 0.64 | 0.06 | 0.9 ± 0.96 | 0.001 * |
| Control | 6.1 ± 1.23 | 5.3 ± 1.87 | 5.6 ± 1.84 | 0.7 ± 0.94 | 0.07 | 0.2 ± 0.48 | 0.45 | 0.5 ± 1.02 | 0.03 * |
| HBX1T | 5.8 ± 1.39 | 4.9 ± 1.65 | 4.7 ± 1.56 | 1.1 ± 1.20 | 0.001 * | 0.2 ± 0.74 | 0.22 | 1.3 ± 1.17 | 0.001 * |
| HBX3T | 6.1 ± 1.29 | 5.0 ± 1.85 | 5.1 ± 1.76 | 1.1 ± 0.88 | 0.98 | 0.0 ± 0.61 | 0.05 * | 1.1 ± 0.82 | 0.001 * |
| p Value | 0.61 | 0.59 | 0.13 | 0.8 | 0.78 | 0.02* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardo, A.; Brancato, G.; Signoriello, A.; Messina, E.; Corrocher, G.; Bellopede, V.; Burlacchini, G.; Signoretto, C.; Lombardo, G. Microbiological and Clinical Evaluation of the Efficacy of a Chemical Desiccant Agent in Non-Surgical Periodontal Treatment: A Randomized Controlled Clinical Trial. Antibiotics 2025, 14, 1050. https://doi.org/10.3390/antibiotics14101050
Pardo A, Brancato G, Signoriello A, Messina E, Corrocher G, Bellopede V, Burlacchini G, Signoretto C, Lombardo G. Microbiological and Clinical Evaluation of the Efficacy of a Chemical Desiccant Agent in Non-Surgical Periodontal Treatment: A Randomized Controlled Clinical Trial. Antibiotics. 2025; 14(10):1050. https://doi.org/10.3390/antibiotics14101050
Chicago/Turabian StylePardo, Alessia, Gabriele Brancato, Annarita Signoriello, Elena Messina, Giovanni Corrocher, Valentina Bellopede, Gloria Burlacchini, Caterina Signoretto, and Giorgio Lombardo. 2025. "Microbiological and Clinical Evaluation of the Efficacy of a Chemical Desiccant Agent in Non-Surgical Periodontal Treatment: A Randomized Controlled Clinical Trial" Antibiotics 14, no. 10: 1050. https://doi.org/10.3390/antibiotics14101050
APA StylePardo, A., Brancato, G., Signoriello, A., Messina, E., Corrocher, G., Bellopede, V., Burlacchini, G., Signoretto, C., & Lombardo, G. (2025). Microbiological and Clinical Evaluation of the Efficacy of a Chemical Desiccant Agent in Non-Surgical Periodontal Treatment: A Randomized Controlled Clinical Trial. Antibiotics, 14(10), 1050. https://doi.org/10.3390/antibiotics14101050






