Targeting Bacterial Biofilms on Medical Implants: Current and Emerging Approaches
Abstract
1. Bacterial Biofilm
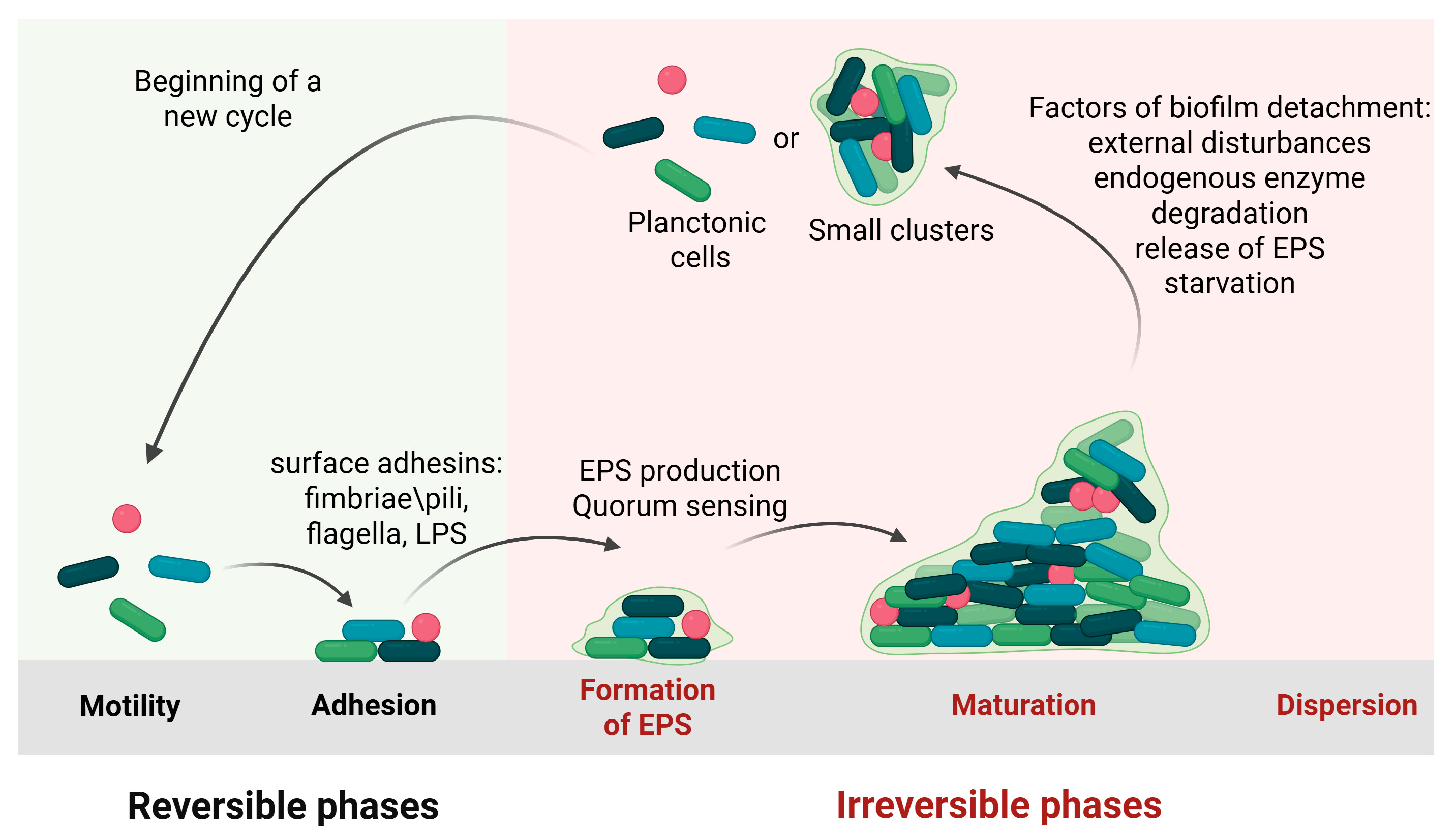
1.1. Biofilm Formation
1.1.1. Initial Attachment
1.1.2. Irreversible Adhesion and Aggregation
1.1.3. Biofilm Maturation
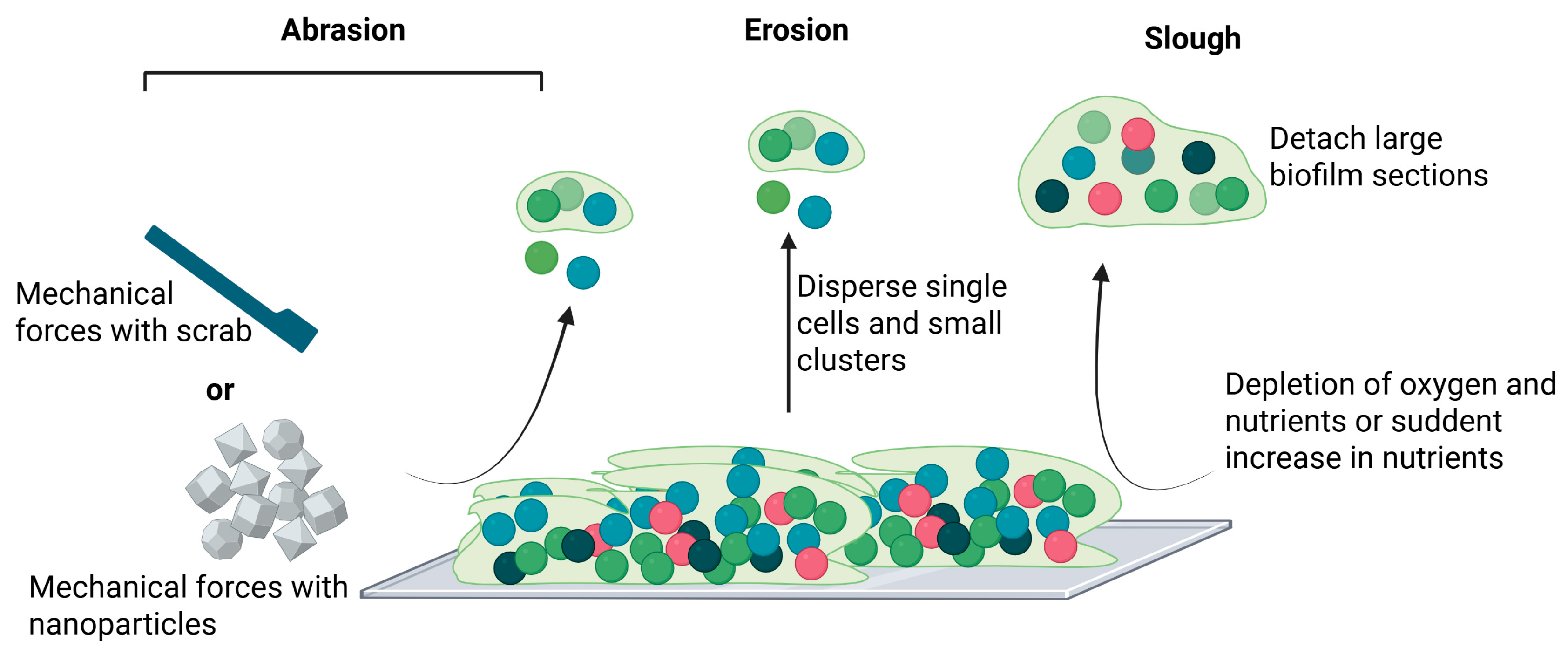
1.1.4. Dispersal/Detachment
2. Clinical Problem of Biofilm
2.1. Biofilm-Mediated Immune Evasion and Virulence Strategies
2.2. Multifactorial Antibiotic Resistance in Biofilm Communities
2.3. Medical Device-Associated Infections
2.3.1. Infections of Prosthetic Hips
2.3.2. Catheter-Associated Infections
2.3.3. Central Venous Catheters (CVCs)
2.3.4. Ventilator-Associated Pneumonia (VAP)
2.3.5. Prosthetic Valve Infections
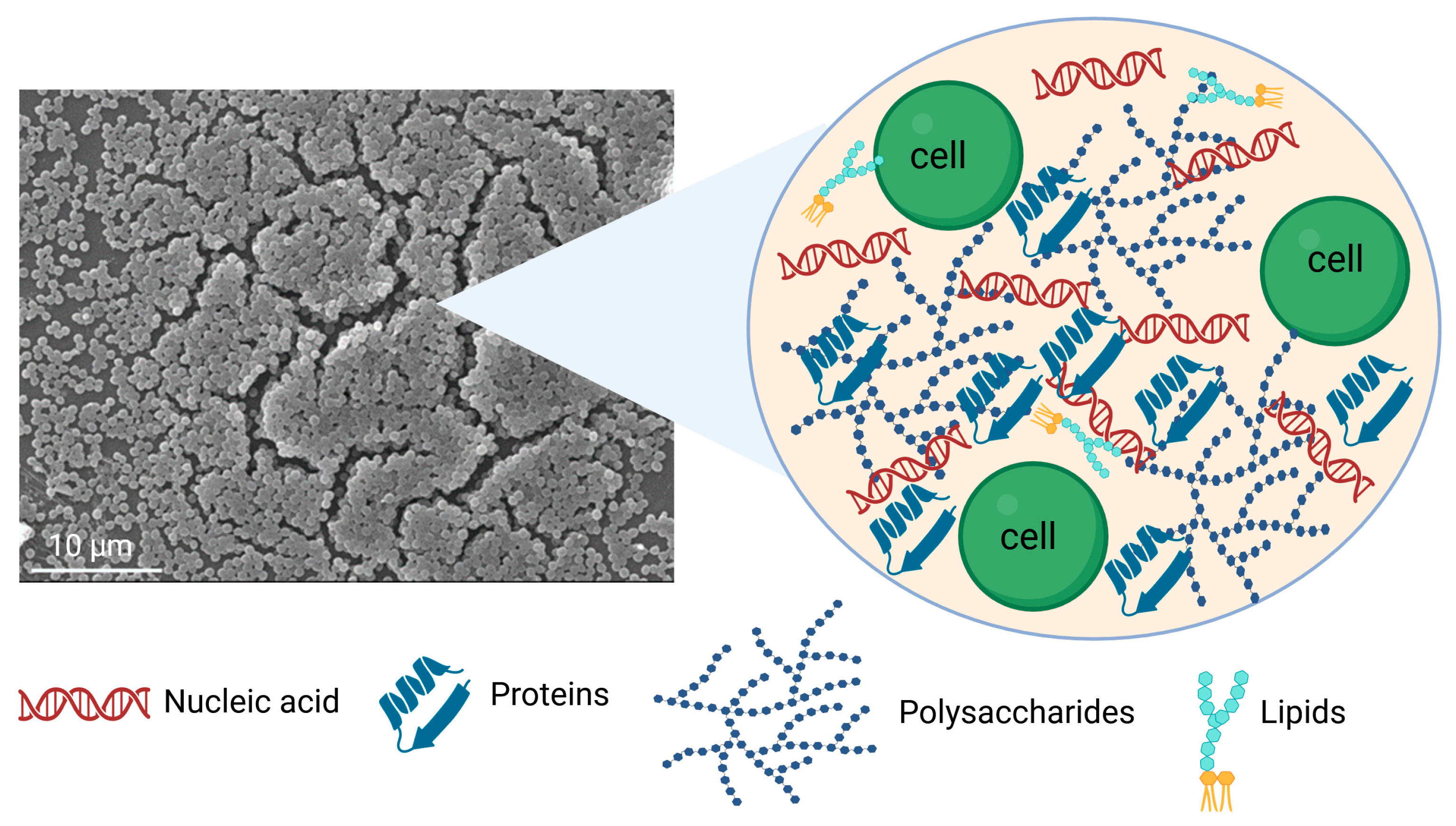
3. Impact of Extracellular Polymeric Substance on Biofilm Formation
3.1. Water
3.2. Polysaccharides
3.3. Proteins
3.4. Nucleic Acids
3.5. Surfactants and Lipids
4. Mitigation of Biofilm Formation on Biomaterials via EPS Matrix Disruption
4.1. Biofilm-Dispersing Enzymes
4.1.1. Glycoside Hydrolysis Enzyme
4.1.2. Proteases
4.1.3. Deoxyribonucleases
4.2. Chelating Agents
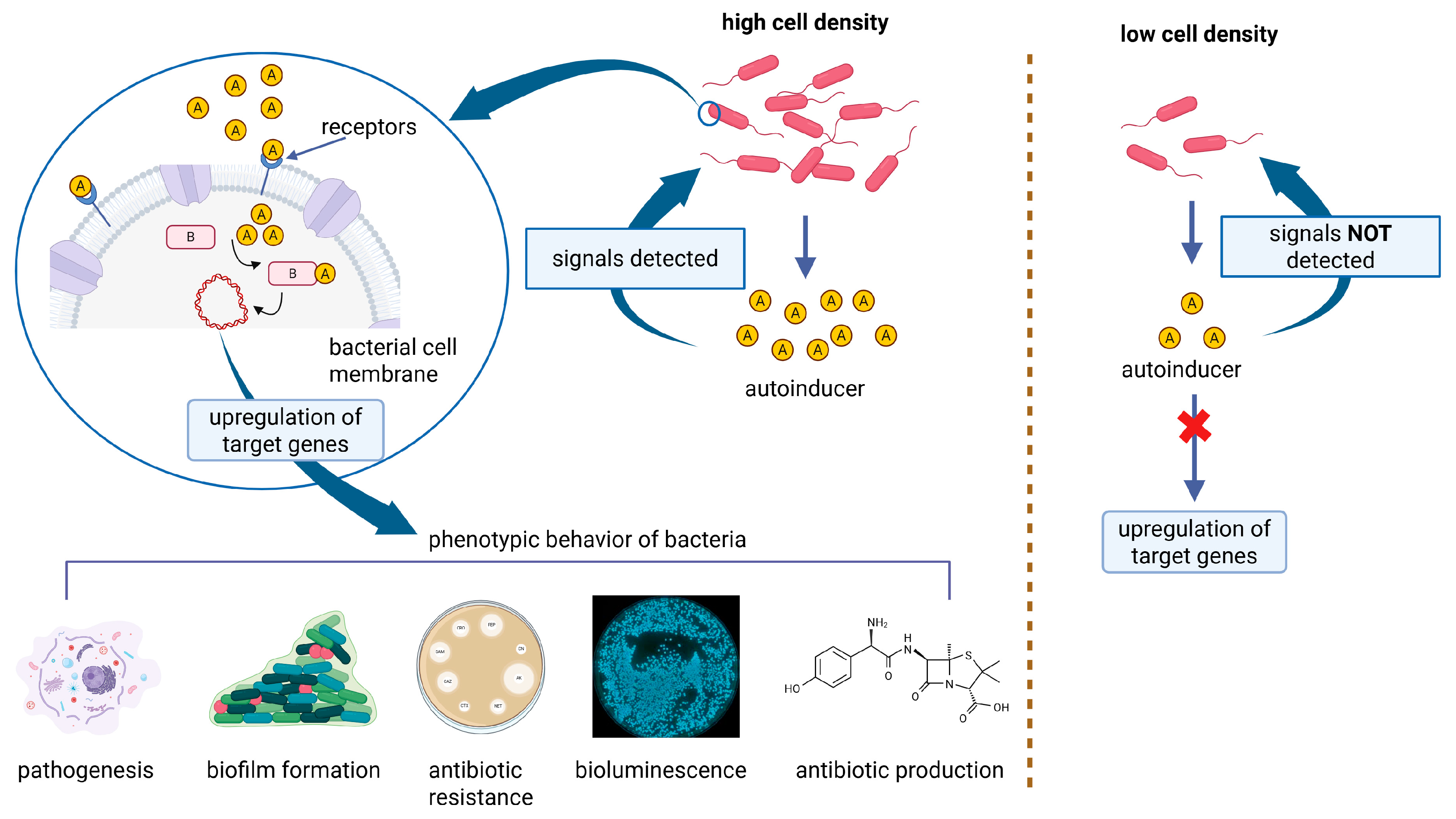
4.3. Quorum Sensing Inhibitors
4.4. Biosurfactants
4.5. Oxidizing Agents
4.5.1. Metal Ions and Metal Nanoparticles
4.5.2. Graphene Oxide
4.5.3. Nitric Oxide
4.5.4. Ozone
4.5.5. Halogens
4.6. Engineered Nanoparticles
Strategies to Penetrate the Biofilm
5. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WT | Wild type |
| EPS | Extracellular polymeric substances |
| C-DI-GMP | Cyclic di-guanosine monophosphate |
| QS | Quorum sensing |
| AHL | N-acylated homoserine lactone |
| AI | Autoinducers |
| SpA | S. Aureus protein a |
| PVL | Panton-Valentine leucocidin |
| CVC | Central venous catheter |
| CAUTI- | Catheter-associated urinary tract infections |
| VAP | Ventilator-associated pneumonia |
| ICU | Intensive care units |
| MRSA | Methicillin-resistant S. Aureus |
| PGA | Poly-N-acetyl-glucosamine (PGA) |
| CA | Colanic acid |
| dPNAG | De-N-acetylated poly β-(1,6)-N-acetyl-d-glucosamine |
| CF | Cystic fibrosis |
| OMV | Outer membrane vescicles |
| DspB | Dispersin B |
| PNAG | Poly-(1→6)-β-N-acetylglucosamine |
| Aap | Accumulation-associated protein |
| EbpS | Elastin-binding protein |
| eDNA | Extracellular DNA |
| PMNs | Polymorphonuclear leukocytes |
| eRNA | Extracellular RNA |
| NTHI | Non-typeable Haemophilus influenzae |
| LPS | Lipopolysaccaride |
| ALU | Aluminium |
| SR | Silicon rubber |
| PET | Polyethylene terephthalate |
| DNases | Deoxyribonucleases |
| GH20 | Glycoside hydrolase family 20 |
| PGA | Poly-N-acetyl glucosamine |
| MagR | Magnetoreceptor |
| LBL | Layer by layer |
| PAH | Poly(allylamine hydrochloride) |
| PMAA | Poly(methacrylic acid) |
| BC | Bacteria cellulose |
| PCD | Phenylboronic acid-modified carbon dots |
| ROS | Reactive oxygen species |
| RhDNAse I | Recombinant human dnase I |
| Ti | Titanium |
| AC-EPD | Alternating current electrophoretic deposition |
| FDA- | Food and Drug Administration |
| EDTA | Ethylenediamine-tetra-acetic acid |
| TSC | Trisodium citrate |
| EGTA | Ethylene glycol-bis (β-aminoethyl ether)-N,N,N’,N’-tetra-acetic acid |
| DFO | Desferrioxamine |
| DFP | Deferiprone |
| AHL | N-acyl homoserine lactones |
| PVD | Pyoverdine |
| PDMS | Polydimethylsiloxane |
| SLA | Silicone discs with lactonic sophrolipids |
| RL | Rhamnolipid |
| NO | Nitric oxide |
| AuNPs- | Gold nanoparticles |
| Ga | Gallium |
| AgNPs | Silver nanoparticles |
| AgNP–PNCs | Agnp–polymer nanocomposites |
| EDS | Dispersive X-ray Spectroscopy |
| Z | Zirconia |
| ZGa | Gallium-doped zirconia |
| GO | Graphene oxide |
| SNAP | S-nitroso-N-acetylpenicillamine |
| QD | Quantum dot |
| PLGA | Poly(lactic-co-glycolic acid) |
References
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial Biofilm: Formation, Architecture, Antibiotic Resistance, and Control Strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef]
- Mugunthan, S.; Wong, L.L.; Winnerdy, F.R.; Summers, S.; Bin Ismail, M.H.; Foo, Y.H.; Jaggi, T.K.; Meldrum, O.W.; Tiew, P.Y.; Chotirmall, S.H.; et al. RNA Is a Key Component of Extracellular DNA Networks in Pseudomonas aeruginosa Biofilms. Nat. Commun. 2023, 14, 7772. [Google Scholar] [CrossRef] [PubMed]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-Chemistry from Initial Bacterial Adhesion to Surface-Programmed Biofilm Growth. Adv. Colloid Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef]
- Riedel, R.; Rani, G.; Sengupta, A. Bacterial Adhesion on Soft Surfaces: The Dual Role of Substrate Stiffness and Bacterial Growth Stage. Microorganisms 2025, 13, 637. [Google Scholar] [CrossRef]
- Chinnaraj, S.B.; Jayathilake, P.G.; Dawson, J.; Ammar, Y.; Portoles, J.; Jakubovics, N.; Chen, J. Modelling the Combined Effect of Surface Roughness and Topography on Bacterial Attachment. J. Mater. Sci. Technol. 2021, 81, 151–161. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Sharifikolouei, E.; Najmi, Z.; Cochis, A.; Scalia, A.C.; Aliabadi, M.; Perero, S.; Rimondini, L. Generation of Cytocompatible Superhydrophobic Zr–Cu–Ag Metallic Glass Coatings with Antifouling Properties for Medical Textiles. Mater. Today Bio 2021, 12, 100148. [Google Scholar] [CrossRef]
- Oh, J.K.; Yegin, Y.; Yang, F.; Zhang, M.; Li, J.; Huang, S.; Verkhoturov, S.V.; Schweikert, E.A.; Perez-Lewis, K.; Scholar, E.A.; et al. The Influence of Surface Chemistry on the Kinetics and Thermodynamics of Bacterial Adhesion. Sci. Rep. 2018, 8, 17247. [Google Scholar] [CrossRef]
- Bhagwat, G.; O’Connor, W.; Grainge, I.; Palanisami, T. Understanding the Fundamental Basis for Biofilm Formation on Plastic Surfaces: Role of Conditioning Films. Front. Microbiol. 2021, 12, 687118. [Google Scholar] [CrossRef]
- Rummel, C.D.; Lechtenfeld, O.J.; Kallies, R.; Benke, A.; Herzsprung, P.; Rynek, R.; Wagner, S.; Potthoff, A.; Jahnke, A.; Schmitt-Jansen, M. Conditioning Film and Early Biofilm Succession on Plastic Surfaces. Environ. Sci. Technol. 2021, 55, 11006–11018. [Google Scholar] [CrossRef]
- Haiko, J.; Westerlund-Wikström, B. The Role of the Bacterial Flagellum in Adhesion and Virulence. Biology 2013, 2, 1242–1267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; He, L.; Qin, J.; Wang, S.; Tong, M. Influence of Flagella and Their Property on the Initial Attachment Behaviors of Bacteria onto Plastics. Water Res. 2023, 231, 119656. [Google Scholar] [CrossRef]
- Hu, H.; Xu, J.; Chen, J.; Tang, C.; Zhou, T.; Wang, J.; Kang, Z. Influence of Flagella on Salmonella Enteritidis Sedimentation, Biofilm Formation, Disinfectant Resistance, and Interspecies Interactions. Foodborne Pathog. Dis. 2024, 22, 1–18. [Google Scholar] [CrossRef]
- Epler Barbercheck, C.R.; Bullitt, E.; Andersson, M. Bacterial Adhesion Pili. In Membrane Protein Complexes: Structure and Function; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–18. [Google Scholar]
- Guerra, M.E.S.; Destro, G.; Vieira, B.; Lima, A.S.; Ferraz, L.F.C.; Hakansson, A.P.; Darrieux, M.; Converso, T.R. Klebsiella Pneumoniae Biofilms and Their Role in Disease Pathogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 877995. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Verma, S.; Bauer, R.; Kumari, M.; Dua, M.; Johri, A.K.; Yadav, V.; Spellerberg, B. Deciphering Streptococcal Biofilms. Microorganisms 2020, 8, 1835. [Google Scholar] [CrossRef]
- McKee, R.W.; Aleksanyan, N.; Garrett, E.M.; Tamayo, R. Type IV Pili Promote Clostridium Difficile Adherence and Persistence in a Mouse Model of Infection. Infect. Immun. 2018, 86, e00943-17. [Google Scholar] [CrossRef] [PubMed]
- Pakharukova, N.; Tuittila, M.; Paavilainen, S.; Malmi, H.; Parilova, O.; Teneberg, S.; Knight, S.D.; Zavialov, A.V. Structural Basis for Acinetobacter Baumannii Biofilm Formation. Proc. Natl. Acad. Sci. USA 2018, 115, 5558–5563. [Google Scholar] [CrossRef]
- Seabaugh, J.A.; Anderson, D.M. Pathogenicity and Virulence of Yersinia. Virulence 2024, 15, 2316439. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Wu, R.X.; Zhang, Y.; Guo, Z.Q.; Zhao, B.; Guo, J.S. Role of Ca2+ and Mg2+ in Changing Biofilm Structure and Enhancing Biofilm Formation of P. Stutzeri Strain XL-2. Colloids Surf. B Biointerfaces 2022, 220, 112972. [Google Scholar] [CrossRef]
- Balducci, E.; Papi, F.; Capialbi, D.E.; Del Bino, L. Polysaccharides’ Structures and Functions in Biofilm Architecture of Antimicrobial-Resistant (AMR) Pathogens. Int. J. Mol. Sci. 2023, 24, 4030. [Google Scholar] [CrossRef]
- Vandana; Das, S. Genetic Regulation, Biosynthesis and Applications of Extracellular Polysaccharides of the Biofilm Matrix of Bacteria. Carbohydr. Polym. 2022, 291, 119536. [Google Scholar] [CrossRef] [PubMed]
- Markowska, K.; Szymanek-Majchrzak, K.; Pituch, H.; Majewska, A. Understanding Quorum-Sensing and Biofilm Forming in Anaerobic Bacterial Communities. Int. J. Mol. Sci. 2024, 25, 12808. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Gurnani, B.; Pippal, B.; Jain, N. Capturing the Micro-Communities: Insights into Biogenesis and Architecture of Bacterial Biofilms. BBA Adv. 2025, 7, 100133. [Google Scholar] [CrossRef]
- Paharik, A.E.; Horswill, A.R. The Staphylococcal Biofilm: Adhesins, Regulation, and Host Response. In Virulence Mechanisms of Bacterial Pathogens; ASM Press: Washington, DC, USA, 2016; pp. 529–566. [Google Scholar]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa Biofilm: A Review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef]
- Sahreen, S.; Mukhtar, H.; Imre, K.; Morar, A.; Herman, V.; Sharif, S. Exploring the Function of Quorum Sensing Regulated Biofilms in Biological Wastewater Treatment: A Review. Int. J. Mol. Sci. 2022, 23, 9751. [Google Scholar] [CrossRef]
- Kaplan, J.B. Biofilm Dispersal: Mechanisms, Clinical Implications, and Potential Therapeutic Uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Langenheder, S.; Jürgens, K. Dispersal Modifies the Diversity and Composition of Active Bacterial Communities in Response to a Salinity Disturbance. Front. Microbiol. 2018, 9, 2188. [Google Scholar] [CrossRef]
- Wille, J.; Coenye, T. Biofilm Dispersion: The Key to Biofilm Eradication or Opening Pandora’s Box? Biofilm 2020, 2, 100027. [Google Scholar] [CrossRef]
- Gupta, A.; Srivastava, A.; Agarwal, V. Biofilm Detachment and Its Implication in Spreading Biofilm-Related Infections. In Proceedings of the Conference BioSangam 2022: Emerging Trends in Biotechnology (BIOSANGAM 2022), Prayagrai, India, 10–12 March 2022; Atlantis Press International BV: Dordrecht, The Netherlands, 2023; pp. 3–13. [Google Scholar]
- Rumbaugh, K.P.; Sauer, K. Biofilm Dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef]
- David, A.; Tahrioui, A.; Tareau, A.-S.; Forge, A.; Gonzalez, M.; Bouffartigues, E.; Lesouhaitier, O.; Chevalier, S. Pseudomonas aeruginosa Biofilm Lifecycle: Involvement of Mechanical Constraints and Timeline of Matrix Production. Antibiotics 2024, 13, 688. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Horswill, A.R. Micrococcal Nuclease Regulates Biofilm Formation and Dispersal in Methicillin-Resistant Staphylococcus aureus USA300. mSphere 2024, 9, e00126-24. [Google Scholar] [CrossRef]
- Alotaibi, G.F.; Bukhari, M.A. Factors Influencing Bacterial Biofilm Formation and Development. Am. J. Biomed. Sci. Res. 2021, 12, 617–626. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Partoazar, A. Targeting Bacterial Biofilm-Related Genes with Nanoparticle-Based Strategies. Front. Microbiol. 2024, 15, 1387114. [Google Scholar] [CrossRef] [PubMed]
- Roilides, E.; Simitsopoulou, M.; Katragkou, A.; Walsh, T.J. How Biofilms Evade Host Defenses. Microbiol. Spectr. 2015, 3, 287–300. [Google Scholar] [CrossRef]
- Merino, N.; Toledo-Arana, A.; Vergara-Irigaray, M.; Valle, J.; Solano, C.; Calvo, E.; Lopez, J.A.; Foster, T.J.; Penadés, J.R.; Lasa, I. Protein A-Mediated Multicellular Behavior in Staphylococcus aureus. J. Bacteriol. 2009, 191, 832–843. [Google Scholar] [CrossRef]
- Bear, A.; Locke, T.; Rowland-Jones, S.; Pecetta, S.; Bagnoli, F.; Darton, T.C. The Immune Evasion Roles of Staphylococcus aureus Protein A and Impact on Vaccine Development. Front. Cell. Infect. Microbiol. 2023, 13, 1242702. [Google Scholar] [CrossRef]
- Müller, E.; Monecke, S.; Armengol Porta, M.; Narvaez Encalada, M.V.; Reissig, A.; Rüttiger, L.; Schröttner, P.; Schwede, I.; Söffing, H.-H.; Thürmer, A.; et al. Rapid Detection of Panton–Valentine Leukocidin Production in Clinical Isolates of Staphylococcus aureus from Saxony and Brandenburg and Their Molecular Characterisation. Pathogens 2025, 14, 238. [Google Scholar] [CrossRef]
- Divyakolu, S.; Chikkala, R.; Ratnakar, K.S.; Sritharan, V. Hemolysins of Staphylococcus aureus—An Update on Their Biology, Role in Pathogenesis and as Targets for Anti-Virulence Therapy. Adv. Infect. Dis. 2019, 9, 80–104. [Google Scholar] [CrossRef]
- Lou, Y.; Liu, Z.; Zhang, Q.; Zhang, L.; Liao, X.; Yang, T.; Liu, D.; Lu, X.; Ahn, J.; Ding, T.; et al. Interspecies and Intraspecies ‘Talk’ Shape the Bacterial Biofilms. Food Qual. Saf. 2025, 9, fyaf008. [Google Scholar] [CrossRef]
- Lan, J.; Zou, J.; Xin, H.; Sun, J.; Han, T.; Sun, M.; Niu, M. Nanomedicines as Disruptors or Inhibitors of Biofilms: Opportunities in Addressing Antimicrobial Resistance. J. Control. Release 2025, 381, 113589. [Google Scholar] [CrossRef]
- Abebe, G.M. The Role of Bacterial Biofilm in Antibiotic Resistance and Food Contamination. Int. J. Microbiol. 2020, 2020, 1705814. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Mancuso, G.; Trinchera, M.; Midiri, A.; Zummo, S.; Vitale, G.; Biondo, C. Novel Antimicrobial Approaches to Combat Bacterial Biofilms Associated with Urinary Tract Infections. Antibiotics 2024, 13, 154. [Google Scholar] [CrossRef]
- Song, Z.; Borgwardt, L.; Høiby, N.; Wu, H.; Sørensen, T.S.; Borgwardt, A. Prosthesis Infections after Orthopedic Joint Replacement: The Possible Role of Bacterial Biofilms. Orthop. Rev. 2013, 5, e14. [Google Scholar] [CrossRef]
- Stewart, S.; Barr, S.; Engiles, J.; Hickok, N.J.; Shapiro, I.M.; Richardson, D.W.; Parvizi, J.; Schaer, T.P. Vancomycin-Modified Implant Surface Inhibits Biofilm Formation and Supports Bone-Healing in an Infected Osteotomy Model in Sheep. J. Bone Jt. Surg. 2012, 94, 1406–1415. [Google Scholar] [CrossRef]
- Oliveira, W.F.; Silva, P.M.S.; Silva, R.C.S.; Silva, G.M.M.; Machado, G.; Coelho, L.C.B.B.; Correia, M.T.S. Staphylococcus aureus and Staphylococcus epidermidis Infections on Implants. J. Hosp. Infect. 2018, 98, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Mussab, R.M.; Khan, S.; Bubak, S.Z.; Madni, A.; Ishaq, U.; Rimsha, S.; Arqam, S.M.; Javed, H. Organisms Causing Postoperative Implant Infection in Orthopedic Patients Presenting at a Tertiary Care Hospital. Cureus 2024, 16, e70821. [Google Scholar] [CrossRef] [PubMed]
- Trampuz, A.; Zimmerli, W. Diagnosis and Treatment of Implant-Associated Septic Arthritis and Osteomyelitis. Curr. Infect. Dis. Rep. 2008, 10, 394–403. [Google Scholar] [CrossRef]
- The McMaster Arthroplasty Collaborative (MAC). Risk Factors for Periprosthetic Joint Infection Following Primary Total Hip Arthroplasty. J. Bone Jt. Surg. 2020, 102, 503–509. [Google Scholar] [CrossRef]
- Natsuhara, K.M.; Shelton, T.J.; Meehan, J.P.; Lum, Z.C. Mortality During Total Hip Periprosthetic Joint Infection. J. Arthroplast. 2019, 34, S337–S342. [Google Scholar] [CrossRef]
- Catheter-Associated Urinary Tract Infection Basics. Available online: https://www.cdc.gov/uti/about/cauti-basics.html (accessed on 27 June 2025).
- Nicastri, E.; Leone, S. Guide to Infection Control in the Healthcare Setting Hospital-Acquired Urinary Tract Infection; The International Society for Infectious Diseases: Brookline, MA, USA, 2018; pp. 1–13. Available online: https://isid.org/wp-content/uploads/2019/07/ISID_GUIDE_HOSPITAL_ACQUIRED_UTI.pdf (accessed on 27 June 2025).
- Alqaissi, N. Nurses’ Knowledge and Behavior in Hospitals Regarding the Prevention of Central Line-Associated Bloodstream Infections: A Systematic Review. SAGE Open Nurs. 2025, 11, 23779608251347119. [Google Scholar] [CrossRef]
- Guggenbichler, J.P.; Assadian, O.; Boeswald, M.; Kramer, A. Incidence and Clinical Implication of Nosocomial Infections Associated with Implantable Biomaterials—Catheters, Ventilator-Associated Pneumonia, Urinary Tract Infections. Dtsch. Ges. Für Allg. Und Krankenh.-Hyg. 2012, 6, Doc18. [Google Scholar]
- Kenaan, M.; Hyzy, R.C. Mechanical Ventilation and Advanced Respiratory Support in the Cardiac Intensive Care Unit. In Cardiac Intensive Care; Elsevier: Amsterdam, The Netherlands, 2019; pp. 548–557. [Google Scholar]
- Howroyd, F.; Chacko, C.; MacDuff, A.; Gautam, N.; Pouchet, B.; Tunnicliffe, B.; Weblin, J.; Gao-Smith, F.; Ahmed, Z.; Duggal, N.A.; et al. Ventilator-Associated Pneumonia: Pathobiological Heterogeneity and Diagnostic Challenges. Nat. Commun. 2024, 15, 6447. [Google Scholar] [CrossRef] [PubMed]
- Sadigov, A.; Mamedova, I.; Mammmadov, K. Ventilator-Associated Pneumonia and In-Hospital Mortality: Which Risk Factors May Predict In-Hospital Mortality in Such Patients? J. Lung Health Dis. 2019, 3, 8–12. [Google Scholar] [CrossRef]
- Thakur, H.K.; Tarai, B.; Bhargava, A.; Soni, P.; Ojha, A.K.; Kancharla, S.; Kolli, P.; Mandadapu, G.; Jena, M.K. Comprehensive Analysis of Etiological Agents and Drug Resistance Patterns in Ventilator-Associated Pneumonia. Microbiol. Res. 2025, 16, 152. [Google Scholar] [CrossRef]
- Weber, C.; Hohmann, C.; Lindner, O.; Wahlers, T.; Jung, N. Patients with Artificial Heart Valves. Dtsch. Arztebl. Int. 2023, 120, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Weng, C.; Zhao, J.; Yuan, D.; Huang, B.; Wang, T. Management and Clinical Outcome of Aortic Graft Infections: A Single-Center Retrospective Study. J. Clin. Med. 2022, 11, 6588. [Google Scholar] [CrossRef]
- Quan, K.; Hou, J.; Zhang, Z.; Ren, Y.; Peterson, B.W.; Flemming, H.-C.; Mayer, C.; Busscher, H.J.; van der Mei, H.C. Water in Bacterial Biofilms: Pores and Channels, Storage and Transport Functions. Crit. Rev. Microbiol. 2022, 48, 283–302. [Google Scholar] [CrossRef]
- Di Martino, P. Extracellular Polymeric Substances, a Key Element in Understanding Biofilm Phenotype. AIMS Microbiol. 2018, 4, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Saharan, B.S.; Beniwal, N.; Duhan, J.S. From Formulation to Function: A Detailed Review of Microbial Biofilms and Their Polymer-Based Extracellular Substances. Microbe 2024, 5, 100194. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, Y.; Li, M.; Wang, X.; Zhu, J.; Liao, L.; Wang, J. Contemporary Strategies and Approaches for Characterizing Composition and Enhancing Biofilm Penetration Targeting Bacterial Extracellular Polymeric Substances. J. Pharm. Anal. 2024, 14, 100906. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M.; Yu, C.; Li, J.; Zhou, X. Biofilm Formation: Mechanistic Insights and Therapeutic Targets. Mol. Biomed. 2023, 4, 49. [Google Scholar] [CrossRef]
- Tukenmez, U.; Aktas, B.; Aslim, B.; Yavuz, S. The Relationship between the Structural Characteristics of Lactobacilli-EPS and Its Ability to Induce Apoptosis in Colon Cancer Cells in Vitro. Sci. Rep. 2019, 9, 8268. [Google Scholar] [CrossRef]
- Li, B.; Chen, H.; Cao, L.; Hu, Y.; Chen, D.; Yin, Y. Effects of an Escherichia coli Exopolysaccharide on Human and Mouse Gut Microbiota in Vitro. Int. J. Biol. Macromol. 2020, 150, 991–999. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Wang, J.; Chen, S.; Wang, Z.; Zhao, L.; Wang, X. Colanic Acid Biosynthesis in Escherichia coli Is Dependent on Lipopolysaccharide Structure and Glucose Availability. Microbiol. Res. 2020, 239, 126527. [Google Scholar] [CrossRef] [PubMed]
- Nhu, N.T.K.; Rahman, M.A.; Goh, K.G.K.; Kim, S.J.; Phan, M.-D.; Peters, K.M.; Alvarez-Fraga, L.; Hancock, S.J.; Ravi, C.; Kidd, T.J.; et al. A Convergent Evolutionary Pathway Attenuating Cellulose Production Drives Enhanced Virulence of Some Bacteria. Nat. Commun. 2024, 15, 1441. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Breslawec, A.P.; Liang, T.; Deng, Z.; Kuperman, L.L.; Yu, Q. Strategy to Combat Biofilms: A Focus on Biofilm Dispersal Enzymes. NPJ Biofilms Microbiomes 2023, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Fleming, D.; Niese, B.; Redman, W.; Vanderpool, E.; Gordon, V.; Rumbaugh, K.P. Contribution of Pseudomonas aeruginosa Exopolysaccharides Pel and Psl to Wound Infections. Front. Cell. Infect. Microbiol. 2022, 12, 835754. [Google Scholar] [CrossRef]
- Van Loon, J.C.; Le Mauff, F.; Vargas, M.A.; Gilbert, S.; Pfoh, R.; Morrison, Z.A.; Razvi, E.; Nitz, M.; Sheppard, D.C.; Howell, P.L. Structural and Functional Analysis of Pseudomonas aeruginosa PelA Provides Insight into the Modification of the Pel Exopolysaccharide. J. Biol. Chem. 2025, 301, 108432. [Google Scholar] [CrossRef]
- Gheorghita, A.A.; Wozniak, D.J.; Parsek, M.R.; Howell, P.L. Pseudomonas aeruginosa Biofilm Exopolysaccharides: Assembly, Function, and Degradation. FEMS Microbiol. Rev. 2023, 47, fuad060. [Google Scholar] [CrossRef]
- Moradali, M.F.; Rehm, B.H.A. The Role of Alginate in Bacterial Biofilm Formation. In Extracellular Sugar-Based Biopolymers Matrices; Springer International Publishing: Cham, Switzerland, 2019; pp. 517–537. [Google Scholar]
- Mahto, K.U.; Vandana; Priyadarshanee, M.; Samantaray, D.P.; Das, S. Bacterial Biofilm and Extracellular Polymeric Substances in the Treatment of Environmental Pollutants: Beyond the Protective Role in Survivability. J. Clean. Prod. 2022, 379, 134759. [Google Scholar] [CrossRef]
- Kaplan, J.B.; Sukhishvili, S.A.; Sailer, M.; Kridin, K.; Ramasubbu, N. Aggregatibacter Actinomycetemcomitans Dispersin B: The Quintessential Antibiofilm Enzyme. Pathogens 2024, 13, 668. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Zhou, C.; Qin, J.; Jiang, Y.; Li, D.; Tang, X.; Luo, J.; Kong, J.; Wang, K. Molecular Mechanisms of DNase Inhibition of Early Biofilm Formation Pseudomonas aeruginosa or Staphylococcus aureus: A Transcriptome Analysis. Biofilm 2024, 7, 100174. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; D’haeseleer, P.; Dill, B.D.; Shah, M.; VerBerkmoes, N.C.; Hettich, R.L.; Banfield, J.F.; Thelen, M.P. Identification of Biofilm Matrix-Associated Proteins from an Acid Mine Drainage Microbial Community. Appl. Environ. Microbiol. 2011, 77, 5230–5237. [Google Scholar] [CrossRef]
- Vo, L.H.; Hong, S.; Stepler, K.E.; Liyanaarachchi, S.M.; Yang, J.; Nemes, P.; Poulin, M.B. Mapping Protein–Exopolysaccharide Binding Interaction in Staphylococcus epidermidis Biofilms by Live Cell Proximity Labeling. bioRxiv 2023. preprint. [Google Scholar] [CrossRef]
- Toyofuku, M.; Roschitzki, B.; Riedel, K.; Eberl, L. Identification of Proteins Associated with the Pseudomonas aeruginosa Biofilm Extracellular Matrix. J. Proteome Res. 2012, 11, 4906–4915. [Google Scholar] [CrossRef]
- Itzek, A.; Zheng, L.; Chen, Z.; Merritt, J.; Kreth, J. Hydrogen Peroxide-Dependent DNA Release and Transfer of Antibiotic Resistance Genes in Streptococcus Gordonii. J. Bacteriol. 2011, 193, 6912–6922. [Google Scholar] [CrossRef]
- Serrage, H.J.; Jepson, M.A.; Rostami, N.; Jakubovics, N.S.; Nobbs, A.H. Understanding the Matrix: The Role of Extracellular DNA in Oral Biofilms. Front. Oral Health 2021, 2, 640129. [Google Scholar] [CrossRef]
- Faddetta, T.; Vassallo, A.; Del Duca, S.; Gallo, G.; Fani, R.; Puglia, A.M. Unravelling the DNA Sequences Carried by Streptomyces Coelicolor Membrane Vesicles. Sci. Rep. 2022, 12, 16651. [Google Scholar] [CrossRef]
- Alhede, M.; Alhede, M.; Qvortrup, K.; Kragh, K.N.; Jensen, P.Ø.; Stewart, P.S.; Bjarnsholt, T. The Origin of Extracellular DNA in Bacterial Biofilm Infections in Vivo. Pathog. Dis. 2020, 78, ftaa018. [Google Scholar] [CrossRef] [PubMed]
- Cherny, K.E.; Sauer, K. Pseudomonas aeruginosa Requires the DNA-Specific Endonuclease EndA to Degrade Extracellular Genomic DNA to Disperse from the Biofilm. J. Bacteriol. 2019, 201, e00059-19. [Google Scholar] [CrossRef]
- Seviour, T.; Winnerdy, F.R.; Wong, L.L.; Shi, X.; Mugunthan, S.; Foo, Y.H.; Castaing, R.; Adav, S.S.; Subramoni, S.; Kohli, G.S.; et al. The Biofilm Matrix Scaffold of Pseudomonas aeruginosa Contains G-Quadruplex Extracellular DNA Structures. NPJ Biofilms Microbiomes 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Chiba, A.; Seki, M.; Suzuki, Y.; Kinjo, Y.; Mizunoe, Y.; Sugimoto, S. Staphylococcus aureus Utilizes Environmental RNA as a Building Material in Specific Polysaccharide-Dependent Biofilms. NPJ Biofilms Microbiomes 2022, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Ragupathi, H.; Pushparaj, M.M.; Gopi, S.M.; Govindarajan, D.K.; Kandaswamy, K. Biofilm Matrix: A Multifaceted Layer of Biomolecules and a Defensive Barrier against Antimicrobials. Arch. Microbiol. 2024, 206, 432. [Google Scholar] [CrossRef]
- Scherbakova, A.; Rykova, V.; Danilova, K.; Solovyev, A.; Egorova, D. Extracellular RNA Isolation from Biofilm Matrix of Pseudomonas aeruginosa. Bio Protoc. 2020, 10, e3810. [Google Scholar] [CrossRef]
- Flemming, A.H.-C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Pinto, R.M.; Soares, F.A.; Reis, S.; Nunes, C.; Van Dijck, P. Innovative Strategies Toward the Disassembly of the EPS Matrix in Bacterial Biofilms. Front. Microbiol. 2020, 11, 952. [Google Scholar] [CrossRef]
- Ron, E.Z.; Rosenberg, E. Natural Roles of Biosurfactants. Environ. Microbiol. 2001, 3, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Rahman, M.A.; Ashrafudoulla, M.; Ha, S.-D. Biofilm Formation and Analysis of EPS Architecture Comprising Polysaccharides and Lipids by Pseudomonas aeruginosa and Escherichia coli on Food Processing Surfaces. Food Res. Int. 2025, 209, 116274. [Google Scholar] [CrossRef] [PubMed]
- Fleming, D.; Rumbaugh, K. Approaches to Dispersing Medical Biofilms. Microorganisms 2017, 5, 15. [Google Scholar] [CrossRef]
- Wang, S.; Breslawec, A.P.; Alvarez, E.; Tyrlik, M.; Li, C.; Poulin, M.B. Differential Recognition of Deacetylated PNAG Oligosaccharides by a Biofilm Degrading Glycosidase. ACS Chem. Biol. 2019, 14, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Breslawec, A.P.; Wang, S.; Monahan, K.N.; Barry, L.L.; Poulin, M.B. The Endoglycosidase Activity of Dispersin B Is Mediated through Electrostatic Interactions with Cationic Poly-β-(1→6)- N-acetylglucosamine. FEBS J. 2023, 290, 1049–1059. [Google Scholar] [CrossRef]
- Al-Madboly, L.A.; Aboulmagd, A.; El-Salam, M.A.; Kushkevych, I.; El-Morsi, R.M. Microbial Enzymes as Powerful Natural Anti-Biofilm Candidates. Microb. Cell Fact. 2024, 23, 343. [Google Scholar] [CrossRef]
- Vivek Ghalsasi, V.; Sourjik, V. Engineering Escherichia coli to Disrupt Poly-N-Acetylglucosamine Containing Bacterial Biofilms. Curr. Synth. Syst. Biol. 2016, 4, 2332-0737. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Z.; Zeng, K.; Xia, Y.; Xu, W.; Wang, R.; Guo, J.; Xie, H. Functional Immobilization of a Biofilm-Releasing Glycoside Hydrolase Dispersin B on Magnetic Nanoparticles. Appl. Biochem. Biotechnol. 2022, 194, 737–747. [Google Scholar] [CrossRef]
- Pavlukhina, S.V.; Kaplan, J.B.; Xu, L.; Chang, W.; Yu, X.; Madhyastha, S.; Yakandawala, N.; Mentbayeva, A.; Khan, B.; Sukhishvili, S.A. Noneluting Enzymatic Antibiofilm Coatings. ACS Appl. Mater. Interfaces 2012, 4, 4708–4716. [Google Scholar] [CrossRef]
- Gawande, P.V.; Leung, K.P.; Madhyastha, S. Antibiofilm and Antimicrobial Efficacy of DispersinB®-KSL-W Peptide-Based Wound Gel Against Chronic Wound Infection Associated Bacteria. Curr. Microbiol. 2014, 68, 635–641. [Google Scholar] [CrossRef]
- Gawande, P.V.; Clinton, A.P.; LoVetri, K.; Yakandawala, N.; Rumbaugh, K.P.; Madhyastha, S. Antibiofilm Efficacy of DispersinB Wound Spray Used in Combination with a Silver Wound Dressing. Microbiol. Insights 2014, 7, MBI-S13914. [Google Scholar] [CrossRef] [PubMed]
- Darouiche, R.O.; Mansouri, M.D.; Gawande, P.V.; Madhyastha, S. Antimicrobial and Antibiofilm Efficacy of Triclosan and DispersinB(R) Combination. J. Antimicrob. Chemother. 2009, 64, 88–93. [Google Scholar] [CrossRef]
- Little, D.J.; Pfoh, R.; Le Mauff, F.; Bamford, N.C.; Notte, C.; Baker, P.; Guragain, M.; Robinson, H.; Pier, G.B.; Nitz, M.; et al. PgaB Orthologues Contain a Glycoside Hydrolase Domain That Cleaves Deacetylated Poly-β(1,6)-N-Acetylglucosamine and Can Disrupt Bacterial Biofilms. PLoS Pathog. 2018, 14, e1006998. [Google Scholar] [CrossRef]
- Forman, A.; Pfoh, R.; Eddenden, A.; Howell, P.L.; Nitz, M. Synthesis of Defined Mono-de-N-Acetylated β-(1→6)-N-Acetyl-d-Glucosamine Oligosaccharides to Characterize PgaB Hydrolase Activity. Org. Biomol. Chem. 2019, 17, 9456–9466. [Google Scholar] [CrossRef]
- Breslawec, A.P.; Wang, S.; Li, C.; Poulin, M.B. The Role of Anionic Amino Acids in Hydrolysis of Poly-β-(1,6)-N-Acetylglucosamine Exopolysaccharides by the Biofilm Dispersing Glycosidase Dispersin B. bioRxiv 2020, preprint. [Google Scholar] [CrossRef]
- Blanco-Cabra, N.; Paetzold, B.; Ferrar, T.; Mazzolini, R.; Torrents, E.; Serrano, L.; LLuch-Senar, M. Characterization of Different Alginate Lyases for Dissolving Pseudomonas aeruginosa Biofilms. Sci. Rep. 2020, 10, 9390. [Google Scholar] [CrossRef]
- Cheng, D.; Jiang, C.; Xu, J.; Liu, Z.; Mao, X. Characteristics and Applications of Alginate Lyases: A Review. Int. J. Biol. Macromol. 2020, 164, 1304–1320. [Google Scholar] [CrossRef]
- Chung, J.; Eisha, S.; Park, S.; Morris, A.J.; Martin, I. How Three Self-Secreted Biofilm Exopolysaccharides of Pseudomonas aeruginosa, Psl, Pel, and Alginate, Can Each Be Exploited for Antibiotic Adjuvant Effects in Cystic Fibrosis Lung Infection. Int. J. Mol. Sci. 2023, 24, 8709. [Google Scholar] [CrossRef]
- Razvi, E.; Whitfield, G.B.; Reichhardt, C.; Dreifus, J.E.; Willis, A.R.; Gluscencova, O.B.; Gloag, E.S.; Awad, T.S.; Rich, J.D.; da Silva, D.P.; et al. Glycoside Hydrolase Processing of the Pel Polysaccharide Alters Biofilm Biomechanics and Pseudomonas aeruginosa Virulence. NPJ Biofilms Microbiomes 2023, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Charęza, M.; Przygrodzka, K.; Żywicka, A.; Grygorcewicz, B.; Sobolewski, P.; Mozia, S.; Śmiglak, M.; Drozd, R. Enhancement of Inhibition of the Pseudomonas Sp. Biofilm Formation on Bacterial Cellulose-Based Wound Dressing by the Combined Action of Alginate Lyase and Gentamicin. Int. J. Mol. Sci. 2023, 24, 4740. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Zhu, Y.; Tao, J.; Zhu, F.; Chen, J.; Li, L.; Zhao, J.; Wang, L.; Sun, S.; Yang, Y.; et al. Alginate Lyase Guided Silver Nanocomposites for Eradicating Pseudomonas aeruginosa from Lungs. ACS Appl. Mater. Interfaces 2020, 12, 9050–9061. [Google Scholar] [CrossRef]
- Asker, D.; Awad, T.S.; Raju, D.; Sanchez, H.; Lacdao, I.; Gilbert, S.; Sivarajah, P.; Andes, D.R.; Sheppard, D.C.; Howell, P.L.; et al. Preventing Pseudomonas aeruginosa Biofilms on Indwelling Catheters by Surface-Bound Enzymes. ACS Appl. Bio Mater. 2021, 4, 8248–8258. [Google Scholar] [CrossRef] [PubMed]
- Pozelli Macedo, M.J.; Xavier-Queiroz, M.; Dabul, A.N.G.; Ricomini-Filho, A.P.; Hamann, P.R.V.; Polikarpov, I. Biochemical Properties of a Flavobacterium johnsoniae Dextranase and Its Biotechnological Potential for Streptococcus mutans Biofilm Degradation. World J. Microbiol. Biotechnol. 2024, 40, 201. [Google Scholar] [CrossRef]
- Ren, Z.; Kim, D.; Paula, A.J.; Hwang, G.; Liu, Y.; Li, J.; Daniell, H.; Koo, H. Dual-Targeting Approach Degrades Biofilm Matrix and Enhances Bacterial Killing. J. Dent. Res. 2019, 98, 322–330. [Google Scholar] [CrossRef]
- Garuba, E.O.; Omoniyi, A.R. Production, Characterization, and Anti-Biofilm Activity of Dextranase from Penicillium citrinum against Streptococcus mutans. EUREKA Life Sci. 2025, 2, 36–46. [Google Scholar] [CrossRef]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3, 223–247. [Google Scholar] [CrossRef]
- Serra, D.O.; Hengge, R. Cellulose in Bacterial Biofilms. In Extracellular Sugar-Based Biopolymers Matrices; Springer International Publishing: Cham, Switzerland, 2019; pp. 355–392. [Google Scholar]
- Lahiri, D.; Nag, M.; Banerjee, R.; Mukherjee, D.; Garai, S.; Sarkar, T.; Dey, A.; Sheikh, H.I.; Pathak, S.K.; Edinur, H.A.; et al. Amylases: Biofilm Inducer or Biofilm Inhibitor? Front. Cell. Infect. Microbiol. 2021, 11, 660048. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Singh, A.K.; Singh, S.; Chakravortty, D.; Das, D. Enzymatic Dispersion of Biofilms: An Emerging Biocatalytic Avenue to Combat Biofilm-Mediated Microbial Infections. J. Biol. Chem. 2022, 298, 102352. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Nair, A.V.; Parmar, K.; Rajmani, R.S.; Chakravortty, D.; Das, D. Combating Biofilm-Associated Klebsiella Pneumoniae Infections Using a Bovine Microbial Enzyme. NPJ Biofilms Microbiomes 2024, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Yu, H.H.; Kim, Y.J.; Lee, N.-K.; Paik, H.-D. The Use of Papain for the Removal of Biofilms Formed by Pathogenic Staphylococcus aureus and Campylobacter jejuni. LWT 2020, 127, 109383. [Google Scholar] [CrossRef]
- Silva, M.P.; Calomino, M.A.; Teixeira, L.A.; Barros, R.R.; Renato de Paula, G.; Teixeira, F.L. Antibiofilm Activity of Bromelain from Pineapple against Staphylococcus aureus. Acta Sci. Biol. Sci. 2023, 45, e65725. [Google Scholar] [CrossRef]
- Baidamshina, D.R.; Koroleva, V.A.; Olshannikova, S.S.; Trizna, E.Y.; Bogachev, M.I.; Artyukhov, V.G.; Holyavka, M.G.; Kayumov, A.R. Biochemical Properties and Anti-Biofilm Activity of Chitosan-Immobilized Papain. Mar. Drugs 2021, 19, 197. [Google Scholar] [CrossRef]
- Kumar, L.; Cox, C.R.; Sarkar, S.K. Matrix Metalloprotease-1 Inhibits and Disrupts Enterococcus faecalis Biofilms. PLoS ONE 2019, 14, e0210218. [Google Scholar] [CrossRef]
- Baidamshina, D.R.; Trizna, E.Y.; Goncharova, S.S.; Sorokin, A.V.; Lavlinskaya, M.S.; Melnik, A.P.; Gafarova, L.F.; Kharitonova, M.A.; Ostolopovskaya, O.V.; Artyukhov, V.G.; et al. The Effect of Ficin Immobilized on Carboxymethyl Chitosan on Biofilms of Oral Pathogens. Int. J. Mol. Sci. 2023, 24, 16090. [Google Scholar] [CrossRef]
- Baidamshina, D.R.; Trizna, E.Y.; Holyavka, M.G.; Bogachev, M.I.; Artyukhov, V.G.; Akhatova, F.S.; Rozhina, E.V.; Fakhrullin, R.F.; Kayumov, A.R. Targeting Microbial Biofilms Using Ficin, a Nonspecific Plant Protease. Sci. Rep. 2017, 7, 46068. [Google Scholar] [CrossRef]
- Krishnaswamy, V.R.; Mintz, D.; Sagi, I. Matrix Metalloproteinases: The Sculptors of Chronic Cutaneous Wounds. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 2220–2227. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. Surface Proteins of Staphylococcus epidermidis. Front. Microbiol. 2020, 11, 1829. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.D.; Bakaletz, L.O. Bacterial Biofilms Utilize an Underlying Extracellular DNA Matrix Structure That Can Be Targeted for Biofilm Resolution. Microorganisms 2022, 10, 466. [Google Scholar] [CrossRef]
- Deng, W.; Lei, Y.; Tang, X.; Li, D.; Liang, J.; Luo, J.; Liu, L.; Zhang, W.; Ye, L.; Kong, J.; et al. DNase Inhibits Early Biofilm Formation in Pseudomonas aeruginosa- or Staphylococcus aureus-Induced Empyema Models. Front. Cell. Infect. Microbiol. 2022, 12, 917038. [Google Scholar] [CrossRef]
- Lander, S.M.; Fisher, G.; Everett, B.A.; Tran, P.; Prindle, A. Secreted Nucleases Reclaim Extracellular DNA during Biofilm Development. NPJ Biofilms Microbiomes 2024, 10, 103. [Google Scholar] [CrossRef]
- Kaplan, J.B.; Florjanczyk, A.P.; Ochiai, M.; Jones, C.D.; Horswill, A.R. Micrococcal Nuclease Regulates Biofilm Formation and Dispersal in Methicillin-Resistant Staphylococcus aureus USA300. bioRxiv 2023, preprint. [Google Scholar] [CrossRef] [PubMed]
- Erskine, E.; MacPhee, C.E.; Stanley-Wall, N.R. Functional Amyloid and Other Protein Fibers in the Biofilm Matrix. J. Mol. Biol. 2018, 430, 3642–3656. [Google Scholar] [CrossRef]
- Tarek, H.; Nam, K.B.; Kim, Y.K.; Suchi, S.A.; Yoo, J.C. Biochemical Characterization and Application of a Detergent Stable, Antimicrobial and Antibiofilm Potential Protease from Bacillus siamensis. Int. J. Mol. Sci. 2023, 24, 5774. [Google Scholar] [CrossRef]
- Sun, H.; Sun, S.; Wang, H.; Cheng, K.; Zhou, Y.; Wang, X.; Gao, S.; Mo, J.; Li, S.; Lin, H.; et al. Phenylboronic Acid-Modified Carbon Dot-Proteinase K Nanohybrids for Enhanced Photodynamic Therapy against Bacterial Biofilm Infections. Acta Biomater. 2025, 194, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Meng, X.; Han, Q.; Huang, Y.; Huo, L.; Lei, Y. An in Vitro Study on the Degradation of Multispecies Biofilm of Periodontitis-Related Microorganisms by Bovine Trypsin. Front. Microbiol. 2022, 13, 951291. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.E.; Thomas, B.C. Reducing Biofilm Infections in Burn Patients’ Wounds and Biofilms on Surfaces in Hospitals, Medical Facilities and Medical Equipment to Improve Burn Care: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 13195. [Google Scholar] [CrossRef]
- Mechmechani, S.; Gharsallaoui, A.; Karam, L.; EL Omari, K.; Fadel, A.; Hamze, M.; Chihib, N.-E. Pepsin and Trypsin Treatment Combined with Carvacrol: An Efficient Strategy to Fight Pseudomonas aeruginosa and Enterococcus faecalis Biofilms. Microorganisms 2023, 11, 143. [Google Scholar] [CrossRef]
- Caro, A.; Humblot, V.; Méthivier, C.; Minier, M.; Barbes, L.; Li, J.; Salmain, M.; Pradier, C.-M. Bioengineering of Stainless Steel Surface by Covalent Immobilization of Enzymes. Physical Characterization and Interfacial Enzymatic Activity. J. Colloid Interface Sci. 2010, 349, 13–18. [Google Scholar] [CrossRef]
- Fu, Z.; Akula, S.; Thorpe, M.; Hellman, L. Marked Difference in Efficiency of the Digestive Enzymes Pepsin, Trypsin, Chymotrypsin, and Pancreatic Elastase to Cleave Tightly Folded Proteins. Biol. Chem. 2021, 402, 861–867. [Google Scholar] [CrossRef]
- Mechmechani, S.; Gharsallaoui, A.; El Omari, K.; Fadel, A.; Hamze, M.; Chihib, N.-E. Hurdle Technology Based on the Use of Microencapsulated Pepsin, Trypsin and Carvacrol to Eradicate Pseudomonas aeruginosa and Enterococcus faecalis Biofilms. Biofouling 2022, 38, 903–915. [Google Scholar] [CrossRef]
- Bowden, L.C.; Finlinson, J.; Jones, B.; Berges, B.K. Beyond the Double Helix: The Multifaceted Landscape of Extracellular DNA in Staphylococcus aureus Biofilms. Front. Cell. Infect. Microbiol. 2024, 14, 1400648. [Google Scholar] [CrossRef]
- Panlilio, H.; Rice, C.V. The Role of Extracellular DNA in the Formation, Architecture, Stability, and Treatment of Bacterial Biofilms. Biotechnol. Bioeng. 2021, 118, 2129–2141. [Google Scholar] [CrossRef]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA Required for Bacterial Biofilm Formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef]
- Terlizzi, V.; Castellani, C.; Taccetti, G.; Ferrari, B. Dornase Alfa in Cystic Fibrosis: Indications, Comparative Studies and Effects on Lung Clearance Index. Ital. J. Pediatr. 2022, 48, 141. [Google Scholar] [CrossRef]
- Ye, J.; Shao, C.; Zhang, X.; Guo, X.; Gao, P.; Cen, Y.; Ma, S.; Liu, Y. Effects of DNase I Coating of Titanium on Bacteria Adhesion and Biofilm Formation. Mater. Sci. Eng. C 2017, 78, 738–747. [Google Scholar] [CrossRef]
- Aktan, M.K.; Van der Gucht, M.; Hendrix, H.; Vande Velde, G.; Baert, K.; Hauffman, T.; Killian, M.S.; Lavigne, R.; Braem, A. Anti-Infective DNase I Coatings on Polydopamine Functionalized Titanium Surfaces by Alternating Current Electrophoretic Deposition. Anal. Chim. Acta 2022, 1218, 340022. [Google Scholar] [CrossRef]
- Wang, T.; Flint, S.; Palmer, J. Magnesium and Calcium Ions: Roles in Bacterial Cell Attachment and Biofilm Structure Maturation. Biofouling 2019, 35, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Jhajharia, K.; Parolia, A.; Shetty, K.V.; Mehta, L. Biofilm in Endodontics: A Review. J. Int. Soc. Prev. Community Dent. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, L.; Zhang, P.; Wang, Y. Novel Approaches to Combat Medical Device-Associated BioFilms. Coatings 2021, 11, 294. [Google Scholar] [CrossRef]
- Lebeaux, D.; Leflon-Guibout, V.; Ghigo, J.-M.; Beloin, C. In Vitro Activity of Gentamicin, Vancomycin or Amikacin Combined with EDTA or Scp/Scp-Arginine as Lock Therapy against a Wide Spectrum of Biofilm-Forming Clinical Strains Isolated from Catheter-Related Infections. J. Antimicrob. Chemother. 2015, 70, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Aboelenin, A.M.; Hassan, R.; Abdelmegeed, E.S. The Effect of EDTA in Combination with Some Antibiotics against Clinical Isolates of Gram Negative Bacteria in Mansoura, Egypt. Microb. Pathog. 2021, 154, 104840. [Google Scholar] [CrossRef]
- Chu, L.; Zhou, X.; Shen, Y.; Yu, Y. Inhibitory Effect of Trisodium Citrate on Biofilms Formed by Klebsiella Pneumoniae. J. Glob. Antimicrob. Resist. 2020, 22, 452–456. [Google Scholar] [CrossRef]
- Liesse Iyamba, J.M.; Seil, M.; Nagant, C.; Dulanto, S.; Deplano, A.; El Khattabi, C.; Takaisi Kikuni, N.B.; Dehaye, J.P. Inhibition by EGTA of the Formation of a Biofilm by Clinical Strains of Staphylococcus aureus. J. Basic Microbiol. 2014, 54, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Leitão, M.M.; Gonçalves, A.S.C.; Moreira, J.; Fernandes, C.; Borges, F.; Simões, M.; Borges, A. Unravelling the Potential of Natural Chelating Agents in the Control of Staphylococcus aureus and Pseudomonas aeruginosa Biofilms. Eur. J. Med. Chem. 2025, 283, 117163. [Google Scholar] [CrossRef]
- Xander, C.; Martinez, E.E.; Toothman, R.G.; Gardner, C.L.; Qiu, J.; Snedeker, J.; Bender, M.H.; Hlubb, C.; Burke, C.W.; Bozue, J.A.; et al. Treatment of Bacterial Biothreat Agents with a Novel Purified Bioactive Lactoferrin Affects Both Growth and Biofilm Formation. Front. Cell. Infect. Microbiol. 2025, 15, 1603689. [Google Scholar] [CrossRef] [PubMed]
- Houshmandyar, S.; Eggleston, I.M.; Bolhuis, A. Biofilm-Specific Uptake of a 4-Pyridone-Based Iron Chelator by Pseudomonas aeruginosa. BioMetals 2021, 34, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A. Combatting Biofilm-Mediated Infections in Clinical Settings by Targeting Quorum Sensing. Cell Surf. 2024, 12, 100133. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bassler, B.L. Bacterial Quorum Sensing in Complex and Dynamically Changing Environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Quadriya, H.; Adeeb Mujtaba Ali, S.; Parameshwar, J.; Manasa, M.; Yahya Khan, M.; Hameeda, B. Microbes Living Together: Exploiting the Art for Making Biosurfactants and Biofilms. In Implication of Quorum Sensing System in Biofilm Formation and Virulence; Springer: Singapore, 2018; pp. 161–177. [Google Scholar]
- Guendouze, A.; Plener, L.; Bzdrenga, J.; Jacquet, P.; Rémy, B.; Elias, M.; Lavigne, J.-P.; Daudé, D.; Chabrière, E. Effect of Quorum Quenching Lactonase in Clinical Isolates of Pseudomonas aeruginosa and Comparison with Quorum Sensing Inhibitors. Front. Microbiol. 2017, 8, 227. [Google Scholar] [CrossRef]
- Vogel, J.; Wakker-Havinga, M.; Setroikromo, R.; Quax, W.J. Immobilized Acylase PvdQ Reduces Pseudomonas aeruginosa Biofilm Formation on PDMS Silicone. Front. Chem. 2020, 8, 54. [Google Scholar] [CrossRef]
- Malakar, C.; Deka, S.; Kalita, M.C. Role of Biosurfactants in Biofilm Prevention and Disruption. In Advancements in Biosurfactants Research; Springer International Publishing: Cham, Switzerland, 2023; pp. 481–501. [Google Scholar]
- Jimoh, A.A.; Booysen, E.; van Zyl, L.; Trindade, M. Do Biosurfactants as Anti-Biofilm Agents Have a Future in Industrial Water Systems? Front. Bioeng. Biotechnol. 2023, 11, 1244595. [Google Scholar] [CrossRef]
- Dhadwal, S.; Handa, S.; Chatterjee, M.; Banat, I.M. Sophorolipid: An Effective Biomolecule for Targeting Microbial Biofilms. Curr. Microbiol. 2024, 81, 388. [Google Scholar] [CrossRef]
- Thakur, B.; Kaur, S.; Dwibedi, V.; Albadrani, G.M.; Al-Ghadi, M.Q.; Abdel-Daim, M.M. Unveiling the Antimicrobial and Antibiofilm Potential of Biosurfactant Produced by Newly Isolated Lactiplantibacillus Plantarum Strain 1625. Front. Microbiol. 2024, 15, 1459388. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, V.; Roelants, S.L.K.W.; Castelein, M.G.; De Maeseneire, S.L.; Soetaert, W.K. Elucidation of the Natural Function of Sophorolipids Produced by Starmerella bombicola. J. Fungi 2021, 7, 917. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, C.; Fracchia, L.; Williams, M.; Banat, I.M.; Díaz De Rienzo, M.A. The Effect of Sophorolipids against Microbial Biofilms on Medical-Grade Silicone. J. Biotechnol. 2020, 309, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, A.K.; Verma, N.; Kaushal, P. Role of Biogenic Capping Agents in the Synthesis of Metallic Nanoparticles and Evaluation of Their Therapeutic Potential. Front. Nanotechnol. 2022, 3, 801620. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Silva, A.F.; Rufino, R.D.; Luna, J.M.; Souza, J.E.G.; Sarubbo, L.A. Synthesis of Silver Nanoparticles Using a Biosurfactant Produced in Low-Cost Medium as Stabilizing Agent. Electron. J. Biotechnol. 2014, 17, 122–125. [Google Scholar] [CrossRef]
- Khalid, H.F.; Tehseen, B.; Sarwar, Y.; Hussain, S.Z.; Khan, W.S.; Raza, Z.A.; Bajwa, S.Z.; Kanaras, A.G.; Hussain, I.; Rehman, A. Biosurfactant Coated Silver and Iron Oxide Nanoparticles with Enhanced Anti-Biofilm and Anti-Adhesive Properties. J. Hazard. Mater. 2019, 364, 441–448. [Google Scholar] [CrossRef]
- Girma, A. Alternative Mechanisms of Action of Metallic Nanoparticles to Mitigate the Global Spread of Antibiotic-Resistant Bacteria. Cell Surf. 2023, 10, 100112. [Google Scholar] [CrossRef]
- Algadi, H.; Alhoot, M.A.; Al-Maleki, A.R.; Purwitasari, N. Effects of Metal and Metal Oxide Nanoparticles against Biofilm-Forming Bacteria: A Systematic Review. J. Microbiol. Biotechnol. 2024, 34, 1748–1756. [Google Scholar] [CrossRef]
- Ferraris, S.; Scalia, A.C.; Nascimben, M.; Perero, S.; Rimondini, L.; Spriano, S.; Cochis, A. Bacteriostatic, Silver-Doped, Zirconia-Based Thin Coatings for Temporary Fixation Devices Tuning Stem Cells’ Expression of Adhesion-Relevant Genes and Proteins. Biomater. Adv. 2025, 176, 214360. [Google Scholar] [CrossRef]
- Qais, F.A.; Ahmad, I.; Altaf, M.; Alotaibi, S.H. Biofabrication of Gold Nanoparticles Using Capsicum Annuum Extract and Its Antiquorum Sensing and Antibiofilm Activity against Bacterial Pathogens. ACS Omega 2021, 6, 16670–16682. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Hassan, M.A.; Britigan, B.E.; Narayanasamy, P. Antimicrobial Activity of Gallium(III) Compounds: Pathogen-Dependent Targeting of Multiple Iron/Heme-Dependent Biological Processes. Curr. Issues Mol. Biol. 2024, 46, 9149–9161. [Google Scholar] [CrossRef]
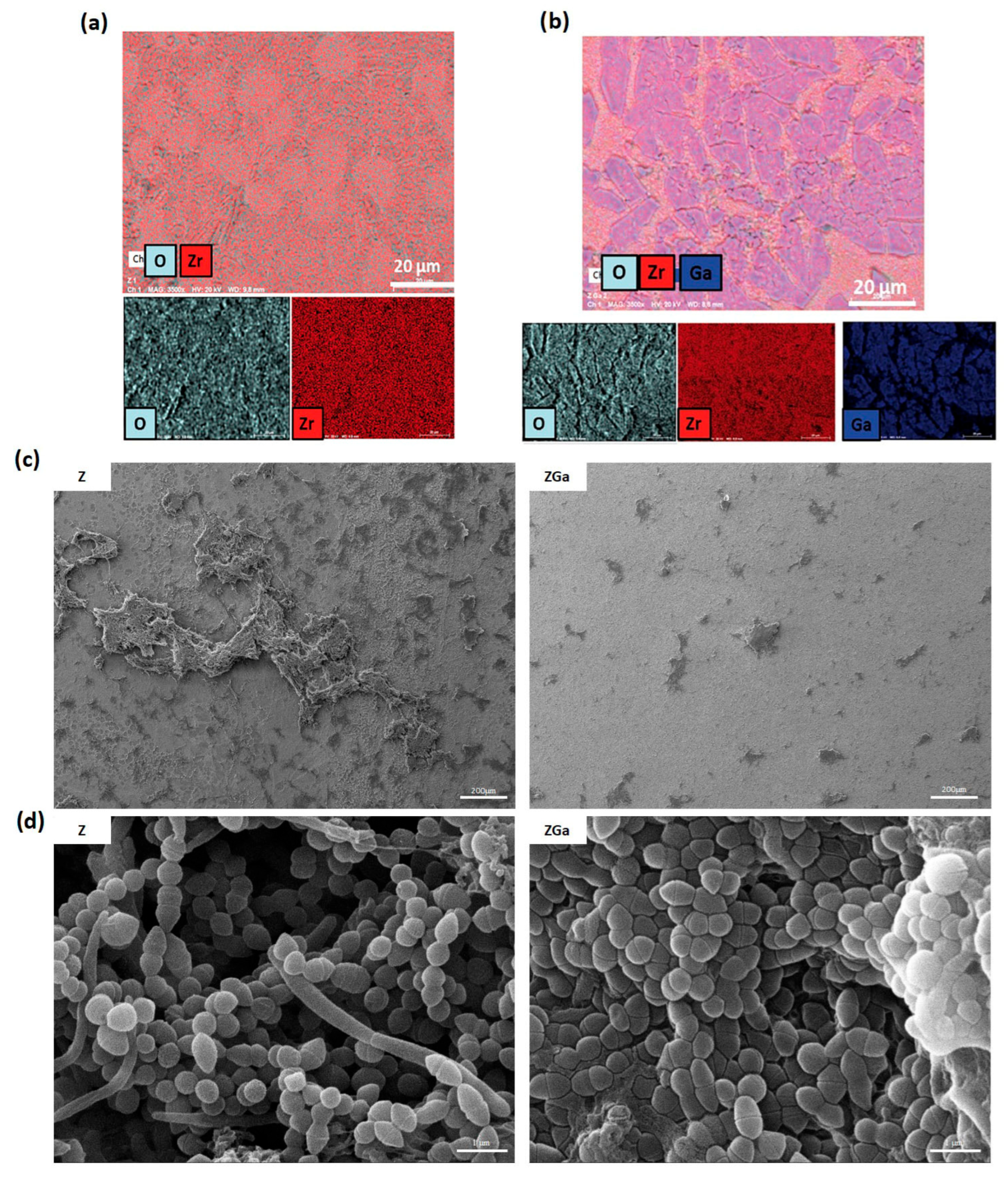
- D’Agostino, A.; Misiti, G.; Scalia, A.C.; Pavarini, M.; Fiorati, A.; Cochis, A.; Rimondini, L.; Borrini, V.F.; Manfredi, M.; Andena, L.; et al. Gallium-doped Zirconia Coatings Modulate Microbiological Outcomes in Dental Implant Surfaces. J. Biomed. Mater. Res. Part A 2024, 112, 2098–2109. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zang, Y.; Qu, J.; Tang, M.; Zhang, T. The Toxicity Of Metallic Nanoparticles On Liver: The Subcellular Damages, Mechanisms, And Outcomes. Int. J. Nanomed. 2019, 14, 8787–8804. [Google Scholar] [CrossRef] [PubMed]
- Wadhawan, S.; Wadhawan, D.; Jain, A.; Mehta, S.K. Toxic Implication of Nanoparticles: A Review of Factors, Mechanism, Exposure and Control Strategies. Int. J. Environ. Sci. Technol. 2025, 22, 1203–1224. [Google Scholar] [CrossRef]
- Jangid, H.; Joshi, H.C.; Dutta, J.; Ahmad, A.; Alshammari, M.B.; Hossain, K.; Pant, G.; Kumar, G. Advancing Food Safety with Biogenic Silver Nanoparticles: Addressing Antimicrobial Resistance, Sustainability, and Commercial Viability. Food Chem. X 2025, 26, 102298. [Google Scholar] [CrossRef]
- Harun-Ur-Rashid, M.; Foyez, T.; Krishna, S.B.N.; Poda, S.; Imran, A. Bin Recent Advances of Silver Nanoparticle-Based Polymer Nanocomposites for Biomedical Applications. RSC Adv. 2025, 15, 8480–8505. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Nadar, S.; Safwat, A.; Qin, N.; Czyż, D.M. An Uphill Path to Commercialization of Silver Nanoparticle Antimicrobials: From Bench to Market. Nanomedicine 2025, 20, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, W.; Zhao, C.; Zhang, W.; Yan, Z. Graphene Coated Ti-6Al-4V Exhibits Antibacterial and Antifungal Properties Against Oral Pathogens. J. Prosthodont. 2023, 32, 505–511. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Lyu, C.; Hu, Y.; Zou, D.; He, Y.-S.; Lu, J. Synergistic Antibacterial Effect of Graphene-Coated Titanium Loaded with Levofloxacin. Colloids Surf. B Biointerfaces 2021, 208, 112090. [Google Scholar] [CrossRef]
- Cheng, Q.; Lu, R.; Wang, X.; Chen, S. Antibacterial Activity and Cytocompatibility Evaluation of the Antimicrobial Peptide Nal-P-113-Loaded Graphene Oxide Coating on Titanium. Dent. Mater. J. 2022, 41, 2022–2094. [Google Scholar] [CrossRef] [PubMed]
- Sadrearhami, Z.; Namivandi-Zangeneh, R.; Price, E.; Krasowska, M.; Al-Bataineh, S.A.; Whittle, J.; Wong, E.H.H.; Blencowe, A.; Boyer, C. S-Nitrosothiol Plasma-Modified Surfaces for the Prevention of Bacterial Biofilm Formation. ACS Biomater. Sci. Eng. 2019, 5, 5881–5887. [Google Scholar] [CrossRef]
- Chug, M.K.; Sapkota, A.; Garren, M.; Brisbois, E.J. Wearable Nitric Oxide-Releasing Antibacterial Insert for Preventing Device-Associated Infections. J. Control. Release 2024, 375, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Failor, K.C.; Silver, B.; Yu, W.; Heindl, J.E. Biofilm Disruption and Bactericidal Activity of Aqueous Ozone Coupled with Ultrasonic Dental Scaling. JADA Found. Sci. 2022, 1, 100003. [Google Scholar] [CrossRef] [PubMed]
- Kovač, B.; Planinić, A.; Planinić, M.; Piletić, K.; Gobin, I. Treatment with Gaseous Ozone Significantly Reduced the Number of Bacteria in Extended-Spectrum-β-Lactamase (ESBL)-Producing Escherichia coli Biofilm. Hygiene 2023, 3, 125–135. [Google Scholar] [CrossRef]
- Tang, M.; Chen, C.; Zhu, J.; Allcock, H.R.; Siedlecki, C.A.; Xu, L.-C. Inhibition of Bacterial Adhesion and Biofilm Formation by a Textured Fluorinated Alkoxyphosphazene Surface. Bioact. Mater. 2021, 6, 447–459. [Google Scholar] [CrossRef]
- Inoue, D.; Kabata, T.; Ohtani, K.; Kajino, Y.; Shirai, T.; Tsuchiya, H. Inhibition of Biofilm Formation on Iodine-Supported Titanium Implants. Int. Orthop. 2017, 41, 1093–1099. [Google Scholar] [CrossRef]
- Aboltins, C.A.; Antoci, V.; Bhattacharyya, S.; Cross, M.; Ducheyne, P.; Freiberg, A.A.; Hailer, N.; Kay, P.; Ketonis, C.; Klement, M.R.; et al. Hip and Knee Section, Prevention, Prosthesis Factors: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S309–S320. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xu, J.; Zou, L.; Luo, S.; Yao, R.; Zheng, B.; Liang, G.; Wu, D.; Li, Y. Long-Lasting Renewable Antibacterial Porous Polymeric Coatings Enable Titanium Biomaterials to Prevent and Treat Peri-Implant Infection. Nat. Commun. 2021, 12, 3303. [Google Scholar] [CrossRef] [PubMed]
- Komnatnyy, V.V.; Chiang, W.; Tolker-Nielsen, T.; Givskov, M.; Nielsen, T.E. Bacteria-Triggered Release of Antimicrobial Agents. Angew. Chem. Int. Ed. 2014, 53, 439–441. [Google Scholar] [CrossRef]
- Powell, L.C.; Abdulkarim, M.; Stokniene, J.; Yang, Q.E.; Walsh, T.R.; Hill, K.E.; Gumbleton, M.; Thomas, D.W. Quantifying the Effects of Antibiotic Treatment on the Extracellular Polymer Network of Antimicrobial Resistant and Sensitive Biofilms Using Multiple Particle Tracking. NPJ Biofilms Microbiomes 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wen, P.; Tian, S.; Zhang, H.; Han, B.; Khan, J.; Xue, Y.; Chen, X.; Li, X.; Li, Y. Enhancing Biofilm Penetration and Antibiofilm Efficacy with Protein Nanocarriers against Pathogenic Biofilms. Int. J. Biol. Macromol. 2024, 256, 128300. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Chakrabartty, I.; Mishra, A.K.; Chopra, H.; Mahanta, S.; Avula, S.K.; Patowary, K.; Ahmed, R.; Mishra, B.; Mohanta, T.K.; et al. Nanotechnology in Combating Biofilm: A Smart and Promising Therapeutic Strategy. Front. Microbiol. 2023, 13, 1028086. [Google Scholar] [CrossRef]
- Li, X.; Yeh, Y.-C.; Giri, K.; Mout, R.; Landis, R.F.; Prakash, Y.S.; Rotello, V.M. Control of Nanoparticle Penetration into Biofilms through Surface Design. Chem. Commun. 2015, 51, 282–285. [Google Scholar] [CrossRef]
- Morris, J.L.; Letson, H.L.; Elliott, L.; Grant, A.L.; Wilkinson, M.; Hazratwala, K.; McEwen, P. Evaluation of Bacteriophage as an Adjunct Therapy for Treatment of Peri-Prosthetic Joint Infection Caused by Staphylococcus aureus. PLoS ONE 2019, 14, e0226574. [Google Scholar] [CrossRef]
- Yazdi, M.; Bouzari, M.; Ghaemi, E.A. Isolation and Characterization of a Lytic Bacteriophage (VB_PmiS-TH) and Its Application in Combination with Ampicillin against Planktonic and Biofilm Forms of Proteus Mirabilis Isolated from Urinary Tract Infection. Microb. Physiol. 2018, 28, 37–46. [Google Scholar] [CrossRef]
- Mayorga-Ramos, A.; Carrera-Pacheco, S.E.; Barba-Ostria, C.; Guamán, L.P. Bacteriophage-Mediated Approaches for Biofilm Control. Front. Cell. Infect. Microbiol. 2024, 14, 1428637. [Google Scholar] [CrossRef]
- Oechslin, F. Resistance Development to Bacteriophages Occurring during Bacteriophage Therapy. Viruses 2018, 10, 351. [Google Scholar] [CrossRef]
- Anomaly, J. The Future of Phage: Ethical Challenges of Using Phage Therapy to Treat Bacterial Infections. Public Health Ethics 2020, 13, 82–88. [Google Scholar] [CrossRef]
- Podlacha, M.; Grabowski, Ł.; Kosznik-Kawśnicka, K.; Zdrojewska, K.; Stasiłojć, M.; Węgrzyn, G.; Węgrzyn, A. Interactions of Bacteriophages with Animal and Human Organisms—Safety Issues in the Light of Phage Therapy. Int. J. Mol. Sci. 2021, 22, 8937. [Google Scholar] [CrossRef]
- Van Belleghem, J.D.; Dąbrowska, K.; Vaneechoutte, M.; Barr, J.J.; Bollyky, P.L. Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System. Viruses 2018, 11, 10. [Google Scholar] [CrossRef]
- Clinical Study of Phage Therapy for Chronic Constipation Efficacy and Safety. Available online: https://www.clinicaltrials.gov/study/NCT05973721?term=phage&viewType=Table&rank=2 (accessed on 1 August 2023).
- Dave, R.; Ahiwale, S. Overcoming Challenges and Regulatory Hurdles for Sustainable Bacteriophage Therapy Products. In Emerging Paradigms for Antibiotic-Resistant Infections: Beyond the Pill; Springer Nature: Berlin/Heidelberg, Germany, 2024; pp. 771–786. [Google Scholar] [CrossRef]
- Redman, W.K.; Welch, G.S.; Williams, A.C.; Damron, A.J.; Northcut, W.O.; Rumbaugh, K.P. Efficacy and Safety of Biofilm Dispersal by Glycoside Hydrolases in Wounds. Biofilm 2021, 3, 100061. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-Y.; Li, F.-L.; Zhang, Y.-W.; Gupta, R.K.; Patel, S.K.S.; Lee, J.-K. Recent Strategies for the Immobilization of Therapeutic Enzymes. Polymers 2022, 14, 1409. [Google Scholar] [CrossRef]
- Fanaei Pirlar, R.; Emaneini, M.; Beigverdi, R.; Banar, M.; van Leeuwen, W.B.; Jabalameli, F. Combinatorial Effects of Antibiotics and Enzymes against Dual-Species Staphylococcus aureus and Pseudomonas aeruginosa Biofilms in the Wound-like Medium. PLoS ONE 2020, 15, e0235093. [Google Scholar] [CrossRef] [PubMed]
- Rigoni, M.; Montecucco, C. Bacterial Protein Toxins as Tools in Cell Biology and Physiology. In Encyclopedia of Cell Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 794–798. [Google Scholar]
- Lordon, B.; Campion, T.; Gibot, L.; Gallot, G. Impact of Trypsin on Cell Cytoplasm during Detachment of Cells Studied by Terahertz Sensing. Biophys. J. 2024, 123, 2476–2483. [Google Scholar] [CrossRef]
- Melander, R.J.; Melander, C. The Challenge of Overcoming Antibiotic Resistance: An Adjuvant Approach? ACS Infect. Dis. 2017, 3, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, V.; Vecchio, G. Prochelator Strategies for Site-Selective Activation of Metal Chelators. J. Inorg. Biochem. 2016, 162, 31–43. [Google Scholar] [CrossRef]
- Muguruza, A.R.; di Maio, A.; Hodges, N.J.; Blair, J.M.A.; Pikramenou, Z. Chelating Silica Nanoparticles for Efficient Antibiotic Delivery and Particle Imaging in Gram-Negative Bacteria. Nanoscale Adv. 2023, 5, 2453–2461. [Google Scholar] [CrossRef]
- Moniz, T.; Dias da Silva, D.; Carmo, H.; de Castro, B.; de Lourdes Bastos, M.; Rangel, M. Insights on the Relationship between Structure vs. Toxicological Activity of Antibacterial Rhodamine-Labelled 3-Hydroxy-4-Pyridinone Iron(III) Chelators in HepG2 Cells. Interdiscip. Toxicol. 2018, 11, 189–199. [Google Scholar] [CrossRef]
- Liu, T.; Wang, X.; Xu, J.; You, X.; Chen, D.; Wang, F.; Li, Y. Biochemical and Genetic Toxicity of Dinotefuran on Earthworms (Eisenia fetida). Chemosphere 2017, 176, 156–164. [Google Scholar] [CrossRef]
- Naga, N.G.; El-Badan, D.E.; Ghanem, K.M.; Shaaban, M.I. It Is the Time for Quorum Sensing Inhibition as Alternative Strategy of Antimicrobial Therapy. Cell Commun. Signal. 2023, 21, 133. [Google Scholar] [CrossRef]
- Patel, K.; Panchal, R.; Sakariya, B.; Gevariya, M.; Raiyani, R.; Soni, R.; Goswami, D. Combatting Antibiotic Resistance by Exploring the Promise of Quorum Quenching in Targeting Bacterial Virulence. Microbe 2025, 6, 100224. [Google Scholar] [CrossRef]
- Elfaky, M.A. Unveiling the Hidden Language of Bacteria: Anti-Quorum Sensing Strategies for Gram-Negative Bacteria Infection Control. Arch. Microbiol. 2024, 206, 124. [Google Scholar] [CrossRef]
- Naga, N.G.; Shaaban, M.I.; El-Metwally, M.M. An Insight on the Powerful of Bacterial Quorum Sensing Inhibition. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 2071–2081. [Google Scholar] [CrossRef]
- Roy, A.; Khan, M.R.; Mukherjee, A.K. Recent Advances in the Application of Microbial Biosurfactants in Food Industries: Opportunities and Challenges. Food Control 2024, 163, 110465. [Google Scholar] [CrossRef]
- Gayathiri, E.; Prakash, P.; Karmegam, N.; Varjani, S.; Awasthi, M.K.; Ravindran, B. Biosurfactants: Potential and Eco-Friendly Material for Sustainable Agriculture and Environmental Safety—A Review. Agronomy 2022, 12, 662. [Google Scholar] [CrossRef]
- da Cruz Nizer, W.S.; Adams, M.E.; Allison, K.N.; Montgomery, M.C.; Mosher, H.; Cassol, E.; Overhage, J. Oxidative Stress Responses in Biofilms. Biofilm 2024, 7, 100203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle–Liver Interactions: Cellular Uptake and Hepatobiliary Elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef] [PubMed]
| Enzyme Classes | Enzyme Name | Component Target | Bacterial Target | Ref. |
|---|---|---|---|---|
| Glycoside hydrolase | Dextranase | α-1,6-glucan (dextran) | Streptococcus mutans (oral biofilm), Lactobacillus spp., | [120,121,122] |
| Mutanase | α-1,3-glucan (mutan) | Streptococcus mutans | [121] | |
| Cellulase | β-1,4-glucan (cellulose) | Pseudomonas fluorescens, Salmonella enterica, E. coli, Komagataeibacter xylinus | [123,124] | |
| Amylase | α-1,4-glucan (amylose) | General polysaccharide degradation (minor components) | [125] | |
| α- or β-Mannosidase | α- or β-mannose-containing polysaccharides | Klebsiella sp., Pseudomonas sp. (minor components) | [126,127] | |
| Proteases | Subtilisin A (serine protease) | Biofilm structural proteins | P. aeruginosa, S. aureus | [128] |
| Papain (cysteine protease) | Surface adhesin, extracellular protein | S. aureus, S. epidermidis | [129,130] | |
| Ficin (cysteine protease) | Biofilm structural and adhesive proteins | S. aureus, S. epidermidis, S. mutans | [131,132] | |
| Bromelain (cysteine protease) | Biofilm structural, adhesins | S. aureus, S. epidermidis, E. coli | [129,133] | |
| Metalloproteinases (zinc-dependent endopeptidase) | Fibronectin-binding proteins (FnBPs), biofilm-associated protein (Bap), accumulation-associated protein (Aap) | S. aureus, S. epidermidis, Enterococcus faecalis | [133,134] | |
| Alkaline protease (serine protease) | FnBPs, Bap, Aap | S. aureus, S. epidermidis, E. coli | [135] | |
| Deoxyribonucleases | Streptodornase (DNase B) | Extracellular DNA (eDNA) | Gram-positive and Gram-negative bacteria | [136,137] |
| NucB | eDNA | E. coli, Bacillus subtilis, Micrococcus luteus | [76,138] | |
| Micrococcal nuclease | Calcium-dependent endo-exonuclease | S. aureus, S. epidermidis, P. aeruginosa, B. subtilis | [139] | |
| Serratia nuclease (NucA) | Single- and double-stranded eDNA | E. coli, Bacillus subtilis | [138] |
| Strengths | Weaknesses | Mitigation Strategies | Toxicity Profile | |
|---|---|---|---|---|
| Biofilm dispersing enzymes | High specificity Less likely to promote resistance development Enhanced antimicrobial efficacy | Enzyme instability and poor retention Limited efficacy against mature biofilms Delivery challenges High cost and limited scalability Narrow spectrum of activity | Enzyme encapsulation in protective carriers can enhance stability, protect against degradation, and improve delivery to infection sites [217] Enzyme engineering to improve stability and activity [218] Combining different enzymes or co-administering with antibiotics can increase effectiveness [219] | Glycoside hydrolases (GHs) may be toxic to human cells in vitro [217] DNases can cause DNA fragmentation in eukaryotic cells [220] Proteases may damage eukaryotic cell membranes and induce apoptosis [221] |
| Chelating agents | Reduced resistance development compared to other methods Inhibition of bacterial growth Biofilm disruption Potential synergistic effects High versatility | Low specificity (cannot distinguish between prokaryotic and eukaryotic cells) Efficacy depends on bacterial species and their metal requirements Delivery challenges | Combination with antibiotics to overcome resistance [222] Development of agents targeting only prokaryotic cells [223] Nanoparticle-based delivery to improve targeting [224] | May deplete essential metals in host cells, leading to dysfunction and toxicity [225] Potential interference with host metal-dependent enzymes, causing oxidative stress [226] |
| Quorum sensing inhibitors | Reduction of antibiotic resistance Targeting of bacterial virulence factors Availability of natural product-based options | Limited specificity Susceptibility to washout Challenges in clinical translation Limited efficacy | Developing QSIs with high specificity can minimize off-target effects and improve efficacy [227] Nanoparticle-based systems can enhance delivery and retention [228] Further research into pathogen-specific QS pathways to design targeted inhibitors [229] | Potential interference with similar signaling pathways in human cells [230] |
| Biosurfactants | Biodegradable Versatile Stable under extreme conditions Modifiable via genetic engineering | Low production yield High production costs Limited understanding and research Lack of production and safety standards | Genetic engineering and nanotechnology approaches to improve production and properties [231] Use of agricultural waste to reduce production costs [232] | Toxicity depends on type, concentration, exposure, and environmental conditions |
| Oxidizing agents | Broad-spectrum antimicrobial activity Low likelihood of resistance development Rapid disinfection High versatility | Formation of harmful by-products Short half-life of some agents Environmental concerns | Combination with other antimicrobials to improve efficacy and reduce resistance risk [233] Encapsulation in delivery systems for controlled release | Induction of oxidative stress Mitochondrial dysfunction Accumulation in tissues or organs [234] Potential to trigger inflammation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalia, A.C.; Najmi, Z. Targeting Bacterial Biofilms on Medical Implants: Current and Emerging Approaches. Antibiotics 2025, 14, 802. https://doi.org/10.3390/antibiotics14080802
Scalia AC, Najmi Z. Targeting Bacterial Biofilms on Medical Implants: Current and Emerging Approaches. Antibiotics. 2025; 14(8):802. https://doi.org/10.3390/antibiotics14080802
Chicago/Turabian StyleScalia, Alessandro Calogero, and Ziba Najmi. 2025. "Targeting Bacterial Biofilms on Medical Implants: Current and Emerging Approaches" Antibiotics 14, no. 8: 802. https://doi.org/10.3390/antibiotics14080802
APA StyleScalia, A. C., & Najmi, Z. (2025). Targeting Bacterial Biofilms on Medical Implants: Current and Emerging Approaches. Antibiotics, 14(8), 802. https://doi.org/10.3390/antibiotics14080802







