Prevalence and Types of Inappropriate Antibiotics Prescribing Among Dialysis Patients: A Systematic Review
Abstract
1. Introduction
2. Results
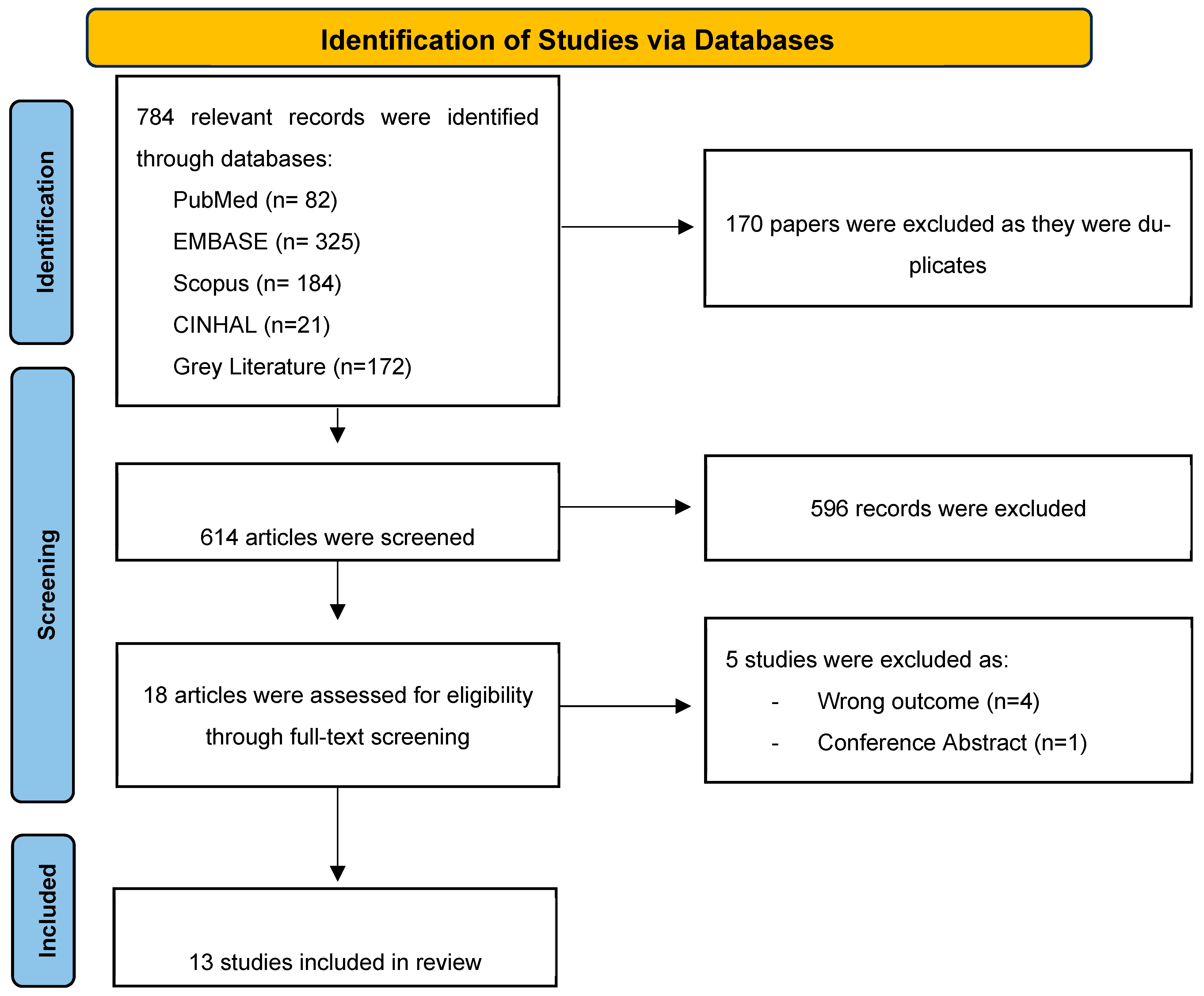
2.1. Search Results and Study Selection
2.2. Study Characteristics
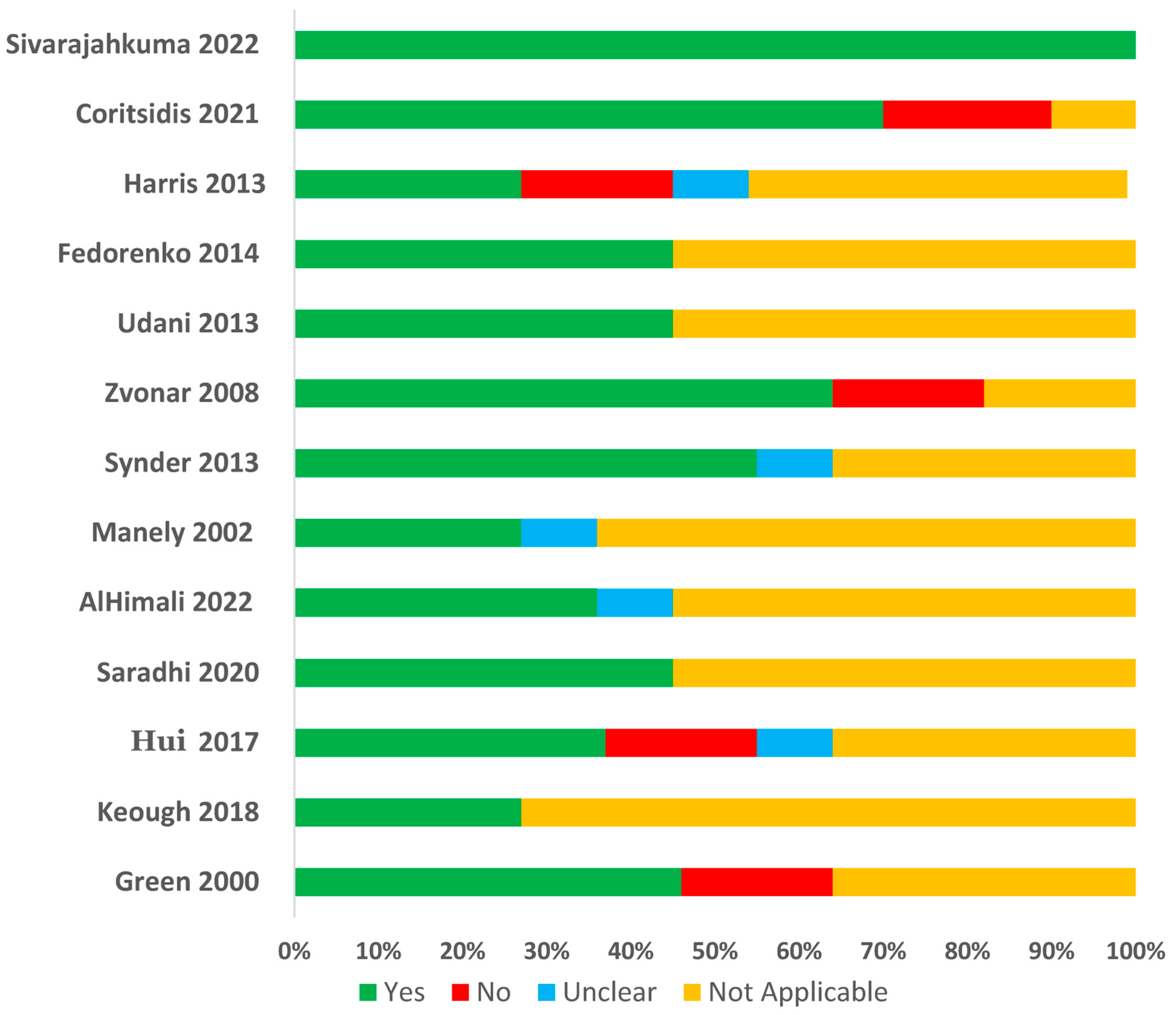
2.3. Quality Assessments of Included Studies
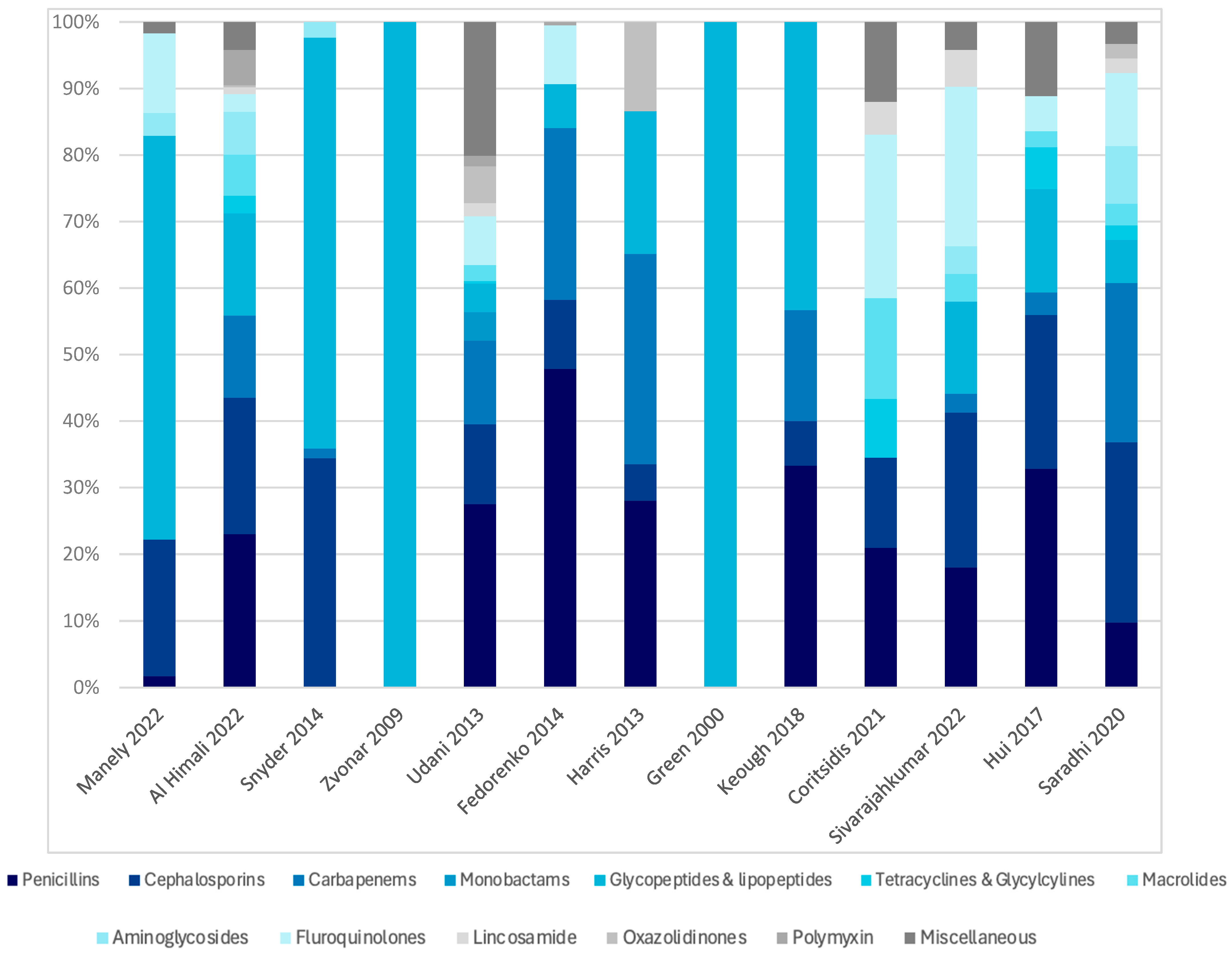
2.4. Prevalence and Types of Antibiotics Used Among Dialysis Patients
2.5. Appropriateness of Antibiotics Prescribed Among Dialysis Patients
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Eligibility Criteria
4.2.1. Inclusion Criteria
- Primary peer-reviewed clinical studies assessing antibiotics prescribing patterns (dosing, indication, route, duration, spectrum) and their appropriateness in dialysis patients.
- Observational studies (both retrospective and prospective) published in the English language.
- Studies that included adult patients (aged 18 years and above) with CKD undergoing chronic or acute dialysis (e.g., intermittent HD “IHD”, continuous renal replacement therapy “CCRT”, peritoneal dialysis “PD”, Prolonged Intermittent Renal Replacement Therapy “PIRRT”).
- Studies conducted in inpatient and outpatient settings, including tertiary hospitals, community healthcare centers, dialysis units, etc.
- No time restriction for studies, with inclusion up to October 2024.
4.2.2. Exclusion Criteria
- Studies focusing on non-clinical participants such as animal models or laboratory-based pharmacokinetics.
- Conference abstracts, editorial commentaries, reviews, case reports, and duplicate studies.
- Unpublished reports, or articles from non-peer-reviewed sources.
- Studies involving exclusively non-dialysis CKD patients, patients with acute kidney injury (AKI), or pediatric population.
4.3. Information Sources
4.4. Search Strategy
4.5. Data Management
4.6. Selection Process
4.7. Data Collection Process
4.8. Data Items
- Study characteristics: Author’s name, year of publication, country, study design, sample size.
- Participants’ characteristics including age, sex, comorbidities, dialysis indication, type of dialysis, and type of infection.
- Antibiotic prescribing details including types, dosing, indication, route of administration, and duration, and the rate and types of inappropriate antibiotic use among the patients. In addition, the appropriateness assessment tool used was extracted. Pre-planned data assumptions include that studies may vary in the definitions of “inappropriate use,” and standardizations will be applied where possible to align these differences across studies.
4.9. Outcomes and Prioritization
- Primary outcome: Rate of antibiotics prescribing, types, dosing, indication, route of administration, duration in patients undergoing dialysis across different healthcare settings.
- Secondary outcome: Prevalence and types of inappropriate antibiotics prescribing in patients undergoing dialysis across different healthcare settings.
4.10. Risk of Bias in Individual Studies
4.11. Data Synthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Couser, W.G.; Remuzzi, G.; Mendis, S.; Tonelli, M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011, 80, 1258–1270. [Google Scholar] [CrossRef]
- Bello, A.K.; Okpechi, I.G.; Levin, A.; Ye, F.; Saad, S.; Zaidi, D.; Houston, G.; Damster, S.; Arruebo, S.; Abu-Alfa, A. A report by the International Society of Nephrology: An assessment of global kidney health care status focussing on capacity, availability, accessibility, affordability and outcomes of kidney disease. In ISN–Global Kidney Health Atlas; International Society of Nephrology: Brussels, NJ, USA, 2023. [Google Scholar]
- Midturi, J.K.; Ranganath, S. Prevention and treatment of multidrug-resistant organisms in end-stage renal disease. Adv. Chronic Kidney Dis. 2019, 26, 51–60. [Google Scholar] [CrossRef]
- Chang, C.H.; Fan, P.C.; Kuo, G.; Lin, Y.S.; Tsai, T.Y.; Chang, S.W.; Tian, Y.C.; Lee, C.C. Infection in advanced chronic kidney disease and subsequent adverse outcomes after dialysis initiation: A nationwide cohort study. Sci. Rep. 2020, 10, 2938. [Google Scholar] [CrossRef]
- Calfee, D.P. Multidrug-resistant organisms in dialysis patients. Semin. Dial. 2013, 26, 447–456. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial resistance in bacteria: Mechanisms, evolution, and persistence. J. Mol. Evol. 2020, 88, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Berns, J.S.; Tokars, J.I. Preventing bacterial infections and antimicrobial resistance in dialysis patients. Am. J. Kidney Dis. 2002, 40, 886–898. [Google Scholar] [CrossRef]
- Cunha, C.B.; D’Agata, E.M. Implementing an antimicrobial stewardship program in out-patient dialysis units. Curr. Opin. Nephrol. Hypertens. 2016, 25, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, U.; Zulkarnain, A.I.; Rodríguez-Baño, J.; Kamarudin, N.; Elrggal, M.E.; Elnaem, M.H.; Harun, S.N. Treatments and predictors of mortality for carbapenem-resistant Gram-negative bacilli infections in Malaysia: A retrospective cohort study. Trop. Med. Infect. Dis. 2022, 7, 415. [Google Scholar] [CrossRef]
- Vilay, A.M. Antibiotic dosing in chronic kidney disease and end-stage renal disease: A focus on contemporary challenges. Adv. Chronic Kidney Dis. 2019, 26, 61–71. [Google Scholar] [CrossRef]
- Hoff, B.M.; Maker, J.H.; Dager, W.E.; Heintz, B.H. Antibiotic dosing for critically ill adult patients receiving intermittent hemodialysis, prolonged intermittent renal replacement therapy, and continuous renal replacement therapy: An update. Ann. Pharmacother. 2020, 54, 43–55. [Google Scholar] [CrossRef]
- D′Agata, E.M. Antimicrobial use and stewardship programs among dialysis centers. InSeminars Dial. 2013, 26, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.; Nalder, M.; Buising, K.; Pefanis, A.; Ooi, K.Y.; Pedagogos, E.; Nelson, C.; Kirkpatrick, C.M.; Kong, D.C. Patterns of use and appropriateness of antibiotics prescribed to patients receiving haemodialysis: An observational study. BMC Nephrol. 2017, 18, 156. [Google Scholar] [CrossRef] [PubMed]
- Manley, H.J.; Huke, M.A.; Dykstra, M.A.; Bedenbaugh, A.V. Antibiotic prescribing evaluation in an outpatient hemodialysis clinic. J. Pharm. Technol. 2002, 18, 128–132. [Google Scholar] [CrossRef]
- Snyder, G.M.; Patel, P.R.; Kallen, A.J.; Strom, J.A.; Tucker, J.K.; D′Agata, E.M. Antimicrobial use in outpatient hemodialysis units. Infect. Control. Hosp. Epidemiol. 2013, 34, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Udani, S.; Chen, L.X.; Daley, M.; Lat, I.; Koyner, J.L. Compliance with antibiotic dosing guidelines in critically ill patients receiving renal replacement therapy. J. Pharm. Technol. 2013, 29, 161–169. [Google Scholar] [CrossRef]
- Fedorenko, M.; Lam, S.W.; Harinstein, L.M.; Neuner, E.A.; Demirjian, S.; Bauer, S.R. Compliance with institutional antimicrobial dosing guidelines in patients receiving continuous venovenous hemodialysis. J. Pharm. Pract. 2015, 28, 380–386. [Google Scholar] [CrossRef]
- Harris, L.E.; Reaves, A.B.; Krauss, A.G.; Griner, J.; Hudson, J.Q. Evaluation of antibiotic prescribing patterns in patients receiving sustained low-efficiency dialysis: Opportunities for pharmacists. Int. J. Pharm. Pract. 2013, 21, 55–61. [Google Scholar] [CrossRef]
- Keough, L.A.; Krauss, A.; Hudson, J.Q. Inadequate antibiotic dosing in patients receiving sustained low efficiency dialysis. Int. J. Clin. Pharm. 2018, 40, 1250–1256. [Google Scholar] [CrossRef]
- Coritsidis, G.N.; Yaphe, S.; Rahkman, I.; Lubowski, T.; Munro, C.; Lee, T.K.; Stern, A.; Bhat, P. Outpatient antibiotic prescribing patterns for adult end-stage renal disease patients in New York state. Clin. Infect. Dis. 2021, 73, e4493-8. [Google Scholar] [CrossRef]
- Green, K.; Schulman, G.; Haas, D.W.; Schaffner, W.; D′Agata, E.M. Vancomycin prescribing practices in hospitalized chronic hemodialysis patients. Am. J. Kidney Dis. 2000, 35, 64–68. [Google Scholar] [CrossRef]
- Sivarajahkumar, S.; So, M.; Morris, A.M.; Lok, C.; Bell, C.M.; Battistella, M. Patterns of antimicrobial use in an outpatient hemodialysis unit. Can. J. Hosp. Pharm. 2022, 75, 15. [Google Scholar] [CrossRef]
- Zvonar, R.; Natarajan, S.; Edwards, C.; Roth, V. Assessment of vancomycin use in chronic haemodialysis patients: Room for improvement. Nephrol. Dial. Transplant. 2008, 23, 3690–3695. [Google Scholar] [CrossRef]
- Al Himali, N.; Al Suleimani, Y.M.; Al-Zakwani, I.; Abdelrahman, A.M. Antibiotics utilization patterns and dosage appropriateness among patients receiving hemodialysis. Saudi Pharm. J. 2022, 30, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Saradhi, S.V.P.; Fatima, M.; Ansari, M.; Fatima, A.; Afreen, S.; Quadri, A.K. spectrum of infections assessment of antibiotic use in maintenance hemodialysis patients. Int. J. Pharm. Sci. Res. 2020, 11, 1327–1332. [Google Scholar]
- Plachouras, D.; Kärki, T.; Hansen, S.; Hopkins, S.; Lyytikäinen, O.; Moro, M.L.; Reilly, J.; Zarb, P.; Zingg, W.; Kinross, P.; et al. Antimicrobial use in European acute care hospitals: Results from the second point prevalence survey (PPS) of healthcare-associated infections and antimicrobial use, 2016 to 2017. Euro Surveill. 2018, 47, 1811222. [Google Scholar] [CrossRef]
- Apata, I.W.; Kabbani, S.; Neu, A.M.; Kear, T.M.; D’Agata, E.M.; Levenson, D.J.; Kliger, A.S.; Hicks, L.A.; Patel, P.R. Opportunities to improve antibiotic prescribing in outpatient hemodialysis facilities: A report from the American Society of Nephrology and Centers for Disease Control and Prevention Antibiotic Stewardship White Paper Writing Group. Am. J. Kidney Dis. 2021, 77, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; Edwards, J.R.; Beldavs, Z.G.; Dumyati, G.; Janelle, S.J.; Kainer, M.A.; Lynfield, R.; Nadle, J.; Neuhauser, M.M.; Susan, M.; et al. Ray Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA 2014, 312, 1438–1446. [Google Scholar] [CrossRef]
- Tham, D.W.J.; Abubakar, U.; Tangiisuran, B. Prevalence and predictors of antibiotic use among children visiting the Emergency Department in a Tertiary Hospital in Malaysia. Eur. J. Pediatr. 2020, 179, 743–748. [Google Scholar] [CrossRef]
- Shamas, N.; Khamis, F.; Eljaaly, K.; Al Salmi, Z.; Al Bahrani, M. Intermittent hemodialysis: A review of the top antimicrobial stewardship practices to be employed. Antimicrob. Steward. Healthc. Epidemiol. 2024, 4, e2. [Google Scholar] [CrossRef] [PubMed]
- Worth, L.J.; Spelman, T.; Holt, S.G.; Brett, J.A.; Bull, A.L.; Richards, M.J. Epidemiology of infections and antimicrobial use in Australian haemodialysis outpatients: Findings from a Victorian surveillance network, 2008–2015. J. Hosp. Infect. 2017, 97, 93–98. [Google Scholar] [CrossRef]
- AbuTaha, S.A.; Al-Kharraz, T.; Belkebir, S.; Taha, A.A.; Zyoud, H. Patterns of microbial resistance in bloodstream infections of hemodialysis patients: A cross-sectional study from Palestine. Sci. Rep. 2022, 12, 18003. [Google Scholar] [CrossRef]
- Snyder, G.M.; Patel, P.R.; Kallen, A.J.; Strom, J.A.; Tucker, J.K.; D’Agata, E.M. Factors associated with the receipt of antimicrobials among chronic hemodialysis patients. Am. J. Infect. Control. 2016, 44, 1269–1274. [Google Scholar] [CrossRef]
- Elshenawy, R.A.; Umaru, N.; Aslanpour, Z. WHO AWaRe classification for antibiotic stewardship: Tackling antimicrobial resistance–a descriptive study from an English NHS Foundation Trust prior to and during the COVID-19 pandemic. Front. Microbiol. 2023, 14, 1298858. [Google Scholar]
- Moja, L.; Zanichelli, V.; Mertz, D.; Gandra, S.; Cappello, B.; Cooke, G.S.; Chuki, P.; Harbarth, S.; Pulcini, C.; Mendelson, M.; et al. WHO′s essential medicines and AWaRe: Recommendations on first-and second-choice antibiotics for empiric treatment of clinical infections. Clin. Microbiol. Infect. 2024, 30, S1–S51. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, U.; Salman, M. Antibiotic use among hospitalized patients in Africa: A systematic review of point prevalence studies. J. Racial Ethn. Health Disparities 2024, 11, 1308–1329. [Google Scholar] [CrossRef]
- Pérez-Galera, S.; Bravo-Ferrer, J.M.; Paniagua, M.; Kostyanev, T.; de Kraker, M.E.; Feifel, J.; Sojo-Dorado, J.; Schotsman, J.; Cantón, R.; Daikos, G.L.; et al. Risk factors for infections caused by carbapenem-resistant Enterobacterales: An international matched case-control-control study (EURECA). EClinicalMedicine 2023, 57, 101871. [Google Scholar] [CrossRef]
- Zhou, H.; Buetti, N.; Pérez-Galera, S.; Bravo-Ferrer, J.; Gutiérrez-Gutiérrez, B.; Paniagua-García, M.; Feifel, J.; Sauser, J.; Kostyanev, T.; Canton, R.; et al. Risk factors for bloodstream infections due to carbapenem-resistant Enterobacterales: A nested case-control-control study. J. Antimicrob. Chemother. 2024, 79, 2132–2141. [Google Scholar]
- Palacios-Baena, Z.R.; Giannella, M.; Manissero, D.; Rodríguez-Baño, J.; Viale, P.; Lopes, S.; Wilson, K.; McCool, R.; Longshaw, C. Risk factors for carbapenem-resistant Gram-negative bacterial infections: A systematic review. Clin. Microbiol. Infect. 2021, 27, 228–235. [Google Scholar] [PubMed]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef]
- Centers for Diseases Control and Prevention (CDC). Core Elements of Hospital Antibiotic Stewardship Programs; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019; Available online: https://www.cdc.gov/antibiotic-use/hcp/core-elements/hospital.html (accessed on 8 March 2025).
- Sanchez Guillermo, V. Core elements of outpatient antibiotic stewardship. MMWR. Recomm. Rep. 2016, 65, 1–12. [Google Scholar] [CrossRef]
- Matusik, E.; Boidin, C.; Friggeri, A.; Richard, J.-C.; Bitker, L.; Roberts, J.A.; Goutelle, S. Therapeutic drug monitoring of antibiotic drugs in patients receiving continuous renal replacement therapy or intermittent hemodialysis: A critical review. Ther. Drug Monit. 2022, 44, 86–102. [Google Scholar] [CrossRef]
- Bogard, K.N.; Peterson, N.T.; Plumb, T.J.; Erwin, M.W.; Fuller, P.D.; Olsen, K.M. Antibiotic dosing during sustained low-efficiency dialysis: Special considerations in adult critically ill patients. Crit. Care Med. 2011, 39, 560–570. [Google Scholar] [CrossRef]
- Roberts, D.M.; Liu, X.; Roberts, J.A.; Nair, P.; Cole, L.; Roberts, M.S.; Lipman, J.; Bellomo, R.; RENAL Replacement Therapy Study Investigators. A multicenter study on the effect of continuous hemodiafiltration intensity on antibiotic pharmacokinetics. Crit. Care 2015, 19, 84. [Google Scholar] [CrossRef]
- Jiang, S.-P.; Xu, Y.-Y.; Yang, P.; Wu, W.-F.; Zhang, X.-G.; Lu, X.-Y.; Xiao, Y.-H.; Liang, W.-F.; Chen, J. Improving antimicrobial dosing in critically ill patients receiving continuous venovenous hemofiltration and the effect of pharmacist dosing adjustment. Eur. J. Intern. Med. 2014, 25, 930–935. [Google Scholar] [CrossRef]
- Abubakar, U.; Tangiisuran, B. Nationwide survey of pharmacists’ involvement in antimicrobial stewardship programs in Nigerian tertiary hospitals. J. Glob. Antimicrob. Resist. 2020, 21, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, U.; Sulaiman, S.A.; Usman, M.N.; Umar, M.D. Nigerian pharmacists′ self-perceived competence and confidence to plan and conduct pharmacy practice research. Pharm. Pract. 2018, 16, 1152. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Wong, T.; Romney, M.; Leung, V. Comparative effectiveness of β-lactam versus vancomycin empiric therapy in patients with methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 27. [Google Scholar] [CrossRef]
- McDanel, J.S.; Perencevich, E.N.; Diekema, D.J.; Herwaldt, L.A.; Smith, T.C.; Chrischilles, E.A.; Dawson, J.D.; Jiang, L.; Goto, M.; Schweizer, M.L. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin. Infect. Dis. 2015, 61, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, C.M.; Lindberg, C.C.; D’Agata, E.; Esposito, B.; Downham, G. Advancing Antimicrobial Stewardship in Outpatient Dialysis Centers Using the Positive Deviance Process. Nephrol. Nurs. J. 2019, 46, 511–518. [Google Scholar]
- D’Agata, E.M.; Lindberg, C.C.; Lindberg, C.M.; Downham, G.; Esposito, B.; Shemin, D.; Rosen, S. The positive effects of an antimicrobial stewardship program targeting outpatient hemodialysis facilities. Infect. Control. Hosp. Epidemiol. 2018, 39, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 29, 372. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
| Author and Year | Country | Study Design | Sample Size | Population | Outcome | Age (Mean ± SD) | Female Gender (%) | Dialysis Type | Dialysis Indication |
|---|---|---|---|---|---|---|---|---|---|
| Al Himali 2022 [26] | Oman | Retrospective cohort | n = 287 | Adult inpatients who received IHD or CVVHD who were admitted under any medical specialty and received at least one antibiotic | Antibiotic prescribing pattern and appropriateness | 58 ± 17 | 107/287 (37.3%) | IHD and (CVVH) | AKI 86/287 (30.0%) CKD 201/287 (70.0%) |
| Manley 2002 [16] | USA | Retrospective cohort | n = 161 | Adult patients who received dialysis at the outpatient hemodialysis center | Antibiotic prescribing pattern and appropriateness | 59.98 ± 14.65 § | 79/161 (49%) | HD | ESRD (100%) |
| Snyder 2013 [17] | USA | Ambispective cohort | n = 278 | Adult patients who received dialysis at either of the outpatient hemodialysis centers | Antibiotic prescribing pattern and appropriateness | 66.7 ± 15.5 | 134/278 (48.2%) ¶ | HD | ESRD (100%) |
| Zvonar 2008 [25] | Canada | Retrospective cohort | n = 105 | Chronic HD patients who received vancomycin at the outpatient hemodialysis center | Antibiotic prescribing pattern and appropriateness | NR | 44/105 (41.9%) ¶ | HD | ESRD (100%) |
| Udani 2013 [18] | USA | Retrospective cohort | n = 723 | Adult patients who received either IHD or CRRT in the ICU | Antibiotic prescribing pattern and appropriateness | 57.33 ± 14.87 § | 230/549 (42%) ¶ | IHD and (CVVH) | AKI 323/723 (58.8%) ESRD 226/723 (41.2%) |
| Fedorenko 2014 [19] | USA | Retrospective cohort | n = 42 | Adult patients receiving CVVHD with a minimum of 24 h of concomitant therapy with one or more IV study antimicrobials * | Antibiotic prescribing pattern and appropriateness | 58.4 ± 14.1 | 22/42 (52.3%) ¶ | IHD and CVVHD | CKD 6/56 (14%) ¶ AKI 36/56 (86%) |
| Harris 2013 [20] | USA | Retrospective cohort | n = 87 | Adult patients with ESRD or AKI who received SLED and at least one of the study antibiotics † | Antibiotic prescribing pattern and appropriateness | 54 ± 14 | 35/87 (40%) | SLED | AKI 61/87 (70%) ESRD 8/87 (9%) Unknown 18/87 (21%) |
| Keough 2018 [21] | USA | Retrospective cohort | n = 89 | Adult patients admitted to any one of the four adult acute care hospitals with either AKI or ESRD who received SLED for at least two sessions and at least one of the select study antibiotics for at least 5 days ‡ | Antibiotic prescribing pattern and appropriateness | 57 ± 14 | 21/87 (41%) | SLED | AKI 35/89 (69%) ESRD 16/89 (31%) |
| Coritsidis 2021 [22] | USA | Retrospective case-control | n = 18,402 | Adult patients with ESRD on HD and non-ESRD patients with at least one oral outpatient antibiotic prescription | Antibiotic prescribing pattern | 64.80 ± 14.80 | 8330/18,402 (45.30%) | HD | ESRD (100%) |
| Sivarajahkuma 2022 [24] | Canada | Retrospective case series | n = 53 | Adult patients who were receiving HD at the outpatient study unit, for whom at least one oral or IV antimicrobial was prescribed by a hospital or community prescriber | Antibiotic prescribing pattern | 61 ± 15 | 25/53 (47%) | HD | ESRD (100%) |
| Hui 2017 [15] | Australia | Prospective cohort | n = 114 | Adult patients receiving IHD in either inpatient or outpatient study units | Antibiotic prescribing pattern and appropriateness | 63.27 ± 18.17 § | 46/114 (40%) | IHD | ESRD (100%) |
| Saradhi 2020 [27] | India | Prospective cohort | n = 110 | Adult patients on IHD who have developed infections or were on antibiotics in either inpatient or outpatient study units | Antibiotic prescribing pattern and appropriateness | 49 ± 18 | 11/110 (37%) | IHD | ESRD (100%) |
| Green 2000 [23] | USA | Prospective cohort | n = 103 | All patients admitted to the study unit who required IHD | Antibiotic prescribing pattern and appropriateness | 56 ± 18 | 52/103 (50.5%) | IHD | ESRD (100%) |
| Variable | Al Himali et al., 2022 [26] | Manley et al., 2002 [16] | Snyder et al., 2013 [17] | Zvonar et al., 2008 [25] | Udani et al., 2013 [18] | Fedorenko et al., 2014 [19] | Harris et al., 2013 [20] | Keough et al., 2018 [21] | Coritsidis et al., 2021 [22] | Sivarajahkuma et al., 2022 [24] | Hui et al., 2017 [15] | Saradhi et al., 2020 [27] | Green et al., 2000 [23] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assessment tool | International guidelines | - | National guidelines | International guidelines + ID specialist | International guidelines + expert opinion (nephrologists and critical care pharmacist) | International guidelines | Literature-based recommendations | Literature-based recommendation | - | - | National and international guidelines + renal drug handbook + expert opinion | Micromedex software | International guidelines |
| Types of inappropriate use | Frequency (%) | ||||||||||||
| Overall inappropriateness | 204/691 (30%) | - | 276/926 (29.8%) | Empiric: 17/163 (10%) Definitive: 53/163 (33%) | 564/1761 (32.03%) * | 41/182 (22.5%) * | 66/115 (57.4%) * | 147/317 (46.4%) * | - | - | 55/224 (24.5%) | 23/35 (65.7%) | 33/164 (20%) |
| Breakdown of types of inappropriateness | |||||||||||||
| Dose | 97/204 (47.5%) | - | - | 239/564 (42.3%) | 41/41 (100%) | 66/66 (100%) | 125/147 (85%) | - | - | 14/55 (25.5%) | 21/23 (91.3%) * | - | |
| Interval | 107/204 (52.5%) | - | - | 325/564 (57.6%) | - | - | 22/147 (15%) | - | - | 29/55 (52.7%) | 2/23 (8.7%) * | - | |
| Duration | - | - | - | - | - | - | - | - | - | 6/55 (10.9%) | - | - | |
| Spectrum/choice | - | - | 74/276 (26.8%) | - | - | - | - | - | - | 13/55 (23.6%) | - | 23/33 (69.7%) | |
| Indication | - | - | 204/276 (73.9%) | - | - | - | - | - | - | 3/55 (5.5%) | - | 10/33 (30.3%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abul-Ola, S.; Alenany, R.; Abubakar, U. Prevalence and Types of Inappropriate Antibiotics Prescribing Among Dialysis Patients: A Systematic Review. Antibiotics 2025, 14, 1049. https://doi.org/10.3390/antibiotics14101049
Abul-Ola S, Alenany R, Abubakar U. Prevalence and Types of Inappropriate Antibiotics Prescribing Among Dialysis Patients: A Systematic Review. Antibiotics. 2025; 14(10):1049. https://doi.org/10.3390/antibiotics14101049
Chicago/Turabian StyleAbul-Ola, Sara, Reem Alenany, and Usman Abubakar. 2025. "Prevalence and Types of Inappropriate Antibiotics Prescribing Among Dialysis Patients: A Systematic Review" Antibiotics 14, no. 10: 1049. https://doi.org/10.3390/antibiotics14101049
APA StyleAbul-Ola, S., Alenany, R., & Abubakar, U. (2025). Prevalence and Types of Inappropriate Antibiotics Prescribing Among Dialysis Patients: A Systematic Review. Antibiotics, 14(10), 1049. https://doi.org/10.3390/antibiotics14101049