Preanalytical Stability of 13 Antibiotics in Biological Samples: A Crucial Factor for Therapeutic Drug Monitoring
Abstract
1. Introduction
2. Results
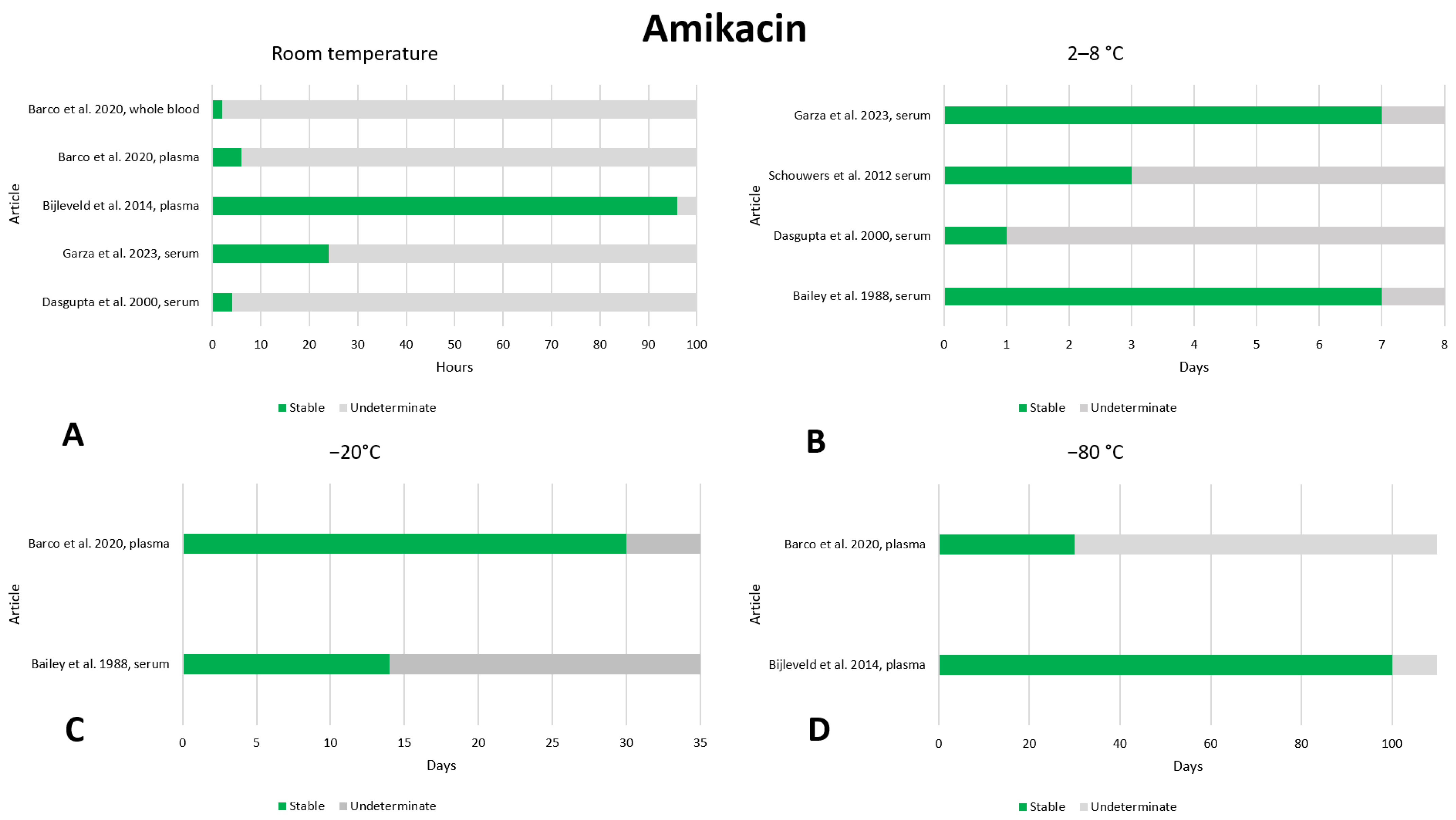
2.1. Amikacin
2.2. Ampicillin
2.3. Cefepime
2.4. Ceftazidime
2.5. Ciprofloxacin
2.6. Daptomycin
2.7. Gentamicin
2.8. Levofloxacin
2.9. Linezolid
2.10. Meropenem
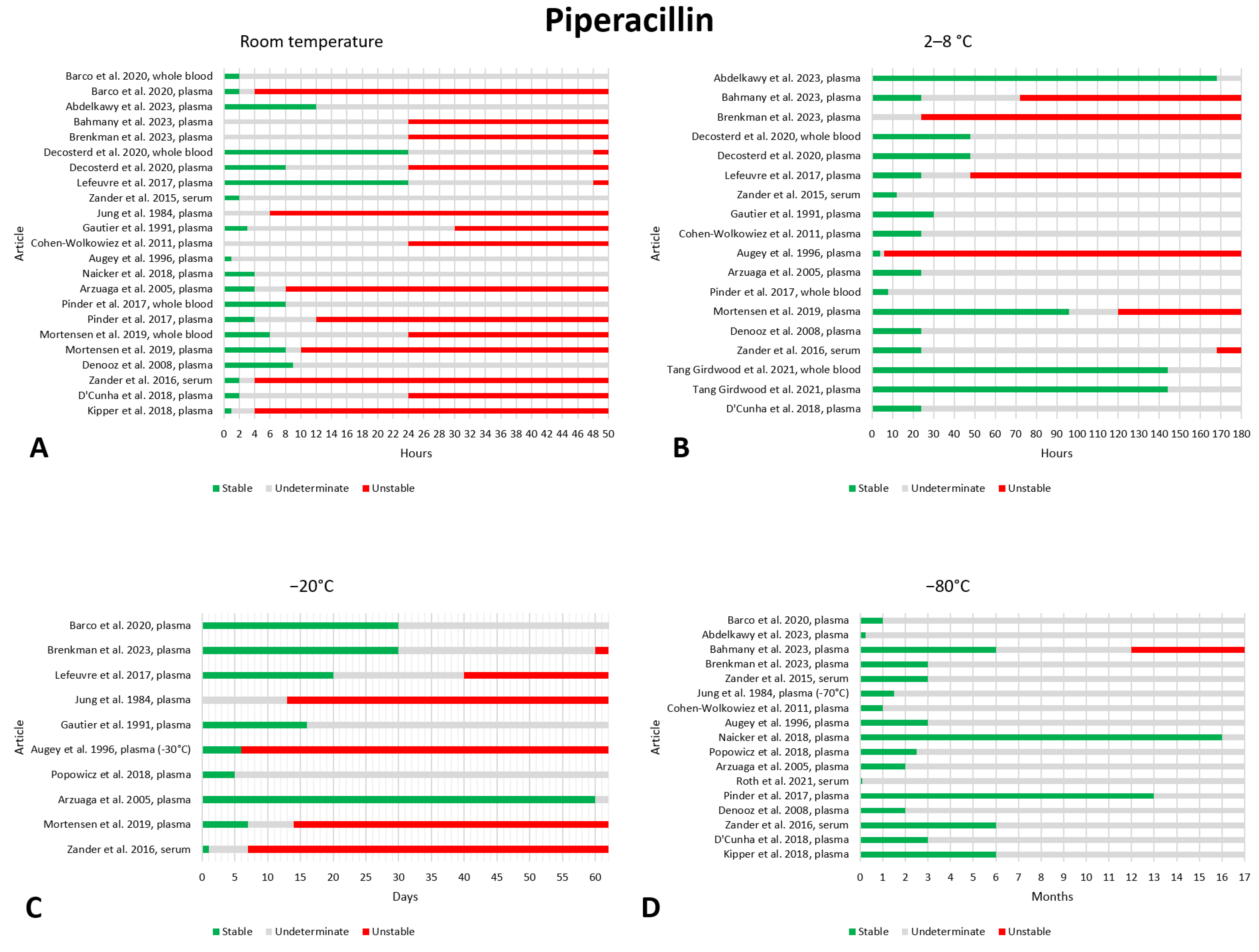
2.11. Piperacillin
2.12. Teicoplanin
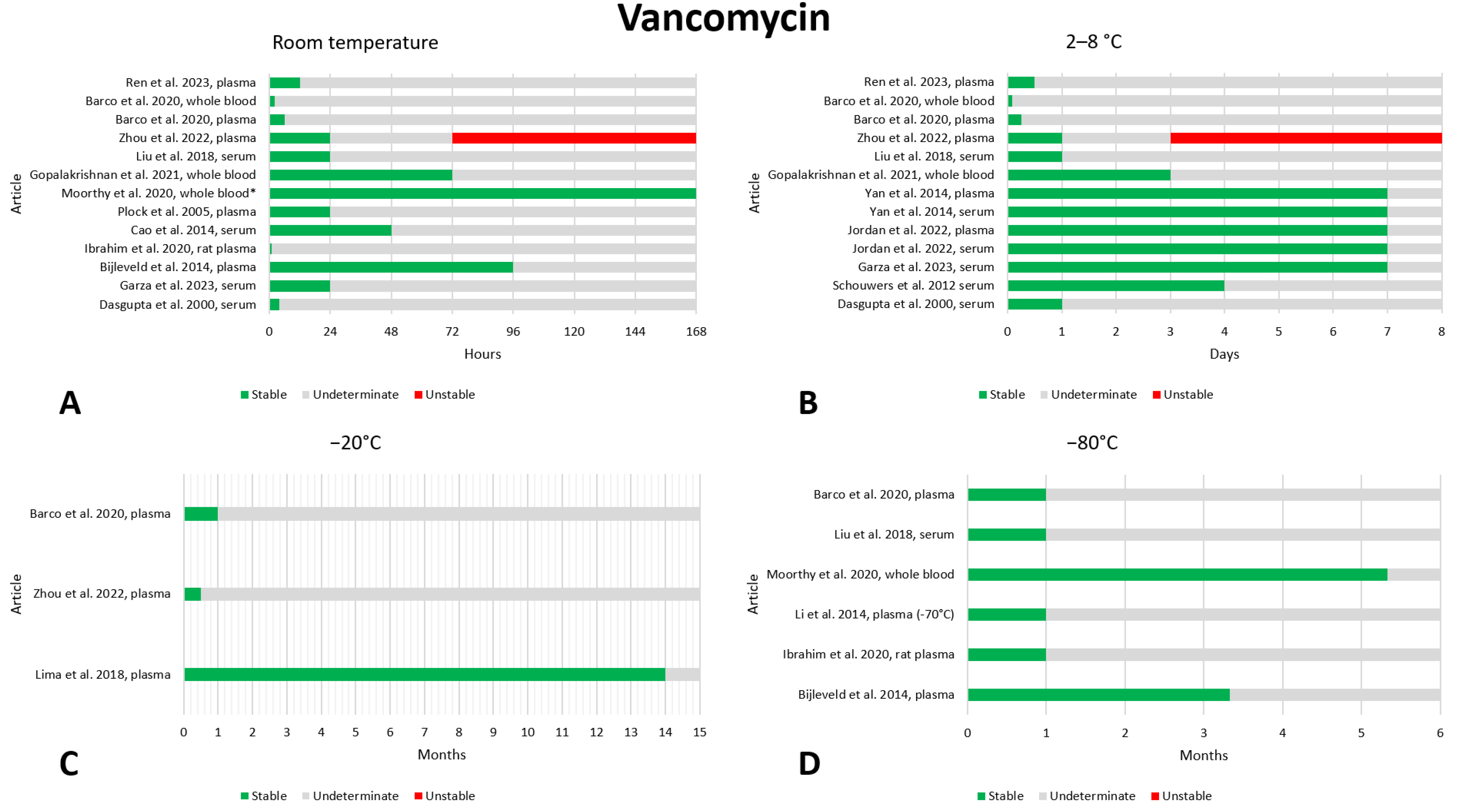
2.13. Vancomycin
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simeoli, R. Editorial: Therapeutic Drug Monitoring (TDM): A Useful Tool for Pediatric Pharmacology Applied to Routine Clinical Practice, Volume II. Front. Pharmacol. 2023, 14, 1250784. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial Therapeutic Drug Monitoring in Critically Ill Adult Patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef] [PubMed]
- Dario Cattaneo, C.G. Therapeutic Drug Monitoring. Giornale Italiano di Farmacoeconomia e Farmacoutilizzazione 2020, 12, 5–14. [Google Scholar]
- Sanz-Codina, M.; Bozkir, H.Ö.; Jorda, A.; Zeitlinger, M. Individualized Antimicrobial Dose Optimization: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Microbiol. Infect. 2023, 29, 845–857. [Google Scholar] [CrossRef]
- Telles, J.P.; Morales, R.; Yamada, C.H.; Marins, T.A.; D’Amaro Juodinis, V.; Sztajnbok, J.; Silva, M.; Bassetti, B.R.; Albiero, J.; Tuon, F.F. Optimization of Antimicrobial Stewardship Programs Using Therapeutic Drug Monitoring and Pharmacokinetics–Pharmacodynamics Protocols: A Cost-Benefit Review. Ther. Drug Monit. 2023, 45, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.S.; Isenschmid, D.S.; Smith, M.L. Drug Stability in Biological Specimens. In Principles of Forensic Toxicology; Levine, B.S., Kerrigan, S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 585–594. [Google Scholar] [CrossRef]
- Chen, J.; Hsieh, Y. Stabilizing Drug Molecules in Biological Samples. Ther. Drug Monit. 2005, 27, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Peters, F.T. Stability of Analytes in Biosamples—An Important Issue in Clinical and Forensic Toxicology? Anal. Bioanal. Chem. 2007, 388, 1505–1519. [Google Scholar] [CrossRef] [PubMed]
- Bahmany, S.; Ewoldt, T.M.J.; Abdulla, A.; Koch, B.C.P. Stability of 10 Beta-Lactam Antibiotics in Human Plasma at Different Storage Conditions. Ther. Drug Monit. 2023, 45, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Kipper, K.; Barker, C.I.S.; Standing, J.F.; Sharland, M.; Johnston, A. Development of a Novel Multipenicillin Assay and Assessment of the Impact of Analyte Degradation: Lessons for Scavenged Sampling in Antimicrobial Pharmacokinetic Study Design. Antimicrob. Agents Chemother. 2018, 62, e01540-17. [Google Scholar] [CrossRef]
- Saxer, C.; Niina, M.; Nakashima, A.; Nagae, Y.; Masuda, N. Simultaneous Determination of Levodopa and 3-O-Methyldopa in Human Plasma by Liquid Chromatography with Electrochemical Detection. J. Chromatogr. B 2004, 802, 299–305. [Google Scholar] [CrossRef]
- Jamieson, C.; Allwood, M.C.; Stonkute, D.; Wallace, A.; Wilkinson, A.-S.; Hills, T. Investigation of Meropenem Stability after Reconstitution: The Influence of Buffering and Challenges to Meet the NHS Yellow Cover Document Compliance for Continuous Infusions in an Outpatient Setting. Eur. J. Hosp. Pharm. 2020, 27, e53–e57. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Guideline M10 on Bioanalytical Method Validation and Study Sample Analysis. 2022. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m10-bioanalytical-method-validation-step-5_en.pdf (accessed on 15 December 2023).
- Mortensen, J.S.; Jensen, B.P.; Doogue, M. Preanalytical Stability of Flucloxacillin, Piperacillin, Tazobactam, Meropenem, Cefalexin, Cefazolin, and Ceftazidime in Therapeutic Drug Monitoring: A Structured Review. Ther. Drug Monit. 2022, 44, 709–719. [Google Scholar] [CrossRef]
- Bailey, D.N.; Coffee, J.J.; Briggs, J.R. Stability of Drug Concentrations in Plasma Stored in Serum Separator Blood Collection Tubes. Ther. Drug Monit. 1988, 10, 352–354. [Google Scholar] [CrossRef]
- Da Silva, A.C.C.; De Lima Feltraco Lizot, L.; Bastiani, M.F.; Venzon Antunes, M.; Brucker, N.; Linden, R. Dried Plasma Spots for Therapeutic Monitoring of Amikacin: Validation of an UHPLC-MS/MS Assay and Pharmacokinetic Application. J. Pharm. Biomed. Anal. 2020, 184, 113201. [Google Scholar] [CrossRef]
- Dasgupta, A.; Yared, M.A.; Wells, A. Time-Dependent Absorption of Therapeutic Drugs by the Gel of the Greiner Vacuette Blood Collection Tube. Ther. Drug Monit. 2000, 22, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Schouwers, S.; Brandt, I.; Willemse, J.; Van Regenmortel, N.; Uyttenbroeck, W.; Wauters, A.; Neels, H. Influence of Separator Gel in Sarstedt S-Monovette® Serum Tubes on Various Therapeutic Drugs, Hormones, and Proteins. Clin. Chim. Acta 2012, 413, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Garza, K.Y.; Carter, J.; Mercer, A.; Jarrar, P.; Martin, J.; Daughtry, S.; Mahomes, A.; Knezevic, C.E. Evaluation of Serum and Rapid Serum Separator Collection Tubes for Therapeutic Drug Assays. Clin. Biochem. 2023, 115, 81–85. [Google Scholar] [CrossRef]
- Bijleveld, Y.; De Haan, T.R.; Toersche, J.; Jorjani, S.; Van Der Lee, J.; Groenendaal, F.; Dijk, P.; Van Heijst, A.; Gavilanes, A.W.D.; De Jonge, R.; et al. A Simple Quantitative Method Analysing Amikacin, Gentamicin, and Vancomycin Levels in Human Newborn Plasma Using Ion-Pair Liquid Chromatography/Tandem Mass Spectrometry and Its Applicability to a Clinical Study. J. Chromatogr. B 2014, 951–952, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Barco, S.; Mesini, A.; Barbagallo, L.; Maffia, A.; Tripodi, G.; Pea, F.; Saffioti, C.; Castagnola, E.; Cangemi, G. A Liquid Chromatography-Tandem Mass Spectrometry Platform for the Routine Therapeutic Drug Monitoring of 14 Antibiotics: Application to Critically Ill Pediatric Patients. J. Pharm. Biomed. Anal. 2020, 186, 113273. [Google Scholar] [CrossRef]
- Do Nascimento, T.G.; Soares Aragao, C.F.; Dantas De Medeiros, F.; De Jesus Oliveira, E.; Oliveira Macedo, R. Validation of a Method for Determination of Ampicillin in Human Plasma Using LC-DAD. J. Chromatogr. Sci. 2009, 47, 749–755. [Google Scholar] [CrossRef]
- Do Nascimento, T.G.; De Jesus Oliveira, E.; Basílio Júnior, I.D.; De Araújo-Júnior, J.X.; Macêdo, R.O. Short-Term Stability Studies of Ampicillin and Cephalexin in Aqueous Solution and Human Plasma: Application of Least Squares Method in Arrhenius Equation. J. Pharm. Biomed. Anal. 2013, 73, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Wolkowiez, M.; White, N.R.; Bridges, A.; Benjamin, D.K.; Kashuba, A.D.M. Development of a Liquid Chromatography–Tandem Mass Spectrometry Assay of Six Antimicrobials in Plasma for Pharmacokinetic Studies in Premature Infants. J. Chromatogr. B 2011, 879, 3497–3506. [Google Scholar] [CrossRef]
- Moorthy, G.S.; Vedar, C.; Zane, N.R.; Downes, K.J.; Prodell, J.L.; DiLiberto, M.A.; Zuppa, A.F. Development and Validation of a Volumetric Absorptive Microsampling- Liquid Chromatography Mass Spectrometry Method for the Analysis of Cefepime in Human Whole Blood: Application to Pediatric Pharmacokinetic Study. J. Pharm. Biomed. Anal. 2020, 179, 113002. [Google Scholar] [CrossRef] [PubMed]
- D’Cunha, R.; Bach, T.; Young, B.A.; Li, P.; Nalbant, D.; Zhang, J.; Winokur, P.; An, G. Quantification of Cefepime, Meropenem, Piperacillin, and Tazobactam in Human Plasma Using a Sensitive and Robust Liquid Chromatography-Tandem Mass Spectrometry Method, Part 2: Stability Evaluation. Antimicrob. Agents Chemother. 2018, 62, e00861-18. [Google Scholar] [CrossRef]
- Cherti, N.; Kinowski, J.-M.; Lefrant, J.Y.; Bressolle, F. High-Performance Liquid Chromatographic Determination of Cefepime in Human Plasma and in Urine and Dialysis Fluid Using a Column-Switching Technique. J. Chromatogr. B Biomed. Sci. Appl. 2001, 754, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Nemutlu, E.; Kır, S.; Katlan, D.; Beksaç, M.S. Simultaneous Multiresponse Optimization of an HPLC Method to Separate Seven Cephalosporins in Plasma and Amniotic Fluid: Application to Validation and Quantification of Cefepime, Cefixime and Cefoperazone. Talanta 2009, 80, 117–126. [Google Scholar] [CrossRef]
- Tang Girdwood, S.C.; Tang, P.H.; Murphy, M.E.; Chamberlain, A.R.; Benken, L.A.; Jones, R.L.; Stoneman, E.M.; Kaplan, J.M.; Vinks, A.A. Demonstrating Feasibility of an Opportunistic Sampling Approach for Pharmacokinetic Studies of β-Lactam Antibiotics in Critically Ill Children. J. Clin. Pharma 2021, 61, 565–573. [Google Scholar] [CrossRef]
- Zander, J.; Maier, B.; Zoller, M.; Döbbeler, G.; Frey, L.; Teupser, D.; Vogeser, M. Effects of Biobanking Conditions on Six Antibiotic Substances in Human Serum Assessed by a Novel Evaluation Protocol. Clin. Chem. Lab. Med. (CCLM) 2016, 54, 265–274. [Google Scholar] [CrossRef]
- Elkhaïli, H.; Linger, L.; Monteil, H.; Jehl, F. High-Performance Liquid Chromatographic Assay for Cefepime in Serum. J. Chromatogr. B Biomed. Sci. Appl. 1997, 690, 181–188. [Google Scholar] [CrossRef]
- Denooz, R.; Charlier, C. Simultaneous Determination of Five β-Lactam Antibiotics (Cefepim, Ceftazidim, Cefuroxim, Meropenem and Piperacillin) in Human Plasma by High-Performance Liquid Chromatography with Ultraviolet Detection. J. Chromatogr. B 2008, 864, 161–167. [Google Scholar] [CrossRef]
- Zander, J.; Maier, B.; Suhr, A.; Zoller, M.; Frey, L.; Teupser, D.; Vogeser, M. Quantification of Piperacillin, Tazobactam, Cefepime, Meropenem, Ciprofloxacin and Linezolid in Serum Using an Isotope Dilution UHPLC-MS/MS Method with Semi-Automated Sample Preparation. Clin. Chem. Lab. Med. (CCLM) 2015, 53, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Lefeuvre, S.; Bois-Maublanc, J.; Hocqueloux, L.; Bret, L.; Francia, T.; Eleout-Da Violante, C.; Billaud, E.M.; Barbier, F.; Got, L. A Simple Ultra-High-Performance Liquid Chromatography-High Resolution Mass Spectrometry Assay for the Simultaneous Quantification of 15 Antibiotics in Plasma. J. Chromatogr. B 2017, 1065–1066, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Isla, A.; Arzuaga, A.; Maynar, J.; Gascón, A.R.; Solinís, M.A.; Corral, E.; Pedraz, J.L. Determination of Ceftazidime and Cefepime in Plasma and Dialysate-Ultrafiltrate from Patients Undergoing Continuous Veno-Venous Hemodiafiltration by HPLC. J. Pharm. Biomed. Anal. 2005, 39, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Decosterd, L.A.; Mercier, T.; Ternon, B.; Cruchon, S.; Guignard, N.; Lahrichi, S.; Pesse, B.; Rochat, B.; Burger, R.; Lamoth, F.; et al. Validation and Clinical Application of a Multiplex High Performance Liquid Chromatography—Tandem Mass Spectrometry Assay for the Monitoring of Plasma Concentrations of 12 Antibiotics in Patients with Severe Bacterial Infections. J. Chromatogr. B 2020, 1157, 122160. [Google Scholar] [CrossRef] [PubMed]
- Brenkman, M.; Cartau, T.; Pape, E.; Kolodziej, A.; Charmillon, A.; Novy, E.; Jouzeau, J.; Gambier, N.; Scala-Bertola, J. In Vitro Stability Study of 10 Beta-lactam Antibiotics in Human Plasma Samples. Fundam. Clin. Pharma 2023, 38, 502–510. [Google Scholar] [CrossRef]
- Martens-Lobenhoffer, J.; Angermair, S.; Bode-Böger, S.M. Quantification of Ceftazidime/Avibactam in Human Plasma and Dried Blood Spots: Implications on Stability and Sample Transport. J. Chromatogr. B 2022, 1193, 123164. [Google Scholar] [CrossRef] [PubMed]
- Rigo-Bonnin, R.; Cobo-Sacristán, S.; Padullés, A.; Ribera, A.; Arbiol-Roca, A.; Murillo, Ó.; Sabater-Riera, J.; Alía, P. Measurement of Ceftazidime Concentration in Human Plasma by Ultra-performance Liquid Chromatography–Tandem Mass Spectrometry. Application to Critically Ill Patients and Patients with Osteoarticular Infections. Biomed. Chromatogr. 2016, 30, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, M.-E.; Gangl, E.T. Bioanalytical Method Validation for the Simultaneous Determination of Ceftazidime and Avibactam in Rat Plasma. Bioanalysis 2016, 8, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Pinder, N.; Brenner, T.; Swoboda, S.; Weigand, M.A.; Hoppe-Tichy, T. Therapeutic Drug Monitoring of Beta-Lactam Antibiotics—Influence of Sample Stability on the Analysis of Piperacillin, Meropenem, Ceftazidime and Flucloxacillin by HPLC-UV. J. Pharm. Biomed. Anal. 2017, 143, 86–93. [Google Scholar] [CrossRef]
- Roth, T.; Weber, L.; Niestroj, M.; Cipa, F.; Löscher, A.; Mihai, S.; Parsch, H. Simultaneous Determination of Six Antibiotics in Human Serum by High-performance Liquid Chromatography with UV Detection. Biomed. Chromatogr. 2021, 35, e5010. [Google Scholar] [CrossRef]
- Humbert, T.; Rümelin, A.; Fauth, U. Ceftazidime Determination in Serum by High-Pressure Liquid Chromatography. Arzneimittelforschung 2011, 54, 320–322. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, J.S.; Jensen, B.P.; Zhang, M.; Doogue, M. Preanalytical Stability of Piperacillin, Tazobactam, Meropenem, and Ceftazidime in Plasma and Whole Blood Using Liquid Chromatography–Tandem Mass Spectrometry. Ther. Drug Monit. 2019, 41, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.; Gorain, B.; Paul, A.; Sarkar, P.; Dan, S.; Chakraborty, P.; Pal, T. Development and Validation of an LC-MS/MS-ESI Method for Comparative Pharmacokinetic Study of Ciprofloxacin in Healthy Male Subjects. Drug Res. 2016, 67, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Muchohi, S.N.; Thuo, N.; Karisa, J.; Muturi, A.; Kokwaro, G.O.; Maitland, K. Determination of Ciprofloxacin in Human Plasma Using High-Performance Liquid Chromatography Coupled with Fluorescence Detection: Application to a Population Pharmacokinetics Study in Children with Severe Malnutrition. J. Chromatogr. B 2011, 879, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Watabe, S.; Yokoyama, Y.; Nakazawa, K.; Shinozaki, K.; Hiraoka, R.; Takeshita, K.; Suzuki, Y. Simultaneous Measurement of Pazufloxacin, Ciprofloxacin, and Levofloxacin in Human Serum by High-Performance Liquid Chromatography with Fluorescence Detection. J. Chromatogr. B 2010, 878, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.; Alves, G.; Fortuna, A.; Pena, A.; Lino, C.; Falcão, A. Development and Validation of a Fast Isocratic Liquid Chromatography Method for the Simultaneous Determination of Norfloxacin, Lomefloxacin and Ciprofloxacin in Human Plasma. Biomed. Chromatogr. 2011, 25, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Van Geijlswijk, I.M.; Van Zanten, A.R.H.; Geert Van Der Meer, Y. Reliable New High-Performance Liquid Chromatographic Method for the Determination of Ciprofloxacin in Human Serum. Ther. Drug Monit. 2006, 28, 278–281. [Google Scholar] [CrossRef]
- Cavazos-Rocha, N.; Carmona-Alvarado, I.; Vera-Cabrera, L.; Waksman-de-Torres, N.; Salazar-Cavazos, M.D.L.L. HPLC Method for the Simultaneous Analysis of Fluoroquinolones and Oxazolidinones in Plasma. J. Chromatogr. Sci. 2014, 52, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Sasongko, L.; Pratiwi, G.K.; Leo, M.; Adiwidjaja, J. Simultaneous HPLC Assay of Gliclazide and Ciprofloxacin in Plasma and Its Implementation for Pharmacokinetic Study in Rats. J. Chromatogr. Sci. 2021, 59, 338–346. [Google Scholar] [CrossRef]
- Yıldırım, S.; Karakoç, H.N.; Yaşar, A.; Köksal, İ. Determination of Levofloxacin, Ciprofloxacin, Moxifloxacin and Gemifloxacin in Urine and Plasma by HPLC–FLD–DAD Using Pentafluorophenyl Core–Shell Column: Application to Drug Monitoring. Biomed. Chromatogr. 2020, 34, e4925. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Z.; Lui, G.; Hirt, D.; Treluyer, J.; Benaboud, S.; Aboura, R.; Gana, I. Simultaneous Quantification of Levofloxacin, Pefloxacin, Ciprofloxacin and Moxifloxacin in Microvolumes of Human Plasma Using High-performance Liquid Chromatography with Ultraviolet Detection. Biomed. Chromatogr. 2019, 33, e4506. [Google Scholar] [CrossRef]
- Grondin, C.; Zhao, W.; Fakhoury, M.; Jacqz-Aigrain, E. Determination of Ciprofloxacin in Plasma by Micro-liquid Chromatography–Mass Spectrometry: An Adapted Method for Neonates. Biomed. Chromatogr. 2011, 25, 827–832. [Google Scholar] [CrossRef]
- Zimmermann, E.S.; Torres, B.G.S.; Dalla Costa, T. Validation of a Sensitive HPLC/Fluorescence Method for Assessment of Ciprofloxacin Levels in Plasma and Prostate Microdialysate Samples from Rats. Biomed. Chromatogr. 2016, 30, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Jourdil, J.-F.; Tonini, J.; Stanke-Labesque, F. Simultaneous Quantitation of Azole Antifungals, Antibiotics, Imatinib, and Raltegravir in Human Plasma by Two-Dimensional High-Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. B 2013, 919–920, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jim, L.K.; El-Sayed, N.; Al-Khamis, K.I. A Simple High-Performance Liquid Chromatographic Assay for Ciprofloxacin in Human Serum. J. Clin. Pharm. Ther. 1992, 17, 111–115. [Google Scholar] [CrossRef]
- Gikas, E.; Bazoti, F.N.; Fanourgiakis, P.; Perivolioti, E.; Roussidis, A.; Skoutelis, A.; Tsarbopoulos, A. Development and Validation of a UPLC-UV Method for the Determination of Daptomycin in Rabbit Plasma. Biomed. Chromatogr. 2010, 24, 522–527. [Google Scholar] [CrossRef]
- Miyadera, Y.; Naito, T.; Yamada, T.; Kawakami, J. Simple LC-MS/MS Methods Using Core–Shell Octadecylsilyl Microparticulate for the Quantitation of Total and Free Daptomycin in Human Plasma. Ther. Drug Monit. 2018, 40, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Baietto, L.; D’Avolio, A.; Pace, S.; Simiele, M.; Marra, C.; Ariaudo, A.; Di Perri, G.; Rosa, F.G.D. Development and Validation of an UPLC–PDA Method to Quantify Daptomycin in Human Plasma and in Dried Plasma Spots. J. Pharm. Biomed. Anal. 2014, 88, 66–70. [Google Scholar] [CrossRef]
- Martenslobenhoffer, J.; Kielstein, J.; Oye, C.; Bodeboger, S. Validated High Performance Liquid Chromatography–UV Detection Method for the Determination of Daptomycin in Human Plasma. J. Chromatogr. B 2008, 875, 546–550. [Google Scholar] [CrossRef]
- Jones, S.M.; Blazevic, D.J.; Balfour, H.H. Stability of Gentamicin in Serum. Antimicrob. Agents Chemother. 1976, 10, 866–867. [Google Scholar] [CrossRef][Green Version]
- Ibrahim, F.; Elgawish, M.S.; Mehana, E.; El-Adl, S.M.; Baraka, M.M.; Ibrahim, S.M.; Sebaiy, M.M. Toxicity Profile and Pharmacokinetic Study of Antibiotic Mixtures, Gentamicin and Vancomycin, in Rat Plasma by Ecofriendly Liquid Chromatography Coupled Tandem Mass Spectrometry. Chem. Res. Toxicol. 2020, 33, 2647–2658. [Google Scholar] [CrossRef] [PubMed]
- Sieradzan, R.P.; Boehler, R.G. Gentamicin Stability with Ciprofloxacin in Human Serum. Drug Intell. Clin. Pharm. 1988, 22, 506–507. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Sherazi, A.; Stevens, A.; Quondam Franks, C.; Sturgeon, K.; Northrup, V.; Shea, J.L. Evaluation of BD BarricorTM and PSTTM Blood Collection Tubes Compared to Serum for Testing 11 Therapeutic Drugs on a Roche Cobas® 8000 Platform. Clin. Biochem. 2022, 100, 60–66. [Google Scholar] [CrossRef]
- Yan, R.; Colantonio, D.; Wong, P.-Y.; Chen, Y. Suitability of Becton Dickinson Vacutainer Rapid Serum Tube for Collecting and Storing Blood Samples for Antibiotic and Anticonvulsant Drug Monitoring. J. Clin. Pathol. 2014, 67, 807–810. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, T.D.; Ratcliff, R.M.; Geary, T.D. Evaluation of an Enzyme Immunoassay for Serum Gentamicin. Antimicrob. Agents Chemother. 1980, 17, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Jongedijk, E.M.; Hu, Y.; Kuhlin, J.; Zheng, R.; Niward, K.; Paues, J.; Xu, B.; Davies Forsman, L.; Schön, T.; et al. Development and Validation of a Simple LC-MS/MS Method for Simultaneous Determination of Moxifloxacin, Levofloxacin, Prothionamide, Pyrazinamide and Ethambutol in Human Plasma. J. Chromatogr. B 2020, 1158, 122397. [Google Scholar] [CrossRef] [PubMed]
- Van Toi, P.; Pouplin, T.; Tho, N.D.K.; Phuong, P.N.; Chau, T.T.H.; Thuong Thuong, N.T.; Heemskerk, D.; Hien, T.T.; Thwaites, G.E. High-Performance Liquid Chromatography with Time-Programmed Fluorescence Detection for the Quantification of Levofloxacin in Human Plasma and Cerebrospinal Fluid in Adults with Tuberculous Meningitis. J. Chromatogr. B 2017, 1061–1062, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.; Alves, G.; Campos, G.; Fortuna, A.; Falcão, A. First Liquid Chromatography Method for the Simultaneous Determination of Levofloxacin, Pazufloxacin, Gatifloxacin, Moxifloxacin and Trovafloxacin in Human Plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 930, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.A.; Juzwin, S.J.; Flor, S.C. Rapid Stereospecific High-Performance Liquid Chromatographic Determination of Levofloxacin in Human Plasma and Urine. J. Pharm. Biomed. Anal. 1997, 15, 765–771. [Google Scholar] [CrossRef]
- Lei, Q.; Zhao, Y.; Wang, H.; Zhou, J.; Lv, X.; Dang, L.; Zhu, C. Simple and Sensitive Method for the Analysis of 14 Antituberculosis Drugs Using Liquid Chromatography/Tandem Mass Spectrometry in Human Plasma. Rapid Commun. Mass. Spectrom. 2020, 34, e8667. [Google Scholar] [CrossRef]
- Zhou, Z.-L.; Yang, M.; Yu, X.-Y.; Peng, H.-Y.; Shan, Z.-X.; Chen, S.-Z.; Lin, Q.-X.; Liu, X.-Y.; Chen, T.-F.; Zhou, S.-F.; et al. A Rapid and Simple High-Performance Liquid Chromatography Method for the Determination of Human Plasma Levofloxacin Concentration and Its Application to Bioequivalence Studies. Biomed. Chromatogr. 2007, 21, 1045–1051. [Google Scholar] [CrossRef]
- Fang, P.-F.; Cai, H.-L.; Li, H.-D.; Zhu, R.-H.; Tan, Q.-Y.; Gao, W.; Xu, P.; Liu, Y.-P.; Zhang, W.-Y.; Chen, Y.-C.; et al. Simultaneous Determination of Isoniazid, Rifampicin, Levofloxacin in Mouse Tissues and Plasma by High Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 2286–2291. [Google Scholar] [CrossRef] [PubMed]
- Cavazos-Rocha, N.; Vera-Cabrera, L.; Welsh-Lozano, O.; Waksman-de-Torres, N.; De La Luz Salazar-Cavazos, M. Simultaneous Determination and Validation of Antimicrobials in Plasma and Tissue of Actinomycetoma by High-Performance Liquid Chromatography with Diode Array and Fluorescence Detection. J. Pharm. Biomed. Anal. 2007, 43, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.H.; Bolhuis, M.S.; Koster, R.A.; Greijdanus, B.; De Lange, W.C.M.; Van Altena, R.; Brouwers, J.R.B.J.; Uges, D.R.A.; Alffenaar, J.W.C. Dried Blood Spot Analysis for Therapeutic Drug Monitoring of Linezolid in Patients with Multidrug-Resistant Tuberculosis. Antimicrob. Agents Chemother. 2012, 56, 5758–5763. [Google Scholar] [CrossRef]
- Sakurai, N.; Nakamura, Y.; Kawaguchi, H.; Abe, J.; Yamada, K.; Nagayama, K.; Kakeya, H. Measurement of Linezolid and Its Metabolites PNU-142300 and PNU-142586 in Human Plasma Using Ultra-Performance Liquid Chromatography Method. Chem. Pharm. Bull. 2019, 67, 439–444. [Google Scholar] [CrossRef]
- Cios, A.; Kuś, K.; Szymura-Oleksiak, J. Determination of Linezolid in Human Serum by Reversed-Phase High-Performance Liquid Chromatography with Ultraviolet and Diode Array Detection. Acta Pol. Pharm. 2013, 70, 631–641. [Google Scholar]
- La Marca, G.; Villanelli, F.; Malvagia, S.; Ombrone, D.; Funghini, S.; De Gaudio, M.; Fallani, S.; Cassetta, M.I.; Novelli, A.; Chiappini, E.; et al. Rapid and Sensitive LC–MS/MS Method for the Analysis of Antibiotic Linezolid on Dried Blood Spot. J. Pharm. Biomed. Anal. 2012, 67–68, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Pan, C.; Xie, Q.; Zheng, Y.; Hu, Y.; Lin, Y. Simultaneous Determination of Tedizolid and Linezolid in Rat Plasma by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry and Its Application to a Pharmacokinetic Study. J. Chromatogr. B 2016, 1011, 94–98. [Google Scholar] [CrossRef]
- Boak, L.M.; Li, J.; Nation, R.L.; Rayner, C.R. High-Performance Liquid Chromatographic Method for Simple and Rapid Determination of Linezolid in Human Plasma. Biomed. Chromatogr. 2006, 20, 782–786. [Google Scholar] [CrossRef]
- Tobin, C.M. A Simple, Isocratic High-Performance Liquid Chromatography Assay for Linezolid in Human Serum. J. Antimicrob. Chemother. 2001, 48, 605–608. [Google Scholar] [CrossRef]
- Mohammed, S.A.; Eissa, M.S.; Ahmed, H.M. Simple Protein Precipitation Extraction Technique Followed by Validated Chromatographic Method for Linezolid Analysis in Real Human Plasma Samples to Study Its Pharmacokinetics. J. Chromatogr. B 2017, 1043, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Berska, J.; Bugajska, J.; Sztefko, K. A Liquid Chromatography-Tandem Mass Spectrometry Method for Simultaneously Determining Meropenem and Linezolid in Blood and Cerebrospinal Fluid. Ann. Lab. Med. 2024, 44, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Deng, Y.; Liu, X.; Liu, Z.; Huang, C.; Miao, L.; Zhang, C. Therapeutic Drug Monitoring Status of Four Common Antibiotics: Vancomycin, Meropenem, Linezolid and Teicoplanin. Scand. J. Clin. Lab. Investig. 2022, 82, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Elkhaïli, H.; Niedergang, S.; Pompei, D.; Linger, L.; Leveque, D.; Jehl, F. High-Performance Liquid Chromatographic Assay for Meropenem in Serum. J. Chromatogr. B Biomed. Sci. Appl. 1996, 686, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, X.; Dang, H. Simultaneous Determination of Meropenem and Imipenem in Rat Plasma by LC–MS/MS and Its Application to a Pharmacokinetic Study. Biomed. Chromatogr. 2021, 35, e5185. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, C.A.; Nicolau, D.P. Development of an HPLC Method for the Determination of Meropenem/Vaborbactam in Biological and Aqueous Matrixes. J. Chromatogr. Sci. 2020, 58, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Jiang, Y.; Wang, S.; Cao, H.; Li, Y.; Huang, J. Dried Plasma Spot Based LC–MS/MS Method for Monitoring of Meropenem in the Blood of Treated Patients. Molecules 2022, 27, 1991. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Meng, F.; Hu, L.; Huang, Q.; Liu, M.; Yin, T. A Novel Reversed-Phase High-Performance Liquid Chromatographic Assay for the Simultaneous Determination of Imipenem and Meropenem in Human Plasma and Its Application in TDM. J. Pharm. Biomed. Anal. 2019, 169, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Robatel, C.; Buclin, T.; Eckert, P.; Schaller, M.D.; Biollaz, J.; Decosterd, L.A. Determination of Meropenem in Plasma and Filtrate-Dialysate from Patients under Continuous Veno-Venous Haemodiafiltration by SPE-LC. J. Pharm. Biomed. Anal. 2002, 29, 17–33. [Google Scholar] [CrossRef]
- Chaursia, B.; Singh, T.; Varshney, B.; Sharma, P.; Iyer, S.; Khuroo, A.; Monif, T. Development and Validation of Liquid Chromato graphy-Tandem Mass Spectrometric Method for Quantification of Meropenem in Rat Plasma and Its Application in a Preclinical Dose Proportionality Study. Drug Res. 2013, 64, 321–329. [Google Scholar] [CrossRef]
- Ahsman, M.J.; Wildschut, E.D.; Tibboel, D.; Mathot, R.A. Microanalysis of β-Lactam Antibiotics and Vancomycin in Plasma for Pharmacokinetic Studies in Neonates. Antimicrob. Agents Chemother. 2009, 53, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Legrand, T.; Chhun, S.; Rey, E.; Blanchet, B.; Zahar, J.; Lanternier, F.; Pons, G.; Jullien, V. Simultaneous Determination of Three Carbapenem Antibiotics in Plasma by HPLC with Ultraviolet Detection. J. Chromatogr. B 2008, 875, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Martens-Lobenhoffer, J.; Bode-Böger, S.M. Quantification of Meropenem in Human Plasma by HILIC—Tandem Mass Spectrometry. J. Chromatogr. B 2017, 1046, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Martens-Lobenhoffer, J.; Monastyrski, D.; Tröger, U.; Bode-Böger, S.M. Stability of Meropenem in Plasma versus Dried Blood Spots (DBS). J. Pharm. Biomed. Anal. 2019, 170, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Dailly, E.; Bouquié, R.; Deslandes, G.; Jolliet, P.; Le Floch, R. A Liquid Chromatography Assay for a Quantification of Doripenem, Ertapenem, Imipenem, Meropenem Concentrations in Human Plasma: Application to a Clinical Pharmacokinetic Study. J. Chromatogr. B 2011, 879, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, J.; He, J.; Zhang, S.; Liu, D.; Shao, H. Quantitation of Meropenem in Dried Blood Spots Using Microfluidic-Based Volumetric Sampling Coupled with LC-MS/MS Bioanalysis in Preterm Neonates. J. Chromatogr. B 2023, 1217, 123625. [Google Scholar] [CrossRef] [PubMed]
- Arzuaga, A.; Isla, A.; Gascón, A.R.; Maynar, J.; Martín, A.; Solinís, M.A.; Toral, D.; Pedraz, J.L. Quantitation and Stability of Piperacillin and Tazobactam in Plasma and Ultrafiltrate from Patients Undergoing Continuous Venovenous Hemofiltration by HPLC. Biomed. Chromatogr. 2005, 19, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Popowicz, N.D.; O’Halloran, S.J.; Fitzgerald, D.; Lee, Y.C.G.; Joyce, D.A. A Rapid, LC-MS/MS Assay for Quantification of Piperacillin and Tazobactam in Human Plasma and Pleural Fluid; Application to a Clinical Pharmacokinetic Study. J. Chromatogr. B 2018, 1081–1082, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Barco, S.; Risso, F.M.; Bruschettini, M.; Bandettini, R.; Ramenghi, L.A.; Tripodi, G.; Castagnola, E.; Cangemi, G. A Validated LC–MS/MS Method for the Quantification of Piperacillin/Tazobactam on Dried Blood Spot. Bioanalysis 2014, 6, 2795–2802. [Google Scholar] [CrossRef]
- Naicker, S.; Guerra Valero, Y.C.; Ordenez Meija, J.L.; Lipman, J.; Roberts, J.A.; Wallis, S.C.; Parker, S.L. A UHPLC–MS/MS Method for the Simultaneous Determination of Piperacillin and Tazobactam in Plasma (Total and Unbound), Urine and Renal Replacement Therapy Effluent. J. Pharm. Biomed. Anal. 2018, 148, 324–333. [Google Scholar] [CrossRef]
- Augey, V.; Grosse, P.-Y.; Albert, G.; Audran, M.; Bressolle, F. High-Performance Liquid Chromatographic Determination of Tazobactam and Piperacillin in Human Plasma and Urine. J. Chromatogr. B Biomed. Sci. Appl. 1996, 682, 125–136. [Google Scholar] [CrossRef]
- Gautier, V.; Demotes-Mainard, F.; Foureau, M.; Vinçon, G. Micro-Method for the Determination of Piperacillin in Plasma by High-Performance Liquid Chromatography. J. Pharm. Biomed. Anal. 1991, 9, 183–186. [Google Scholar] [CrossRef]
- Jung, D.; Mahajan, N.K. An Improved Micro-Scale Liquid-Chromatographic Assay for Piperacillin in Plasma and Urine. Clin. Chem. 1984, 30, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Abdelkawy, K.; Le, T.; Mahmoud, S.H. Simple HPLC-UV Method for Piperacillin/Tazobactam Assay in Human Plasma. Antibiotics 2023, 12, 321. [Google Scholar] [CrossRef]
- Rybak, M.J.; Bailey, E.M.; Reddy, V.N. Clinical Evaluation of Teicoplanin Fluorescence Polarization Immunoassay. Antimicrob. Agents Chemother. 1991, 35, 1586–1590. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, J.; Chung, E.-K.; Kang, S.-W.; Lee, H.-J.; Rhie, S.-J. Quantification of Teicoplanin Using the HPLC-UV Method for Clinical Applications in Critically Ill Patients in Korea. Pharmaceutics 2021, 13, 572. [Google Scholar] [CrossRef]
- Li, X.; Wang, F.; Xu, B.; Yu, X.; Yang, Y.; Zhang, L.; Li, H. Determination of the Free and Total Concentrations of Vancomycin by Two-Dimensional Liquid Chromatography and Its Application in Elderly Patients. J. Chromatogr. B 2014, 969, 181–189. [Google Scholar] [CrossRef]
- Plock, N.; Buerger, C.; Kloft, C. Successful Management of Discovered pH Dependence in Vancomycin Recovery Studies: Novel HPLC Method for Microdialysis and Plasma Samples. Biomed. Chromatogr. 2005, 19, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, G.S.; Downes, K.J.; Vedar, C.; Zuppa, A.F. A Whole Blood Microsampling Assay for Vancomycin: Development, Validation and Application for Pediatric Clinical Study. Bioanalysis 2020, 12, 1295–1310. [Google Scholar] [CrossRef]
- Lima, T.D.M.; Seba, K.S.; Gonçalves, J.C.S.; Cardoso, F.L.L.; Estrela, R.D.C.E. A Rapid and Simple HPLC Method for Therapeutic Monitoring of Vancomycin. J. Chromatogr. Sci. 2018, 56, 115–121. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.V.; Sunish, S.C.; Mathew, B.S.; Prabha, R.; Mathew, S.K. Stability of Vancomycin in Whole Blood. Ther. Drug Monit. 2021, 43, 443–444. [Google Scholar] [CrossRef]
- Liu, M.; Yang, Z.-H.; Li, G.-H. A Novel Method for the Determination of Vancomycin in Serum by High-Performance Liquid Chromatography-Tandem Mass Spectrometry and Its Application in Patients with Diabetic Foot Infections. Molecules 2018, 23, 2939. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Liu, Y.; Li, S.; Li, X.; Wu, X.; Li, Y.; Zhang, Z. Therapeutic Drug Monitoring of Free Vancomycin Concentration in Practice: A New Analytical Technique Based on the HFCF–UF Sample Separation Method. Biomed. Chromatogr. 2023, 37, e5559. [Google Scholar] [CrossRef]
- Cao, Y.; Yu, J.; Chen, Y.; Zhang, J.; Wu, X.; Zhang, Y.; Li, G. Development and Validation of a New Ultra-Performance Liquid Chromatographic Method for Vancomycin Assay in Serum and Its Application to Therapeutic Drug Monitoring. Ther. Drug Monit. 2014, 36, 175–181. [Google Scholar] [CrossRef]
- Reed, G.A. Stability of Drugs, Drug Candidates, and Metabolites in Blood and Plasma. CP Pharmacol. 2016, 75. [Google Scholar] [CrossRef]
- Deshpande, A.D.; Baheti, K.G.; Chatterjee, N.R. Degradation of β-Lactam Antibiotics. Curr. Sci. 2004, 87, 1684–1695. Available online: https://www.jstor.org/stable/24109765 (accessed on 15 June 2024).
- Hubicka, U.; Krzek, J.; Walczak, M. Stability of Ciprofloxacin and Norfloxacin in the Presence and Absence of Metal Ions in Acidic Solution. Pharm. Dev. Technol. 2010, 15, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Love, I.; Smith, G.T.; Hill, H.M. Assessment of Whole Blood Stability and Blood/Plasma Distribution of Drugs. In Handbook of LC-MS Bioanalysis; Li, W., Zhang, J., Tse, F.L.S., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 127–138. [Google Scholar] [CrossRef]
- Zailani, N.N.B.; Ho, P.C.-L. Dried Blood Spots—A Platform for Therapeutic Drug Monitoring (TDM) and Drug/Disease Response Monitoring (DRM). Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 467–494. [Google Scholar] [CrossRef]
- Mensitieri, F.; Coglianese, A.; Giudice, V.; Charlier, B.; De Rosa, F.; Filippelli, A.; Filippelli, A.; Del Piaz, F.; Izzo, V. Effects of Selected Preanalytical Variables on Dried Blood Spot (DBS) and Volumetric Adsorptive Microsampling (VAMS) Based Bioanalytical Methods for the Determination of Four β-Lactam Antibiotics. Biochim. Clin. 2022, 46, 141–153. [Google Scholar] [CrossRef]
- Wagner, M.; Tonoli, D.; Varesio, E.; Hopfgartner, G. The Use of Mass Spectrometry to Analyze Dried Blood Spots. Mass. Spectrom. Rev. 2016, 35, 361–438. [Google Scholar] [CrossRef]











| Room Temperature (Hours) | 2–8 °C (Days) | −20 °C (Days) | −80 °C (Months) | |
|---|---|---|---|---|
| Amikacin | 96 | 7 | 30 | 3.3 |
| Ampicillin | 24 | 5 | 20 | 1 (6) |
| Cefepime | 6 | 1 | 30 | 3 (6) |
| Ceftazidime | 12 | 3 | 30 | 12 |
| Ciprofloxacin | 24 | 7 | 180 | 180 |
| Daptomycin | 48 | 2 | 8 (30) | 1 |
| Gentamicin | 48 (96) | 7 | 30 (60) | 1 (3.3) |
| Levofloxacin | 24 (72) | 2 (7) | 60 | 3 (6) |
| Linezolid | 24 | 7 | 180 | 6 (12) |
| Meropenem | 6 | 1 | 7 | 6 (12) |
| Piperacillin | 4 | 1 | 5 (7) | 6 |
| Teicoplanin | 24 | 1.5 | 365 | 1 |
| Vancomycin | 96 | 7 | 30 (420) | 1 (5.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalla Zuanna, P.; Curci, D.; Lucafò, M.; Addobbati, R.; Fabretto, A.; Stocco, G. Preanalytical Stability of 13 Antibiotics in Biological Samples: A Crucial Factor for Therapeutic Drug Monitoring. Antibiotics 2024, 13, 675. https://doi.org/10.3390/antibiotics13070675
Dalla Zuanna P, Curci D, Lucafò M, Addobbati R, Fabretto A, Stocco G. Preanalytical Stability of 13 Antibiotics in Biological Samples: A Crucial Factor for Therapeutic Drug Monitoring. Antibiotics. 2024; 13(7):675. https://doi.org/10.3390/antibiotics13070675
Chicago/Turabian StyleDalla Zuanna, Paolo, Debora Curci, Marianna Lucafò, Riccardo Addobbati, Antonella Fabretto, and Gabriele Stocco. 2024. "Preanalytical Stability of 13 Antibiotics in Biological Samples: A Crucial Factor for Therapeutic Drug Monitoring" Antibiotics 13, no. 7: 675. https://doi.org/10.3390/antibiotics13070675
APA StyleDalla Zuanna, P., Curci, D., Lucafò, M., Addobbati, R., Fabretto, A., & Stocco, G. (2024). Preanalytical Stability of 13 Antibiotics in Biological Samples: A Crucial Factor for Therapeutic Drug Monitoring. Antibiotics, 13(7), 675. https://doi.org/10.3390/antibiotics13070675






