Artificial Intelligence to Close the Gap between Pharmacokinetic/Pharmacodynamic Targets and Clinical Outcomes in Critically Ill Patients: A Narrative Review on Beta Lactams
Abstract
1. Introduction
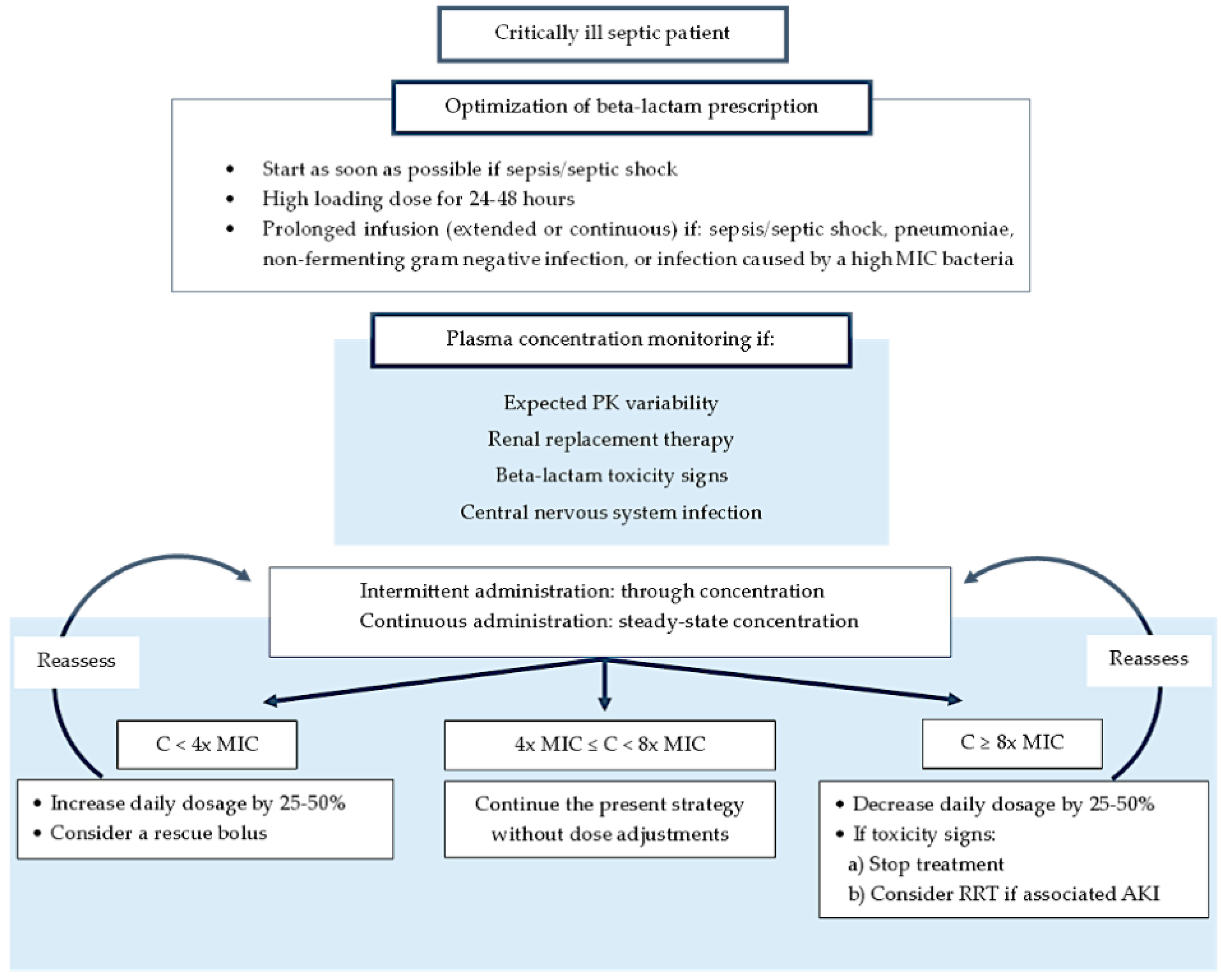
Impact of Therapeutic Drug Monitoring on the Outcomes
2. Reasons for the Absence of Response Despite Adequate Plasma Concentrations
- β-lactam tissue penetration may be influenced by tissue perfusion, adequate microcirculation availability, tissue inflammation, or necrosis;
- the PD value, which mainly reflects the antibiotic time-kill kinetics, is strongly influenced by the phenotypical expression of bacteria, namely the presence of biofilms;
- bacterial tolerance and acquisition of resistance during therapy may lead to poor bacterial clearance and therapeutic failure.
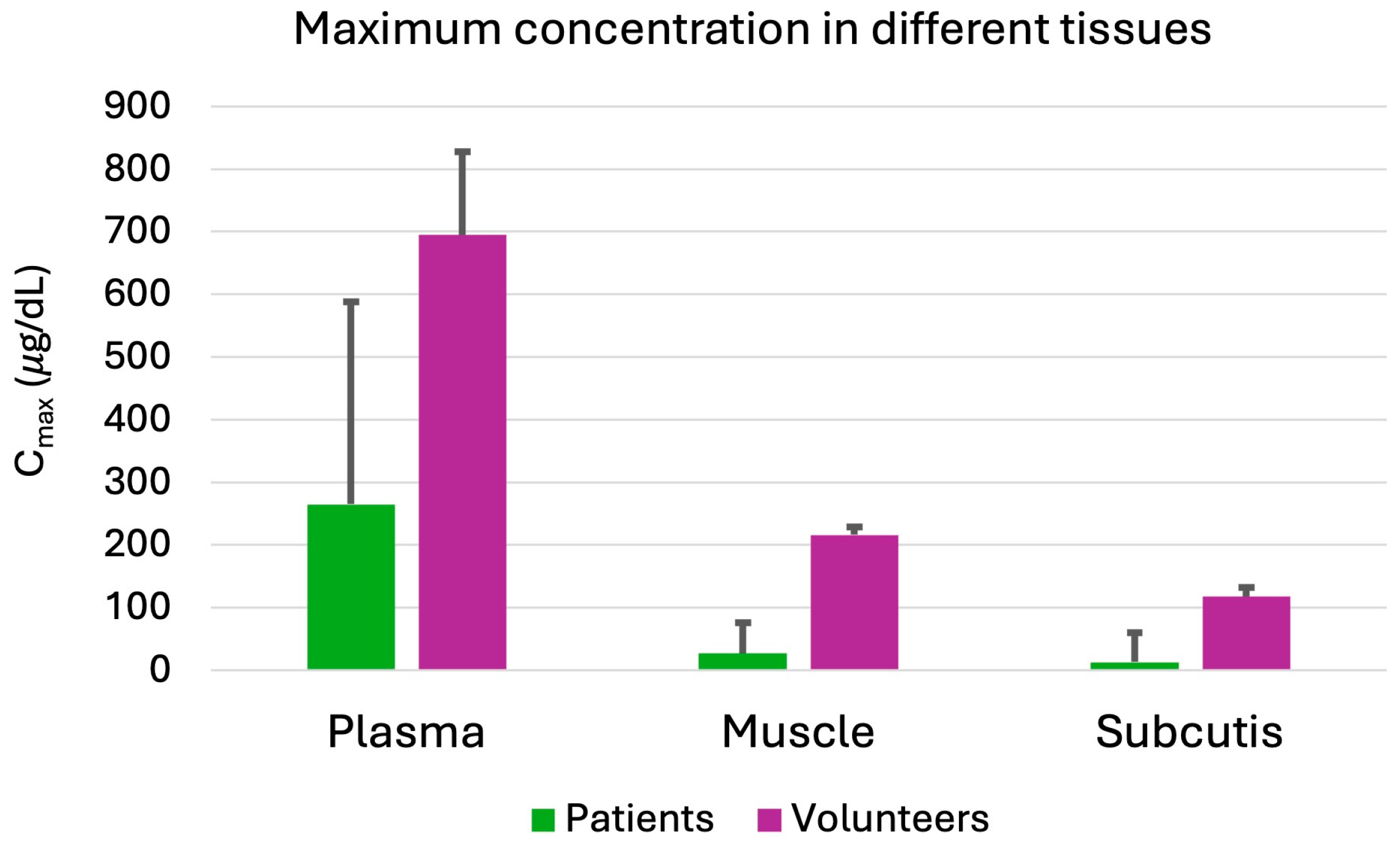
2.1. Tissue Penetration
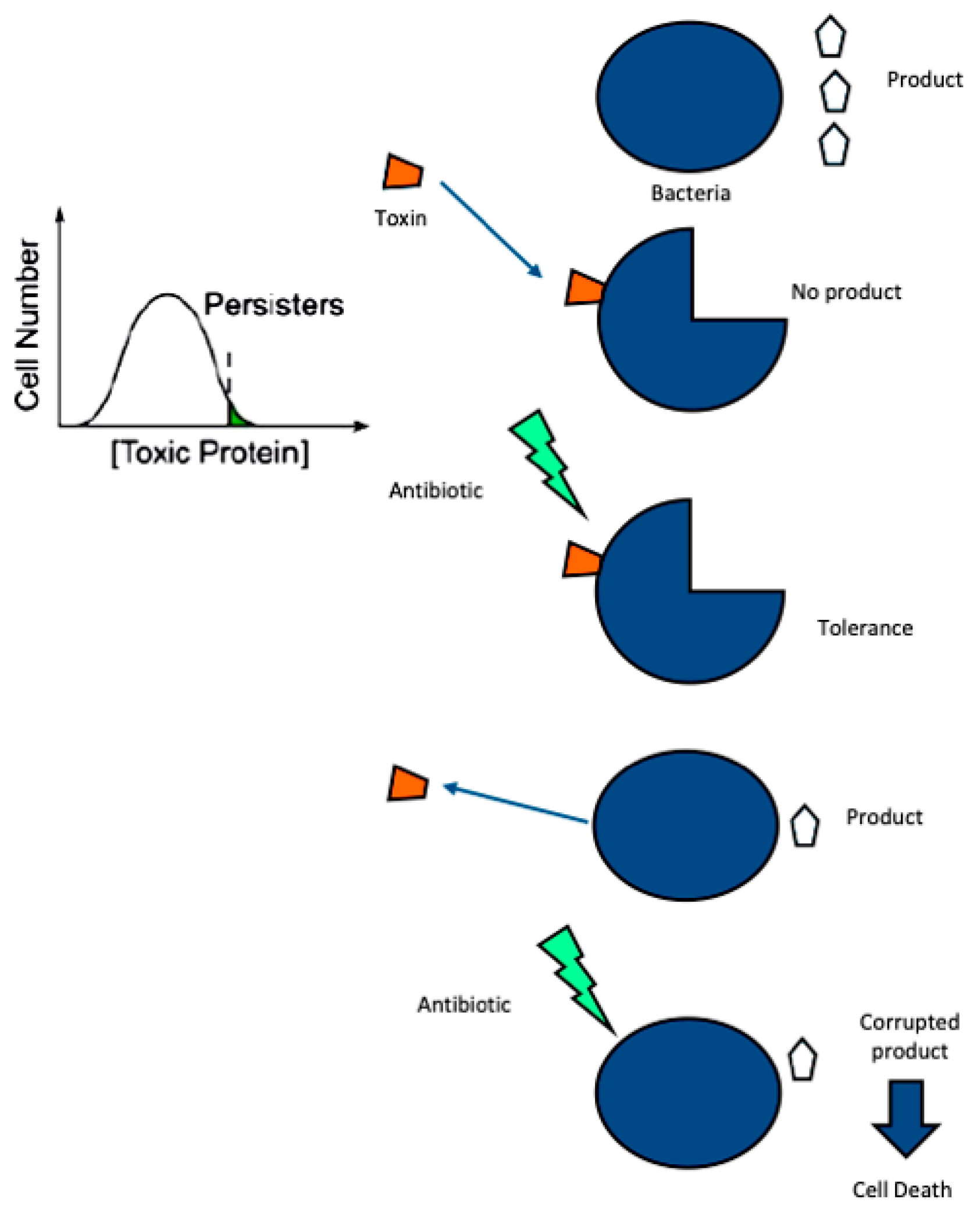
2.2. Biofilms
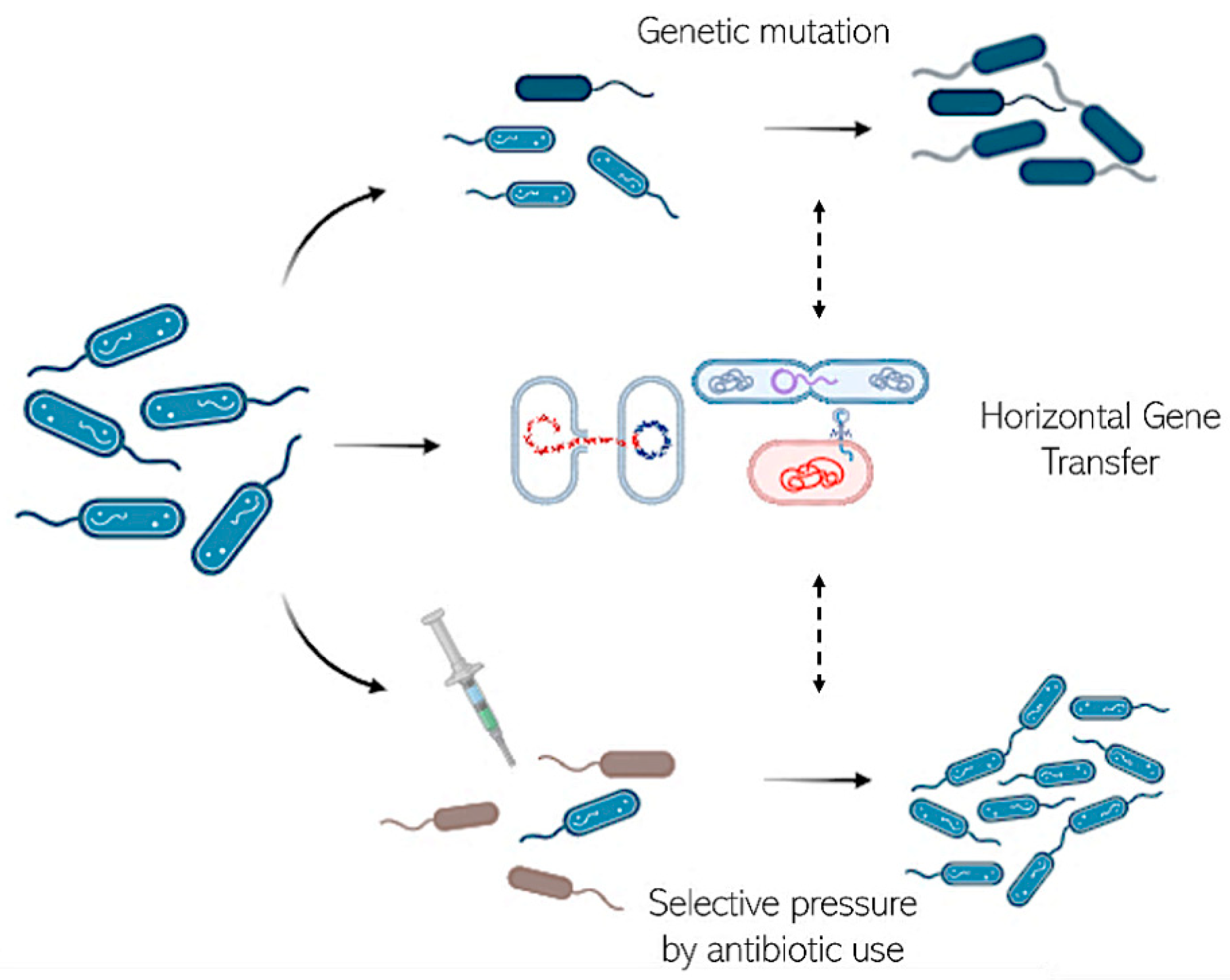
2.3. Acquisition of Resistance: Bet-Hedging
2.4. Acquisition of Resistance during Therapy
2.5. Compensatory Mutations
3. Therapeutic Response to β-Lactams to Guide Antibiotic Dosing
3.1. Clinical Response and Monitoring
3.2. Inflammatory Biomarkers
3.3. Microdialysis Is the Window into the Place Where the Infection Is
4. The Use of Machine Learning to Improve Antibiotic Dosing
4.1. Machine Learning
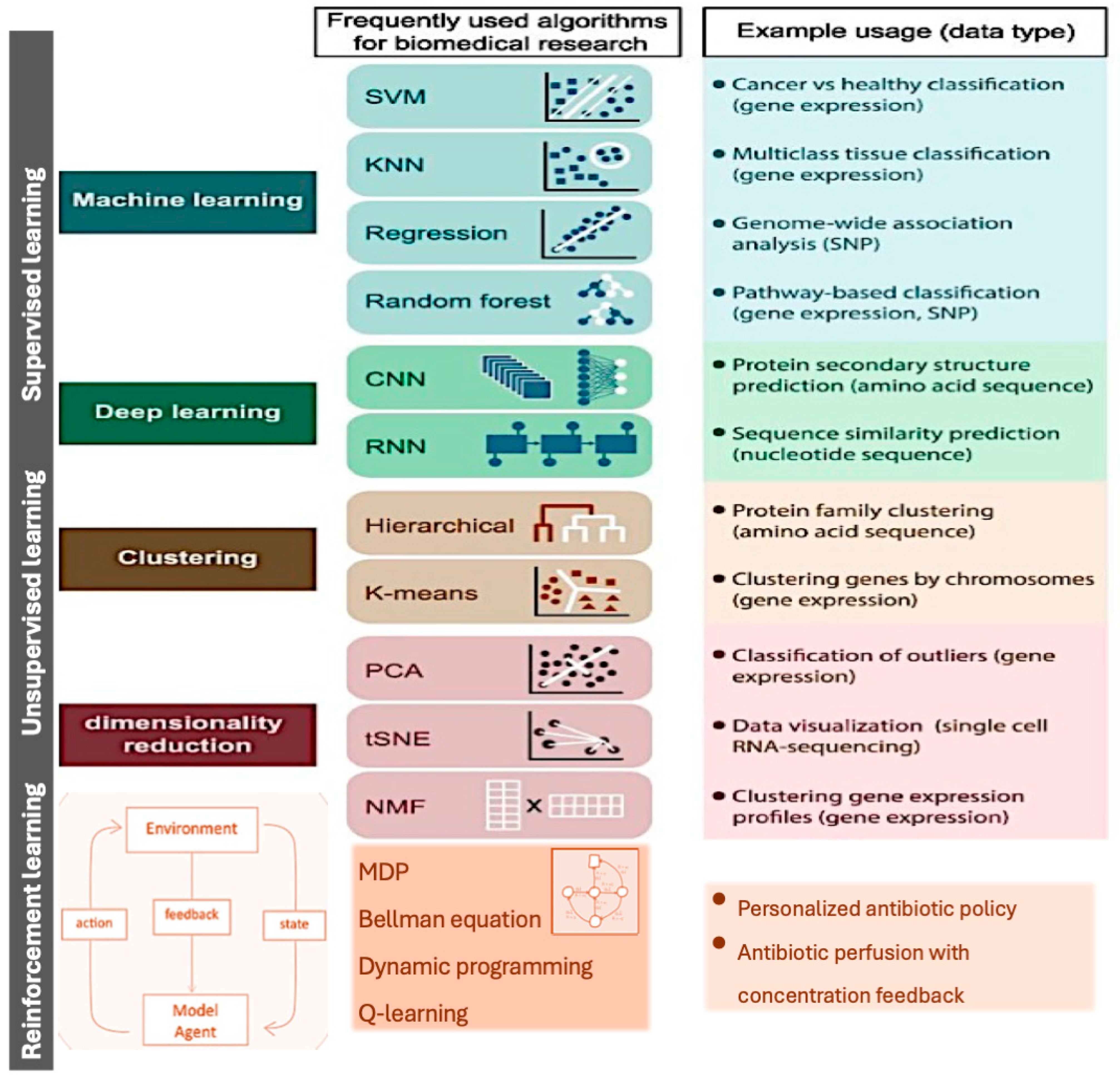
4.2. Selection of Data for Machine Learning Models
4.3. Application of Machine Learning Models to Antibiotic Pharmacokinetics and Pharmacodynamics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawson, T.M.; Wilson, R.C.; O’Hare, D.; Herrero, P.; Kambugu, A.; Lamorde, M.; Ellington, M.; Georgiou, P.; Cass, A.; Hope, W.W.; et al. Optimizing Antimicrobial Use: Challenges, Advances and Opportunities. Nat. Rev. Microbiol. 2021, 19, 747–758. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining Antibiotic Levels in Intensive Care Unit Patients: Are Current ß-Lactam Antibiotic Doses Sufficient for Critically Ill Patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- Goncalves-Pereira, J.; Fernandes, J.; Duarte, A.R.; Fernandes, S.M. β-Lactam Dosing in Critical Patients: A Narrative Review of Optimal Efficacy and the Prevention of Resistance and Toxicity. Antibiotics 2022, 11, 1839. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised Antibiotic Dosing for Patients Who Are Critically Ill: Challenges and Potential Solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.; Lipman, J.; Mouton, J.; Hope, W.; Roberts, J. Applying Pharmacokinetic/Pharmacodynamic Principles in Critically Ill Patients: Optimizing Efficacy and Reducing Resistance Development. Semin. Respir. Crit. Care Med. 2015, 36, 136–153. [Google Scholar] [CrossRef]
- Sumi, C.D.; Heffernan, A.J.; Lipman, J.; Roberts, J.A.; Sime, F.B. What Antibiotic Exposures Are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A Systematic Review. Clin. Pharmacokinet. 2019, 58, 1407–1443. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial Therapeutic Drug Monitoring in Critically Ill Adult Patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- McKinnon, P.S.; Paladino, J.A.; Schentag, J.J. Evaluation of Area under the Inhibitory Curve (AUIC) and Time above the Minimum Inhibitory Concentration (T>MIC) as Predictors of Outcome for Cefepime and Ceftazidime in Serious Bacterial Infections. Int. J. Antimicrob. Agents 2008, 31, 345–351. [Google Scholar] [CrossRef]
- Li, C.; Du, X.; Kuti, J.L.; Nicolau, D.P. Clinical Pharmacodynamics of Meropenem in Patients with Lower Respiratory Tract Infections. Antimicrob. Agents Chemother. 2007, 51, 1725–1730. [Google Scholar] [CrossRef]
- Aitken, S.L.; Altshuler, J.; Guervil, D.J.; Hirsch, E.B.; Ostrosky-Zeichner, L.L.; Ericsson, C.D.; Tam, V.H. Cefepime Free Minimum Concentration to Minimum Inhibitory Concentration (FCmin/MIC) Ratio Predicts Clinical Failure in Patients with Gram-Negative Bacterial Pneumonia. Int. J. Antimicrob. Agents 2015, 45, 541–544. [Google Scholar] [CrossRef]
- Tam, V.H.; McKinnon, P.S.; Akins, R.L.; Rybak, M.J.; Drusano, G.L. Pharmacodynamics of Cefepime in Patients with Gram-Negative Infections. J. Antimicrob. Chemother. 2002, 50, 425–428. [Google Scholar] [CrossRef]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the Treatment with Beta-Lactam Antibiotics in Critically Ill Patients—Guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique—SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation-SFAR). Crit. Care 2019, 23, 104. [Google Scholar] [CrossRef]
- Ulldemolins, M.; Roberts, J.A.; Lipman, J.; Rello, J. Antibiotic Dosing in Multiple Organ Dysfunction Syndrome. Chest 2011, 139, 1210–1220. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.H.; Davis, J.S.; Dulhunty, J.M.; Cotta, M.O.; Myburgh, J.; Bellomo, R.; Lipman, J. Continuous versus Intermittent β-Lactam Infusion in Severe Sepsis: A Meta-Analysis of Individual Patient Data from Randomized Trials. Am. J. Respir. Crit. Care Med. 2016, 194, 681–691. [Google Scholar] [CrossRef]
- Stašek, J.; Keller, F.; Kočí, V.; Klučka, J.; Klabusayová, E.; Wiewiorka, O.; Strašilová, Z.; Beňovská, M.; Škardová, M.; Maláska, J. Update on Therapeutic Drug Monitoring of Beta-Lactam Antibiotics in Critically Ill Patients—A Narrative Review. Antibiotics 2023, 12, 568. [Google Scholar] [CrossRef]
- Takahashi, N.; Kondo, Y.; Kubo, K.; Egi, M.; Kano, K.; Ohshima, Y.; Nakada, T. Efficacy of Therapeutic Drug Monitoring-Based Antibiotic Regimen in Critically Ill Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Intensive Care 2023, 11, 48. [Google Scholar] [CrossRef]
- Hagel, S.; Bach, F.; Brenner, T.; Bracht, H.; Brinkmann, A.; Annecke, T.; Hohn, A.; Weigand, M.A.; Michels, G.; Kluge, S.; et al. Correction to: Effect of Therapeutic Drug Monitoring-Based Dose Optimization of Piperacillin/Tazobactam on Sepsis-Related Organ Dysfunction in Patients with Sepsis: A Randomized Controlled Trial. Intensive Care Med. 2022, 48, 646–647. [Google Scholar] [CrossRef]
- Wong, G.; Briscoe, S.; McWhinney, B.; Ally, M.; Ungerer, J.; Lipman, J.; Roberts, J.A. Therapeutic Drug Monitoring of β-Lactam Antibiotics in the Critically Ill: Direct Measurement of Unbound Drug Concentrations to Achieve Appropriate Drug Exposures. J. Antimicrob. Chemother. 2018, 73, 3087–3094. [Google Scholar] [CrossRef]
- De Waele, J.J.; Carrette, S.; Carlier, M.; Stove, V.; Boelens, J.; Claeys, G.; Leroux-Roels, I.; Hoste, E.; Depuydt, P.; Decruyenaere, J.; et al. Therapeutic Drug Monitoring-Based Dose Optimisation of Piperacillin and Meropenem: A Randomised Controlled Trial. Intensive Care Med. 2014, 40, 380–387. [Google Scholar] [CrossRef]
- Roggeveen, L.F.; Guo, T.; Fleuren, L.M.; Driessen, R.; Thoral, P.; van Hest, R.M.; Mathot, R.A.A.; Swart, E.L.; de Grooth, H.-J.; van den Bogaard, B.; et al. Right Dose, Right Now: Bedside, Real-Time, Data-Driven, and Personalised Antibiotic Dosing in Critically Ill Patients with Sepsis or Septic Shock—A Two-Centre Randomised Clinical Trial. Crit. Care 2022, 26, 265. [Google Scholar] [CrossRef]
- Sime, F.B.; Roberts, M.S.; Tiong, I.S.; Gardner, J.H.; Lehman, S.; Peake, S.L.; Hahn, U.; Warner, M.S.; Roberts, J.A. Can Therapeutic Drug Monitoring Optimize Exposure to Piperacillin in Febrile Neutropenic Patients with Haematological Malignancies? A Randomized Controlled Trial. J. Antimicrob. Chemother. 2015, 70, 2369–2375. [Google Scholar] [CrossRef]
- Ewoldt, T.M.J.; Abdulla, A.; Rietdijk, W.J.R.; Muller, A.E.; de Winter, B.C.M.; Hunfeld, N.G.M.; Purmer, I.M.; van Vliet, P.; Wils, E.-J.; Haringman, J.; et al. Model-Informed Precision Dosing of Beta-Lactam Antibiotics and Ciprofloxacin in Critically Ill Patients: A Multicentre Randomised Clinical Trial. Intensive Care Med. 2022, 48, 1760–1771. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Oliveira, A.; Vidal, R.; Gonçalves-Pereira, J. Infectious Foci, Comorbidities and Its Influence on the Outcomes of Septic Critically Ill Patients. Microorganisms 2024, 12, 1705. [Google Scholar] [CrossRef]
- Duncan, C.F.; Youngstein, T.; Kirrane, M.D.; Lonsdale, D.O. Diagnostic Challenges in Sepsis. Curr. Infect. Dis. Rep. 2021, 23, 22. [Google Scholar] [CrossRef]
- Tilanus, A.; Drusano, G. Optimizing the Use of Beta-Lactam Antibiotics in Clinical Practice: A Test of Time. Open Forum Infect. Dis. 2023, 10, ofad305. [Google Scholar] [CrossRef]
- Goncalves-Pereira, J.; Paiva, J.-A. Dose Modulation: A New Concept of Antibiotic Therapy in the Critically Ill Patient? J. Crit. Care 2013, 28, 341–346. [Google Scholar] [CrossRef]
- Goncalves-Pereira, J.; Póvoa, P. Antibiotics in Critically Ill Patients: A Systematic Review of the Pharmacokinetics of β-Lactams. Crit. Care 2011, 15, R206. [Google Scholar] [CrossRef]
- Veiga, R.P.; Paiva, J.-A. Pharmacokinetics–Pharmacodynamics Issues Relevant for the Clinical Use of Beta-Lactam Antibiotics in Critically Ill Patients. Crit. Care 2018, 22, 233. [Google Scholar] [CrossRef]
- Scheetz, M.H.; Lodise, T.P.; Downes, K.J.; Drusano, G.; Neely, M. The Case for Precision Dosing: Medical Conservatism Does Not Justify Inaction. J. Antimicrob. Chemother. 2021, 76, 1661–1665. [Google Scholar] [CrossRef]
- Economou, C.J.P.; Wong, G.; McWhinney, B.; Ungerer, J.P.J.; Lipman, J.; Roberts, J.A. Impact of β-Lactam Antibiotic Therapeutic Drug Monitoring on Dose Adjustments in Critically Ill Patients Undergoing Continuous Renal Replacement Therapy. Int. J. Antimicrob. Agents 2017, 49, 589–594. [Google Scholar] [CrossRef]
- Póvoa, P.; Moniz, P.; Pereira, J.G.; Coelho, L. Optimizing Antimicrobial Drug Dosing in Critically Ill Patients. Microorganisms 2021, 9, 1401. [Google Scholar] [CrossRef] [PubMed]
- De Sutter, P.J.; De Cock, P.; Johnson, T.N.; Musther, H.; Gasthuys, E.; Vermeulen, A. Predictive Performance of Physiologically Based Pharmacokinetic Modelling of Beta-Lactam Antibiotic Concentrations in Adipose, Bone, and Muscle Tissues. Drug Metab. Dispos. 2023, 51, 499–508. [Google Scholar] [CrossRef]
- Finazzi, S.; Luci, G.; Olivieri, C.; Langer, M.; Mandelli, G.; Corona, A.; Viaggi, B.; Di Paolo, A. Tissue Penetration of Antimicrobials in Intensive Care Unit Patients: A Systematic Review—Part I. Antibiotics 2022, 11, 1164. [Google Scholar] [CrossRef] [PubMed]
- Munroe, E.S.; Hyzy, R.C.; Semler, M.W.; Shankar-Hari, M.; Young, P.J.; Zampieri, F.G.; Prescott, H.C. Evolving Management Practices for Early Sepsis-Induced Hypoperfusion: A Narrative Review. Am. J. Respir. Crit. Care Med. 2023, 207, 1283–1299. [Google Scholar] [CrossRef]
- Klekner, A.; Bagyi, K.; Bognar, L.; Gaspar, A.; Andrasi, M.; Szabo, J. Effectiveness of Cephalosporins in the Sputum of Patients with Nosocomial Bronchopneumonia. J. Clin. Microbiol. 2006, 44, 3418–3421. [Google Scholar] [CrossRef][Green Version]
- Boselli, E.; Breilh, D.; Saux, M.C.; Gordien, J.B.; Allaouchiche, B. Pharmacokinetics and Lung Concentrations of Ertapenem in Patients with Ventilator-Associated Pneumonia. Intensive Care Med. 2006, 32, 2059–2062. [Google Scholar] [CrossRef]
- Boselli, E.; Breilh, D.; Rimmelé, T.; Djabarouti, S.; Saux, M.-C.; Chassard, D.; Allaouchiche, B. Pharmacokinetics and Intrapulmonary Diffusion of Levofloxacin in Critically Ill Patients with Severe Community-Acquired Pneumonia. Crit. Care Med. 2005, 33, 104–109. [Google Scholar] [CrossRef]
- Boselli, E.; Breilh, D.; Rimmelé, T.; Guillaume, C.; Xuereb, F.; Saux, M.-C.; Bouvet, L.; Chassard, D.; Allaouchiche, B. Alveolar Concentrations of Piperacillin/Tazobactam Administered in Continuous Infusion to Patients with Ventilator-Associated Pneumonia. Crit. Care Med. 2008, 36, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Dalley, A.J.; Deans, R.; Lipman, J.; Venkatesh, B.; Rudd, M.; Roberts, M.S.; Cross, S.E. Unbound Cephalothin Pharmacokinetics in Adult Burn Patients Are Related to the Elapsed Time after Injury. Antimicrob. Agents Chemother. 2009, 53, 5303–5305. [Google Scholar] [CrossRef]
- Roberts, J.; Roberts, M.S.; Robertson, T.; Dalley, A.J.; Lipman, J. Piperacillin Penetration into Tissue of Critically Ill Patients with Sepsis—Bolus versus Continuous Administration? Crit. Care Med. 2009, 37, 926–933. [Google Scholar] [CrossRef]
- Dahyot, C.; Marchand, S.; Bodin, M.; Debeane, B.; Mimoz, O.; Couet, W. Application of Basic Pharmacokinetic Concepts to Analysis of Microdialysis Data: Illustration with Imipenem Muscle Distribution. Clin. Pharmacokinet. 2008, 47, 181–189. [Google Scholar] [CrossRef]
- Joukhadar, C.; Frossard, M.; Mayer, B.X.; Brunner, M.; Klein, N.; Siostrzonek, P.; Eichler, H.G.; Müller, M. Impaired Target Site Penetration of Beta-Lactams May Account for Therapeutic Failure in Patients with Septic Shock. Crit. Care Med. 2001, 29, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, I.; Schmidtko, A.; Bräutigam, L.; Kirschbaum, A.; Geisslinger, G.; Lötsch, J. Tissue Distribution of Imipenem in Critically Ill Patients. Clin. Pharmacol. Ther. 2002, 71, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Karjagin, J.; Lefeuvre, S.; Oselin, K.; Kipper, K.; Marchand, S.; Tikkerberi, A.; Starkopf, J.; Couet, W.; Sawchuk, R.J. Pharmacokinetics of Meropenem Determined by Microdialysis in the Peritoneal Fluid of Patients with Severe Peritonitis Associated with Septic Shock. Clin. Pharmacol. Ther. 2008, 83, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Buijk, S.L.C.E.; Gyssens, I.C.; Mouton, J.W.; Van Vliet, A.; Verbrugh, H.A.; Bruining, H.A. Pharmacokinetics of Ceftazidime in Serum and Peritoneal Exudate during Continuous versus Intermittent Administration to Patients with Severe Intra-Abdominal Infections. J. Antimicrob. Chemother. 2002, 49, 121–128. [Google Scholar] [CrossRef]
- Sanz Codina, M.; Zeitlinger, M. Biomarkers Predicting Tissue Pharmacokinetics of Antimicrobials in Sepsis: A Review. Clin. Pharmacokinet. 2022, 61, 593–617. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, M.I.; Keyt, H.; Reyes, L.F. Aerosolized Antibiotics. Respir. Care 2015, 60, 762–773. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Morawska, L.P.; Hernandez-Valdes, J.A.; Kuipers, O.P. Diversity of Bet-hedging Strategies in Microbial Communities—Recent Cases and Insights. WIREs Mech. Dis. 2022, 14, e1544. [Google Scholar] [CrossRef]
- Rumbaugh, K.P.; Bjarnsholt, T. Microbial Primer: In Vivo Biofilm. Microbiology 2023, 169, 001407. [Google Scholar] [CrossRef]
- Boisvert, A.A.; Cheng, M.P.; Sheppard, D.C.; Nguyen, D. Microbial Biofilms in Pulmonary and Critical Care Diseases. Ann. Am. Thorac. Soc. 2016, 13, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Sun, J.; Liu, Y. Understanding Bacterial Biofilms: From Definition to Treatment Strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic Resistance of Bacterial Biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Amanatidou, E.; Matthews, A.C.; Kuhlicke, U.; Neu, T.R.; McEvoy, J.P.; Raymond, B. Biofilms Facilitate Cheating and Social Exploitation of β-Lactam Resistance in Escherichia Coli. NPJ Biofilms Microbiomes 2019, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Hengzhuang, W.; Ciofu, O.; Yang, L.; Wu, H.; Song, Z.; Oliver, A.; Høiby, N. High β-Lactamase Levels Change the Pharmacodynamics of β-Lactam Antibiotics in Pseudomonas Aeruginosa Biofilms. Antimicrob. Agents Chemother. 2013, 57, 196–204. [Google Scholar] [CrossRef]
- Lopatkin, A.J.; Bening, S.C.; Manson, A.L.; Stokes, J.M.; Kohanski, M.A.; Badran, A.H.; Earl, A.M.; Cheney, N.J.; Yang, J.H.; Collins, J.J. Clinically Relevant Mutations in Core Metabolic Genes Confer Antibiotic Resistance. Science 2021, 371, eaba0862. [Google Scholar] [CrossRef]
- de Groot, D.H.; Tjalma, A.J.; Bruggeman, F.J.; van Nimwegen, E. Effective Bet-Hedging through Growth Rate Dependent Stability. Proc. Natl. Acad. Sci. USA 2023, 120, e2211091120. [Google Scholar] [CrossRef]
- Veening, J.W.; Stewart, E.J.; Berngruber, T.W.; Taddei, F.; Kuipers, O.P.; Hamoen, L.W. Bet-Hedging and Epigenetic Inheritance in Bacterial Cell Development. Proc. Natl. Acad. Sci. USA 2008, 105, 4393–4398. [Google Scholar] [CrossRef]
- Keren, I.; Shah, D.; Spoering, A.; Kaldalu, N.; Lewis, K. Specialized Persister Cells and the Mechanism of Multidrug Tolerance in Escherichia Coli. J. Bacteriol. 2004, 186, 8172–8180. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.-A.; Klugman, K.; Davies, S. Access to Effective Antimicrobials: A Worldwide Challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
- Cillóniz, C.; Ewig, S.; Ferrer, M.; Polverino, E.; Gabarrús, A.; Puig de la Bellacasa, J.; Mensa, J.; Torres, A. Community-Acquired Polymicrobial Pneumonia in the Intensive Care Unit: Aetiology and Prognosis. Crit. Care 2011, 15, R209. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Carattoli, A. Plasmids and the Spread of Resistance. Int. J. Med. Microbiol. 2013, 303, 298–304. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Microbiological Effects of Sublethal Levels of Antibiotics. Nat. Rev. Microbiol. 2014, 12, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Andreescu, M. Molecular Insights into the Role of Gut Microbiota in Antibiotic Therapy Selection and Resistance Mitigation. Cureus 2023, 15, e50318. [Google Scholar] [CrossRef] [PubMed]
- Gianvecchio, C.; Lozano, N.A.; Henderson, C.; Kalhori, P.; Bullivant, A.; Valencia, A.; Su, L.; Bello, G.; Wong, M.; Cook, E.; et al. Variation in Mutant Prevention Concentrations. Front. Microbiol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Corbin, C.K.; Sung, L.; Chattopadhyay, A.; Noshad, M.; Chang, A.; Deresinksi, S.; Baiocchi, M.; Chen, J.H. Personalized Antibiograms for Machine Learning Driven Antibiotic Selection. Commun. Med. 2022, 2, 38. [Google Scholar] [CrossRef]
- Pinto-de-Sá, R.; Sousa-Pinto, B.; Costa-de-Oliveira, S. Brave New World of Artificial Intelligence: Its Use in Antimicrobial Stewardship—A Systematic Review. Antibiotics 2024, 13, 307. [Google Scholar] [CrossRef]
- Chang, A.; Chen, J.H. BSAC Vanguard Series: Artificial Intelligence and Antibiotic Stewardship. J. Antimicrob. Chemother. 2022, 77, 1216–1217. [Google Scholar] [CrossRef]
- Revitt-Mills, S.A.; Robinson, A. Antibiotic-Induced Mutagenesis: Under the Microscope. Front. Microbiol. 2020, 11, 585175. [Google Scholar] [CrossRef]
- Palleja, A.; Mikkelsen, K.H.; Forslund, S.K.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Liang, S.; Feng, Q.; Zhang, C.; et al. Recovery of Gut Microbiota of Healthy Adults Following Antibiotic Exposure. Nat. Microbiol. 2018, 3, 1255–1265. [Google Scholar] [CrossRef]
- Ubeda, C.; Taur, Y.; Jenq, R.R.; Equinda, M.J.; Son, T.; Samstein, M.; Viale, A.; Socci, N.D.; Van Den Brink, M.R.M.; Kamboj, M.; et al. Vancomycin-Resistant Enterococcus Domination of Intestinal Microbiota Is Enabled by Antibiotic Treatment in Mice and Precedes Bloodstream Invasion in Humans. J. Clin. Investig. 2010, 120, 4332–4341. [Google Scholar] [CrossRef] [PubMed]
- Laubitz, D.; Typpo, K.; Midura-Kiela, M.; Brown, C.; Barberán, A.; Ghishan, F.K.; Kiela, P.R. Dynamics of Gut Microbiota Recovery after Antibiotic Exposure in Young and Old Mice (A Pilot Study). Microorganisms 2021, 9, 647. [Google Scholar] [CrossRef]
- Saddler, C.A.; Wu, Y.; Valckenborgh, F.; Tanaka, M.M. Epidemiological Control of Drug Resistance and Compensatory Mutation under Resistance Testing and Second-Line Therapy. Epidemics 2013, 5, 164–173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loftie-Eaton, W.; Bashford, K.; Quinn, H.; Dong, K.; Millstein, J.; Hunter, S.; Thomason, M.K.; Merrikh, H.; Ponciano, J.M.; Top, E.M. Compensatory Mutations Improve General Permissiveness to Antibiotic Resistance Plasmids. Nat. Ecol. Evol. 2017, 1, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.E.; MacLean, C.; Papkou, A.; Pritchard, M.; Powell, L.; Thomas, D.; Andrey, D.O.; Li, M.; Spiller, B.; Yang, W.; et al. Compensatory Mutations Modulate the Competitiveness and Dynamics of Plasmid-Mediated Colistin Resistance in Escherichia Coli Clones. ISME J. 2020, 14, 861–865. [Google Scholar] [CrossRef] [PubMed]
- van Hecke, O.; Wang, K.; Lee, J.J.; Roberts, N.W.; Butler, C.C. Implications of Antibiotic Resistance for Patients’ Recovery From Common Infections in the Community: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2017, 65, 371–382. [Google Scholar] [CrossRef]
- Drekonja, D.M.; Trautner, B.; Amundson, C.; Kuskowski, M.; Johnson, J.R. Effect of 7 vs 14 Days of Antibiotic Therapy on Resolution of Symptoms Among Afebrile Men with Urinary Tract Infection. JAMA 2021, 326, 324. [Google Scholar] [CrossRef]
- Kaye, K.S.; Bhowmick, T.; Metallidis, S.; Bleasdale, S.C.; Sagan, O.S.; Stus, V.; Vazquez, J.; Zaitsev, V.; Bidair, M.; Chorvat, E.; et al. Effect of Meropenem-Vaborbactam vs Piperacillin-Tazobactam on Clinical Cure or Improvement and Microbial Eradication in Complicated Urinary Tract Infection the TANGO I Randomized Clinical Trial. JAMA 2018, 319, 788–799. [Google Scholar] [CrossRef]
- Llor, C.; Moragas, A.; Bayona, C.; Cots, J.M.; Hernández, S.; Calviño, O.; Rodríguez, M.; Miravitlles, M. Efficacy and Safety of Discontinuing Antibiotic Treatment for Uncomplicated Respiratory Tract Infections When Deemed Unnecessary. A Multicentre, Randomized Clinical Trial in Primary Care. Clin. Microbiol. Infect. 2022, 28, 241–247. [Google Scholar] [CrossRef]
- Dennesen, P.J.W.; Van der Ven, A.J.A.M.; Kessels, A.G.H.; Ramsay, G.; Bonten, M.J.M. Resolution of Infectious Parameters after Antimicrobial Therapy in Patients with Ventilator-Associated Pneumonia. Am. J. Respir. Crit. Care Med. 2001, 163, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, P.; Coelho, L.; Dal-Pizzol, F.; Ferrer, R.; Huttner, A.; Conway Morris, A.; Nobre, V.; Ramirez, P.; Rouze, A.; Salluh, J.; et al. How to Use Biomarkers of Infection or Sepsis at the Bedside: Guide to Clinicians. Intensive Care Med. 2023, 49, 142–153. [Google Scholar] [CrossRef]
- Póvoa, P.R.; Teixeira-Pinto, A.M.; Carneiro, A.H.A.H.; Póvoa, P.; Teixeira-Pinto, A.M.; Carneiro, A.H.A.H. C-Reactive Protein, an Early Marker of Community-Acquired Sepsis Resolution: A Multi-Center Prospective Observational Study. Crit. Care 2011, 15, R169. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.F.; de Paula, A.C.R.B.; Hasparyk, U.G.; de Oliveira Rabelo Bassalo Coutinho, M.; Alderete, J.R.A.; Kanjongo, J.C.; Silva, R.A.M.; Guimarães, N.S.; Simões e Silva, A.C.; Nobre, V. Use of C-Reactive Protein to Guide the Antibiotic Therapy in Hospitalized Patients: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2023, 23, 276. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Wirz, Y.; Sager, R.; Christ-Crain, M.; Stolz, D.; Tamm, M.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; et al. Effect of Procalcitonin-Guided Antibiotic Treatment on Mortality in Acute Respiratory Infections: A Patient Level Meta-Analysis. Lancet Infect. Dis. 2018, 18, 95–107. [Google Scholar] [CrossRef]
- Siriwardena, A.K.; Jegatheeswaran, S.; Mason, J.M.; Siriwardena, A.K.; Jegatheeswaran, S.; Mason, J.M.; Baltatzis, M.; Sheen, A.J.; O’Reilly, D.A.; Jamdar, S.; et al. A Procalcitonin-Based Algorithm to Guide Antibiotic Use in Patients with Acute Pancreatitis (PROCAP): A Single-Centre, Patient-Blinded, Randomised Controlled Trial. Lancet Gastroenterol. Hepatol. 2022, 7, 913–921. [Google Scholar] [CrossRef]
- Eggimann, P.; Que, Y.A.; Rebeaud, F. Measurement of Pancreatic Stone Protein in the Identification and Management of Sepsis. Biomark. Med. 2019, 13, 135–145. [Google Scholar] [CrossRef]
- Serrano, M.A.; Gomes, A.M.C.; Fernandes, S.M. Monitoring of the Forgotten Immune System during Critical Illness—A Narrative Review. Medicina 2022, 59, 61. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Leong, C.L.; Burnish, R.A.; Hassan, S.; Zhang, Y.; Clough, G.F.; Boutelle, M.G.; Voegeli, D.; Niu, X. Monitoring Biomolecule Concentrations in Tissue Using a Wearable Droplet Microfluidic-Based Sensor. Nat. Commun. 2019, 10, 2741. [Google Scholar] [CrossRef]
- Sabroe, J.E.; Axelsen, A.R.; Ellebæk, M.B.; Dahler-Eriksen, B.; Qvist, N. Intraperitoneal Lactate/Pyruvate Ratio and the Level of Glucose and Glycerol Concentration Differ between Patients Surgically Treated for Upper and Lower Perforations of the Gastrointestinal Tract: A Pilot Study. BMC Res. Notes 2017, 10, 302. [Google Scholar] [CrossRef][Green Version]
- Tůma, P.; Jaček, M.; Sommerová, B.; Dlouhý, P.; Jarošíková, R.; Husáková, J.; Wosková, V.; Fejfarová, V. Monitoring of Amoxicilline and Ceftazidime in the Microdialysate of Diabetic Foot and Serum by Capillary Electrophoresis with Contactless Conductivity Detection. Electrophoresis 2022, 43, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Dhanani, J.; Roberts, J.A.; Monsel, A.; Torres, A.; Kollef, M.; Rouby, J.-J.; Arvaniti, K.; Assefi, M.; Bassetti, M.; Blot, S.; et al. Understanding the Nebulisation of Antibiotics: The Key Role of Lung Microdialysis Studies. Crit. Care 2024, 28, 49. [Google Scholar] [CrossRef] [PubMed]
- Bowler, R.P.; Wendt, C.H.; Fessler, M.B.; Foster, M.W.; Kelly, R.S.; Lasky-Su, J.; Rogers, A.J.; Stringer, K.A.; Winston, B.W. New Strategies and Challenges in Lung Proteomics and Metabolomics. An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2017, 14, 1721–1743. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Wu, X.; Tang, S. Statistical Analysis of One-Compartment Pharmacokinetic Models with Drug Adherence. J. Pharmacokinet. Pharmacodyn. 2022, 49, 209–225. [Google Scholar] [CrossRef]
- González, P.; Mesa, P.; Maldonado, C.; Rojas, D.; Gómez, S.; Trujillo, S.; David Berti, A.; Kebriaei, R.; Parra González, D.; Alejandro Pérez Mesa, J.; et al. Pharmacokinetics of Vancomycin among Patients with Chemotherapy-Associated Febrile Neutropenia: Which Would Be the Best Dosing to Obtain Appropriate Exposure? Antibiotics 2022, 11, 1523. [Google Scholar] [CrossRef]
- Chasseloup, E.; Hooker, A.C.; Karlsson, M.O. Generation and Application of Avatars in Pharmacometric Modelling. J. Pharmacokinet. Pharmacodyn. 2023, 50, 411–423. [Google Scholar] [CrossRef]
- Cellina, M.; Cè, M.; Alì, M.; Irmici, G.; Ibba, S.; Caloro, E.; Fazzini, D.; Oliva, G.; Papa, S. Digital Twins: The New Frontier for Personalized Medicine? Appl. Sci. 2023, 13, 7940. [Google Scholar] [CrossRef]
- Neural Networks History. Available online: https://cs.stanford.edu/people/eroberts/courses/soco/projects/neural-networks/History/history1.html (accessed on 20 March 2022).
- Rajkomar, A.; Dean, J.; Kohane, I. Machine Learning in Medicine. N. Eng. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef]
- Lecun, Y.; Bengio, Y.; Hinton, G. Deep Learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Greco, M.; Caruso, P.F.; Cecconi, M. Artificial Intelligence in the Intensive Care Unit. Semin. Respir. Crit. Care Med. 2021, 42, 2–9. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V.; Saitta, L. Support-Vector Networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Auslander, N.; Gussow, A.B.; Koonin, E.V. Incorporating Machine Learning into Established Bioinformatics Frameworks. Int. J. Mol. Sci. 2021, 22, 2903. [Google Scholar] [CrossRef] [PubMed]
- Panch, T.; Szolovits, P.; Atun, R. Artificial Intelligence, Machine Learning and Health Systems. J. Glob. Health 2018, 8, 020303. [Google Scholar] [CrossRef]
- van der Maaten, L.; Hinton, G. Visualizing Data Using T-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Sutton, R.S.; Barto, A.G. Reinforcement Learning: An Introduction; A Bradford Book; The MIT Press Cambridge: Cambridge, MA, USA, 2014. [Google Scholar]
- Wallach, J.D.; Boyack, K.W.; Ioannidis, J.P.A. Reproducible Research Practices, Transparency, and Open Access Data in the Biomedical Literature, 2015–2017. PLoS Biol. 2018, 16, e2006930. [Google Scholar] [CrossRef] [PubMed]
- Mamdani, M.; Slutsky, A.S. Artificial Intelligence in Intensive Care Medicine. Intensive Care Med. 2021, 47, 147–149. [Google Scholar] [CrossRef]
- Moseley, E.T.; Hsu, D.J.; Stone, D.J.; Celi, L.A. Beyond Open Big Data: Addressing Unreliable Research. J. Med. Internet Res. 2014, 16, e259. [Google Scholar] [CrossRef]
- Cismondi, F.; Fialho, A.S.; Vieira, S.M.; Reti, S.R.; Sousa, J.M.C.; Finkelstein, S.N. Missing Data in Medical Databases: Impute, Delete or Classify? Artif. Intell. Med. 2013, 58, 63–72. [Google Scholar] [CrossRef]
- Heitjan, D.F. Annotation: What Can Be Done about Missing Data? Approaches to Imputation. Am. J. Public Health 2011, 87, 548–550. [Google Scholar] [CrossRef]
- García, S.; Ramírez-Gallego, S.; Luengo, J.; Benítez, J.M.; Herrera, F. Big Data Preprocessing: Methods and Prospects. Big Data Analytics 2016, 1, 9. [Google Scholar] [CrossRef]
- Han, J.; Kamber, M.; Pei, J. Data Mining: Concepts and Techniques, 3rd ed.; Elsevier: Walton, KY, USA, 2012. [Google Scholar]
- Guyon, I.; Elisseeff, A. An Introduction to Variable and Feature Selection. J. Mach. Learn. Res. 2003, 3, 1157–1182. [Google Scholar]
- Moehring, R.W.; Phelan, M.; Lofgren, E.; Nelson, A.; Dodds Ashley, E.; Anderson, D.J.; Goldstein, B.A. Development of a Machine Learning Model Using Electronic Health Record Data to Identify Antibiotic Use among Hospitalized Patients. JAMA Netw. Open 2021, 4, e213460. [Google Scholar] [CrossRef]
- Cios, K.J.; Kurgan, L.A. Trends in Data Mining and Knowledge Discovery. In Advanced Techniques in Knowledge Discovery and Data Mining; Springer: London, UK, 2007; pp. 1–26. [Google Scholar] [CrossRef]
- Komorowski, M.; Celi, L.A.; Badawi, O.; Gordon, A.C.; Faisal, A.A. The Artificial Intelligence Clinician Learns Optimal Treatment Strategies for Sepsis in Intensive Care. Nat. Med. 2018, 24, 1716–1720. [Google Scholar] [CrossRef]
- Hewamalage, H.; Bergmeir, C.; Bandara, K. Recurrent Neural Networks for Time Series Forecasting: Current Status and Future Directions. Int. J. Forecast. 2021, 37, 388–427. [Google Scholar] [CrossRef]
- Khoshnevisan, F.; Ivy, J.; Capan, M.; Arnold, R.; Huddleston, J.; Chi, M. Recent Temporal Pattern Mining for Septic Shock Early Prediction. In Proceedings of the IEEE International Conference on Healthcare Informatics, New York, NY, USA, 4–7 June 2018; pp. 229–240. [Google Scholar] [CrossRef]
- Horta, A.B.; Salgado, C.; Fernandes, M.; Vieira, S.; Sousa, J.M.; Papoila, A.L.; Xavier, M. Clinical Decision Support Tool for Co-Management Signalling. Int. J. Med. Inform. 2018, 113, 56–62. [Google Scholar] [CrossRef]
- Nair, S.; Hsu, D.; Celi, L.A. Challenges and Opportunities in Secondary Analyses of Electronic Health Record Data. In Secondary Analysis of Electronic Health Records; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Stower, H. Transparency in Medical AI. Nat. Med. 2020, 26, 1804. [Google Scholar] [CrossRef]
- Haibe-Kains, B.; Adam, G.A.; Hosny, A.; Khodakarami, F.; Shraddha, T.; Kusko, R.; Sansone, S.-A.; Tong, W.; Wolfinger, R.D.; Mason, C.E.; et al. Transparency and Reproducibility in Artificial Intelligence. Nature 2020, 586, E14–E16. [Google Scholar] [CrossRef]
- Blischak, J.D.; Davenport, E.R.; Wilson, G. A Quick Introduction to Version Control with Git and GitHub. PLoS Comput. Biol. 2016, 12, e1004668. [Google Scholar] [CrossRef]
- Peiffer-Smadja, N.; Rawson, T.M.; Ahmad, R.; Buchard, A.; Pantelis, G.; Lescure, F.X.; Birgand, G.; Holmes, A.H. Machine Learning for Clinical Decision Support in Infectious Diseases: A Narrative Review of Current Applications. Clin. Microbiol. Infect. 2020, 26, 584–595. [Google Scholar] [CrossRef]
- Zhou, H.; Hartford, A.; Tsai, K. A Bayesian Approach for PK/PD Modeling with PD Data below Limit of Quantification. J. Biopharm. Stat. 2012, 22, 1220–1243. [Google Scholar] [CrossRef]
- Dansirikul, C.; Morris, R.G.; Tett, S.E.; Duffull, S.B. A Bayesian Approach for Population Pharmacokinetic Modelling of Sirolimus. Br. J. Clin. Pharmacol. 2006, 62, 420–434. [Google Scholar] [CrossRef]
- You, W.; Widmer, N.; De Micheli, G. Example-Based Support Vector Machine for Drug Concentration Analysis. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August 2011–3 September 2011; pp. 153–157. [Google Scholar] [CrossRef]
- You, W.; Simalatsar, A.; Widmer, N.; Micheli, G. De Personalized Drug Administrations Using Support Vector Machine: A New Approach in Computer-Aided Dose Analysis. Bionanoscience 2013, 3, 378–393. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Chung, C.-R.; Chen, C.-J.; Lu, K.-P.; Tseng, Y.-J.; Chang, T.-H.; Wu, M.-H.; Huang, W.-T.; Lin, T.-W.; Liu, T.-P.; et al. Clinically Applicable System for Rapidly Predicting Enterococcus Faecium Susceptibility to Vancomycin. Microbiol. Spectr. 2021, 9, e00913-21. [Google Scholar] [CrossRef]
- Wang, G.; Giannakeas, P.; Schmelcher, P.; Farhana, N.A.; Afendi, F.M.; Fitrianto, A.; Wijaya, S.H. Classification Modeling of Support Vector Machine (SVM) and Random Forest in Predicting Pharmacodynamics Interactions. J. Phys. Conf. Ser. 2021, 1863, 012067. [Google Scholar] [CrossRef]
- Keutzer, L.; You, H.; Farnoud, A.; Nyberg, J.; Wicha, S.G.; Maher-Edwards, G.; Vlasakakis, G.; Moghaddam, G.K.; Svensson, E.M.; Menden, M.P.; et al. Machine Learning and Pharmacometrics for Prediction of Pharmacokinetic Data: Differences, Similarities and Challenges Illustrated with Rifampicin. Pharmaceutics 2022, 14, 1530. [Google Scholar] [CrossRef]
- Kim, D.; Choi, H.S.; Lee, D.H.; Kim, M.; Kim, Y.; Han, S.S.; Heo, Y.; Park, J.H.; Park, J. A Deep Learning-Based Approach for Prediction of Vancomycin Treatment Monitoring: Retrospective Study among Patients with Critical Illness. JMIR Form. Res. 2024, 8, e45202. [Google Scholar] [CrossRef]
- Ng, C.; Xiao, Y.; Putnam, W.; Lum, B.; Tropsha, A. Quantitative Structure-Pharmacokinetic Parameters Relationships (QSPKR) Analysis of Antimicrobial Agents in Humans Using Simulated Annealing k-Nearest-Neighbor and Partial Least-Square Analysis Methods. J. Pharm. Sci. 2004, 93, 2535–2544. [Google Scholar] [CrossRef]
- Peng, J.; Li, J.; Shang, X. A Learning-Based Method for Drug-Target Interaction Prediction Based on Feature Representation Learning and Deep Neural Network. BMC Bioinform. 2020, 21, 394. [Google Scholar] [CrossRef]
- Wang, Y.B.; You, Z.H.; Yang, S.; Yi, H.C.; Chen, Z.H.; Zheng, K. A Deep Learning-Based Method for Drug-Target Interaction Prediction Based on Long Short-Term Memory Neural Network. BMC Med. Inform. Decis. Mak. 2020, 20, 49. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef]
- Kim, J.I.; Maguire, F.; Tsang, K.K.; Gouliouris, T.; Peacock, S.J.; McAllister, T.A.; McArthur, A.G.; Beiko, R.G. Machine Learning for Antimicrobial Resistance Prediction: Current Practice, Limitations, and Clinical Perspective. Clin. Microbiol. Rev. 2022, 35, e0017921. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, H.; Shen, M. Identifying Antibiotic Resistance in Pathogenic Bacteria through SVM and Neural Network Predictive Models. In Proceedings of the 4th International Conference on Computer, Big Data and Artificial Intelligence, Guiyang, China, 15–17 December 2023; pp. 571–575. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Allen, P.G.; Lee, S.-I. A Unified Approach to Interpreting Model Predictions. In Advances in Neural Information Processing Systems 30 (NIPS 2017); Guyon, I., Von Luxburg, U., Bengio, S., Wallach, H., Fergus, R., Vishwanathan, S., Garnett, R., Eds.; NeurIPS Proceedings: Long Beach, CA, USA, 2017. [Google Scholar]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.I. From Local Explanations to Global Understanding with Explainable AI for Trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef]
- Topol, E.J. High-Performance Medicine: The Convergence of Human and Artificial Intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Dunn, J.; Runge, R.; Snyder, M. Wearables and the Medical Revolution. Per Med. 2018, 15, 429–448. [Google Scholar] [CrossRef]
- Wieringa, A.; Ewoldt, T.M.J.; Gangapersad, R.N.; Gijsen, M.; Parolya, N.; Kats, C.J.A.R.; Spriet, I.; Endeman, H.; Haringman, J.J.; van Hest, R.M.; et al. Predicting Beta-Lactam Target Non-Attainment in ICU Patients at Treatment Initiation: Development and External Validation of Three Novel (Machine Learning) Models. Antibiotics 2023, 12, 1674. [Google Scholar] [CrossRef]
- Bachmann, F.; Koch, G.; Pfister, M.; Szinnai, G.; Schropp, J. OptiDose: Computing the Individualized Optimal Drug Dosing Regimen Using Optimal Control. J. Optim. Theory Appl. 2021, 189, 46. [Google Scholar] [CrossRef]
- Sadiq, M.W.; Nielsen, E.I.; Khachman, D.; Conil, J.M.; Georges, B.; Houin, G.; Laffont, C.M.; Karlsson, M.O.; Friberg, L.E. A Whole-Body Physiologically Based Pharmacokinetic (WB-PBPK) Model of Ciprofloxacin: A Step towards Predicting Bacterial Killing at Sites of Infection. J. Pharmacokinet. Pharmacodyn. 2017, 44, 69–79. [Google Scholar] [CrossRef]

| Algorithm | Aim/Target/Study | Advantages | Limitations |
|---|---|---|---|
| Bayesian/WinBUGS version 1.4 | To handle data below the limit of quantification [127] | Prior information from the literature can be used directly for model-fitting; easy implementation. | Long computational time. Negative data in certain PK/PD models, which are not possible. |
| Bayesian/PKBUGS (version 1.1)/WinBUGS (version 1.3) | Pharmacokinetic analysis of sirolimus concentration data for therapeutic drug monitoring [128] | Easy incorporation of prior information with current data; identification of possible covariate relationship. | A limited number of datasets and poorly informative data. |
| Support Vector Machine/Least-Square SVM | Drug concentration analysis of sample drug based on individual patient profile [129] | Personalized model for every new patient. SVM-based approaches are more accurate than the PK modeling method for predicting drug concentration. | Outliers in samples greatly affect the model, limiting its accuracy. |
| Support Vector Machine/Drug Administration Decision Support System (DADSS) and Random Sample Consensus (RANSAC) | Prediction of drug concentration, ideal dose, and dose intervals for a new patient [130] | More flexible and structurally adjustable. | The noise of the dataset impacts the overall predictivity of the algorithm. |
| Support Vector Machine/Random Forest Model/K means | A predictive model was developed and validated to distinguish Enterococcus faecium vancomycin-resistant strains using SVM, K means, and random forest (RF) [131] | Overall good classification performances for the isolates from the specimens, with mean accuracy, sensitivity, and specificity of 0.78, 0.79, and 0.77. | Susceptibility results must be confirmed by routine methods. |
| Support Vector System + Random Forrest Model | Pharmacodynamic drug interaction (PDI) based on side-effect similarity (SES), Chemical Similarity (CS), and target protein connectedness (TPC) [132] | PDI was predicted with an accuracy of 89.93% and an AUC value of 79.96%. | Requires more data processing and filtration. |
| Linear Regression (LASSO)/Gradient Boosting Machines/ XGBoost/Random Forest | Prediction of the plasma concentration-time series and area under the concentration vs. time curve from 0 to 24 h after repeated dosing of rifampicin [133] | Time-efficient analysis; improved method for covariate selection. | Risk of results not being clinically relevant. |
| XGBoost | Joint multilayer perceptron (JointMLP), a new deep-learning model for predicting vancomycin therapeutic drug monitoring (TDM) levels, comparing its performance with population pharmacokinetic models, extreme gradient boosting (XGBoost), and TabNet [134] | JointMLP model outperformed other models in predicting vancomycin TDM levels in internal and external datasets. | Further research is needed to compare the AUC/MIC range with this approach. |
| Simulated Annealing k-Nearest-Neighbor (SA-kNN)/Partial Least-Square (PLS)/Multiple Linear Regression (MLR)/Sybyl version 6.7 | Prediction of pharmacokinetic parameters of antimicrobial agents in humans, based on their molecular structure [135] | Cost-effective; requires smaller sample size. | Requires multiple model-generation methods. Interpretation of individual descriptors is almost impossible. |
| Drug Target Interaction Convolutional Neural Network (DTICNN) | Identification of the drug–target interactions and predict potential drug molecules [136] | Cost-effective; time-saving | Large datasets are required. |
| Deep Long Short-Term Memory (DeepLSTM) | Computational methods to validate the interaction between drugs and target [137] | Based on position-specific scoring matrix (PSSM) and Legendre moment (LM) (drug molecular substructure fingerprints). | Large datasets are required. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves Pereira, J.; Fernandes, J.; Mendes, T.; Gonzalez, F.A.; Fernandes, S.M. Artificial Intelligence to Close the Gap between Pharmacokinetic/Pharmacodynamic Targets and Clinical Outcomes in Critically Ill Patients: A Narrative Review on Beta Lactams. Antibiotics 2024, 13, 853. https://doi.org/10.3390/antibiotics13090853
Gonçalves Pereira J, Fernandes J, Mendes T, Gonzalez FA, Fernandes SM. Artificial Intelligence to Close the Gap between Pharmacokinetic/Pharmacodynamic Targets and Clinical Outcomes in Critically Ill Patients: A Narrative Review on Beta Lactams. Antibiotics. 2024; 13(9):853. https://doi.org/10.3390/antibiotics13090853
Chicago/Turabian StyleGonçalves Pereira, João, Joana Fernandes, Tânia Mendes, Filipe André Gonzalez, and Susana M. Fernandes. 2024. "Artificial Intelligence to Close the Gap between Pharmacokinetic/Pharmacodynamic Targets and Clinical Outcomes in Critically Ill Patients: A Narrative Review on Beta Lactams" Antibiotics 13, no. 9: 853. https://doi.org/10.3390/antibiotics13090853
APA StyleGonçalves Pereira, J., Fernandes, J., Mendes, T., Gonzalez, F. A., & Fernandes, S. M. (2024). Artificial Intelligence to Close the Gap between Pharmacokinetic/Pharmacodynamic Targets and Clinical Outcomes in Critically Ill Patients: A Narrative Review on Beta Lactams. Antibiotics, 13(9), 853. https://doi.org/10.3390/antibiotics13090853







