Synergistic Antimicrobial Activity of Biogenic Silver Nanoparticles and Acanthospermum australe Essential Oil against Skin Infection Pathogens
Abstract
1. Introduction
2. Results
2.1. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis of Essential Oil
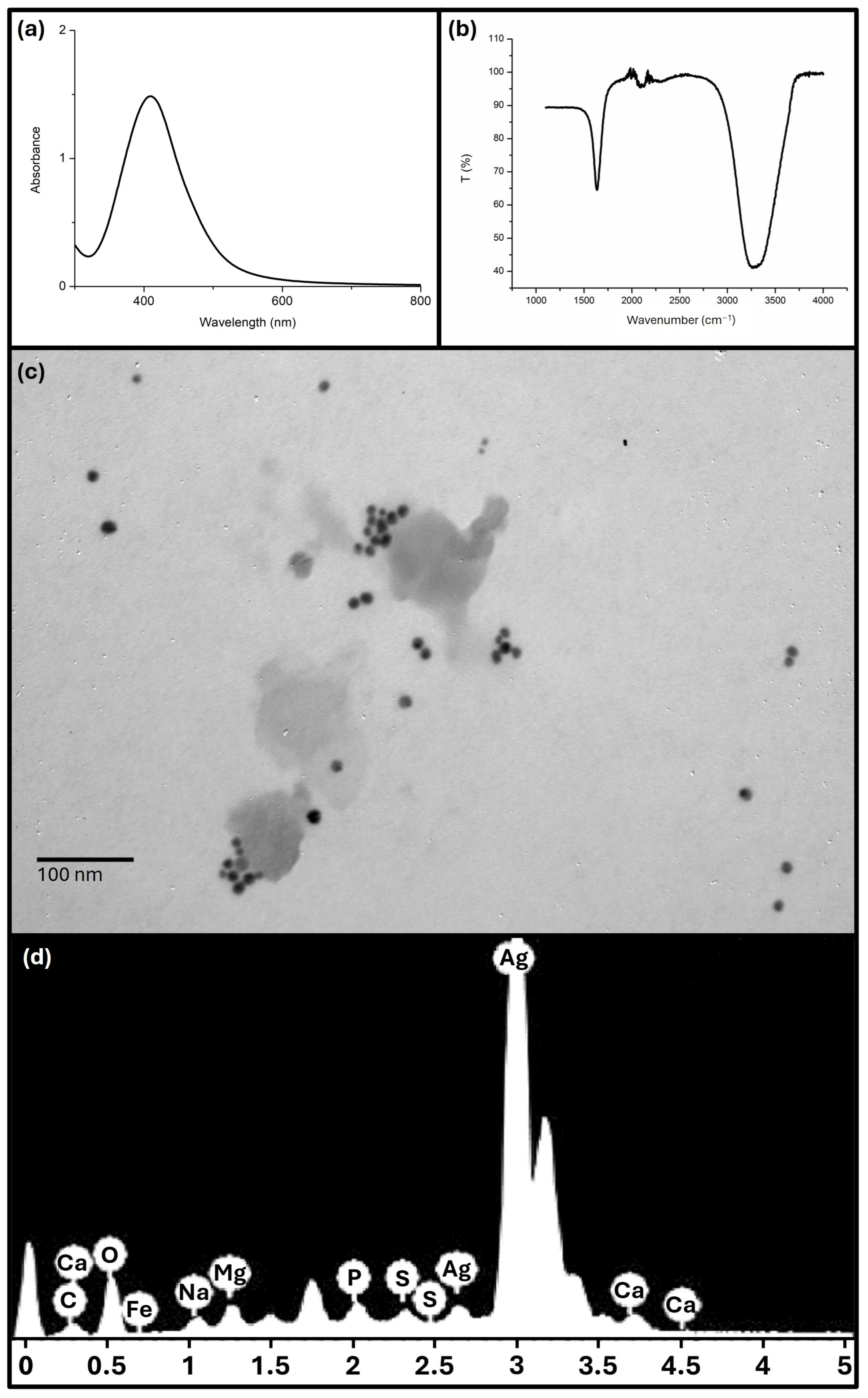
2.2. Synthesis and Characterisation of Silver Nanoparticles
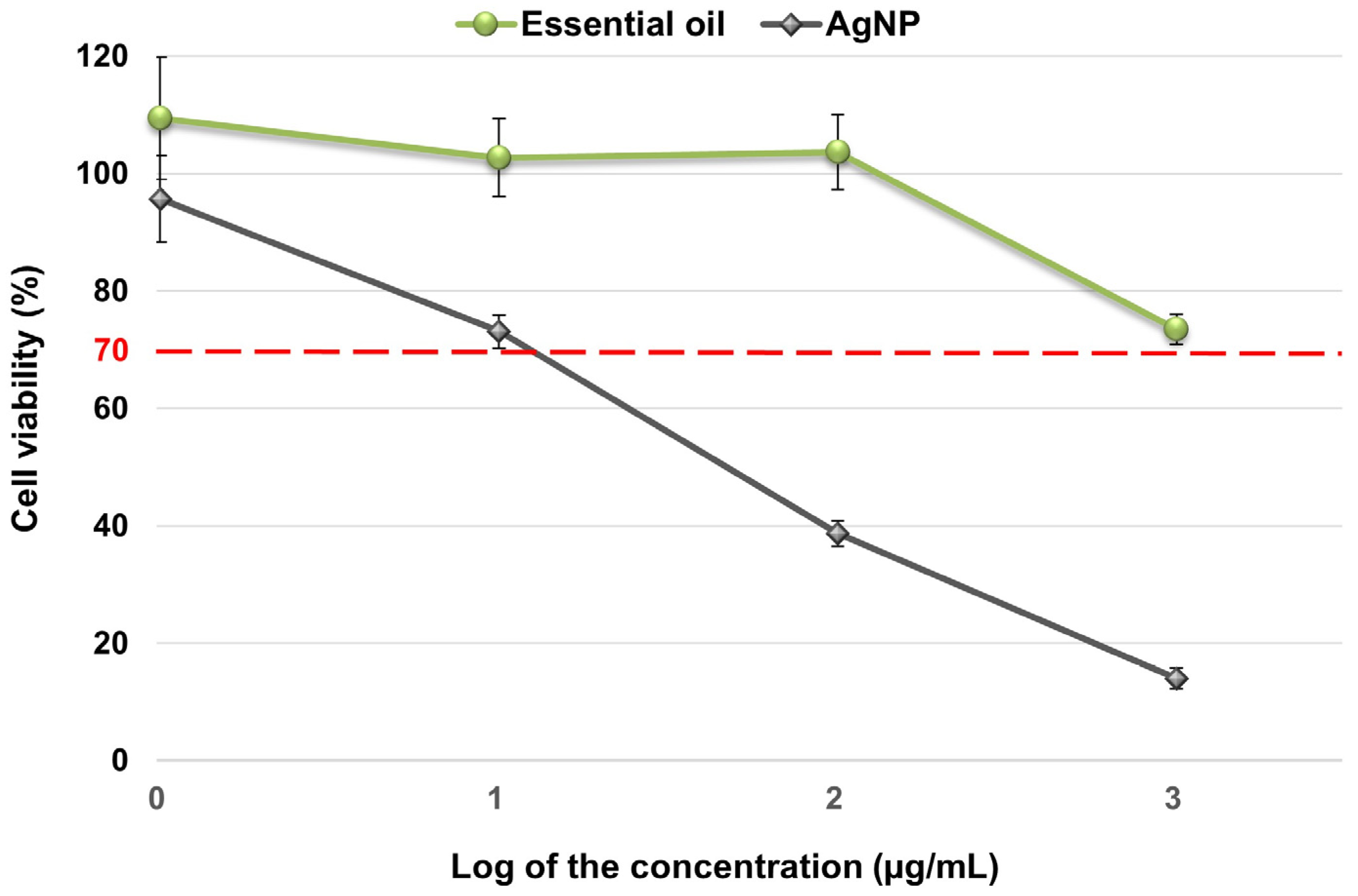
2.3. Cytotoxicity Assay of the Essential Oil and Silver Nanoparticles
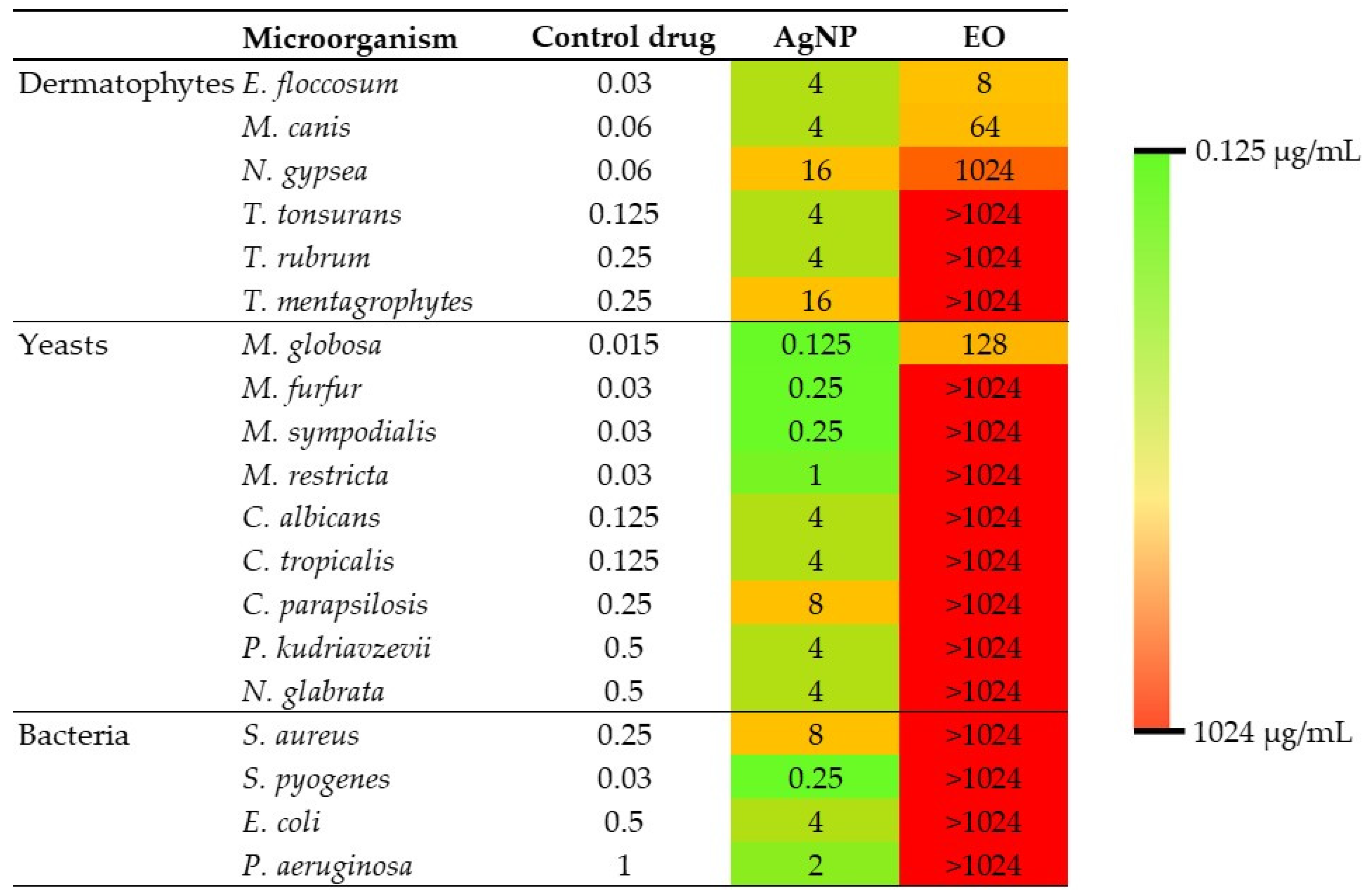
2.4. Antimicrobial Activity of Essential Oil and Silver Nanoparticles
2.5. Synergy Testing
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Acanthospermum Australe Essential Oil
4.3. GC–MS Analysis
4.4. Green Synthesis of Silver Nanoparticles
4.5. Characterisation of Silver Nanoparticles
4.6. Cytotoxicity Assay
4.7. Microorganisms
4.8. Antimicrobial Activity
4.9. Synergy Testing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clebak, K.T.; Malone, M.A. Skin Infections. Prim. Care—Clin. Off. Pract. 2018, 45, 433–454. [Google Scholar] [CrossRef]
- Moffarah, A.S.; Mohajer, M.A.; Hurwitz, B.L.; Armstrong, D.G. Skin and Soft Tissue Infections. Microbiol. Spectr. 2016, 4, 1–16. [Google Scholar] [CrossRef]
- Kaushik, N.; Pujalte, G.G.A.; Reese, S.T. Superficial Fungal Infections. Prim. Care—Clin. Off. Pract. 2015, 42, 501–516. [Google Scholar] [CrossRef]
- Theelen, B.; Cafarchia, C.; Gaitanis, G.; Bassukas, I.D.; Boekhout, T.; Dawson, T.L. Malassezia Ecology, Pathophysiology, and Treatment. Med. Mycol. 2018, 56, S10–S25. [Google Scholar] [CrossRef]
- Mussin, J.E.; Roldán, M.V.; Rojas, F.; de Los Ángeles Sosa, M.; Pellegri, N.; Giusiano, G. Antifungal Activity of Silver Nanoparticles in Combination with Ketoconazole against Malassezia Furfur. AMB Express 2019, 9, 131. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022.
- Mussin, J.; Giusiano, G. Biogenic Silver Nanoparticles as Antifungal Agents. Front. Chem. 2022, 10, 1023542. [Google Scholar] [CrossRef]
- Banti, C.N.; Hadjikakou, S.K. Anti-Proliferative and Anti-Tumor Activity of Silver(I) Compounds. Metallomics 2013, 5, 569–596. [Google Scholar] [CrossRef]
- Mukkavalli, S.; Chalivendra, V.; Singh, B.R. Physico-Chemical Analysis of Herbally Prepared Silver Nanoparticles and Its Potential as a Drug Bioenhancer. OpenNano 2017, 2, 19–27. [Google Scholar] [CrossRef]
- Mussin, J.; Giusiano, G. Ethno–Phytopharmacology: Product Validation Process Based on Traditional Knowledge of Medicinal Plants. In Agricultural, Forestry and Bioindustry Biotechnology and Biodiscovery; Chong, P.A., Newman, D.J., Steinmacher, D.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 331–353. ISBN 9783030513580. [Google Scholar]
- Cantero-González, G.; Alvarenga, N.; Florentín-Pavía, M.M.; Gonzalez-Maldonado, P.; Sotelo, P.H. Antiviral Activity of Two Acanthospermum Species against Herpes Simplex Virus 1. J. Ethnopharmacol. 2023, 303, 115958. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Hilgert, N.I.; Keller, H.A.; Gil, G. Medicinal Plant Diversity and Inter-Cultural Interactions between Indigenous Guarani, Criollos and Polish Migrants in the Subtropics of Argentina. PLoS ONE 2017, 12, e0169373. [Google Scholar] [CrossRef]
- Svetaz, L.A.; Postigo, A.; Butassi, E.; Zacchino, S.A.; Sortino, M.A. Antifungal Drugs Combinations: A Patent Review 2000–2015. Expert Opin. Ther. Pat. 2016, 26, 439–453. [Google Scholar] [CrossRef]
- Dryden, M.; Lye, D.C.; Chan, M.; De Simone, G.; Bouza, E.; Esposito, S.; Segreti, J.; Yalcin, A.N.; Unal, S.; Bassetti, M.; et al. Hot Topics in the Diagnosis and Management of Skin and Soft-Tissue Infections. Int. J. Antimicrob. Agents 2016, 48, 19–26. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between Essential Oil Components and Antibiotics: A Review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar] [CrossRef]
- Ncube, B.; Finnie, J.F.; Van Staden, J. In Vitro Antimicrobial Synergism within Plant Extract Combinations from Three South African Medicinal Bulbs. J. Ethnopharmacol. 2012, 139, 81–89. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Angiolella, L.; Rojas, F.; Mussin, J.; Giusiano, G. Modulatory Effect of Origanum Vulgare Essential Oil and Carvacrol on Malassezia Spp. Virulence Factors. Med. Mycol. 2023, 61, myad026. [Google Scholar] [CrossRef]
- Morais, S.M.D.; Machado, M.I.L.; Machado, S.F.M.; Facundo, V.A. Essential Oil of Acanthospermum Australe DC. J. Essent. Oil Res. 1997, 9, 601–602. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid.-Based Complement Altern. Med. 2016, 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Michel, L.; Chaumont, J.-P.; Millet-Clerc, J. Use of Caryophyllene Oxide as an Antifungal Agent in an in Vitro Experimental Model of Onychomycosis. Mycopathologia 2000, 148, 79–82. [Google Scholar] [CrossRef]
- Doungchawee, J.; Kulsing, C.; Suekaew, N.; Pombejra, S.N.; Chavasiri, W.; Plabutong, N.; Thammahong, A.; Khotavivattana, T. Volatile Chemical Composition, Antibacterial and Antifungal Activities of Extracts from Different Parts of Globba Schomburgkii Hook. F. Chem. Biodivers. 2019, 16, e1900057. [Google Scholar] [CrossRef]
- Delgado-Altamirano, R.; López-Palma, R.I.; Monzote, L.; Delgado-Domínguez, J.; Becker, I.; Rivero-Cruz, J.F.; Esturau-Escofet, N.; Vázquez-Landaverde, P.A.; Rojas-Molina, A. Chemical Constituents with Leishmanicidal Activity from a Pink-Yellow Cultivar of Lantana Camara Var. Aculeata (L.) Collected in Central Mexico. Int. J. Mol. Sci. 2019, 20, 872. [Google Scholar] [CrossRef]
- Mussin, J.; Botero, V.R.; Pimentel, R.C.; Rojas, F.; Angiolella, L.; San, E.; Martínez, M.; Giusiano, G. Antimicrobial and Cytotoxic Activity of Green Synthesis Silver Nanoparticles Targeting Skin and Soft Tissue Infectious Agents. Sci. Rep. 2021, 11, 14566. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and Their Enhanced Antibacterial Property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef]
- Scandorieiro, S.; Rodrigues, B.C.D.; Nishio, E.K.; Panagio, L.A.; de Oliveira, A.G.; Durán, N.; Nakazato, G.; Kobayashi, R.K.T. Biogenic Silver Nanoparticles Strategically Combined With Origanum Vulgare Derivatives: Antibacterial Mechanism of Action and Effect on Multidrug-Resistant Strains. Front. Microbiol. 2022, 13, 842600. [Google Scholar] [CrossRef]
- McLafferty, F.W.; Stauffer, D.B.; Stenhagen, E.; Heller, S.R. National Bureau of Standards. In The Wiley/NBS Registry of Mass Spectral Data, 5th ed.; Wiley: Hoboken, NJ, USA, 1994; ISBN 0471628867. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Pub. Corp: Carol Stream, IL, USA, 2007; ISBN 1932633219. [Google Scholar]
- Mondello, L. Mass Spectra of Flavors and Fragrances of Natural and Synthetic Compounds; John Wiley & Sons Inc: Hoboken, NJ, USA, 2015; ISBN 111906984X. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. The NIST Chemistry WebBook: A Chemical Data Resource on the Internet. J. Chem. Eng. Data 2001, 46, 1059–1063. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, M27, 4th ed.; Clinical and Laboratory Standards Institute (CLSI): Malvern, PA, USA, 2017; ISBN 1-56238-826-6. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, M38, 3rd ed.; Clinical and Laboratory Standards Institute (CLSI): Malvern, PA, USA, 2017; ISBN 1-56238-830-4. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, M07, 11th ed.; Clinical and Laboratory Standards Institute (CLSI): Malvern, PA, USA, 2018; ISBN 1-56238-836-3. [Google Scholar]
- Moody, J.A. Synergism Testing: Broth Microdilution Checkerboard and Broth Macrodilution Methods. In Clinical Microbiology Procedures Handbook, 4th ed.; ASM Press: Washington, DC, USA, 2016; pp. 5.16.1–5.16.23. ISBN 9781555815271. [Google Scholar]
- Odds, F.C. Synergy, Antagonism, and What the Chequerboard Puts between Them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
| RT | LRI | Identified Compound Name | Area (%) |
|---|---|---|---|
| 6.811 | 933 | α-Thujene | 0.01 |
| 7.204 | 936 | α-Pinene | 5.53 |
| 7.724 | 950 | Camphene | 0.1 |
| 8.935 | 973 | Sabinene | 0.15 |
| 9.088 | 978 | β-Pinene | 1.26 |
| 9.682 | 986 | 6-Methyl-5-heptene-2-one | 0.03 |
| 9.908 | 989 | Myrcene | 0.61 |
| 11.23 | 1017 | α-Terpinene | 0.02 |
| 11.775 | 1024 | para-Cymene | 1.99 |
| 12.092 | 1030 | Limonene | 4.28 |
| 12.61 | 1038 | (Z)-β-Ocimene | 0.03 |
| 13.22 | 1048 | (E)-β-Ocimene | 0.07 |
| 13.54 | 1038 | 2,6-Dimethyl-5-heptenal | 0.03 |
| 13.778 | 1060 | γ-Terpinene | 0.03 |
| 15.119 | 1192 | Myrtanal | 0.01 |
| 15.637 | 1087 | Terpinolene | 0.04 |
| 16.52 | 1099 | Linalool | 0.04 |
| 16.814 | 1103 | Nonanal | 0.02 |
| 17.493 | 1119 | Octen-1-ol, acetate | 0.26 |
| 18.288 | 1110 | 3-Octyl acetate | 0.02 |
| 18.578 | 1134 | Limonene oxide <cis-> | 0.01 |
| 18.712 | 1134 | Mentha-2,8-dien-1-ol <cis-, para-> | 0.01 |
| 18.91 | 1138 | Limonene oxide <trans-> | 0.02 |
| 19.354 | 1149 | Isopulegol | 0.04 |
| 20.008 | 1151 | Menthone | 0.03 |
| 20.314 | 1154 | Citronellal | 3.19 |
| 23.736 | 1205 | Decanal | 0.02 |
| 24.478 | 1217 | trans-Carveol | 0.02 |
| 25.35 | 1228 | Citronellol | 0.06 |
| 25.713 | 1234 | Thymol methyl ether | 0.17 |
| 26.13 | 1242 | Carvone | 0.04 |
| 27.139 | 1255 | Geraniol | 0.09 |
| 28.176 | 1242 | Neral | 0.02 |
| 29.421 | 1290 | Thymol | 0.04 |
| 29.815 | 1300 | Carvacrol | 0.14 |
| 31.567 | 1329 | Silphiperfol-5-ene | 0.07 |
| 32.617 | 1337 | δ-Elemene | 0.56 |
| 33.842 | 1378 | Silphiperfol-6-ene | 0.34 |
| 35.238 | 1380 | β-Patchoulene | 0.44 |
| 35.606 | 1384 | β-Bourbonene | 0.04 |
| 36.08 | 1433 | β-Copaene | 0.1 |
| 36.242 | 1390 | β-Elemene | 0.59 |
| 38.324 | 1420 | trans-Caryophyllene | 14.97 |
| 38.701 | 1387 | β-Cubebene | 1.81 |
| 39.135 | 1494 | Bicyclogermacrene | 0.15 |
| 39.598 | 1481 | Germacrene D | 0.57 |
| 40.248 | 1453 | α-Humulene | 4.34 |
| 42.502 | 1515 | γ-Cadinene | 21.47 |
| 43.217 | 1501 | Germacrene A | 24.07 |
| 44.921 | 1523 | δ-Cadinene | 1.15 |
| 45.431 | 1550 | (E)-γ-Bisabolene | 0.83 |
| 51.784 | 1651 | Intermedeol<neo-> | 0.54 |
| 54.382 | 1681 | Germacra-4(15),5,10(14)-trien-1-α-ol | 0.97 |
| 63.13 | 1849 | Phytone | 0.97 |
| 69.397 | 1970 | Palmitic acid | 2.16 |
| 71.684 | 2116 | Phytol | 1.96 |
| 71.98 | 2134 | Linoleic acid | 0.73 |
| 72.248 | 2200 | Stearic acid | 0.16 |
| Microorganism | AgNP | EO | FICi | Interpretation | ||||
|---|---|---|---|---|---|---|---|---|
| MICc | FICAgNP | fd | MICc | FICEO | fd | |||
| E. floccosum | 2 | 0.5 | 2× | 4 | 0.5 | 2× | 1 | No interaction |
| M. canis | 1 | 0.25 | 4× | 8 | 0.125 | 8× | 0.375 | Synergism |
| N. gypsea | 2 | 0.125 | 8× | 256 | 0.25 | 4× | 0.375 | Synergism |
| M. globosa | 0.03 | 0.25 | 4× | 32 | 0.25 | 4× | 0.5 | Synergism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mussin, J.; Giusiano, G. Synergistic Antimicrobial Activity of Biogenic Silver Nanoparticles and Acanthospermum australe Essential Oil against Skin Infection Pathogens. Antibiotics 2024, 13, 674. https://doi.org/10.3390/antibiotics13070674
Mussin J, Giusiano G. Synergistic Antimicrobial Activity of Biogenic Silver Nanoparticles and Acanthospermum australe Essential Oil against Skin Infection Pathogens. Antibiotics. 2024; 13(7):674. https://doi.org/10.3390/antibiotics13070674
Chicago/Turabian StyleMussin, Javier, and Gustavo Giusiano. 2024. "Synergistic Antimicrobial Activity of Biogenic Silver Nanoparticles and Acanthospermum australe Essential Oil against Skin Infection Pathogens" Antibiotics 13, no. 7: 674. https://doi.org/10.3390/antibiotics13070674
APA StyleMussin, J., & Giusiano, G. (2024). Synergistic Antimicrobial Activity of Biogenic Silver Nanoparticles and Acanthospermum australe Essential Oil against Skin Infection Pathogens. Antibiotics, 13(7), 674. https://doi.org/10.3390/antibiotics13070674









