Longitudinal Trends in In-Patient Antibiotic Consumption According to the WHO Access, Watch, Reserve (AWaRe) Antibiotic Groups and Cost: An Analysis of Data at a National Antimicrobial Consumption Network (NAC-NET) Site in North India over 7 Years (2017–2023)
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Setting
4.2. Data Collection
4.3. Data Management and Analysis
- Number of defined daily doses (DDDs): DDD is the assumed average maintenance dose per day for a drug used for its main indication in adults. The number of DDDs is calculated as per the following formula:Number of DDDs = Number of items issued × amount of drug per item/WHO DDD measure.
- The bed occupancy rate (%) is calculated as follows:Bed occupancy rate = (Total number of in-patient days for a given period × 100)/(Number of available beds × number of days in that period).
- DDD/100 bed-days is calculated as follows:DDD/100 bed-days = (Number of DDDs × 100)/(Number of beds × number of days in the period × occupancy index).
4.4. Measures of Antibiotic Consumption
4.5. Statistical Analysis
4.5.1. Average Annual Percent Change (AAPC)
4.5.2. Average Monthly Percent Change (AMPC)
4.5.3. Average Quarterly Percent Change (AQPC)
4.6. Ethical and Administrative Approvals
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Cizman, M. The use and resistance to antibiotics in the community. Int. J. Antimicrob. Agents 2003, 21, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Iwamoto, K.; Hoxha, I.; Ghazaryan, L.; Abilova, V.; Cvijanovic, A.; Pyshnik, H.; Darakhvelidze, M.; Makalkina, L.; Jakupi, A.; et al. Antimicrobial Medicines Consumption in Eastern Europe and Central Asia—An Updated Cross-National Study and Assessment of Quantitative Metrics for Policy Action. Front. Pharmacol. 2019, 9, 1156. [Google Scholar] [CrossRef]
- Mölstad, S.; Erntell, M.; Hanberger, H.; Melander, E.; Norman, C.; Skoog, G.; Lundborg, C.S.; Söderström, A.; Torell, E.; Cars, O. Sustained reduction of antibiotic use and low bacterial resistance: 10-year follow-up of the Swedish Strama programme. Lancet Infect. Dis. 2008, 8, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Coenen, S.; Ferech, M.; Haaijer-Ruskamp, F.M.; Butler, C.C.; Stichele, R.H.V.; Verheij, T.J.M.; Monnet, D.L.; Little, P.; Goossens, P.L.H.; The ESAC Project Group. European Surveillance of Antimicrobial Consumption (ESAC): Quality indicators for outpatient antibiotic use in Europe. Qual. Saf. Health Care 2007, 16, 440–445. [Google Scholar] [CrossRef]
- Metz-Gercek, S.; Maieron, A.; Strauss, R.; Wieninger, P.; Apfalter, P.; Mittermayer, H. Ten years of antibiotic consumption in ambulatory care: Trends in prescribing practice and antibiotic resistance in Austria. BMC Infect. Dis. 2009, 9, 61. [Google Scholar] [CrossRef][Green Version]
- Kim, B.; Hwang, H.; Kim, J.; Lee, M.-J.; Pai, H. Ten-year trends in antibiotic usage at a tertiary care hospital in Korea, 2004 to 2013. Korean J. Intern. Med. 2020, 35, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Schultz, L.; Lowe, T.J.; Srinivasan, A.; Neilson, D.; Pugliese, G. Economic impact of redundant antimicrobial therapy in US hospitals. Infect. Control Hosp. Epidemiol. 2014, 35, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Gandra, S.; Ashok, A.; Caudron, Q.; Grenfell, B.T.; Levin, S.; Laxminarayan, R. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect. Dis. 2014, 14, 742–750. [Google Scholar] [CrossRef]
- Sulis, G.; Daniels, B.; Kwan, A.; Gandra, S.; Daftary, A.; Das, J.; Pai, M. Antibiotic overuse in the primary health care setting: A secondary data analysis of standardised patient studies from India, China and Kenya. BMJ Glob. Health 2020, 5, e003393. [Google Scholar] [CrossRef]
- Patel, V.; Vaidya, R.; Naik, D.; Borker, P. Irrational drug use in India: A prescription survey from Goa. J. Postgrad. Med. 2005, 51, 9–12. [Google Scholar] [PubMed]
- Kumari, I.K.S.; Chandy, S.J.; Jeyaseelan, L.; Kumar, R.; Suresh, S. Antimicrobial prescription patterns for common acute infections in some rural & urban health facilities of India. Indian J. Med. Res. 2008, 128, 165–171. [Google Scholar] [PubMed]
- Chandy, S.J.; Thomas, K.; Mathai, E.; Antonisamy, B.; Holloway, K.A.; Stalsby Lundborg, C. Patterns of antibiotic use in the community and challenges of antibiotic surveillance in a lower-middle-income country setting: A repeated cross-sectional study in Vellore, South India. J. Antimicrob. Chemother. 2013, 68, 229–236. [Google Scholar] [CrossRef] [PubMed]
- National Programme on AMR Containment. Available online: https://ncdc.mohfw.gov.in/national-programme-on-amr-containment/ (accessed on 15 June 2023).
- Ministry of Health and Family Welfare, Government of India. National Action Plan on AMR (NAP-AMR), 2017–2021. 2017. Available online: https://ncdc.mohfw.gov.in/national-action-plan-on-amr-nap-amr/ (accessed on 15 June 2023).
- WHO. Global Action Plan on Antimicrobial Resistance. 2015. Available online: https://apps.who.int/iris/handle/10665/193736 (accessed on 15 June 2023).
- Kotwani, A.; Holloway, K. Trends in antibiotic use among outpatients in New Delhi, India. BMC Infect. Dis. 2011, 11, 99. [Google Scholar] [CrossRef]
- Farooqui, H.H.; Selvaraj, S.; Mehta, A.; Heymann, D.L. Community level antibiotic utilization in India and its comparison vis-à-vis European countries: Evidence from pharmaceutical sales data. PLoS ONE 2018, 13, e0204805. [Google Scholar] [CrossRef]
- Mugada, V.; Mahato, V.; Andhavaram, D.; Vajhala, S.M. Evaluation of Prescribing Patterns of Antibiotics Using Selected Indicators for Antimicrobial Use in Hospitals and the Access, Watch, Reserve (AWaRe) Classification by the World Health Organization. Turk. J. Pharm. Sci. 2021, 18, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Fondevilla, E.; Grau, S.; Echeverría-Esnal, D.; Gudiol, F.; VINCat Program Group. Antibiotic consumption trends among acute care hospitals in Catalonia (2008–2016): Impact of different adjustments on the results. Expert. Rev. Anti-Infect. Ther. 2021, 19, 245–251. [Google Scholar] [CrossRef]
- Filius, P.M.; Liem, T.B.; van der Linden, P.D.; Janknegt, R.; Natsch, S.; Vulto, A.G.; Verbrugh, H.A. An additional measure for quantifying antibiotic use in hospitals. J. Antimicrob. Chemother. 2005, 55, 805–808. [Google Scholar] [CrossRef]
- Vandael, E.; Magerman, K.; Coenen, S.; Goossens, H.; Catry, B. Antibiotic consumption in Belgian acute care hospitals: Analysis of the surveillance methodology, consumption evolution 2003 to 2016 and future perspectives. Eurosurveillance 2019, 24, 1900098. [Google Scholar] [CrossRef]
- Neves e Castro, P.B.; da Silva Rodrigues, D.A.; Roeser, H.M.P.; da Fonseca Santiago, A.; de Cássia Franco Afonso, R.J. Antibiotic consumption in developing countries defies global commitments: An overview on Brazilian growth in consumption. Environ. Sci. Pollut. Res. Int. 2020, 27, 21013–21020. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, Y.; Temkin, E.; Ben-David, D.; Carmeli, Y.; Schwaber, M.J. Antimicrobial use trends, Israel, 2012 to 2017. Eurosurveillance 2019, 24, 1900022. [Google Scholar] [CrossRef]
- Chen, J.; Min, R.; Wang, H.; Zhao, S.; Li, H.; Fang, P. Trends and Drivers of Inpatient Antibiotic Consumption among 89 China Tertiary General Hospitals from 2011Q1 to 2015Q4. BioMed Res. Int. 2018, 2018, 5968653. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Global Health Observatory. Target ≥ 60% of Total Antibiotic Consumption Being Access Group Antibiotics (GPW 13 Target 4b). Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/5767 (accessed on 13 February 2024).
- WHO 2021 AwaRe Classification. Available online: https://www.who.int/publications/i/item/2021-aware-classification (accessed on 13 February 2024).
- Mittal, N.; Mittal, R.; Parmar, A.; Bahl, A.; Kaur, S.; Gudibanda, K.R.; Dudhraj, V.; Singh, S.K. WHO-Point Prevalence Survey of Antibiotic Use Among Inpatients at a Core National Antimicrobial Consumption Network Site in North India: Findings and Implications. Microb. Drug Resist. 2023, 29, 1–9. [Google Scholar] [CrossRef]
- Sulis, G.; Batomen, B.; Kotwani, A.; Pai, M.; Gandra, S. Sales of antibiotics and hydroxychloroquine in India during the COVID-19 epidemic: An interrupted time series analysis. PLoS Med. 2021, 18, e1003682. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Papanas, N.; Kioumis, I.; Chatzaki, E.; Maltezos, E.; Zarogoulidis, K. Macrolides: From in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur. J. Clin. Pharmacol. 2012, 68, 479–503. [Google Scholar] [CrossRef]
- Al Sulaiman, K.; Aljuhani, O.; Korayem, G.B.; Alnajjar, L.I.; Altebainawi, A.F.; AlFaifi, M.; Vishwakarma, R.; Alenazi, A.A.; Alalawi, M.; Alissa, A.; et al. Doxycycline’s Potential Role in Reducing Thrombosis and Mortality in Critically Ill Patients with COVID-19: A Multicenter Cohort Study. Clin. Appl. Thromb. Hemost. 2023, 29, 10760296231177017. [Google Scholar] [CrossRef] [PubMed]
- Padhan, S.; Pugazhenthan, T.; Chandrakar, R.; Galhotra, A.; Borkar, N.B. Assessment of the Impact of COVID-19 on Drug Store Management in a Tertiary Care Teaching Hospital of Central India. Cureus 2021, 13, e19723. [Google Scholar] [CrossRef]
- Fukushige, M.; Ngo, N.H.; Lukmanto, D.; Fukuda, S.; Ohneda, O. Effect of the COVID-19 pandemic on antibiotic consumption: A systematic review comparing 2019 and 2020 data. Front. Public Health 2022, 10, 946077. [Google Scholar] [CrossRef]
- Morris, A.M. Antimicrobial Stewardship Programs: Appropriate Measures and Metrics to Study their Impact. Curr. Treat. Options Infect. Dis. 2014, 6, 101–112. [Google Scholar] [CrossRef]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Mittal, R.; Singh, S.; Godara, S. The Availability of Essential Antimicrobials in Public and Private Sector Facilities: A Cross-Sectional Survey in a District of North India. Antibiotics 2024, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- AMC Tool: The Antimicrobial Consumption Tool. Available online: https://amu-tools.org/amctool/amctool.html (accessed on 20 June 2023).
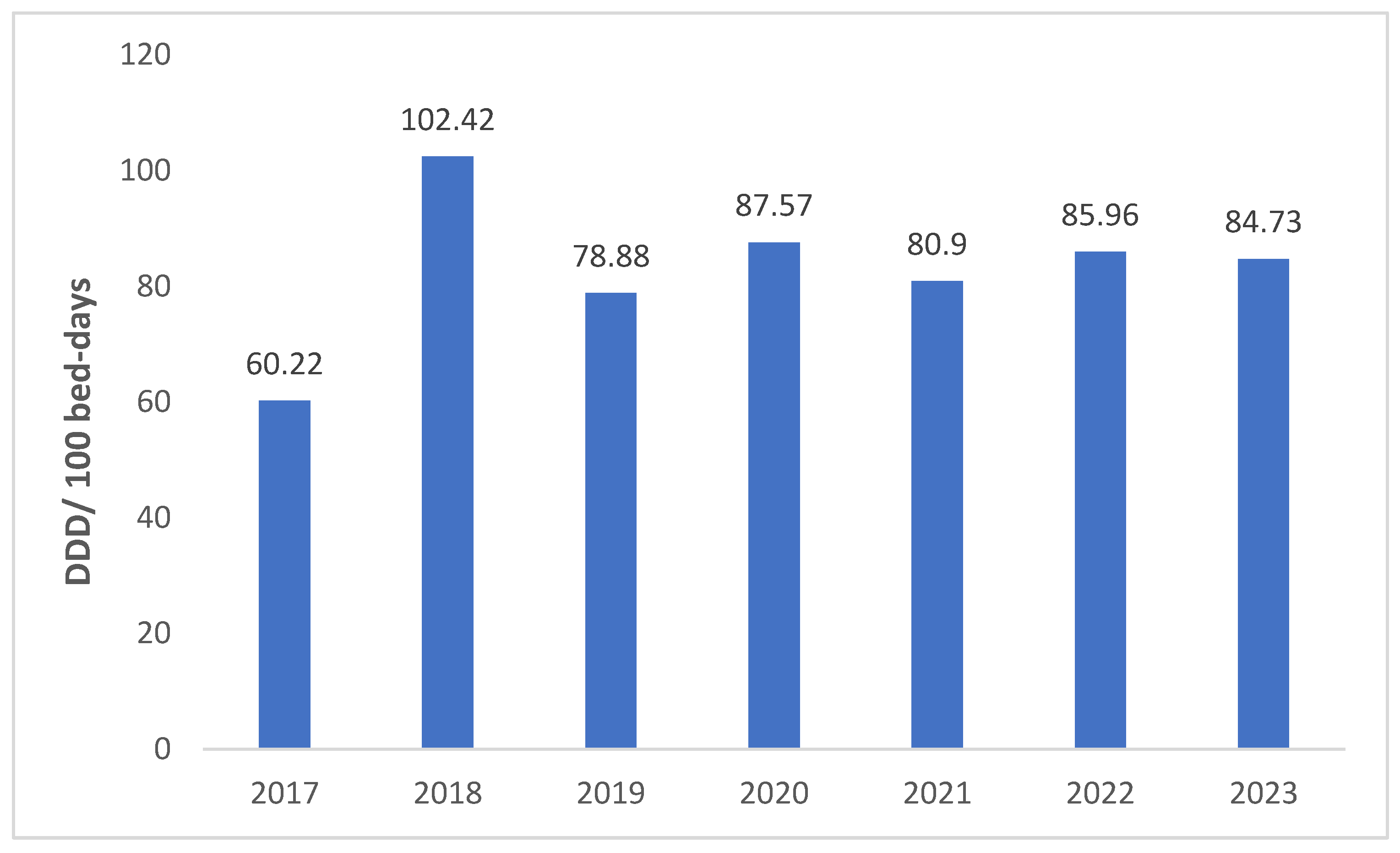
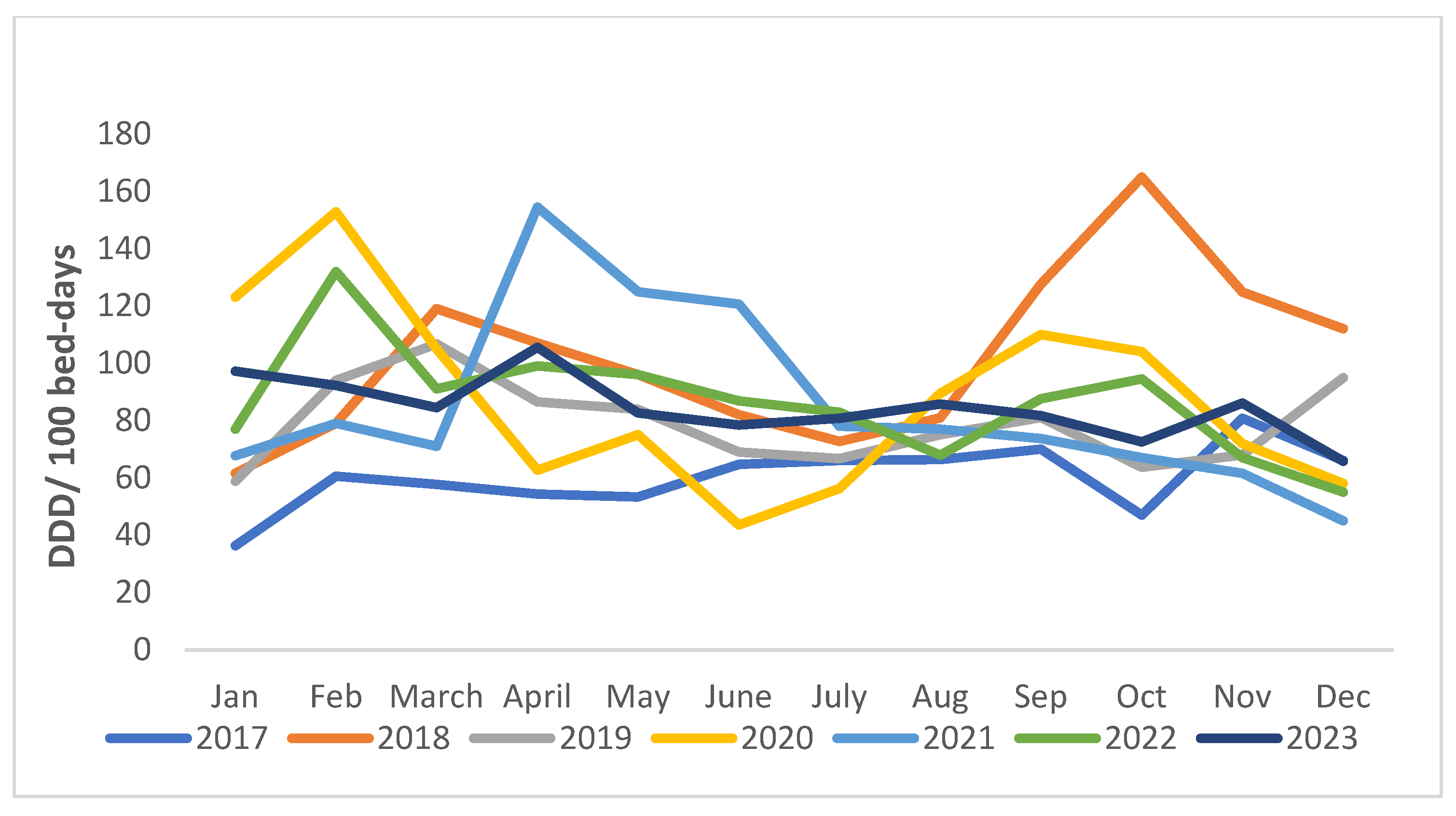
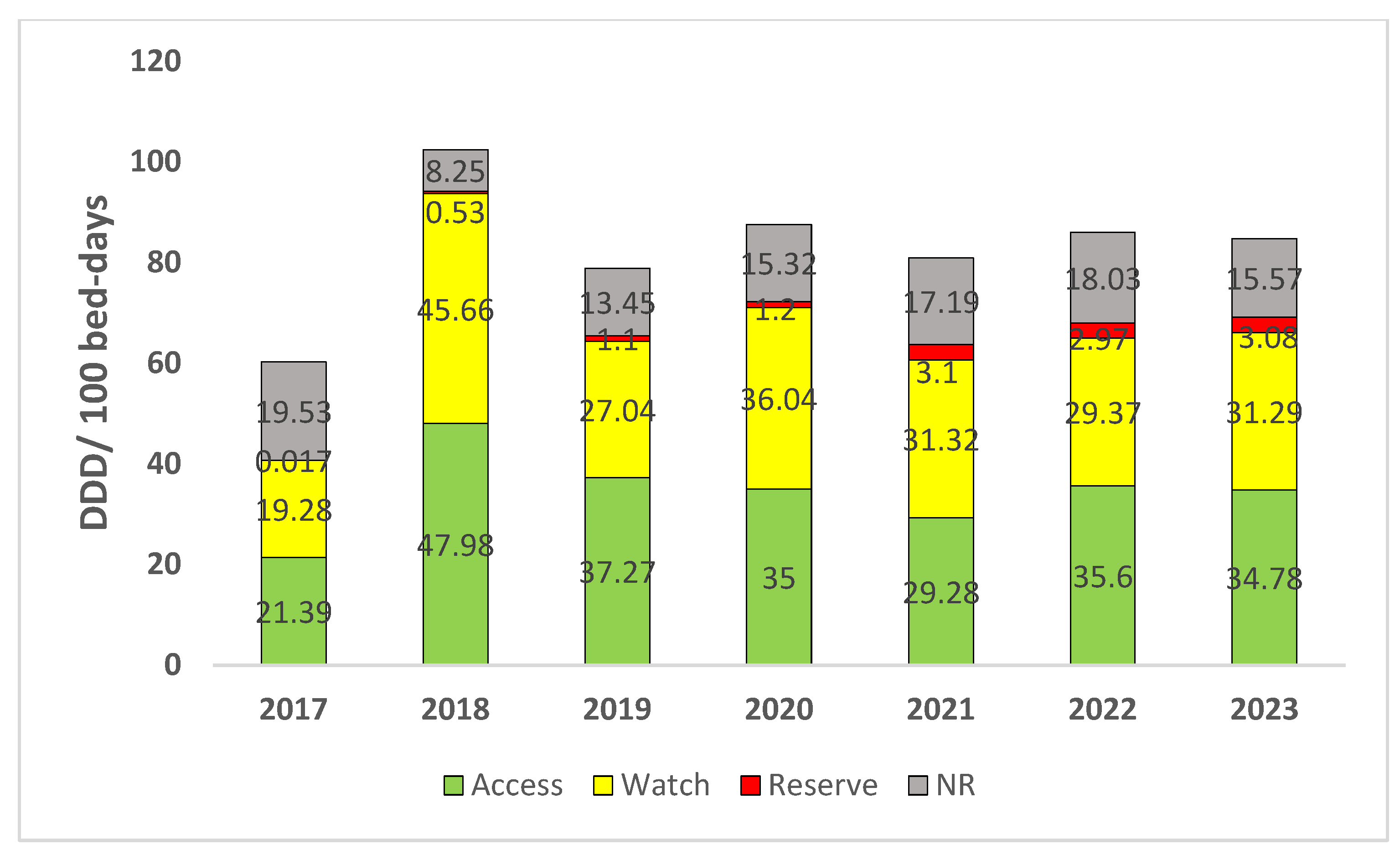
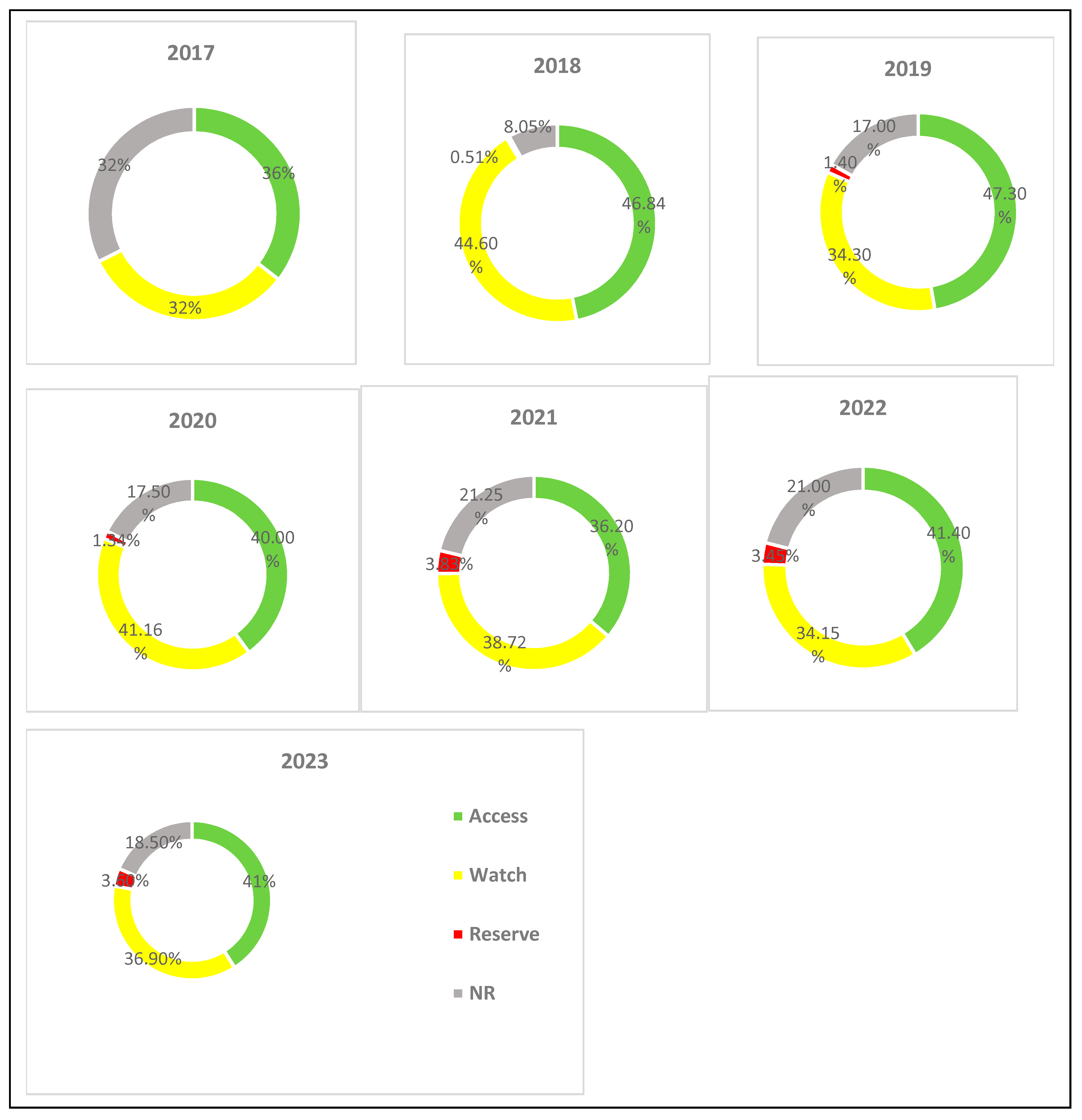
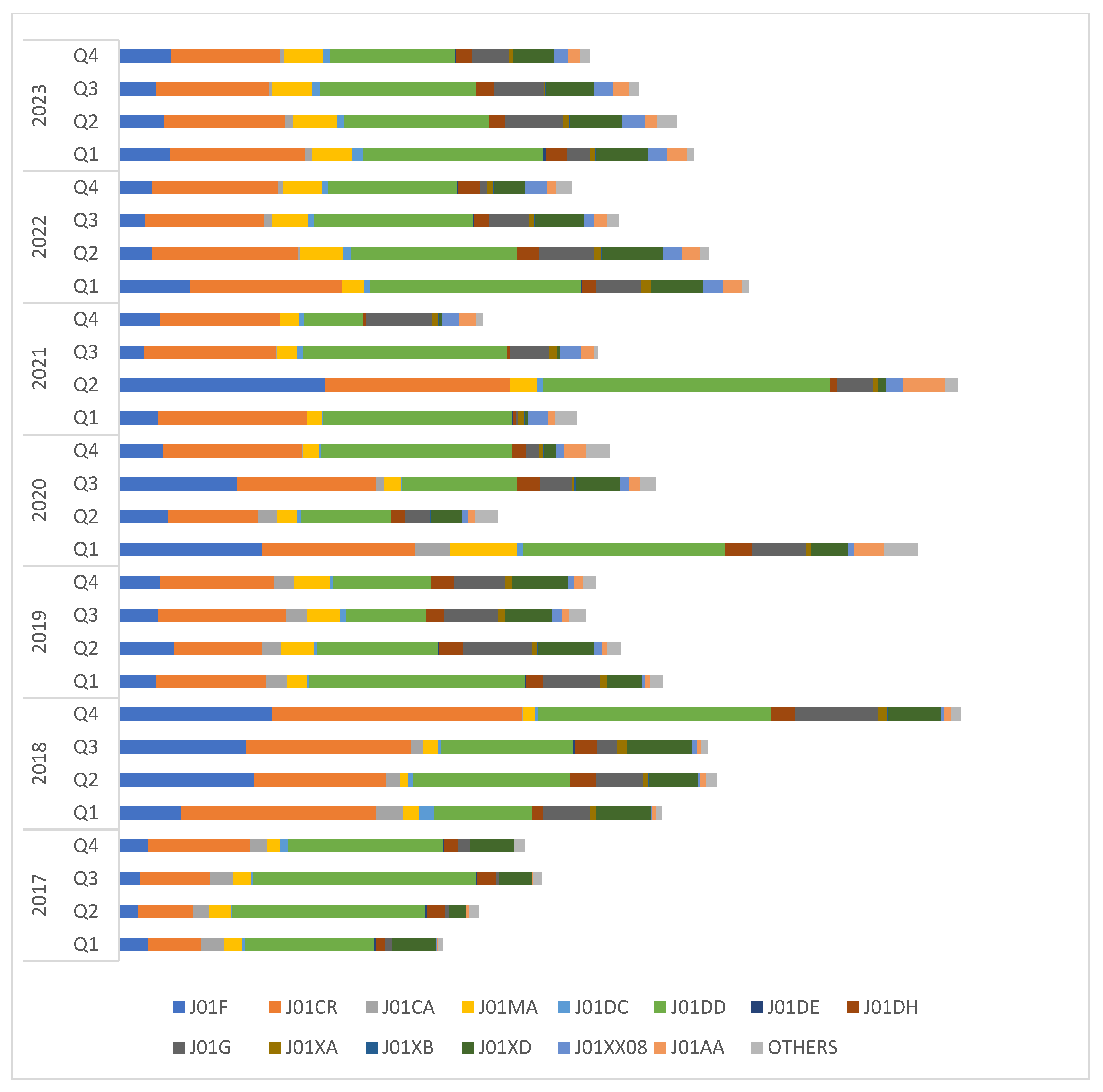
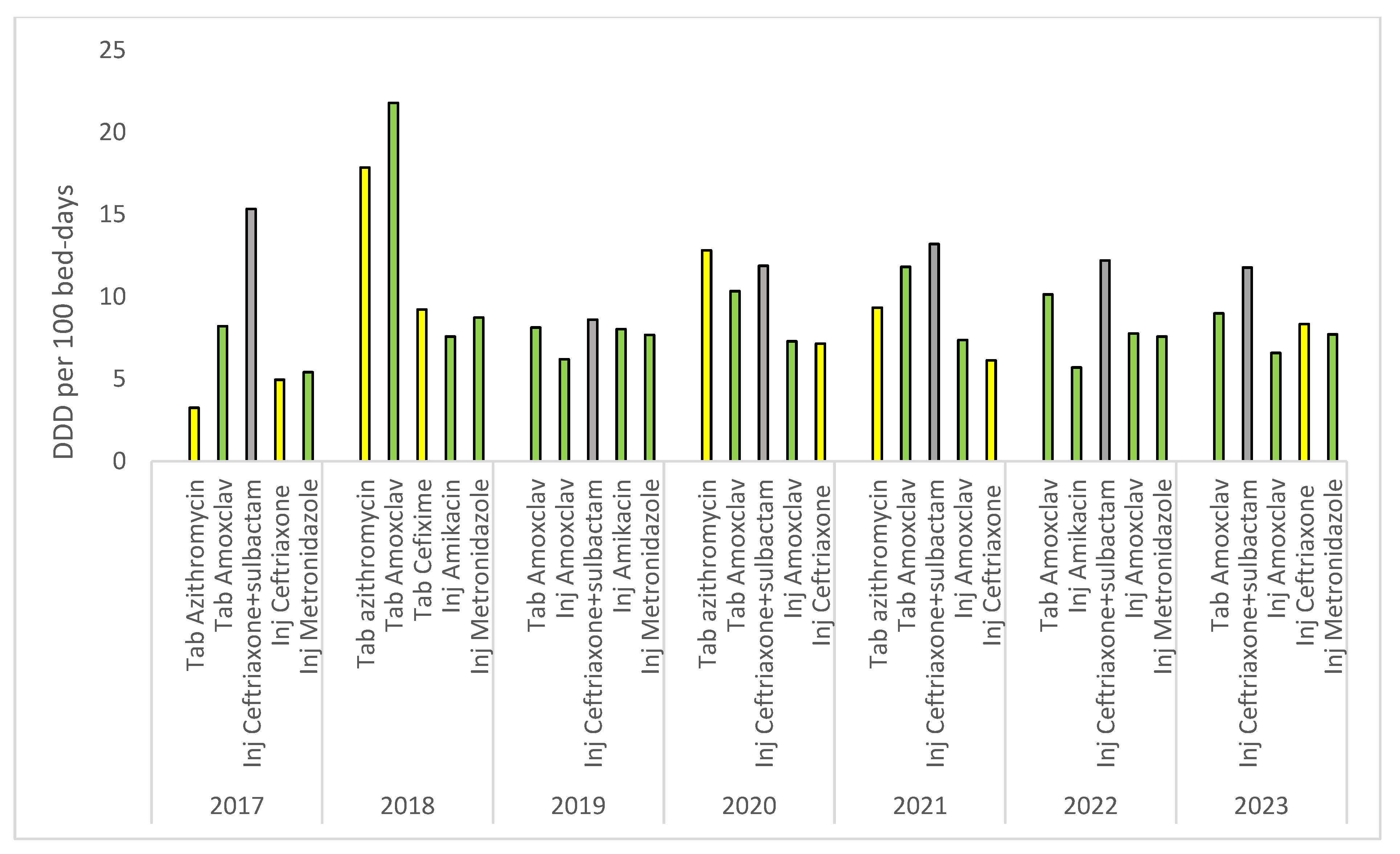
| Class of Antibiotics | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | Overall Consumption | AAPC (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| J01F: Macrolides and lincosamides | 46.8 | 228.7 | 83.5 | 169.6 | 149.4 | 78.1 | 89 | 845.1 | 63.77 (−61.92, 189.46) |
| J01CR: Penicillin/beta-lactamase inhibitors | 134 | 353.4 | 209.5 | 248 | 279.2 | 259.2 | 228.2 | 1711.5 | 22.47 (−31.07, 76.02) |
| J01CA: Extended spectrum penicillin | 38 | 25.9 | 38 | 29.96 | 0.1 | 6.5 | 10.3 | 148.76 | 1058.75 (−880.13, 2997.64) |
| J01MA: Fluoroquinolones | 34 | 24.1 | 58 | 57.76 | 38.6 | 67.4 | 77.2 | 357.06 | 27.85 (−22.24, 77.94) |
| J01DC: Second-generation cephalosporins | 6.4 | 12 | 7.3 | 6.29 | 9.05 | 12.7 | 16.4 | 70.14 | 24.64 (−8.72, 58) |
| J01DD: Third-generation cephalosporins | 334 | 295.3 | 245.1 | 284.3 | 351.5 | 316.8 | 288.1 | 2115.1 | −1.31 (−13.74, 11.11) |
| J01DE: Fourth-generation cephalosporins | 2.12 | 0.97 | 1.2 | 0 | 0.9 | 0.96 | 2.48 | 8.63 | -- |
| J01DH: Carbapenems | 28.8 | 40.1 | 39.13 | 37.5 | 6.8 | 35.5 | 33.4 | 221.23 | 61.15 (−73.01, 195.32) |
| J01G: Aminoglycosides | 12.76 | 93.3 | 109.8 | 59.75 | 69.05 | 69.4 | 80.2 | 494.26 | 105.82 (−85.67, 297.31) |
| J01XA: Glycopeptide anti-bacterials | 0 | 14.4 | 12.5 | 5.17 | 11.47 | 13.8 | 8 | 65.34 | -- |
| J01XB: Polymyxins | 0.14 | 1.6 | 0.26 | 1.47 | 1.42 | 1.8 | 1.28 | 7.97 | 236.49 (−90.99, 563.98) |
| J01XD: Imidazole derivatives | 65.6 | 106.2 | 92.5 | 59.45 | 7.69 | 91.2 | 92.1 | 514.74 | 168.86 (−165.91, 503.62) |
| J01XX08: Linezolid | 0.6 | 4.63 | 13 | 12.93 | 36.1 | 33.4 | 35.7 | 136.36 | 171.75 (−21.15, 364.65) |
| J01AA: Tetracyclines | 2.03 | 9.7 | 12.3 | 33.86 | 37.8 | 28.5 | 28.3 | 152.49 | 94.38 (−21.1, 209.85) |
| Others | 16.4 | 15.5 | 26.5 | 45.8 | 21.3 | 20.3 | 21.5 | 167.3 | 14.34 (−22.04, 50.71) |
| Class of Antibiotics | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 |
|---|---|---|---|---|---|---|---|
| J01F | 4.47 (−32.09, 41.02) | 43.26 (−18.94, 105.46) | 8.06 (−29.02, 45.14) | 4.38 (−111.97, 120.72) | 131.57 (−123.68, 386.82) | −15.27 (−56.16, 25.62) | 3.54 (−26.2, 33.28) |
| J01CR | 26 (5.44, 46.57) | 14.58 (−27.18, 56.34) | 4.59 (−30.16, 39.34) | 4.44 (−41.86, 50.74) | −4.58 (−31.11, 21.95) | −5.45 (−17.31, 6.40) | −6.93 (−10.45, −3.41) |
| J01CA | −4.15 (−47.35, 39.05) | −49.66 (−90.8, −8.51) | −1.81 (−9.98, 6.36) | −66.49 (−94.39, −38.58) | -- | -- | −5.19 (−58.26, 47.88) |
| J01MA | −6.97 (−33.61, 19.67) | 5.21 (−63.58, 74) | 26.17 (−9.34, 61.68) | −28.72 (−65.15, 7.72) | 17.42 (−39.98, 74.82) | 25.65 (−26.03, 77.34) | 0.11 (−9.1, 9.32) |
| J01DC | 105.24 (−74.54, 285.02) | −35.27 (−68.1, −2.44) | 34.72 (−32.88, 102.32) | −27.15 (−66.19, 11.89) | 94.21 (−80.46, 268.87) | 8.46 (−24.98, 41.89) | −10.93 (−36.4, 14.54) |
| J01DD | 11.42 (−27.65, 50.5) | 40.53 (−8.43, 89.48) | −18.35 (−53.68, 16.97) | 12.96 (−48.23, 74.16) | −16.14 (−77.28, 45.01) | −14.84 (−24.08, −5.6) | −10.73 (−25.99, 4.53) |
| J01DE | −15.01 (−53.29, 23.27) | -- | -- | -- | 38.89 (−98.09, 175.87) | 13.33 (−65.88, 92.54) | −2.22 (−79.31, 74.87) |
| J01DH | 24.72 (−37.74, 87.19) | 38.52 (−34.22, 111.26) | 13.58 (−17.62, 44.77) | −7.86 (−70.74, 55.02) | 10.79 (−77.87, 99.46) | 25.4 (−25.5, 76.31) | −7.05 (−28.55, 14.45) |
| J01G | 89.33 (−124.45, 303.12) | 87.32 (−113.04, 287.69) | −3.08 (−22.77, 16.61) | −27.51 (−74.22, 19.2) | 499.66 (−283.53, 1282.86) | −29.21 (−81.4, 22.97) | 39.96 (−61.94, 141.87) |
| J01XA | -- | 23.74 (−22.66, 70.13) | 6.82 (−3.64, 17.28) | 301.26 (−215.9, 818.42) | 14.97 (−57.24, 87.17) | −13.53 (−42.71, 15.64) | 185.07 (−198.58, 568.72) |
| J01XB | -- | 44.44 (−30.99, 119.88) | -- | -- | -- | -- | −24.47 (−91.03, 42.08) |
| J01XD | 23.23 (−57.21, 103.68) | 0.96 (−24.74, 26.65) | 20.67 (−16.74, 58.08) | −16.28 (−71.69, 39.14) | 62.89 (−103.06, 228.83) | −13.35 (−39.91, 13.22) | −7.63 (−14.73, −0.54) |
| J01XX08 | -- | 177.32 (−5.6, 360.24) | 35.6 (−45.37, 116.57) | 16.37 (−27.86, 60.6) | −3.84 (−25.76, 18.08) | 23.42 (−62.44, 109.29) | −6.66 (−34.97, 21.65) |
| J01AA | 80.49 (−219, 379.97) | 33.39 (−38.25, 105.04) | 29.29 (20.13, 38.44) | 25.63 (−67.93, 119.19) | 155.91 (−145.17, 456.98) | −21.6 (−37.6, −5.6) | −8.34 (−53.49, 37.11) |
| 0thers | 36.37 (−25.34, 98.08) | 32.26 (−34.64, 99.17) | 2.78 (−25.21, 30.76) | −4.49 (−49.62, 40.65) | −19.47 (−79.68, 40.74) | 35.75 (32.14, 39.37) | 46.6 (−83.1, 176.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mittal, N.; Tayal, A.; Kumar, S.; Dhawan, R.; Goel, N.; Mittal, R. Longitudinal Trends in In-Patient Antibiotic Consumption According to the WHO Access, Watch, Reserve (AWaRe) Antibiotic Groups and Cost: An Analysis of Data at a National Antimicrobial Consumption Network (NAC-NET) Site in North India over 7 Years (2017–2023). Antibiotics 2024, 13, 673. https://doi.org/10.3390/antibiotics13070673
Mittal N, Tayal A, Kumar S, Dhawan R, Goel N, Mittal R. Longitudinal Trends in In-Patient Antibiotic Consumption According to the WHO Access, Watch, Reserve (AWaRe) Antibiotic Groups and Cost: An Analysis of Data at a National Antimicrobial Consumption Network (NAC-NET) Site in North India over 7 Years (2017–2023). Antibiotics. 2024; 13(7):673. https://doi.org/10.3390/antibiotics13070673
Chicago/Turabian StyleMittal, Niti, Ashish Tayal, Suneel Kumar, Reevanshi Dhawan, Nidhi Goel, and Rakesh Mittal. 2024. "Longitudinal Trends in In-Patient Antibiotic Consumption According to the WHO Access, Watch, Reserve (AWaRe) Antibiotic Groups and Cost: An Analysis of Data at a National Antimicrobial Consumption Network (NAC-NET) Site in North India over 7 Years (2017–2023)" Antibiotics 13, no. 7: 673. https://doi.org/10.3390/antibiotics13070673
APA StyleMittal, N., Tayal, A., Kumar, S., Dhawan, R., Goel, N., & Mittal, R. (2024). Longitudinal Trends in In-Patient Antibiotic Consumption According to the WHO Access, Watch, Reserve (AWaRe) Antibiotic Groups and Cost: An Analysis of Data at a National Antimicrobial Consumption Network (NAC-NET) Site in North India over 7 Years (2017–2023). Antibiotics, 13(7), 673. https://doi.org/10.3390/antibiotics13070673






