When to Stop Antibiotics in the Critically Ill?
Abstract
1. Introduction
2. Three Approaches to Determining When to Stop Antibiotics
2.1. Fixed Duration
2.1.1. Bacteremia
2.1.2. Pneumonia
2.1.3. Intra-Abdominal Infection
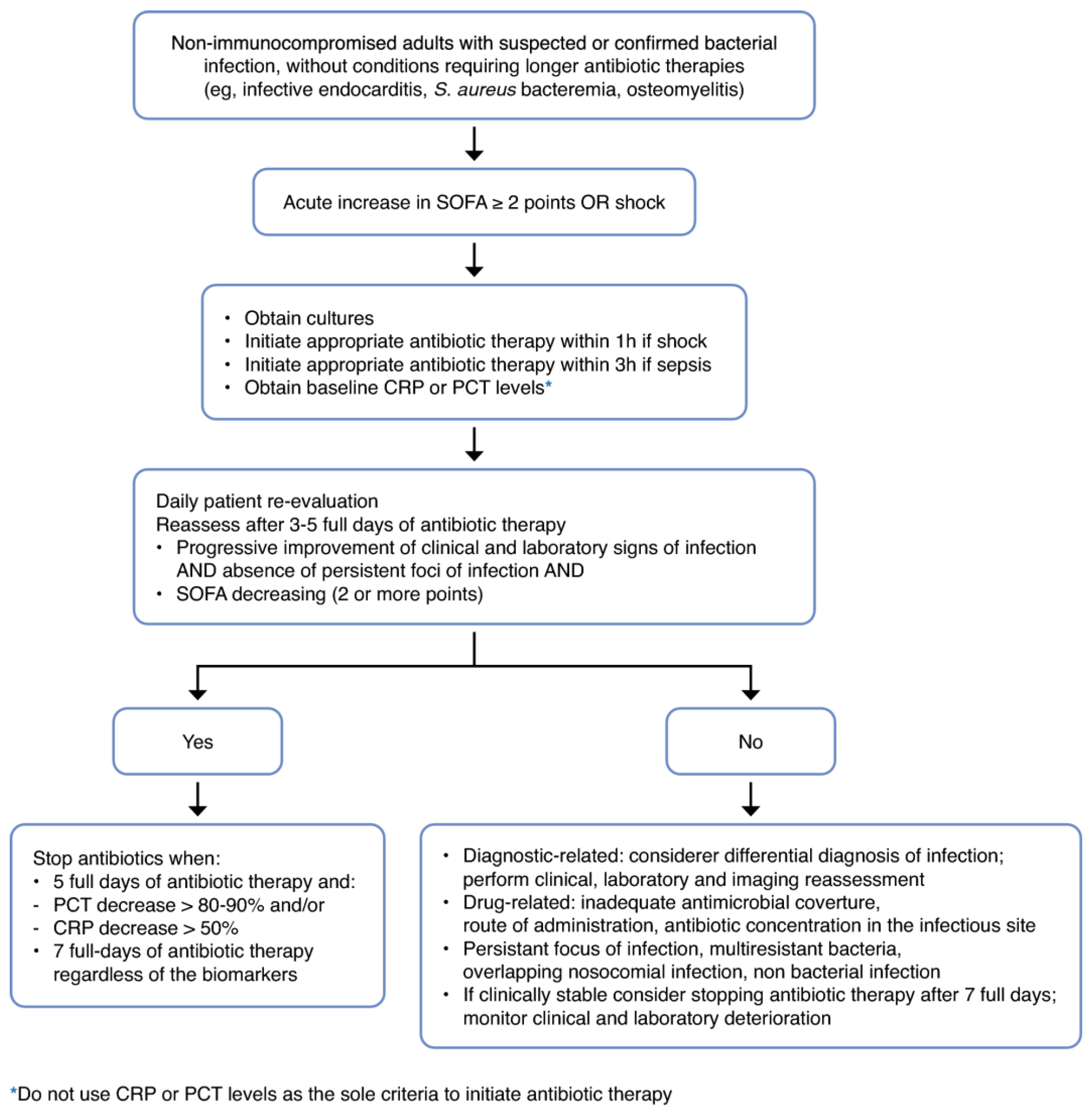
2.2. Clinical Criteria
2.3. Biomarker-Guided
3. The Final Piece: Implementation
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Carlet, J.M.; Masur, H.; Gerlach, H.; Calandra, T.; Cohen, J.; Gea-Banacloche, J.; Keh, D.; Marshall, J.C.; Parker, M.M.; et al. Surviving Sepsis Campaign Guidelines for Management of Severe Sepsis and Septic Shock. Crit. Care Med. 2004, 32, 858. [Google Scholar] [CrossRef] [PubMed]
- Povoa, P.; Kalil, A.C. Any Role for Biomarker-Guide Algorithms in Antibiotic Stewardship Programs? Crit. Care Med. 2020, 48, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Lanckohr, C.; Boeing, C.; De Waele, J.J.; de Lange, D.W.; Schouten, J.; Prins, M.; Nijsten, M.; Povoa, P.; Morris, A.C.; Bracht, H. Antimicrobial Stewardship, Therapeutic Drug Monitoring and Infection Management in the ICU: Results from the International A- TEAMICU Survey. Ann. Intensive Care 2021, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Hellen, G.; Molly, M.P.; Suraj, P.; Sumanth, G.; Jordan, L.; Devra, B.; Andrea, W.; Ramanan, L. The State of the World’s Antibiotics 2015. Wound Heal. South. Afr. 2015, 8, 30–34. [Google Scholar]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Bassetti, S.; Tschudin-Sutter, S.; Egli, A.; Osthoff, M. Optimizing Antibiotic Therapies to Reduce the Risk of Bacterial Resistance. Eur. J. Intern. Med. 2022, 99, 7–12. [Google Scholar] [CrossRef]
- Bozcal, E.; Dagdeviren, M. Toxicity of β-Lactam Antibiotics: Pathophysiology, Molecular Biology and Possible Recovery Strategies. In Poisoning—From Specific Toxic Agents to Novel Rapid and Simplified Techniques for Analysis; Malangu, N., Ed.; InTech: Nappanee, IN, USA, 2017; ISBN 978-953-51-3681-1. [Google Scholar]
- Baggio, D.; Ananda-Rajah, M.R. Fluoroquinolone Antibiotics and Adverse Events. Aust. Prescr. 2021, 44, 161–164. [Google Scholar] [CrossRef]
- Johanesen, P.A.; Mackin, K.E.; Hutton, M.L.; Awad, M.M.; Larcombe, S.; Amy, J.M.; Lyras, D. Disruption of the Gut Microbiome: Clostridium Difficile Infection and the Threat of Antibiotic Resistance. Genes 2015, 6, 1347–1360. [Google Scholar] [CrossRef]
- Chastre, J.; Wolff, M.; Fagon, J.-Y.; Chevret, S.; Thomas, F.; Wermert, D.; Clementi, E.; Gonzalez, J.; Jusserand, D.; Asfar, P.; et al. Comparison of 8 vs. 15 Days of Antibiotic Therapy for Ventilator-Associated Pneumonia in Adults: A Randomized Trial. JAMA 2003, 290, 2588. [Google Scholar] [CrossRef]
- Llewelyn, M.J.; Fitzpatrick, J.M.; Darwin, E.; SarahTonkin-Crine; Gorton, C.; Paul, J.; Peto, T.E.A.; Yardley, L.; Hopkins, S.; Walker, A.S. The Antibiotic Course Has Had Its Day. BMJ 2017, 358, j3418. [Google Scholar] [CrossRef]
- Spellberg, B. The New Antibiotic Mantra—“Shorter Is Better”. JAMA Intern. Med. 2016, 176, 1254. [Google Scholar] [CrossRef]
- Fowler, V.G.; Sanders, L.L.; Sexton, D.J.; Kong, L.; Marr, K.A.; Gopal, A.K.; Gottlieb, G.; McClelland, R.S.; Corey, G.R. Outcome of Staphylococcus Aureus Bacteremia According to Compliance with Recommendations of Infectious Diseases Specialists: Experience with 244 Patients. Clin. Infect. Dis. 1998, 27, 478–486. [Google Scholar] [CrossRef]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus Aureus Infections in Adults and Children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [PubMed]
- Kouijzer, I.J.E.; Fowler, V.G.; Ten Oever, J. Redefining Staphylococcus Aureus Bacteremia: A Structured Approach Guiding Diagnostic and Therapeutic Management. J. Infect. 2023, 86, 9–13. [Google Scholar] [CrossRef]
- Yahav, D.; Franceschini, E.; Koppel, F.; Turjeman, A.; Babich, T.; Bitterman, R.; Neuberger, A.; Ghanem-Zoubi, N.; Santoro, A.; Eliakim-Raz, N.; et al. Seven Versus 14 Days of Antibiotic Therapy for Uncomplicated Gram-Negative Bacteremia: A Noninferiority Randomized Controlled Trial. Clin. Infect. Dis. 2019, 69, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Von Dach, E.; Albrich, W.C.; Brunel, A.-S.; Prendki, V.; Cuvelier, C.; Flury, D.; Gayet-Ageron, A.; Huttner, B.; Kohler, P.; Lemmenmeier, E.; et al. Effect of C-Reactive Protein–Guided Antibiotic Treatment Duration, 7-Day Treatment, or 14-Day Treatment on 30-Day Clinical Failure Rate in Patients with Uncomplicated Gram-Negative Bacteremia: A Randomized Clinical Trial. JAMA 2020, 323, 2160. [Google Scholar] [CrossRef]
- Molina, J.; Montero-Mateos, E.; Praena-Segovia, J.; León-Jiménez, E.; Natera, C.; López-Cortés, L.E.; Valiente, L.; Rosso-Fernández, C.M.; Herrero, M.; Aller-García, A.I.; et al. Seven-versus 14-Day Course of Antibiotics for the Treatment of Bloodstream Infections by Enterobacterales: A Randomized, Controlled Trial. Clin. Microbiol. Infect. 2022, 28, 550–557. [Google Scholar] [CrossRef]
- Turjeman, A.; Von Dach, E.; Molina, J.; Franceschini, E.; Koppel, F.; Yelin, D.; Dishon-Benattar, Y.; Mussini, C.; Rodríguez-Baño, J.; Cisneros, J.M.; et al. Duration of Antibiotic Treatment for Gram-Negative Bacteremia—Systematic Review and Individual Participant Data (IPD) Meta-Analysis. eClinicalMedicine 2023, 55, 101750. [Google Scholar] [CrossRef] [PubMed]
- Moussaoui, R.E.; De Borgie, C.A.J.M.; Van Den Broek, P.; Hustinx, W.N.; Bresser, P.; Van Den Berk, G.E.L.; Poley, J.-W.; Van Den Berg, B.; Krouwels, F.H.; Bonten, M.J.M.; et al. Effectiveness of Discontinuing Antibiotic Treatment after Three Days versus Eight Days in Mild to Moderate-Severe Community Acquired Pneumonia: Randomised, Double Blind Study. BMJ 2006, 332, 1355. [Google Scholar] [CrossRef]
- Dinh, A.; Ropers, J.; Duran, C.; Davido, B.; Deconinck, L.; Matt, M.; Senard, O.; Lagrange, A.; Makhloufi, S.; Mellon, G.; et al. Discontinuing β-Lactam Treatment after 3 Days for Patients with Community-Acquired Pneumonia in Non-Critical Care Wards (PTC): A Double-Blind, Randomised, Placebo-Controlled, Non-Inferiority Trial. Lancet 2021, 397, 1195–1203. [Google Scholar] [CrossRef]
- Uranga, A.; España, P.P.; Bilbao, A.; Quintana, J.M.; Arriaga, I.; Intxausti, M.; Lobo, J.L.; Tomás, L.; Camino, J.; Nuñez, J.; et al. Duration of Antibiotic Treatment in Community-Acquired Pneumonia: A Multicenter Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 1257. [Google Scholar] [CrossRef]
- Schönwald, S.; Kuzman, I.; Orešković, K.; Burek, V.; Škerk, V.; Car, V.; Božinović, D.; Čulig, J.; Radošević, S. Azithromycin: Single 1.5g Dose in the Treatment of Patients with Atypical Pneumonia Syndrome—A Randomized Study. Infection 1999, 27, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Capellier, G.; Mockly, H.; Charpentier, C.; Annane, D.; Blasco, G.; Desmettre, T.; Roch, A.; Faisy, C.; Cousson, J.; Limat, S.; et al. Early-Onset Ventilator-Associated Pneumonia in Adults Randomized Clinical Trial: Comparison of 8 versus 15 Days of Antibiotic Treatment. PLoS ONE 2012, 7, e41290. [Google Scholar] [CrossRef]
- Bouglé, A.; Tuffet, S.; Federici, L.; Leone, M.; Monsel, A.; Dessalle, T.; Amour, J.; Dahyot-Fizelier, C.; Barbier, F.; Luyt, C.-E.; et al. Comparison of 8 versus 15 Days of Antibiotic Therapy for Pseudomonas Aeruginosa Ventilator-Associated Pneumonia in Adults: A Randomized, Controlled, Open-Label Trial. Intensive Care Med. 2022, 48, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, R.G.; Claridge, J.A.; Nathens, A.B.; Rotstein, O.D.; Duane, T.M.; Evans, H.L.; Cook, C.H.; O’Neill, P.J.; Mazuski, J.E.; Askari, R.; et al. Trial of Short-Course Antimicrobial Therapy for Intraabdominal Infection. N. Engl. J. Med. 2015, 372, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Srinu, D.; Shah, J.; Jena, A.; Jearth, V.; Singh, A.K.; Mandavdhare, H.S.; Sharma, V.; Irrinki, S.; Sakaray, Y.R.; Gupta, R.; et al. Conventional vs Short Duration of Antibiotics in Patients with Moderate or Severe Cholangitis: Noninferiority Randomized Trial. Am. J. Gastroenterol. 2024, 119, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Montravers, P.; Tubach, F.; Lescot, T.; Veber, B.; Esposito-Farèse, M.; Seguin, P.; Paugam, C.; Lepape, A.; Meistelman, C.; Cousson, J.; et al. Short-Course Antibiotic Therapy for Critically Ill Patients Treated for Postoperative Intra-Abdominal Infection: The DURAPOP Randomised Clinical Trial. Intensive Care Med. 2018, 44, 300–310. [Google Scholar] [CrossRef]
- Curran, J.; Lo, J.; Leung, V.; Brown, K.; Schwartz, K.L.; Daneman, N.; Garber, G.; Wu, J.H.C.; Langford, B.J. Estimating Daily Antibiotic Harms: An Umbrella Review with Individual Study Meta-Analysis. Clin. Microbiol. Infect. 2022, 28, 479–490. [Google Scholar] [CrossRef]
- Rice, L.B. The Maxwell Finland Lecture: For the Duration—Rational Antibiotic Administration in an Era of Antimicrobial Resistance and Clostridium Difficile. Clin. Infect. Dis. 2008, 46, 491–496. [Google Scholar] [CrossRef]
- Kollef, M.H.; Chastre, J.; Clavel, M.; Restrepo, M.I.; Michiels, B.; Kaniga, K.; Cirillo, I.; Kimko, H.; Redman, R. A Randomized Trial of 7-Day Doripenem versus 10-Day Imipenem-Cilastatin for Ventilator-Associated Pneumonia. Crit. Care 2012, 16, R218. [Google Scholar] [CrossRef]
- Jeffrey, M.; Denny, K.J.; Lipman, J.; Conway Morris, A. Differentiating Infection, Colonisation, and Sterile Inflammation in Critical Illness: The Emerging Role of Host-Response Profiling. Intensive Care Med. 2023, 49, 760–771. [Google Scholar] [CrossRef]
- Pandolfo, A.M.; Horne, R.; Jani, Y.; Reader, T.W.; Bidad, N.; Brealey, D.; Enne, V.I.; Livermore, D.M.; Gant, V.; Brett, S.J.; et al. Understanding Decisions about Antibiotic Prescribing in ICU: An Application of the Necessity Concerns Framework. BMJ Qual. Saf. 2022, 31, 199–210. [Google Scholar] [CrossRef]
- Arulkumaran, N.; Khpal, M.; Tam, K.; Baheerathan, A.; Corredor, C.; Singer, M. Effect of Antibiotic Discontinuation Strategies on Mortality and Infectious Complications in Critically Ill Septic Patients: A Meta-Analysis and Trial Sequential Analysis. Crit. Care Med. 2020, 48, 757–764. [Google Scholar] [CrossRef]
- Montravers, P.; Fagon, J.Y.; Chastre, J.; Lecso, M.; Dombret, M.C.; Trouillet, J.L.; Gibert, C. Follow-up Protected Specimen Brushes to Assess Treatment in Nosocomial Pneumonia. Am. Rev. Respir. Dis. 1993, 147, 38–44. [Google Scholar] [CrossRef]
- Dennesen, P.J.; van der Ven, A.J.; Kessels, A.G.; Ramsay, G.; Bonten, M.J. Resolution of Infectious Parameters after Antimicrobial Therapy in Patients with Ventilator-Associated Pneumonia. Am. J. Respir. Crit. Care Med. 2001, 163, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Rogers, P.; Atwood, C.W.; Wagener, M.M.; Yu, V.L. Short-Course Empiric Antibiotic Therapy for Patients with Pulmonary Infiltrates in the Intensive Care Unit: A Proposed Solution for Indiscriminate Antibiotic Prescription. Am. J. Respir. Crit. Care Med. 2000, 162, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Lennard, E.S.; Dellinger, E.P.; Wertz, M.J.; Minshew, B.H. Implications of Leukocytosis and Fever at Conclusion of Antibiotic Therapy for Intra-Abdominal Sepsis. Ann. Surg. 1982, 195, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, T.L.; Evans, H.L.; Smith, R.L.; McElearney, S.T.; Schulman, A.S.; Chong, T.W.; Pruett, T.L.; Sawyer, R.G. Can We Define the Ideal Duration of Antibiotic Therapy? Surg. Infect. 2006, 7, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Cevik, M.; Kuppalli, K.; Kindrachuk, J.; Peiris, M. Virology, Transmission, and Pathogenesis of SARS-CoV-2. BMJ 2020, 371, m3862. [Google Scholar] [CrossRef]
- Vidaur, L.; Gualis, B.; Rodriguez, A.; Ramírez, R.; Sandiumenge, A.; Sirgo, G.; Diaz, E.; Rello, J. Clinical Resolution in Patients with Suspicion of Ventilator-Associated Pneumonia: A Cohort Study Comparing Patients with and without Acute Respiratory Distress Syndrome. Crit. Care Med. 2005, 33, 1248–1253. [Google Scholar] [CrossRef]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults with Hospital-Acquired and Ventilator-Associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Torres, A.; Nagavci, B.; Aliberti, S.; Antonelli, M.; Bassetti, M.; Bos, L.D.; Chalmers, J.D.; Derde, L.; de Waele, J.; et al. ERS/ESICM/ESCMID/ALAT Guidelines for the Management of Severe Community-Acquired Pneumonia. Intensive Care Med. 2023, 49, 615–632. [Google Scholar] [CrossRef]
- Mazuski, J.E.; Tessier, J.M.; May, A.K.; Sawyer, R.G.; Nadler, E.P.; Rosengart, M.R.; Chang, P.K.; O’Neill, P.J.; Mollen, K.P.; Huston, J.M.; et al. The Surgical Infection Society Revised Guidelines on the Management of Intra-Abdominal Infection. Surg. Infect. 2017, 18, 1–76. [Google Scholar] [CrossRef]
- Roope, L.S.J.; Buchanan, J.; Morrell, L.; Pouwels, K.B.; Sivyer, K.; Mowbray, F.; Abel, L.; Cross, E.L.A.; Yardley, L.; Peto, T.; et al. Why Do Hospital Prescribers Continue Antibiotics When It Is Safe to Stop? Results of a Choice Experiment Survey. BMC Med. 2020, 18, 196. [Google Scholar] [CrossRef]
- Washington University (Saint Louis, Missouri), Department of Medicine. Manual of Medical Therapeutics, 27th ed.; Little, Brown: Boston, MA, USA, 1992; ISBN 978-0-316-92420-7. [Google Scholar]
- Zilahi, G.; McMahon, M.A.; Povoa, P.; Martin-Loeches, I. Duration of Antibiotic Therapy in the Intensive Care Unit. J. Thorac. Dis. 2016, 8, 3774–3780. [Google Scholar] [CrossRef]
- Salluh, J.I.F.; Souza-Dantas, V.C.; Póvoa, P. The Current Status of Biomarkers for the Diagnosis of Nosocomial Pneumonias. Curr. Opin. Crit. Care 2017, 23, 391–397. [Google Scholar] [CrossRef]
- Póvoa, P.; Coelho, L.; Dal-Pizzol, F.; Ferrer, R.; Huttner, A.; Conway Morris, A.; Nobre, V.; Ramirez, P.; Rouze, A.; Salluh, J.; et al. How to Use Biomarkers of Infection or Sepsis at the Bedside: Guide to Clinicians. Intensive Care Med. 2023, 49, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Papp, M.; Kiss, N.; Baka, M.; Trásy, D.; Zubek, L.; Fehérvári, P.; Harnos, A.; Turan, C.; Hegyi, P.; Molnár, Z. Procalcitonin-Guided Antibiotic Therapy May Shorten Length of Treatment and May Improve Survival—A Systematic Review and Meta-Analysis. Crit. Care 2023, 27, 394. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, T.; Salluh, J.; Povoa, P. Do We Need New Trials of Procalcitonin-Guided Antibiotic Therapy? Crit. Care 2018, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, P.; Salluh, J.I.F.; Lisboa, T. Biomarkers in the ICU: Less Is More? Not Sure. Intensive Care Med. 2021, 47, 101–103. [Google Scholar] [CrossRef]
- Pepper, D.J.; Sun, J.; Rhee, C.; Welsh, J.; Powers, J.H.; Danner, R.L.; Kadri, S.S. Procalcitonin-Guided Antibiotic Discontinuation and Mortality in Critically Ill Adults. Chest 2019, 155, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Bloos, F.; Trips, E.; Nierhaus, A.; Briegel, J.; Heyland, D.K.; Jaschinski, U.; Moerer, O.; Weyland, A.; Marx, G.; Gründling, M.; et al. Effect of Sodium Selenite Administration and Procalcitonin-Guided Therapy on Mortality in Patients with Severe Sepsis or Septic Shock: A Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 1266. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.U.; Hein, L.; Lundgren, B.; Bestle, M.H.; Mohr, T.T.; Andersen, M.H.; Thornberg, K.J.; Løken, J.; Steensen, M.; Fox, Z.; et al. Procalcitonin-Guided Interventions against Infections to Increase Early Appropriate Antibiotics and Improve Survival in the Intensive Care Unit: A Randomized Trial. Crit. Care Med. 2011, 39, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.C.; Mehta, A.B.; Walkey, A.J. Practice Patterns and Outcomes Associated with Procalcitonin Use in Critically Ill Patients with Sepsis. Clin. Infect. Dis. 2017, 64, 1509–1515. [Google Scholar] [CrossRef]
- Lawandi, A.; Oshiro, M.; Warner, S.; Diao, G.; Strich, J.R.; Babiker, A.; Rhee, C.; Klompas, M.; Danner, R.L.; Kadri, S.S. Reliability of Admission Procalcitonin Testing for Capturing Bacteremia Across the Sepsis Spectrum: Real-World Utilization and Performance Characteristics, 65 U.S. Hospitals, 2008–2017. Crit. Care Med. 2023, 51, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Balk, R.A.; Kadri, S.S.; Cao, Z.; Robinson, S.B.; Lipkin, C.; Bozzette, S.A. Effect of Procalcitonin Testing on Health-Care Utilization and Costs in Critically Ill Patients in the United States. Chest 2017, 151, 23–33. [Google Scholar] [CrossRef]
- Borges, I.; Carneiro, R.; Bergo, R.; Martins, L.; Colosimo, E.; Oliveira, C.; Saturnino, S.; Andrade, M.V.; Ravetti, C.; Nobre, V.; et al. Duration of Antibiotic Therapy in Critically Ill Patients: A Randomized Controlled Trial of a Clinical and C-Reactive Protein-Based Protocol versus an Evidence-Based Best Practice Strategy without Biomarkers. Crit. Care 2020, 24, 281. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.F.; Botoni, F.A.; Oliveira, C.R.A.; Silva, C.B.; Pereira, H.A.; Serufo, J.C.; Nobre, V. Procalcitonin Versus C-Reactive Protein for Guiding Antibiotic Therapy in Sepsis: A Randomized Trial. Crit. Care Med. 2013, 41, 2336–2343. [Google Scholar] [CrossRef]
- Dias, R.F.; De Paula, A.C.R.B.; Hasparyk, U.G.; De Oliveira Rabelo Bassalo Coutinho, M.; Alderete, J.R.A.; Kanjongo, J.C.; Silva, R.A.M.; Guimarães, N.S.; Simões E Silva, A.C.; Nobre, V. Use of C-Reactive Protein to Guide the Antibiotic Therapy in Hospitalized Patients: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2023, 23, 276. [Google Scholar] [CrossRef]
- De Santis, V.; Gresoiu, M.; Corona, A.; Wilson, A.P.R.; Singer, M. Bacteraemia Incidence, Causative Organisms and Resistance Patterns, Antibiotic Strategies and Outcomes in a Single University Hospital ICU: Continuing Improvement between 2000 and 2013. J. Antimicrob. Chemother. 2015, 70, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Tabah, A.; Koulenti, D.; Laupland, K.; Misset, B.; Valles, J.; Bruzzi de Carvalho, F.; Paiva, J.A.; Cakar, N.; Ma, X.; Eggimann, P.; et al. Characteristics and Determinants of Outcome of Hospital-Acquired Bloodstream Infections in Intensive Care Units: The EUROBACT International Cohort Study. Intensive Care Med. 2012, 38, 1930–1945. [Google Scholar] [CrossRef] [PubMed]
- Zahar, J.-R.; Lesprit, P.; Ruckly, S.; Eden, A.; Hikombo, H.; Bernard, L.; Harbarth, S.; Timsit, J.-F.; Brun-Buisson, C.; BacterCom Study Group. Predominance of Healthcare-Associated Cases among Episodes of Community-Onset Bacteraemia Due to Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae. Int. J. Antimicrob. Agents 2017, 49, 67–73. [Google Scholar] [CrossRef] [PubMed]
- de Jong, E.; van Oers, J.A.; Beishuizen, A.; Vos, P.; Vermeijden, W.J.; Haas, L.E.; Loef, B.G.; Dormans, T.; van Melsen, G.C.; Kluiters, Y.C.; et al. Efficacy and Safety of Procalcitonin Guidance in Reducing the Duration of Antibiotic Treatment in Critically Ill Patients: A Randomised, Controlled, Open-Label Trial. Lancet Infect. Dis. 2016, 16, 819–827. [Google Scholar] [CrossRef]
- Janssen, R.M.E.; Oerlemans, A.J.M.; Van Der Hoeven, J.G.; Ten Oever, J.; Schouten, J.A.; Hulscher, M.E.J.L. Why We Prescribe Antibiotics for Too Long in the Hospital Setting: A Systematic Scoping Review. J. Antimicrob. Chemother. 2022, 77, 2105–2119. [Google Scholar] [CrossRef]
- Schouten, J.; De Angelis, G.; De Waele, J.J. A Microbiologist Consultant Should Attend Daily ICU Rounds. Intensive Care Med. 2020, 46, 372–374. [Google Scholar] [CrossRef]
- Janssen, R.M.E.; Oerlemans, A.J.M.; van der Hoeven, J.G.; Oostdijk, E.A.N.; Derde, L.P.G.; Ten Oever, J.; Wertheim, H.F.L.; Hulscher, M.E.J.L.; Schouten, J.A. Decision-Making Regarding Antibiotic Therapy Duration: An Observational Study of Multidisciplinary Meetings in the Intensive Care Unit. J. Crit. Care 2023, 78, 154363. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, N.D.; Dean, J.T., III; Shald, E.A.; Conway Morris, A.; Povoa, P.; Schouten, J.; Parchim, N. When to Stop Antibiotics in the Critically Ill? Antibiotics 2024, 13, 272. https://doi.org/10.3390/antibiotics13030272
Nielsen ND, Dean JT III, Shald EA, Conway Morris A, Povoa P, Schouten J, Parchim N. When to Stop Antibiotics in the Critically Ill? Antibiotics. 2024; 13(3):272. https://doi.org/10.3390/antibiotics13030272
Chicago/Turabian StyleNielsen, Nathan D., James T. Dean, III, Elizabeth A. Shald, Andrew Conway Morris, Pedro Povoa, Jeroen Schouten, and Nicholas Parchim. 2024. "When to Stop Antibiotics in the Critically Ill?" Antibiotics 13, no. 3: 272. https://doi.org/10.3390/antibiotics13030272
APA StyleNielsen, N. D., Dean, J. T., III, Shald, E. A., Conway Morris, A., Povoa, P., Schouten, J., & Parchim, N. (2024). When to Stop Antibiotics in the Critically Ill? Antibiotics, 13(3), 272. https://doi.org/10.3390/antibiotics13030272





