Comparative Impact of an Optimized PK/PD Target Attainment of Piperacillin-Tazobactam vs. Meropenem on the Trend over Time of SOFA Score and Inflammatory Biomarkers in Critically Ill Patients Receiving Continuous Infusion Monotherapy for Treating Documented Gram-Negative BSIs and/or VAP
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Inclusion Criteria
4.2. Data Collection
4.3. Outcome Definition
baseline SOFA score value—48-h SOFA score value;
baseline SOFA score value—7-days SOFA score value
4.4. Antibiotic Dosing Regimens, Sampling Procedure, Definition of Optimal PK/PD Target Attainment, and Procedure for Optimizing PK/PD Target Attainment
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaukonen, K.-M.; Bailey, M.; Suzuki, S.; Pilcher, D.; Bellomo, R. Mortality Related to Severe Sepsis and Septic Shock among Critically Ill Patients in Australia and New Zealand, 2000–2012. JAMA 2014, 311, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; van der Poll, T. Severe Sepsis and Septic Shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- MacVane, S.H. Antimicrobial Resistance in the Intensive Care Unit: A Focus on Gram-Negative Bacterial Infections. J. Intensive Care Med. 2017, 32, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Taccone, F.S.; Lipman, J. Understanding PK/PD. Intensive Care Med. 2016, 42, 1797–1800. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2023 Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections. Clin Infect Dis 2023, ciad428. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli (Endorsed by European Society of Intensive Care Medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef]
- Gatti, M.; Viaggi, B.; Rossolini, G.M.; Pea, F.; Viale, P. An Evidence-Based Multidisciplinary Approach Focused on Creating Algorithms for Targeted Therapy of Infection-Related Ventilator-Associated Complications (IVACs) Caused by Pseudomonas Aeruginosa and Acinetobacter Baumannii in Critically Ill Adult Patients. Antibiotics 2021, 11, 33. [Google Scholar] [CrossRef]
- Gatti, M.; Viaggi, B.; Rossolini, G.M.; Pea, F.; Viale, P. An Evidence-Based Multidisciplinary Approach Focused at Creating Algorithms for Targeted Therapy of BSIs, cUTIs, and cIAIs Caused by Enterobacterales in Critically Ill Adult Patients. Infect. Drug Resist. 2021, 14, 2461–2498. [Google Scholar] [CrossRef]
- Gutiérrez-Gutiérrez, B.; Rodríguez-Baño, J. Current Options for the Treatment of Infections Due to Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae in Different Groups of Patients. Clin. Microbiol. Infect. 2019, 25, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Karaiskos, I.; Giamarellou, H. Carbapenem-Sparing Strategies for ESBL Producers: When and How. Antibiotics 2020, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Munch, M.W.; Granholm, A.; Jonsson, A.B.; Sjövall, F.; Helleberg, M.; Hertz, F.B.; Andersen, J.S.; Steensen, M.; Achiam, M.P.; Perner, A.; et al. Piperacillin/Tazobactam versus Carbapenems in Patients with Severe Bacterial Infections: A Systematic Review with Meta-analysis. Acta Anaesthesiol. Scand. 2023, 67, 853–868. [Google Scholar] [CrossRef]
- Benanti, G.E.; Brown, A.R.T.; Shigle, T.L.; Tarrand, J.J.; Bhatti, M.M.; McDaneld, P.M.; Shelburne, S.A.; Aitken, S.L. Carbapenem versus Cefepime or Piperacillin-Tazobactam for Empiric Treatment of Bacteremia Due to Extended-Spectrum-β-Lactamase-Producing Escherichia Coli in Patients with Hematologic Malignancy. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef]
- Harris, P.N.A.; Yin, M.; Jureen, R.; Chew, J.; Ali, J.; Paynter, S.; Paterson, D.L.; Tambyah, P.A. Comparable Outcomes for β-Lactam/β-Lactamase Inhibitor Combinations and Carbapenems in Definitive Treatment of Bloodstream Infections Caused by Cefotaxime-Resistant Escherichia Coli or Klebsiella Pneumoniae. Antimicrob. Resist. Infect. Control 2015, 4, 14. [Google Scholar] [CrossRef]
- Ng, T.M.; Khong, W.X.; Harris, P.N.A.; De, P.P.; Chow, A.; Tambyah, P.A.; Lye, D.C. Empiric Piperacillin-Tazobactam versus Carbapenems in the Treatment of Bacteraemia Due to Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae. PLoS ONE 2016, 11, e0153696. [Google Scholar] [CrossRef]
- Seo, Y.B.; Lee, J.; Kim, Y.K.; Lee, S.S.; Lee, J.-A.; Kim, H.Y.; Uh, Y.; Kim, H.-S.; Song, W. Randomized Controlled Trial of Piperacillin-Tazobactam, Cefepime and Ertapenem for the Treatment of Urinary Tract Infection Caused by Extended-Spectrum Beta-Lactamase-Producing Escherichia Coli. BMC Infect. Dis. 2017, 17, 404. [Google Scholar] [CrossRef]
- Rodríguez-Baño, J.; Gutiérrez-Gutiérrez, B.; Kahlmeter, G. Antibiotics for Ceftriaxone-Resistant Gram-Negative Bacterial Bloodstream Infections. JAMA 2019, 321, 612–613. [Google Scholar] [CrossRef]
- Gutiérrez-Gutiérrez, B.; Pérez-Galera, S.; Salamanca, E.; de Cueto, M.; Calbo, E.; Almirante, B.; Viale, P.; Oliver, A.; Pintado, V.; Gasch, O.; et al. A Multinational, Preregistered Cohort Study of β-Lactam/β-Lactamase Inhibitor Combinations for Treatment of Bloodstream Infections Due to Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2016, 60, 4159–4169. [Google Scholar] [CrossRef]
- Gutiérrez-Gutiérrez, B.; Bonomo, R.A.; Carmeli, Y.; Paterson, D.L.; Almirante, B.; Martínez-Martínez, L.; Oliver, A.; Calbo, E.; Peña, C.; Akova, M.; et al. Ertapenem for the Treatment of Bloodstream Infections Due to ESBL-Producing Enterobacteriaceae: A Multinational Pre-Registered Cohort Study. J. Antimicrob. Chemother. 2016, 71, 1672–1680. [Google Scholar] [CrossRef]
- Gudiol, C.; Royo-Cebrecos, C.; Abdala, E.; Akova, M.; Álvarez, R.; Maestro-de la Calle, G.; Cano, A.; Cervera, C.; Clemente, W.T.; Martín-Dávila, P.; et al. Efficacy of β-Lactam/β-Lactamase Inhibitor Combinations for the Treatment of Bloodstream Infection Due to Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae in Hematological Patients with Neutropenia. Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef]
- Ko, J.-H.; Lee, N.R.; Joo, E.-J.; Moon, S.-Y.; Choi, J.-K.; Park, D.A.; Peck, K.R. Appropriate Non-Carbapenems Are Not Inferior to Carbapenems as Initial Empirical Therapy for Bacteremia Caused by Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae: A Propensity Score Weighted Multicenter Cohort Study. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Sharara, S.L.; Amoah, J.; Pana, Z.D.; Simner, P.J.; Cosgrove, S.E.; Tamma, P.D. Is Piperacillin-Tazobactam Effective for the Treatment of Pyelonephritis Caused by Extended-Spectrum β-Lactamase-Producing Organisms? Clin. Infect. Dis. 2020, 71, e331–e337. [Google Scholar] [CrossRef] [PubMed]
- John, R.; Colley, P.; Nguyen, H.L.; Berhe, M. Outcomes Analysis in Patients with Extended-Spectrum Beta-Lactamase Bacteremia Empirically Treated with Piperacillin/Tazobactam versus Carbapenems. J. Bayl. Scott White Health 2019, 32, 187–191. [Google Scholar] [CrossRef]
- Kang, C.-I.; Park, S.Y.; Chung, D.R.; Peck, K.R.; Song, J.-H. Piperacillin-Tazobactam as an Initial Empirical Therapy of Bacteremia Caused by Extended-Spectrum β-Lactamase-Producing Escherichia Coli and Klebsiella Pneumoniae. J. Infect. 2012, 64, 533–534. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, C.-Y.; Li, S.-R.; Fan, T.-T.; Cao, S.-S.; Cui, B.; Li, M.-Y.; Fan, B.-Y.; Ji, B.; Wang, L.; et al. Efficacy and Safety of Piperacillin-Tazobactam Compared with Meropenem in Treating Complicated Urinary Tract Infections Including Acute Pyelonephritis Due to Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae. Front. Cell Infect. Microbiol. 2023, 13, 1093842. [Google Scholar] [CrossRef]
- Hoashi, K.; Hayama, B.; Suzuki, M.; Sakurai, A.; Takehana, K.; Enokida, T.; Takeda, K.; Ohkushi, D.; Doi, Y.; Harada, S. Comparison of the Treatment Outcome of Piperacillin-Tazobactam versus Carbapenems for Patients with Bacteremia Caused by Extended-Spectrum β-Lactamase-Producing Escherichia Coli in Areas with Low Frequency of Coproduction of OXA-1: A Preliminary Analysis. Microbiol. Spectr. 2022, 10, e0220622. [Google Scholar] [CrossRef]
- Gatti, M.; Cojutti, P.G.; Bartoletti, M.; Tonetti, T.; Bianchini, A.; Ramirez, S.; Pizzilli, G.; Ambretti, S.; Giannella, M.; Mancini, R.; et al. Expert Clinical Pharmacological Advice May Make an Antimicrobial TDM Program for Emerging Candidates More Clinically Useful in Tailoring Therapy of Critically Ill Patients. Crit. Care 2022, 26, 178. [Google Scholar] [CrossRef]
- Roberts, J.A.; Croom, K.; Adomakoh, N. Continuous Infusion of Beta-Lactam Antibiotics: Narrative Review of Systematic Reviews, and Implications for Outpatient Parenteral Antibiotic Therapy. Expert. Rev. Anti Infect. Ther. 2023, 21, 375–385. [Google Scholar] [CrossRef]
- Sumi, C.D.; Heffernan, A.J.; Lipman, J.; Roberts, J.A.; Sime, F.B. What Antibiotic Exposures Are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A Systematic Review. Clin. Pharmacokinet. 2019, 58, 1407–1443. [Google Scholar] [CrossRef]
- Gatti, M.; Cojutti, P.G.; Pascale, R.; Tonetti, T.; Laici, C.; Dell’Olio, A.; Siniscalchi, A.; Giannella, M.; Viale, P.; Pea, F. Assessment of a PK/PD Target of Continuous Infusion Beta-Lactams Useful for Preventing Microbiological Failure and/or Resistance Development in Critically Ill Patients Affected by Documented Gram-Negative Infections. Antibiotics 2021, 10, 1311. [Google Scholar] [CrossRef]
- Alshaer, M.H.; Maranchick, N.; Alexander, K.M.; Manigaba, K.; Shoulders, B.R.; Felton, T.W.; Mathew, S.K.; Peloquin, C.A. Beta-Lactam Target Attainment and Associated Outcomes in Patients with Bloodstream Infections. Int. J. Antimicrob. Agents 2023, 61, 106727. [Google Scholar] [CrossRef]
- Alshaer, M.H.; Maranchick, N.; Bai, C.; Maguigan, K.L.; Shoulders, B.; Felton, T.W.; Mathew, S.K.; Mardini, M.T.; Peloquin, C.A. Using Machine Learning To Define the Impact of Beta-Lactam Early and Cumulative Target Attainment on Outcomes in Intensive Care Unit Patients with Hospital-Acquired and Ventilator-Associated Pneumonia. Antimicrob. Agents Chemother. 2022, 66, e0056322. [Google Scholar] [CrossRef]
- Abdulla, A.; Dijkstra, A.; Hunfeld, N.G.M.; Endeman, H.; Bahmany, S.; Ewoldt, T.M.J.; Muller, A.E.; van Gelder, T.; Gommers, D.; Koch, B.C.P. Failure of Target Attainment of Beta-Lactam Antibiotics in Critically Ill Patients and Associated Risk Factors: A Two-Center Prospective Study (EXPAT). Crit. Care 2020, 24, 558. [Google Scholar] [CrossRef]
- Ferreira, F.L.; Bota, D.P.; Bross, A.; Mélot, C.; Vincent, J.L. Serial Evaluation of the SOFA Score to Predict Outcome in Critically Ill Patients. JAMA 2001, 286, 1754–1758. [Google Scholar] [CrossRef]
- de Grooth, H.-J.; Geenen, I.L.; Girbes, A.R.; Vincent, J.-L.; Parienti, J.-J.; Oudemans-van Straaten, H.M. SOFA and Mortality Endpoints in Randomized Controlled Trials: A Systematic Review and Meta-Regression Analysis. Crit. Care 2017, 21, 38. [Google Scholar] [CrossRef]
- Soni, N.J.; Samson, D.J.; Galaydick, J.L.; Vats, V.; Huang, E.S.; Aronson, N.; Pitrak, D.L. Procalcitonin-Guided Antibiotic Therapy: A Systematic Review and Meta-Analysis. J. Hosp. Med. 2013, 8, 530–540. [Google Scholar] [CrossRef]
- von Dach, E.; Albrich, W.C.; Brunel, A.-S.; Prendki, V.; Cuvelier, C.; Flury, D.; Gayet-Ageron, A.; Huttner, B.; Kohler, P.; Lemmenmeier, E.; et al. Effect of C-Reactive Protein-Guided Antibiotic Treatment Duration, 7-Day Treatment, or 14-Day Treatment on 30-Day Clinical Failure Rate in Patients With Uncomplicated Gram-Negative Bacteremia: A Randomized Clinical Trial. JAMA 2020, 323, 2160–2169. [Google Scholar] [CrossRef]
- Vincent, J.L.; de Mendonça, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M.; Sprung, C.L.; Colardyn, F.; Blecher, S. Use of the SOFA Score to Assess the Incidence of Organ Dysfunction/Failure in Intensive Care Units: Results of a Multicenter, Prospective Study. Working Group on “Sepsis-Related Problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef]
- Albrich, W.C.; Harbarth, S. Pros and Cons of Using Biomarkers versus Clinical Decisions in Start and Stop Decisions for Antibiotics in the Critical Care Setting. Intensive Care Med. 2015, 41, 1739–1751. [Google Scholar] [CrossRef]
- Bouadma, L.; Luyt, C.-E.; Tubach, F.; Cracco, C.; Alvarez, A.; Schwebel, C.; Schortgen, F.; Lasocki, S.; Veber, B.; Dehoux, M.; et al. Use of Procalcitonin to Reduce Patients’ Exposure to Antibiotics in Intensive Care Units (PRORATA Trial): A Multicentre Randomised Controlled Trial. Lancet 2010, 375, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Bréchot, N.; Hékimian, G.; Chastre, J.; Luyt, C.-E. Procalcitonin to Guide Antibiotic Therapy in the ICU. Int. J. Antimicrob. Agents 2015, 46 (Suppl. S1), S19–S24. [Google Scholar] [CrossRef] [PubMed]
- de Jong, E.; van Oers, J.A.; Beishuizen, A.; Vos, P.; Vermeijden, W.J.; Haas, L.E.; Loef, B.G.; Dormans, T.; van Melsen, G.C.; Kluiters, Y.C.; et al. Efficacy and Safety of Procalcitonin Guidance in Reducing the Duration of Antibiotic Treatment in Critically Ill Patients: A Randomised, Controlled, Open-Label Trial. Lancet Infect. Dis. 2016, 16, 819–827. [Google Scholar] [CrossRef]
- Galli, F.; Bindo, F.; Motos, A.; Fernández-Barat, L.; Barbeta, E.; Gabarrús, A.; Ceccato, A.; Bermejo-Martin, J.F.; Ferrer, R.; Riera, J.; et al. Procalcitonin and C-Reactive Protein to Rule out Early Bacterial Coinfection in COVID-19 Critically Ill Patients. Intensive Care Med. 2023, 49, 934–945. [Google Scholar] [CrossRef]
- Papp, M.; Kiss, N.; Baka, M.; Trásy, D.; Zubek, L.; Fehérvári, P.; Harnos, A.; Turan, C.; Hegyi, P.; Molnár, Z. Procalcitonin-Guided Antibiotic Therapy May Shorten Length of Treatment and May Improve Survival-a Systematic Review and Meta-Analysis. Crit. Care 2023, 27, 394. [Google Scholar] [CrossRef]
- Perrella, A.; Giuliani, A.; De Palma, M.; Castriconi, M.; Molino, C.; Vennarecci, G.; Antropoli, C.; Esposito, C.; Calise, F.; Frangiosa, A.; et al. C-Reactive Protein but Not Procalcitonin May Predict Antibiotic Response and Outcome in Infections Following Major Abdominal Surgery. Updates Surg. 2022, 74, 765–771. [Google Scholar] [CrossRef]
- Jolivet, P.; Christen, G.; Seematter, G.; Que, Y.-A.; Eggimann, P. [Usefulness of biomarkers of sepsis in the ICU]. Rev. Med. Suisse 2011, 7, 2430–2434. [Google Scholar]
- Plata-Menchaca, E.P.; Ferrer, R. Procalcitonin Is Useful for Antibiotic Deescalation in Sepsis. Crit. Care Med. 2021, 49, 693–696. [Google Scholar] [CrossRef]
- Walker, C. Procalcitonin-Guided Antibiotic Therapy Duration in Critically Ill Adults. AACN Adv. Crit. Care 2015, 26, 99–106. [Google Scholar] [CrossRef]
- Wirz, Y.; Meier, M.A.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; Tubach, F.; Schroeder, S.; Nobre, V.; Annane, D.; et al. Effect of Procalcitonin-Guided Antibiotic Treatment on Clinical Outcomes in Intensive Care Unit Patients with Infection and Sepsis Patients: A Patient-Level Meta-Analysis of Randomized Trials. Crit. Care 2018, 22, 191. [Google Scholar] [CrossRef]
- Gatti, M.; Rinaldi, M.; Tonetti, T.; Siniscalchi, A.; Viale, P.; Pea, F. Could an Optimized Joint Pharmacokinetic/Pharmacodynamic Target Attainment of Continuous Infusion Piperacillin-Tazobactam Be a Valuable Innovative Approach for Maximizing the Effectiveness of Monotherapy Even in the Treatment of Critically Ill Patients with Documented Extended-Spectrum Beta-Lactamase-Producing Enterobacterales Bloodstream Infections and/or Ventilator-Associated Pneumonia? Antibiotics 2023, 12, 1736. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Wang, J.; Cui, C. The Effect of Meropenem versus Piperacillin-Tazobactam in Critically Ill Patients with Sepsis and Septic Shock. Heliyon 2023, 9, e16542. [Google Scholar] [CrossRef]
- Shiber, S.; Yahav, D.; Avni, T.; Leibovici, L.; Paul, M. β-Lactam/β-Lactamase Inhibitors versus Carbapenems for the Treatment of Sepsis: Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Antimicrob. Chemother. 2015, 70, 41–47. [Google Scholar] [CrossRef]
- Tam, V.H.; Chang, K.-T.; Zhou, J.; Ledesma, K.R.; Phe, K.; Gao, S.; Van Bambeke, F.; Sánchez-Díaz, A.M.; Zamorano, L.; Oliver, A.; et al. Determining β-Lactam Exposure Threshold to Suppress Resistance Development in Gram-Negative Bacteria. J. Antimicrob. Chemother. 2017, 72, 1421–1428. [Google Scholar] [CrossRef]
- Felton, T.W.; Goodwin, J.; O’Connor, L.; Sharp, A.; Gregson, L.; Livermore, J.; Howard, S.J.; Neely, M.N.; Hope, W.W. Impact of Bolus Dosing versus Continuous Infusion of Piperacillin and Tazobactam on the Development of Antimicrobial Resistance in Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 5811–5819. [Google Scholar] [CrossRef]
- Al-Shaer, M.H.; Rubido, E.; Cherabuddi, K.; Venugopalan, V.; Klinker, K.; Peloquin, C. Early Therapeutic Monitoring of β-Lactams and Associated Therapy Outcomes in Critically Ill Patients. J. Antimicrob. Chemother. 2020, 75, 3644–3651. [Google Scholar] [CrossRef]
- Chua, N.G.; Loo, L.; Hee, D.K.H.; Lim, T.P.; Ng, T.M.; Hoo, G.S.R.; Soong, J.L.; Ong, J.C.L.; Tang, S.S.L.; Zhou, Y.P.; et al. Therapeutic Drug Monitoring of Meropenem and Piperacillin-Tazobactam in the Singapore Critically Ill Population—A Prospective, Multi-Center, Observational Study (BLAST 1). J. Crit. Care 2022, 68, 107–113. [Google Scholar] [CrossRef]
- Carrié, C.; Petit, L.; d’Houdain, N.; Sauvage, N.; Cottenceau, V.; Lafitte, M.; Foumenteze, C.; Hisz, Q.; Menu, D.; Legeron, R.; et al. Association between Augmented Renal Clearance, Antibiotic Exposure and Clinical Outcome in Critically Ill Septic Patients Receiving High Doses of β-Lactams Administered by Continuous Infusion: A Prospective Observational Study. Int. J. Antimicrob. Agents 2018, 51, 443–449. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Lipman, J.; Roberts, J.A. Identifying “at-Risk” Patients for Sub-Optimal Beta-Lactam Exposure in Critically Ill Patients with Severe Infections. Crit. Care 2017, 21, 283. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised Antibiotic Dosing for Patients Who Are Critically Ill: Challenges and Potential Solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Joynt, G.M.; Choi, G.Y.S.; Gomersall, C.D.; Lipman, J. How to Optimise Antimicrobial Prescriptions in the Intensive Care Unit: Principles of Individualised Dosing Using Pharmacokinetics and Pharmacodynamics. Int. J. Antimicrob. Agents 2012, 39, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Roberts, J.A.; Boots, R.J.; Paterson, D.L.; Lipman, J. Augmented Renal Clearance: Implications for Antibacterial Dosing in the Critically Ill. Clin. Pharmacokinet. 2010, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial Therapeutic Drug Monitoring in Critically Ill Adult Patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the Treatment with Beta-Lactam Antibiotics in Critically Ill Patients-Guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation-SFAR). Crit. Care 2019, 23, 104. [Google Scholar] [CrossRef]
- Alshaer, M.H.; Williams, R.; Mousa, M.J.; Alexander, K.M.; Maguigan, K.L.; Manigaba, K.; Maranchick, N.; Shoulders, B.R.; Felton, T.W.; Mathew, S.K.; et al. Cefepime Daily Exposure and the Associated Impact on the Change in Sequential Organ Failure Assessment Scores and Vasopressors Requirement in Critically Ill Patients Using Repeated-Measures Mixed-Effect Modeling. Crit. Care Explor. 2023, 5, e0993. [Google Scholar] [CrossRef]
- Hagel, S.; Bach, F.; Brenner, T.; Bracht, H.; Brinkmann, A.; Annecke, T.; Hohn, A.; Weigand, M.; Michels, G.; Kluge, S.; et al. Effect of Therapeutic Drug Monitoring-Based Dose Optimization of Piperacillin/Tazobactam on Sepsis-Related Organ Dysfunction in Patients with Sepsis: A Randomized Controlled Trial. Intensive Care Med. 2022, 48, 311–321. [Google Scholar] [CrossRef]
- Hoff, B.M.; Maker, J.H.; Dager, W.E.; Heintz, B.H. Antibiotic Dosing for Critically Ill Adult Patients Receiving Intermittent Hemodialysis, Prolonged Intermittent Renal Replacement Therapy, and Continuous Renal Replacement Therapy: An Update. Ann. Pharmacother. 2020, 54, 43–55. [Google Scholar] [CrossRef]
- Cook, A.M.; Hatton-Kolpek, J. Augmented Renal Clearance. Pharmacotherapy 2019, 39, 346–354. [Google Scholar] [CrossRef]
- Hefny, F.; Stuart, A.; Kung, J.Y.; Mahmoud, S.H. Prevalence and Risk Factors of Augmented Renal Clearance: A Systematic Review and Meta-Analysis. Pharmaceutics 2022, 14, 445. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN Surveillance Definition of Health Care-Associated Infection and Criteria for Specific Types of Infections in the Acute Care Setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- EUCAST—European Committee on Antimicrobial Susceptibility Testing European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs and Zone Diameters Version 12.0, Valid from 2022-01-01. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_14.0_Breakpoint_Tables.pdf (accessed on 1 February 2024).
- Shields, R.K.; Nguyen, M.H.; Chen, L.; Press, E.G.; Kreiswirth, B.N.; Clancy, C.J. Pneumonia and Renal Replacement Therapy Are Risk Factors for Ceftazidime-Avibactam Treatment Failures and Resistance among Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob. Agents Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Vena, A.; Giacobbe, D.R.; Castaldo, N.; Cattelan, A.; Mussini, C.; Luzzati, R.; Rosa, F.G.D.; Del Puente, F.; Mastroianni, C.M.; Cascio, A.; et al. Clinical Experience with Ceftazidime-Avibactam for the Treatment of Infections Due to Multidrug-Resistant Gram-Negative Bacteria Other than Carbapenem-Resistant Enterobacterales. Antibiotics 2020, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Rinaldi, M.; Laici, C.; Siniscalchi, A.; Viale, P.; Pea, F. Role of a Real-Time TDM-Based Expert Clinical Pharmacological Advice Program in Optimizing the Early Pharmacokinetic/Pharmacodynamic Target Attainment of Continuous Infusion Beta-Lactams among Orthotopic Liver Transplant Recipients with Documented or Suspected Gram-Negative Infections. Antibiotics 2023, 12, 1599. [Google Scholar] [CrossRef]


| Demographics and Clinical Variables | Piperacillin-Tazobactam (n = 43) | Meropenem (n = 32) | p Value |
|---|---|---|---|
| Patient demographics | |||
| Age (years) (median (IQR)) | 69 (57–74) | 71.5 (61.25–76.25) | 0.28 |
| Gender (male/female) (n (%)) | 25/18 (58.1/41.9) | 24/8 (75.0/25.0) | 0.13 |
| Body weight (Kg) (median (IQR)) | 80 (65–90) | 80 (70–90) | 0.52 |
| Body mass index (Kg/m2) (median (IQR)) | 26.1 (23.1–29.4) | 27.6 (24.2–32.5) | 0.31 |
| Severity of clinical conditions | |||
| Mechanical ventilation (n (%)) | 35 (81.4) | 24 (75.0) | 0.51 |
| Vasopressors (n (%)) | 27 (62.8) | 20 (62.5) | 0.98 |
| Continuous renal replacement therapy (n (%)) | 11 (25.6) | 10 (31.3) | 0.59 |
| Augmented renal clearance (n (%)) | 3 (7.0) | 3 (9.4) | 0.99 |
| Baseline SOFA score (median (IQR)) | 8 (4–11) | 9 (5.75–13) | 0.56 |
| Baseline serum PCT levels (median (IQR)) | 4.7 (0.6–34.0) | 8.7 (2.0–58.8) | 0.14 |
| Baseline serum CRP levels (median (IQR)) | 14.9 (7.1–23.3) | 16.1 (9.1–26.7) | 0.33 |
| Site of infection (n (%)) | |||
| BSI | 24 (55.8) | 21 (65.6) | 0.39 |
| VAP | 16 (37.2) | 5 (15.6) | 0.04 |
| VAP + BSI | 3 (7.0) | 6 (18.8) | 0.16 |
| Gram-negative clinical isolatesa (n (%)) | |||
| Escherichia coli | 18 (37.5) | 7 (17.9) | 0.046 |
| Pseudomonas aeruginosa | 14 (29.0) | 4 (10.3) | 0.04 |
| Klebsiella pneumoniae | 6 (12.5) | 14 (35.9) | 0.01 |
| Klebsiella aerogenes | 2 (4.2) | 3 (7.7) | 0.65 |
| Proteus mirabilis | 2 (4.2) | 1 (2.6) | 0.99 |
| Proteus vulgaris | 2 (4.2) | 0 (0.0) | 0.50 |
| Serratia marcescens | 1 (2.1) | 2 (5.1) | 0.58 |
| Citrobacter koseri | 1 (2.1) | 0 (0.0) | 0.99 |
| Citrobacter braakii | 1 (2.1) | 0 (0.0) | 0.99 |
| Klebsiella oxytoca | 1 (2.1) | 2 (5.1) | 0.58 |
| Enterobacter cloacae | 0 (0.0) | 2 (5.1) | 0.20 |
| Enterobacter bugadensis | 0 (0.0) | 1 (2.6) | 0.45 |
| Morganella morganii | 0 (0.0) | 1 (2.6) | 0.45 |
| Acinetobacter baumannii | 0 (0.0) | 1 (2.6) | 0.45 |
| Hafnia alvei | 0 (0.0) | 1 (2.6) | 0.45 |
| ESBL-producing Enterobacterales | 7 (14.6) | 12 (30.8) | 0.07 |
| AmpC-producing Enterobacterales | 3 (6.3) | 4 (10.3) | 0.70 |
| Beta-lactam treatment | |||
| Daily dose (mg) (median (IQR)) | 18 g/day (13.5–18 g/day) | 2 g/day (1.5–4 g/day) | |
| Piperacillin/Meropenem fCss (mg/L) (median (IQR)) | 54.6 (41.0–91.2) | 14.9 (10.5–24.8) | |
| Tazobactam fCss (mg/L) (median (IQR)) | 7.2 (4.6–11.6) | - | |
| Piperacillin/Meropenem fCss/MIC ratio (median (IQR)) | 7.6 (4.8–13.0) | 92.3 (20.3–166.5) | |
| Tazobactam fCss/CT ratio (median (IQR)) | 1.8 (1.2–2.9) | - | |
| PK/PD target attainment | |||
| Overall optimal joint PK/PD target (n (%)) | 36 (83.7) | 32 (100.0) | |
| Overall quasi-optimal joint PK/PD target (n (%)) | 6 (14.0) | 0 (0.0) | 0.06 |
| Overall suboptimal joint PK/PD target (n (%)) | 1 (2.3) | 0 (0.0) | |
| ECPA program | |||
| Overall TDM-based ECPAs | 93 | 80 | |
| N. of TDM-based ECPA per treatment course (median (IQR)) | 2 (1–2.5) | 2 (1–3.25) | 0.48 |
| N. of dosage confirmations at first TDM assessment (n (%)) | 15 (34.9) | 7 (21.9) | 0.22 |
| N. of dosage increases at first TDM assessment (n (%)) | 1 (2.3) | 0 (0.0) | 0.99 |
| N. of dosage decreases at first TDM assessment (n (%)) | 27 (62.8) | 25 (78.1) | 0.16 |
| Overall n. of dosage confirmations (n (%)) | 49 (52.7) | 38 (47.5) | 0.50 |
| Overall n. of dosage increases (n (%)) | 5 (5.4) | 4 (5.0) | 0.99 |
| Overall n. of dosage decreases (n (%)) | 39 (41.9) | 38 (47.5) | 0.46 |
| Outcome | Piperacillin-Tazobactam (n = 43) | Meropenem (n = 32) | p Value |
|---|---|---|---|
| Primary outcomes | |||
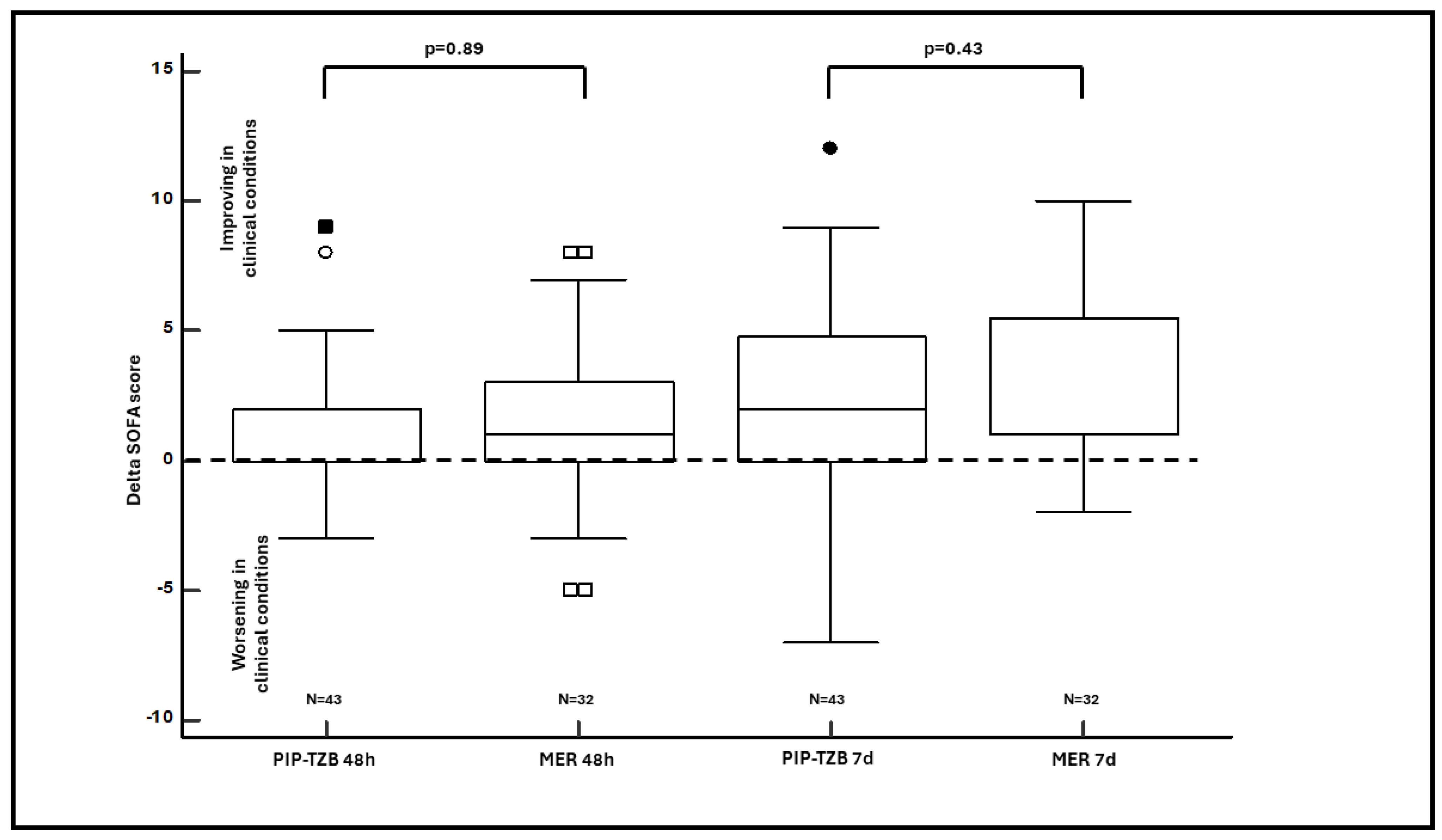
| Delta 48-h SOFA (median (IQR)) | 0 (0–2) | 1 (0–3) | 0.89 |
| Delta 7-days SOFA (median (IQR)) | 2 (0–4.5) | 1 (1–5.25) | 0.43 |
| Secondary outcomes | |||
| Delta 48-h CRP (median (IQR)) * | 18.3% (−9.2–43.2%) | 18.0% (−47.9–41.1%) | 0.64 |
| Delta 7-days CRP (median (IQR)) ** | 50.3% (14.4–72.3%) | 51.8% (27.8–67.9%) | 0.86 |
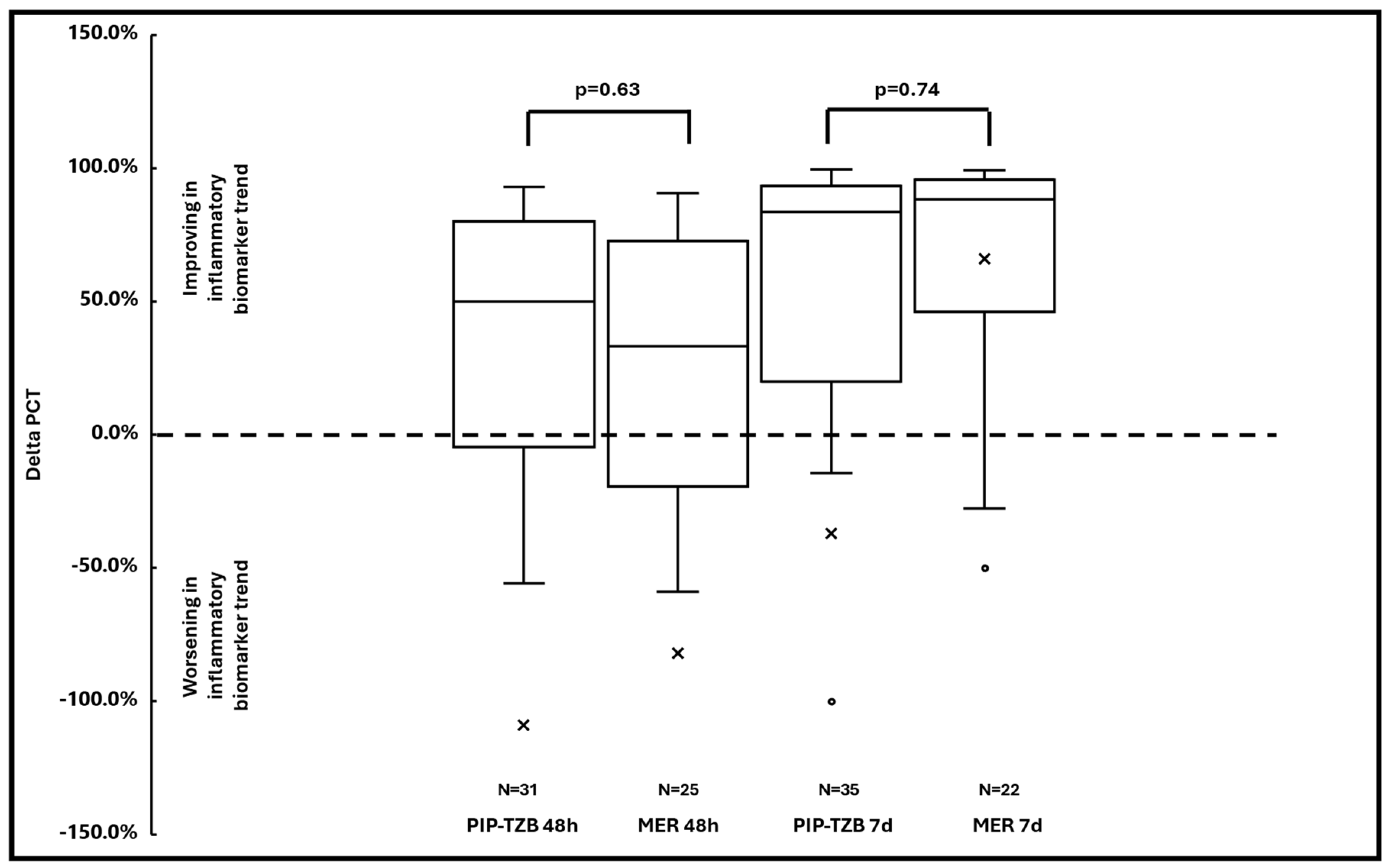
| Delta 48-h PCT (median (IQR)) *** | 50.0% (2.6–77.6%) | 33.3% (−11.5–70.9%) | 0.63 |
| Delta 7-days PCT (median (IQR)) **** | 83.5% (35.0–93.2%) | 88.1% (52.3–94.7%) | 0.74 |
| Microbiological eradication (n (%)) | 32 (74.4) | 27 (84.4) | 0.30 |
| Resistance occurrence (n (%)) | 3 (7.0) | 2 (6.3) | 0.99 |
| Clinical cure (n (%)) | 29 (67.4) | 23 (71.9) | 0.68 |
| 90-days MDR colonization (n (%)) | 4 (9.3) | 5 (15.6) | 0.48 |
| ICU mortality (n (%)) | 4 (9.3) | 6 (18.8) | 0.31 |
| 30-day mortality (n (%)) | 6 (14.0) | 7 (21.9) | 0.37 |
| Outcome | Piperacillin-Tazobactam (n = 43) | Meropenem (n = 32) | p Value |
|---|---|---|---|
| Delta 48-h cardiovascular SOFA subscore (median (IQR)) | 0 (0–1) | 0 (0–1) | 0.40 |
| Delta 7-days cardiovascular SOFA subscore (median (IQR)) | 1 (0–4) | 0 (0–4) | 0.98 |
| Delta 48-h respiratory SOFA subscore (median (IQR)) | 0 (0–1) | 0 (0–1) | 0.57 |
| Delta 7-days respiratory SOFA subscore (median (IQR)) | 0 (0–1) | 1 (0–1) | 0.17 |
| Delta 48-h coagulation SOFA subscore (median (IQR)) | 0 (−1–0) | 0 (0–0) | 0.16 |
| Delta 7-days coagulation SOFA subscore (median (IQR)) | 0 (−0.5–0) | 0 (0–0.25) | 0.13 |
| Delta 48-h renal SOFA subscore (median (IQR)) | 0 (0–0) | 0 (−0.25–0) | 0.46 |
| Delta 7-days renal SOFA subscore (median (IQR)) | 0 (0–0.5) | 0 (0–1) | 0.78 |
| Delta 48-h hepatic SOFA subscore (median (IQR)) | 0 (0–0) | 0 (0–0) | 0.49 |
| Delta 7-days hepatic SOFA subscore (median (IQR)) | 0 (0–0) | 0 (0–0.25) | 0.61 |
| Delta 48-h neurological SOFA subscore (median (IQR)) | 0 (0–0) | 0 (0–0) | 0.27 |
| Delta 7-days neurological SOFA subscore (median (IQR)) | 0 (0–1) | 0 (0–0) | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatti, M.; Rinaldi, M.; Tonetti, T.; Siniscalchi, A.; Viale, P.; Pea, F. Comparative Impact of an Optimized PK/PD Target Attainment of Piperacillin-Tazobactam vs. Meropenem on the Trend over Time of SOFA Score and Inflammatory Biomarkers in Critically Ill Patients Receiving Continuous Infusion Monotherapy for Treating Documented Gram-Negative BSIs and/or VAP. Antibiotics 2024, 13, 296. https://doi.org/10.3390/antibiotics13040296
Gatti M, Rinaldi M, Tonetti T, Siniscalchi A, Viale P, Pea F. Comparative Impact of an Optimized PK/PD Target Attainment of Piperacillin-Tazobactam vs. Meropenem on the Trend over Time of SOFA Score and Inflammatory Biomarkers in Critically Ill Patients Receiving Continuous Infusion Monotherapy for Treating Documented Gram-Negative BSIs and/or VAP. Antibiotics. 2024; 13(4):296. https://doi.org/10.3390/antibiotics13040296
Chicago/Turabian StyleGatti, Milo, Matteo Rinaldi, Tommaso Tonetti, Antonio Siniscalchi, Pierluigi Viale, and Federico Pea. 2024. "Comparative Impact of an Optimized PK/PD Target Attainment of Piperacillin-Tazobactam vs. Meropenem on the Trend over Time of SOFA Score and Inflammatory Biomarkers in Critically Ill Patients Receiving Continuous Infusion Monotherapy for Treating Documented Gram-Negative BSIs and/or VAP" Antibiotics 13, no. 4: 296. https://doi.org/10.3390/antibiotics13040296
APA StyleGatti, M., Rinaldi, M., Tonetti, T., Siniscalchi, A., Viale, P., & Pea, F. (2024). Comparative Impact of an Optimized PK/PD Target Attainment of Piperacillin-Tazobactam vs. Meropenem on the Trend over Time of SOFA Score and Inflammatory Biomarkers in Critically Ill Patients Receiving Continuous Infusion Monotherapy for Treating Documented Gram-Negative BSIs and/or VAP. Antibiotics, 13(4), 296. https://doi.org/10.3390/antibiotics13040296







