Antimicrobial Properties of Colostrum and Milk
Abstract
1. Introduction
2. Antibacterial Components of Milk/Colostrum
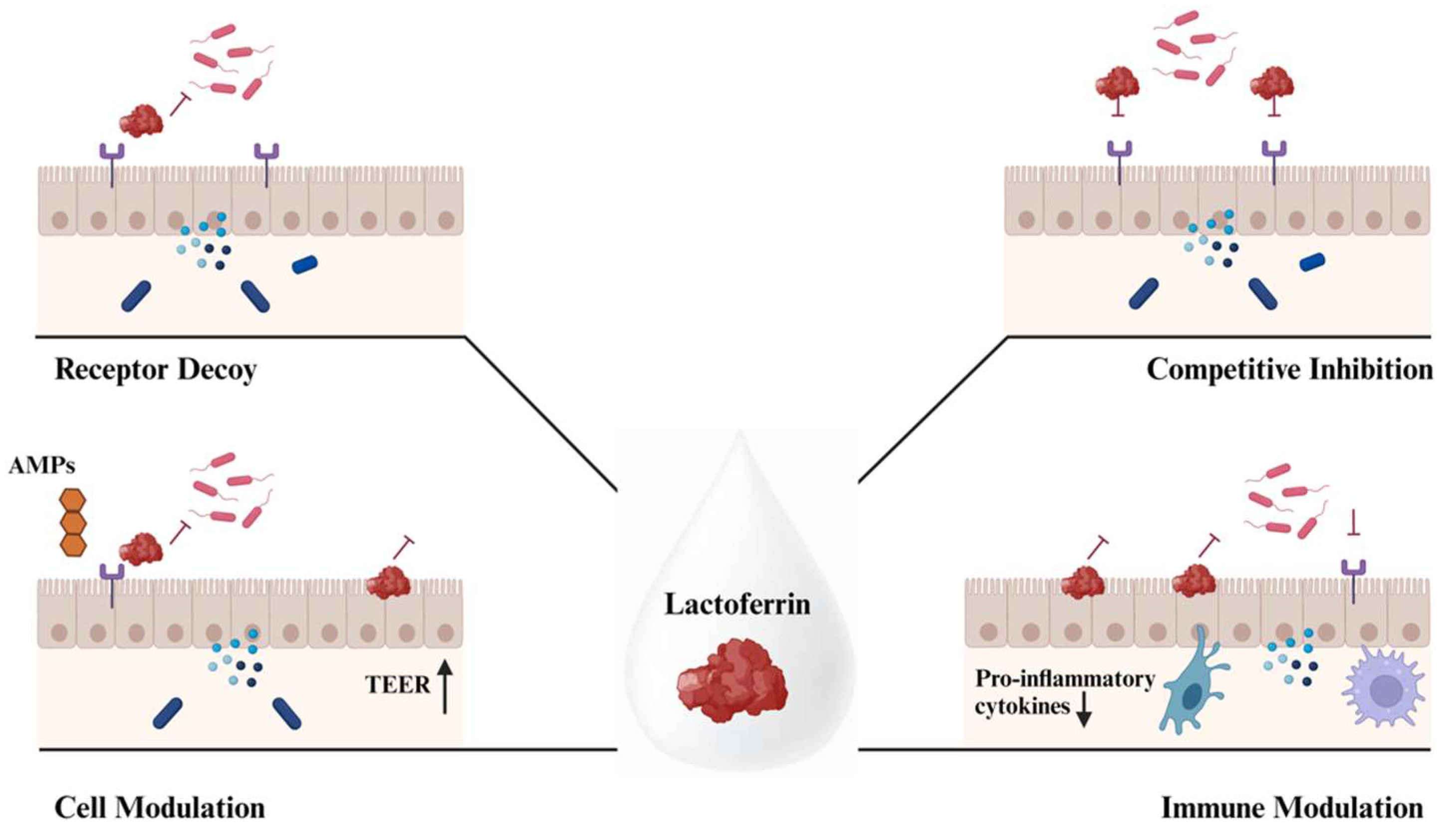
2.1. Lactoferrin
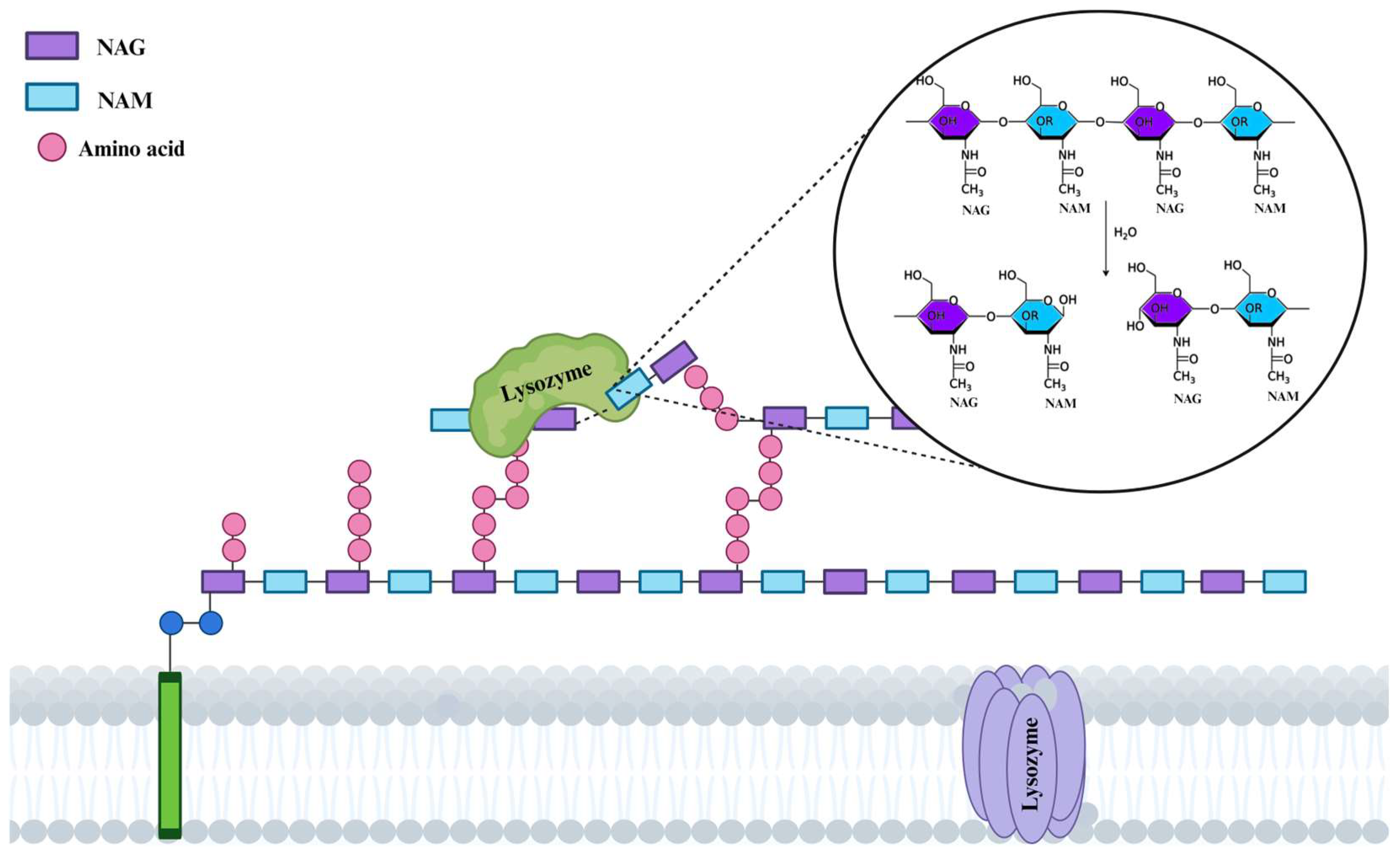
2.2. Lysozyme
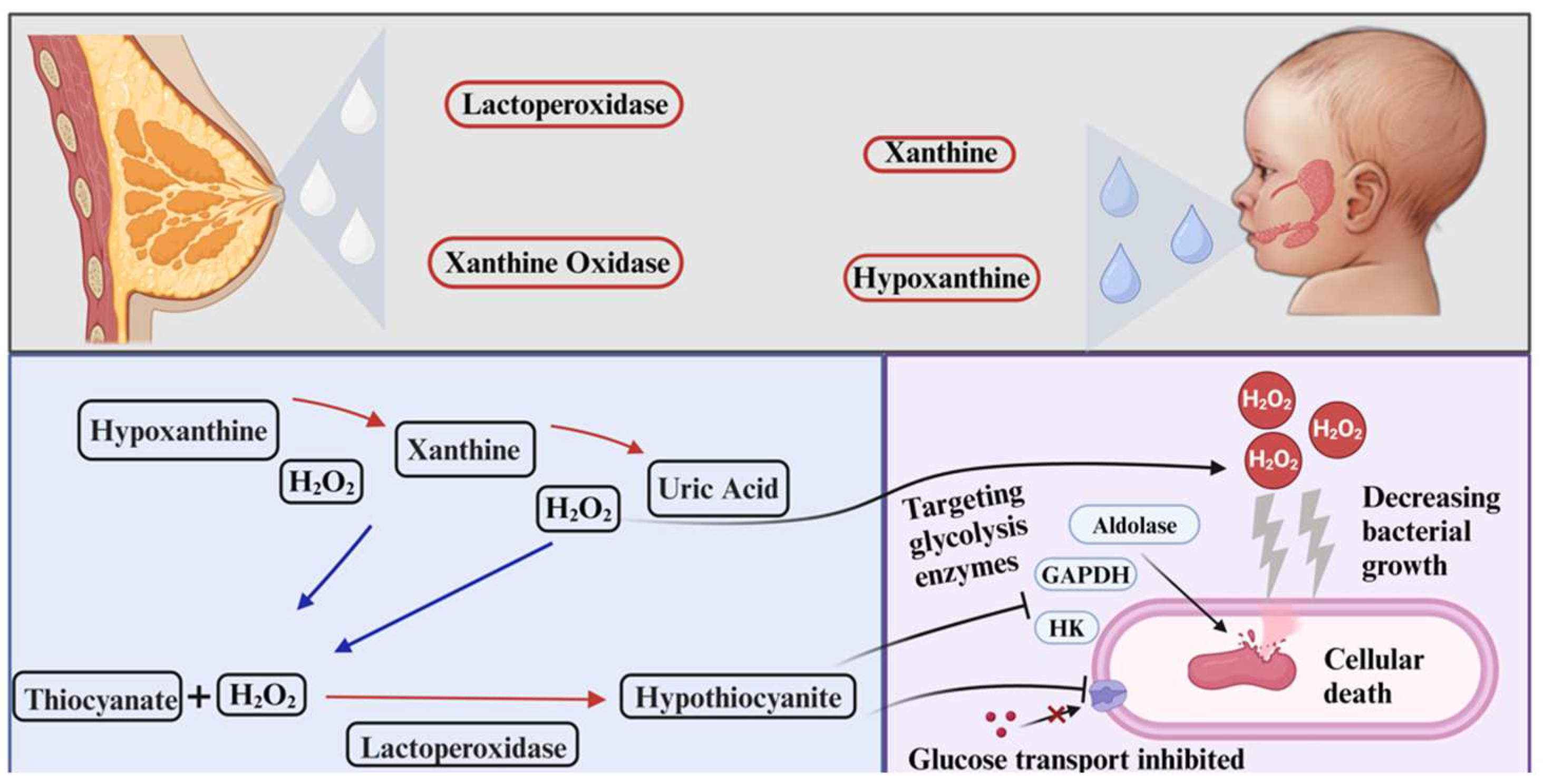
2.3. Xanthine Oxidase
2.4. Lactoperoxidase
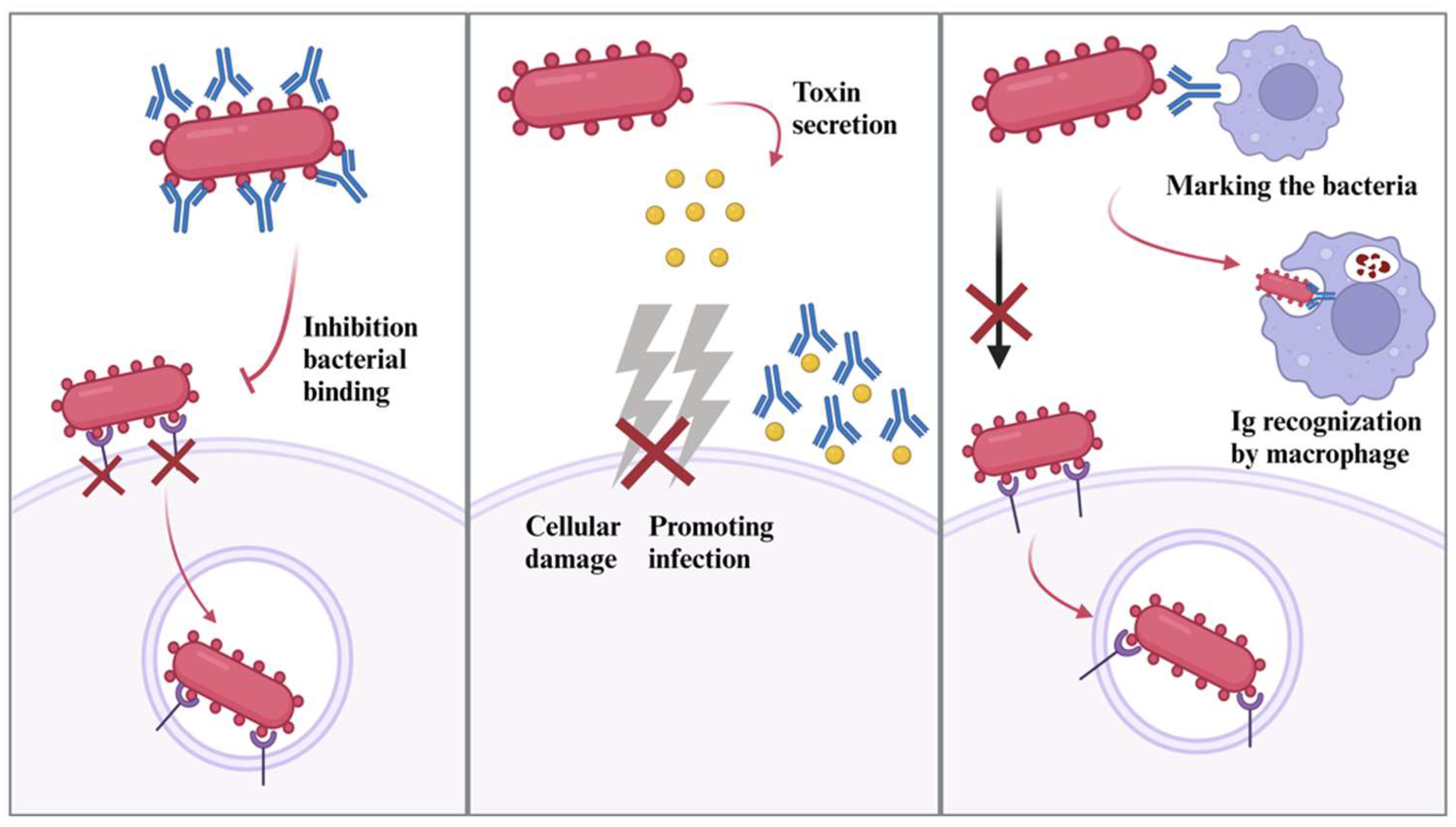
2.5. Immunoglobulins
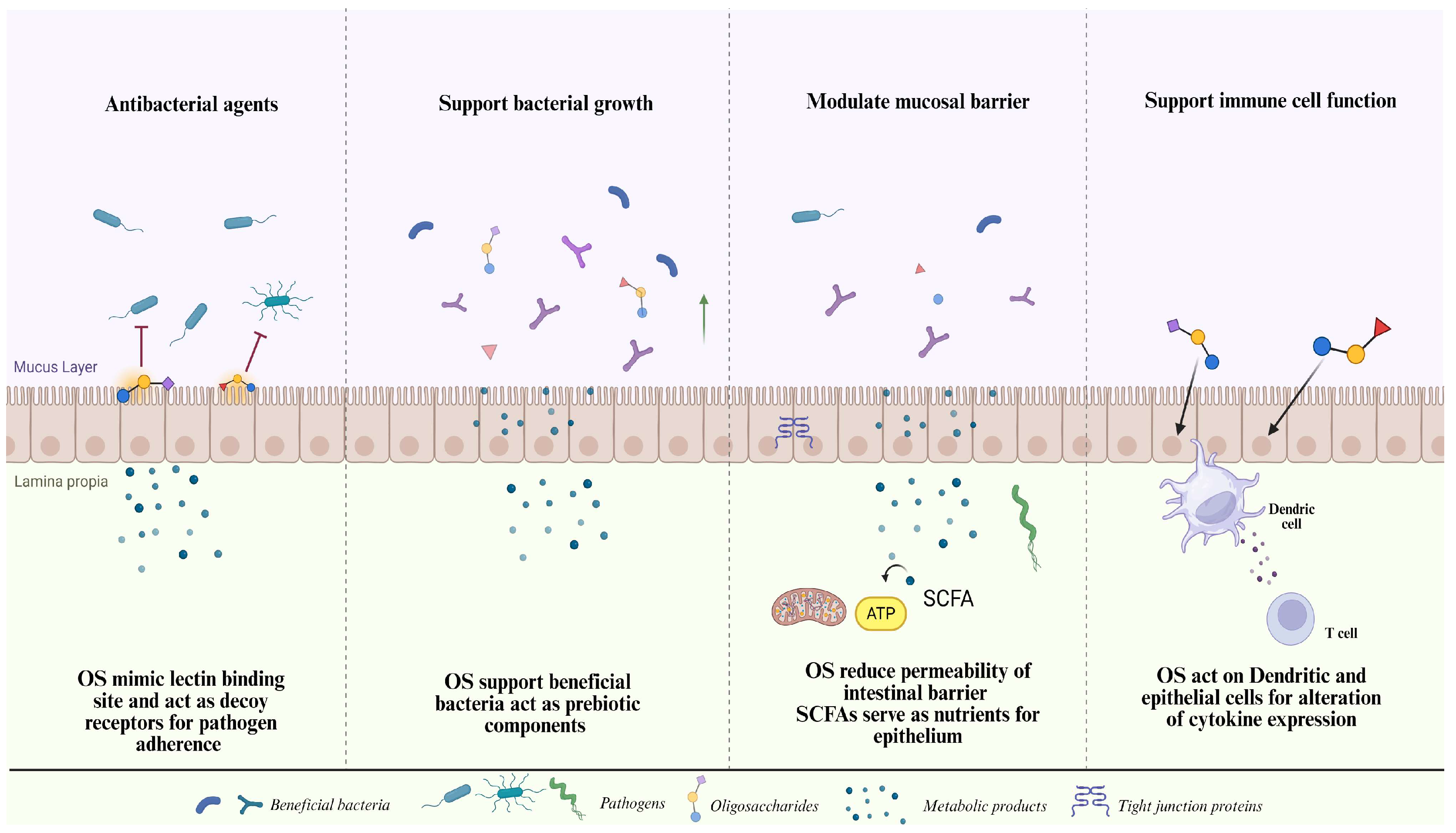
2.6. Oligosaccharides
3. Other Antibacterial Components
3.1. α-Lactalbumin
3.2. Epidermal Growth Factor
3.3. Glycomacropeptide
3.4. Glycosaminoglycans
4. Limitations, Advantages, and Disadvantages of Milk and Colostrum Compound Development and Usage
5. Discussion and Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Crowley, S.V.; Kelly, A.L.; Lucey, J.A.; O’Mahony, J.A. Potential Applications of Non-Bovine Mammalian Milk in Infant Nutrition. In Handbook of Milk of Non-Bovine Mammals; Park, Y.W., Haenlein, G.F.W., Wendorff, W.L., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 625–654. ISBN 978-1-119-11027-9. [Google Scholar]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Biologically Active Compounds in Milk. In Dairy Chemistry and Biochemistry; Springer: Cham, Switzerland, 2015; pp. 415–497. ISBN 978-3-319-14891-5. [Google Scholar]
- Beermann, C.; Hartung, J. Physiological Properties of Milk Ingredients Released by Fermentation. Food Funct. 2013, 4, 185–199. [Google Scholar] [CrossRef]
- Thum, C.; Ozturk, G.; McNabb, W.C.; Roy, N.C.; Leite Nobrega De Moura Bell, J.M. Effects of Microwave Processing Conditions on Microbial Safety and Antimicrobial Proteins in Bovine Milk. J. Food Process. Preserv. 2020, 44, 14348. [Google Scholar] [CrossRef]
- Kaplan, M.; Arslan, A.; Duman, H.; Karyelioğlu, M.; Baydemir, B.; Günar, B.B.; Alkan, M.; Bayraktar, A.; Tosun, H.İ.; Ertürk, M.; et al. Production of Bovine Colostrum for Human Consumption to Improve Health. Front. Pharmacol. 2021, 12, 796824. [Google Scholar] [CrossRef] [PubMed]
- Playford, R.J.; Weiser, M.J. Bovine Colostrum: Its Constituents and Uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.D.S.; Anaya, K.; Galdino, A.B.S.; Oliveira, J.P.F.; Gama, M.A.S.; Medeiros, C.A.C.X.; Gavioli, E.C.; Porto, A.L.F.; Rangel, A.H.N. Bovine Colostrum: A Source of Bioactive Compounds for Prevention and Treatment of Gastrointestinal Disorders. NFS J. 2021, 25, 1–11. [Google Scholar] [CrossRef]
- McGrath, B.A.; Fox, P.F.; McSweeney, P.L.H.; Kelly, A.L. Composition and Properties of Bovine Colostrum: A Review. Dairy Sci. Technol. 2016, 96, 133–158. [Google Scholar] [CrossRef]
- Duman, H.; Karav, S. Bovine Colostrum and Its Potential Contributions for Treatment and Prevention of COVID-19. Front. Immunol. 2023, 14, 1214514. [Google Scholar] [CrossRef]
- Arslan, A.; Kaplan, M.; Duman, H.; Bayraktar, A.; Ertürk, M.; Henrick, B.M.; Frese, S.A.; Karav, S. Bovine Colostrum and Its Potential for Human Health and Nutrition. Front. Nutr. 2021, 8, 651721. [Google Scholar] [CrossRef]
- Playford, R.J.; Macdonald, C.E.; Calnan, D.P.; Floyd, D.N.; Podas, T.; Johnson, W.; Wicks, A.C.; Bashir, O.; Marchbank, T. Co-Administration of the Health Food Supplement, Bovine Colostrum, Reduces the Acute Non-Steroidal Anti-Inflammatory Drug-Induced Increase in Intestinal Permeability. Clin. Sci. 2001, 100, 627–633. [Google Scholar] [CrossRef]
- Pereira, P.C. Milk Nutritional Composition and Its Role in Human Health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, A.; Lai, S.; Yuan, Q.; Jia, X.; Wang, P.; Zhang, Y. Longitudinal Changes in the Concentration of Major Human Milk Proteins in the First Six Months of Lactation and Their Effects on Infant Growth. Nutrients 2021, 13, 1476. [Google Scholar] [CrossRef]
- Czosnykowska-Łukacka, M.; Lis-Kuberka, J.; Królak-Olejnik, B.; Orczyk-Pawiłowicz, M. Changes in Human Milk Immunoglobulin Profile During Prolonged Lactation. Front. Pediatr. 2020, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Al-Shehri, S.S.; Duley, J.A.; Bansal, N. Xanthine Oxidase-Lactoperoxidase System and Innate Immunity: Biochemical Actions and Physiological Roles. Redox Biol. 2020, 34, 101524. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, G.G.; Mucci, N.C.; González, V.; Sánchez, L.; Parrón, J.A.; Pérez, M.D.; Calvo, M.; Aller, J.F.; Hozbor, F.A.; Mutto, A.A. Detection of Recombinant Human Lactoferrin and Lysozyme Produced in a Bitransgenic Cow. J. Dairy Sci. 2017, 100, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, J.; Tang, B.; Liu, Y.; Guo, C.; Yang, P.; Yu, T.; Li, R.; Zhao, J.; Zhang, L.; et al. Characterization of Bioactive Recombinant Human Lysozyme Expressed in Milk of Cloned Transgenic Cattle. PLoS ONE 2011, 6, e17593. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Meletharayil, G.; Kapoor, R.; Abbaspourrad, A. Bioactives in Bovine Milk: Chemistry, Technology, and Applications. Nutr. Rev. 2021, 79, 48–69. [Google Scholar] [CrossRef] [PubMed]
- Urashima, T.; Asakuma, S.; Leo, F.; Fukuda, K.; Messer, M.; Oftedal, O. The Predominance of Type I Oligosaccharides Is a Feature Specific to Human Breast Milk. Adv. Nutr. 2012, 3, 473S–482S. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, M.; Caorsi, C.; Ceruti, P.; Varadhachary, A.; Forni, G.; Pericle, F.; Giovarelli, M. Lactoferrin, a Major Defense Protein of Innate Immunity, Is a Novel Maturation Factor for Human Dendritic Cells. FASEB J. 2008, 22, 2747–2757. [Google Scholar] [CrossRef]
- Katunuma, N.; Shiota, H.; Le, Q.T. Medical Significance of Cysteine Protease Inhibitors in Mammalian Secretory Fluids. J. Med. Investig. 2003, 50, 154–161. [Google Scholar]
- Kowalczyk, P.; Kaczyńska, K.; Kleczkowska, P.; Bukowska-Ośko, I.; Kramkowski, K.; Sulejczak, D. The Lactoferrin Phenomenon—A Miracle Molecule. Molecules 2022, 27, 2941. [Google Scholar] [CrossRef]
- Embleton, N.D.; Berrington, J.E.; McGuire, W.; Stewart, C.J.; Cummings, S.P. Lactoferrin: Antimicrobial Activity and Therapeutic Potential. Semin. Fetal Neonatal Med. 2013, 18, 143–149. [Google Scholar] [CrossRef]
- Alves de Queiroz, V.O.; Assis, A.M.O.; da Costa, R.J.H. Protective Effect of Human Lactoferrin in the Gastrointestinal Tract. Rev. Paul Pediatr. 2013, 31, 90–95. [Google Scholar] [CrossRef]
- García-Montoya, I.A.; Cendón, T.S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin a Multiple Bioactive Protein: An Overview. Biochim. Biophys. Acta 2012, 1820, 226–236. [Google Scholar] [CrossRef]
- Da Vieira, D.S.; Polveiro, R.C.; Butler, T.J.; Hackett, T.A.; Braga, C.P.; Lal Puniya, B.; Teixeira, W.F.P.; de Padilha, P.M.; Adamec, J.; Feirosa, F.L.F. An in Silico, Structural, and Biological Analysis of Lactoferrin of Different Mammals. Int. J. Biol. Macromol. 2021, 187, 119–126. [Google Scholar] [CrossRef]
- Arnold, R.R.; Brewer, M.; Gauthier, J.J. Bactericidal Activity of Human Lactoferrin: Sensitivity of a Variety of Microorganisms. Infect. Immun. 1980, 28, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory Effects of Lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Drago-Serrano, M.E.; Campos-Rodríguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin: Balancing Ups and Downs of Inflammation Due to Microbial Infections. Int. J. Mol. Sci. 2017, 18, 501. [Google Scholar] [CrossRef] [PubMed]
- Actor, J.K.; Hwang, S.-A.; Kruzel, M.L. Lactoferrin as a Natural Immune Modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef] [PubMed]
- Artym, J.; Kocięba, M.; Zaczyńska, E.; Adamik, B.; Kübler, A.; Zimecki, M.; Kruzel, M. Immunomodulatory Properties of Human Recombinant Lactoferrin in Mice: Implications for Therapeutic Use in Humans. Adv. Clin. Exp. Med. 2018, 27, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.D. Iron, Infection, and Neoplasia. Clin. Physiol. Biochem. 1986, 4, 50–60. [Google Scholar]
- Morrin, S.T.; Buck, R.H.; Farrow, M.; Hickey, R.M. Milk-Derived Anti-Infectives and Their Potential to Combat Bacterial and Viral Infection. J. Funct. Foods 2021, 81, 104442. [Google Scholar] [CrossRef]
- Manzoni, P.; Stolfi, I.; Messner, H.; Cattani, S.; Laforgia, N.; Romeo, M.G.; Bollani, L.; Rinaldi, M.; Gallo, E.; Quercia, M.; et al. Bovine Lactoferrin Prevents Invasive Fungal Infections in Very Low Birth Weight Infants: A Randomized Controlled Trial. Pediatrics 2012, 129, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.N.; Jiang, P.; Stensballe, A.; Bendixen, E.; Sangild, P.T.; Chatterton, D.E.W. Bovine Lactoferrin Regulates Cell Survival, Apoptosis and Inflammation in Intestinal Epithelial Cells and Preterm Pig Intestine. J. Proteom. 2016, 139, 95–102. [Google Scholar] [CrossRef]
- Singh, A.; Duche, R.T.; Wandhare, A.G.; Sian, J.K.; Singh, B.P.; Sihag, M.K.; Singh, K.S.; Sangwan, V.; Talan, S.; Panwar, H. Milk-Derived Antimicrobial Peptides: Overview, Applications, and Future Perspectives. Probiotics Antimicrob. Proteins 2023, 15, 44–62. [Google Scholar] [CrossRef]
- Kim, J. Microbiome Engineering Using Probiotic Yeast: Saccharomyces Boulardii and the Secreted Human Lysozyme Lead to Changes in the Gut Microbiome and Metabolome of Mice. Microbiol. Spectr. 2022, 11, e0078023. [Google Scholar] [CrossRef]
- Gingerich, A.D.; Doja, F.; Thomason, R.; Tóth, E.; Bradshaw, J.L.; Douglass, M.V.; McDaniel, L.S.; Rada, B. Oxidative Killing of Encapsulated and Nonencapsulated Streptococcus pneumoniae by Lactoperoxidase-Generated Hypothiocyanite. PLoS ONE 2020, 15, e0236389. [Google Scholar] [CrossRef]
- Sheikh, I.A.; Yasir, M.; Khan, I.; Khan, S.B.; Azum, N.; Jiffri, E.H.; Kamal, M.A.; Ashraf, G.M.; Beg, M.A. Lactoperoxidase Immobilization on Silver Nanoparticles Enhances Its Antimicrobial Activity. J. Dairy Res. 2018, 85, 460–464. [Google Scholar] [CrossRef]
- Maga, E.A.; Desai, P.T.; Weimer, B.C.; Dao, N.; Kültz, D.; Murray, J.D. Consumption of Lysozyme-Rich Milk Can Alter Microbial Fecal Populations. Appl. Environ. Microbiol. 2012, 78, 6153–6160. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Li, X.; Lu, D.; Liu, S.; Suo, X.; Li, Q.; Li, N. Lysozyme Improves Gut Performance and Protects against Enterotoxigenic Escherichia coli Infection in Neonatal Piglets. Vet. Res. 2018, 49, 20. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zhou, J.; Liu, H.; Tang, Y.; Tan, B.; Yin, Y. Dietary Lysozyme Supplementation Contributes to Enhanced Intestinal Functions and Gut Microflora of Piglets. Food Funct. 2019, 10, 1696–1706. [Google Scholar] [CrossRef]
- Rybarczyk, J.; Kieckens, E.; Vanrompay, D.; Cox, E. In Vitro and in Vivo Studies on the Antimicrobial Effect of Lactoferrin against Escherichia coli O157:H7. Vet. Microbiol. 2017, 202, 23–28. [Google Scholar] [CrossRef]
- Diarra, M.S.; Petitclerc, D.; Deschênes, E.; Lessard, N.; Grondin, G.; Talbot, B.G.; Lacasse, P. Lactoferrin against Staphylococcus aureus Mastitis. Lactoferrin Alone or in Combination with Penicillin G on Bovine Polymorphonuclear Function and Mammary Epithelial Cells Colonisation by Staphylococcus aureus. Vet. Immunol. Immunopathol. 2003, 95, 33–42. [Google Scholar] [CrossRef]
- Flores-Villaseñor, H.; Canizalez-Román, A.; Reyes-Lopez, M.; Nazmi, K.; de la Garza, M.; Zazueta-Beltrán, J.; León-Sicairos, N.; Bolscher, J.G.M. Bactericidal Effect of Bovine Lactoferrin, LFcin, LFampin and LFchimera on Antibiotic-Resistant Staphylococcus aureus and Escherichia coli. Biometals 2010, 23, 569–578. [Google Scholar] [CrossRef]
- Théolier, J.; Fliss, I.; Jean, J.; Hammami, R. MilkAMP: A Comprehensive Database of Antimicrobial Peptides of Dairy Origin. Dairy Sci. Technol. 2014, 94, 181–193. [Google Scholar] [CrossRef]
- León-Calvijo, M.A.; Leal-Castro, A.L.; Almanzar-Reina, G.A.; Rosas-Pérez, J.E.; García-Castañeda, J.E.; Rivera-Monroy, Z.J. Antibacterial Activity of Synthetic Peptides Derived from Lactoferricin against Escherichia coli ATCC 25922 and Enterococcus faecalis ATCC 29212. Biomed. Res. Int. 2015, 2015, 453826. [Google Scholar] [CrossRef]
- Björck, L.; Rosén, C.; Marshall, V.; Reiter, B. Antibacterial Activity of the Lactoperoxidase System in Milk against Pseudomonads and Other Gram-Negative Bacteria. Appl. Microbiol. 1975, 30, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Alustiza, F.; Bellingeri, R.; Picco, N.; Motta, C.; Grosso, M.C.; Barbero, C.A.; Acevedo, D.F.; Vivas, A. IgY against Enterotoxigenic Escherichia coli Administered by Hydrogel-Carbon Nanotubes Composites to Prevent Neonatal Diarrhoea in Experimentally Challenged Piglets. Vaccine 2016, 34, 3291–3297. [Google Scholar] [CrossRef]
- Chaneton, L.; Pérez Sáez, J.M.; Bussmann, L.E. Antimicrobial Activity of Bovine β-Lactoglobulin against Mastitis-Causing Bacteria. J. Dairy Sci. 2011, 94, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Pantanella, F.; Natalizi, T.; Frioni, A.; Paesano, R.; Polimeni, A.; Valenti, P. Antiviral Properties of Lactoferrin—A Natural Immunity Molecule. Molecules 2011, 16, 6992–7018. [Google Scholar] [CrossRef]
- Redwan, E.M.; Uversky, V.N.; El-Fakharany, E.M.; Al-Mehdar, H. Potential Lactoferrin Activity against Pathogenic Viruses. Comptes Rendus Biol. 2014, 337, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Telang, S. Lactoferrin: A Critical Player in Neonatal Host Defense. Nutrients 2018, 10, 1228. [Google Scholar] [CrossRef]
- Ward, P.P.; Conneely, O.M. Lactoferrin: Role in Iron Homeostasis and Host Defense against Microbial Infection. Biometals 2004, 17, 203–208. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Erdmann, P.; Thakkar, S.K.; Sauser, J.; Destaillats, F. Longitudinal Evolution of True Protein, Amino Acids and Bioactive Proteins in Breast Milk: A Developmental Perspective. J. Nutr. Biochem. 2017, 41, 1–11. [Google Scholar] [CrossRef]
- Bruni, N.; Capucchio, M.T.; Biasibetti, E.; Pessione, E.; Cirrincione, S.; Giraudo, L.; Corona, A.; Dosio, F. Antimicrobial Activity of Lactoferrin-Related Peptides and Applications in Human and Veterinary Medicine. Molecules 2016, 21, 752. [Google Scholar] [CrossRef] [PubMed]
- Walzem, R.L.; Dillard, C.J.; German, J.B. Whey Components: Millennia of Evolution Create Functionalities for Mammalian Nutrition: What We Know and What We May Be Overlooking. Crit. Rev. Food Sci. Nutr. 2002, 42, 353–375. [Google Scholar] [CrossRef]
- Tossi, A.; Sandri, L.; Giangaspero, A. Amphipathic, α-Helical Antimicrobial Peptides. Pept. Sci. 2000, 55, 4–30. [Google Scholar] [CrossRef]
- Chen, Y.; Guarnieri, M.T.; Vasil, A.I.; Vasil, M.L.; Mant, C.T.; Hodges, R.S. Role of Peptide Hydrophobicity in the Mechanism of Action of α-Helical Antimicrobial Peptides. Antimicrob. Agents Chemother. 2007, 51, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Baindara, P.; Mandal, S.M. Gut-Antimicrobial Peptides: Synergistic Co-Evolution with Antibiotics to Combat Multi-Antibiotic Resistance. Antibiotics 2023, 12, 1732. [Google Scholar] [CrossRef] [PubMed]
- Pammi, M.; Abrams, S.A. Oral Lactoferrin for the Prevention of Sepsis and Necrotizing Enterocolitis in Preterm Infants. Cochrane Database Syst. Rev. 2015, 20, CD007137. [Google Scholar] [CrossRef]
- Manzoni, P.; Meyer, M.; Stolfi, I.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Decembrino, L.; Laforgia, N.; et al. Bovine Lactoferrin Supplementation for Prevention of Necrotizing Enterocolitis in Very-Low-Birth-Weight Neonates: A Randomized Clinical Trial. Early Hum. Dev. 2014, 90 (Suppl. S1), S60–S65. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, H.; Hancock, R.E.W. Antimicrobial Properties of Lactoferrin. Biochimie 2009, 91, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Lizzi, A.R.; Carnicelli, V.; Clarkson, M.M.; Di Giulio, A.; Oratore, A. Lactoferrin Derived Peptides: Mechanisms of Action and Their Perspectives as Antimicrobial and Antitumoral Agents. Mini Rev. Med. Chem. 2009, 9, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Kutila, T.; Pyörälä, S.; Saloniemi, H.; Kaartinen, L. Antibacterial Effect of Bovine Lactoferrin against Udder Pathogens. Acta Vet. Scand. 2003, 44, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Jahani, S.; Shakiba, A.; Jahani, L. The Antimicrobial Effect of Lactoferrin on Gram-Negative and Gram-Positive Bacteria. Int. J. Infect. 2015, 2, e27954. [Google Scholar] [CrossRef]
- Wang, Y.; El-Din Bekhit, A.A.; Mason, S.L.; Morton, J.D. Lactoferrin Isolation and Hydrolysis from Red Deer (Cervus elaphus) Milk and the Antibacterial Activity of Deer Lactoferrin and Its Hydrolysates. Foods 2020, 9, 1711. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Morton, J.D.; El-Din Bekhit, A.A.; Carne, A.; Mason, S.L. Amino Acid Sequences of Lactoferrin from Red Deer (Cervus elaphus) Milk and Antimicrobial Activity of Its Derived Peptides Lactoferricin and Lactoferrampin. Foods 2021, 10, 1305. [Google Scholar] [CrossRef]
- Harouna, S.; Carramiñana, J.J.; Navarro, F.; Pérez, M.D.; Calvo, M.; Sánchez, L. Antibacterial Activity of Bovine Milk Lactoferrin on the Emerging Foodborne Pathogen Cronobacter Sakazakii: Effect of Media and Heat Treatment. Food Control 2015, 47, 520–525. [Google Scholar] [CrossRef]
- Ripolles, D.; Harouna, S.; Parrón, J.A.; Calvo, M.; Pérez, M.D.; Carramiñana, J.J.; Sánchez, L. Antibacterial Activity of Bovine Milk Lactoferrin and Its Hydrolysates Prepared with Pepsin, Chymosin and Microbial Rennet against Foodborne Pathogen Listeria Monocytogenes. Int. Dairy J. 2015, 45, 15–22. [Google Scholar] [CrossRef]
- Conesa, C.; García, C.; Pérez, M.; Calvo, M.; Sanchez, L. Antimicrobial Activity of Recombinant Human Lactoferrin from Aspergillus Awamori, Human Milk Lactoferrin and Their Hydrolysates. Eur. Food Res. Technol. 2008, 228, 205–211. [Google Scholar] [CrossRef]
- Conesa, C.; Sánchez, L.; Rota, C.; Pérez, M.-D.; Calvo, M.; Farnaud, S.; Evans, R.W. Isolation of Lactoferrin from Milk of Different Species: Calorimetric and Antimicrobial Studies. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 150, 131–139. [Google Scholar] [CrossRef]
- Ellison, R.T.; Giehl, T.J. Killing of Gram-Negative Bacteria by Lactoferrin and Lysozyme. J. Clin. Investig. 1991, 88, 1080–1091. [Google Scholar] [CrossRef]
- Diarra, M.S.; Petitclerc, D.; Lacasse, P. Effect of Lactoferrin in Combination with Penicillin on the Morphology and the Physiology of Staphylococcus Aureus Isolated from Bovine Mastitis1, 2. J. Dairy Sci. 2002, 85, 1141–1149. [Google Scholar] [CrossRef]
- Khorshidian, N.; Khanniri, E.; Koushki, M.R.; Sohrabvandi, S.; Yousefi, M. An Overview of Antimicrobial Activity of Lysozyme and Its Functionality in Cheese. Front. Nutr. 2022, 9, 833618. [Google Scholar] [CrossRef] [PubMed]
- Ragland, S.A.; Criss, A.K. From Bacterial Killing to Immune Modulation: Recent Insights into the Functions of Lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef] [PubMed]
- Ferraboschi, P.; Ciceri, S.; Grisenti, P. Applications of Lysozyme, an Innate Immune Defense Factor, as an Alternative Antibiotic. Antibiotics 2021, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Callewaert, L.; Michiels, C.W. Lysozymes in the Animal Kingdom. J. Biosci. 2010, 35, 127–160. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, S.; Xia, J.; Shao, P.; Sun, P.; Xiang, N. Enhanced Antibacterial Activity of Hen Egg-White Lysozyme against Staphylococcus aureus and Escherichia coli Due to Protein Fibrillation. Biomacromolecules 2021, 22, 890–897. [Google Scholar] [CrossRef]
- Yu, L.; Sun, B.; Li, J.; Sun, L. Characterization of a C-Type Lysozyme of Scophthalmus Maximus: Expression, Activity, and Antibacterial Effect. Fish Shellfish. Immunol. 2013, 34, 46–54. [Google Scholar] [CrossRef]
- Ling, X.; Lv, J.; Chen, F.; Qin, X.; Wu, M.; Bai, F.; Luo, H. Expression Characteristics and in Vitro Antibacterial Properties of C-Type Lysozyme in Crucian Carp Infected with Aeromonas Salmonicida. Heliyon 2024, 10, e24044. [Google Scholar] [CrossRef]
- Venkataramani, S.; Truntzer, J.; Coleman, D. Thermal Stability of High Concentration Lysozyme across Varying pH: A Fourier Transform Infrared Study. J. Pharm. Bioall. Sci. 2013, 5, 148. [Google Scholar] [CrossRef]
- Cosentino, C.; Labella, C.; Elshafie, H.S.; Camele, I.; Musto, M.; Paolino, R.; D’Adamo, C.; Freschi, P. Effects of Different Heat Treatments on Lysozyme Quantity and Antimicrobial Activity of Jenny Milk. J. Dairy Sci. 2016, 99, 5173–5179. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, N.; Wen, S.; Wang, F.; Nawaz, S.; Raza, J.; Iftikhar, M.; Usman, M. Lysozyme and Its Application as Antibacterial Agent in Food Industry. Molecules 2022, 27, 6305. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.M. Antimicrobials in Food; Routledge: London, UK, 2021. [Google Scholar]
- Ibrahim, H.R.; Matsuzaki, T.; Aoki, T. Genetic Evidence That Antibacterial Activity of Lysozyme Is Independent of Its Catalytic Function. FEBS Lett. 2001, 506, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; Salari, F.; Licitra, R.; La Motta, C.; Altomonte, I. Lysozyme Activity in Donkey Milk. Int. Dairy J. 2019, 96, 98–101. [Google Scholar] [CrossRef]
- Eladawy, M.; El-Mowafy, M.; El-Sokkary, M.M.A.; Barwa, R. Effects of Lysozyme, Proteinase K, and Cephalosporins on Biofilm Formation by Clinical Isolates of Pseudomonas Aeruginosa. Interdiscip. Perspect. Infect. Dis. 2020, 2020, e6156720. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Imazato, K.; Ono, H. Human Lysozyme Possesses Novel Antimicrobial Peptides within Its N-Terminal Domain That Target Bacterial Respiration. J. Agric. Food Chem. 2011, 59, 10336–10345. [Google Scholar] [CrossRef]
- Shibuya, S.; Watanabe, K.; Ozawa, Y.; Shimizu, T. Xanthine Oxidoreductase-Mediated Superoxide Production Is Not Involved in the Age-Related Pathologies in Sod1-Deficient Mice. Int. J. Mol. Sci. 2021, 22, 3542. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine Oxidoreductase-Derived Reactive Species: Physiological and Pathological Effects. Oxidative Med. Cell. Longev. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Mehmood, A.; Ishaq, M.; Zhao, L.; Safdar, B.; Rehman, A.; Munir, M.; Raza, A.; Nadeem, M.; Iqbal, W.; Wang, C. Natural Compounds with Xanthine Oxidase Inhibitory Activity: A Review. Chem. Biol. Drug Des. 2019, 93, 387–418. [Google Scholar] [CrossRef]
- Mi, S.; Gong, L.; Sui, Z. Friend or Foe? An Unrecognized Role of Uric Acid in Cancer Development and the Potential Anticancer Effects of Uric Acid-Lowering Drugs. J. Cancer 2020, 11, 5236–5244. [Google Scholar] [CrossRef]
- Furuhashi, M. New Insights into Purine Metabolism in Metabolic Diseases: Role of Xanthine Oxidoreductase Activity. Am. J. Physiol.-Endocrinol. Metab. 2020, 319, E827–E834. [Google Scholar] [CrossRef] [PubMed]
- Gadave, K.S.; Panda, S.; Singh, S.; Kalra, S.; Malakar, D.; Mohanty, A.K.; Kaushik, J.K. Structural and Functional Insights into the Catalytic Inactivity of the Major Fraction of Buffalo Milk Xanthine Oxidoreductase. PLoS ONE 2014, 9, e87618. [Google Scholar] [CrossRef] [PubMed]
- Kostić, D.A.; Dimitrijević, D.S.; Stojanović, G.S.; Palić, I.R.; Đorđević, A.S.; Ickovski, J.D. Xanthine Oxidase: Isolation, Assays of Activity, and Inhibition. J. Chem. 2015, volume 2015, 1–8. [Google Scholar] [CrossRef]
- Ozturk, G.; German, J.B.; De Moura Bell, J.M.L.N. Effects of Industrial Heat Treatments on the Kinetics of Inactivation of Antimicrobial Bovine Milk Xanthine Oxidase. NPJ Sci. Food 2019, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, G.; Liang, N.; Bhattacharya, M.; Robinson, R.C.; Shankar, S.; Huang, Y.-P.; Paviani, B.; Taha, A.Y.; Barile, D. Glycoproteomic and Lipidomic Characterization of Industrially Produced Whey Protein Phospholipid Concentrate with Emphasis on Antimicrobial Xanthine Oxidase, Oxylipins and Small Milk Fat Globules. Dairy 2022, 3, 277–302. [Google Scholar] [CrossRef]
- Ozturk, G.; Shah, I.M.; Mills, D.A.; German, J.B.; De Moura Bell, J.M.L.N. The Antimicrobial Activity of Bovine Milk Xanthine Oxidase. Int. Dairy J. 2020, 102, 104581. [Google Scholar] [CrossRef] [PubMed]
- Al-Shehri, S.S.; Knox, C.L.; Liley, H.G.; Cowley, D.M.; Wright, J.R.; Henman, M.G.; Hewavitharana, A.K.; Charles, B.G.; Shaw, P.N.; Sweeney, E.L.; et al. Breastmilk-Saliva Interactions Boost Innate Immunity by Regulating the Oral Microbiome in Early Infancy. PLoS ONE 2015, 10, e0135047. [Google Scholar] [CrossRef]
- Sweeney, E.L.; Al-Shehri, S.S.; Cowley, D.M.; Liley, H.G.; Bansal, N.; Charles, B.G.; Shaw, P.N.; Duley, J.A.; Knox, C.L. The Effect of Breastmilk and Saliva Combinations on the in Vitro Growth of Oral Pathogenic and Commensal Microorganisms. Sci. Rep. 2018, 8, 15112. [Google Scholar] [CrossRef]
- Musilova, S.; Rada, V.; Vlkova, E.; Bunesova, V. Beneficial Effects of Human Milk Oligosaccharides on Gut Microbiota. Benef. Microbes 2014, 5, 273–283. [Google Scholar] [CrossRef]
- Walsh, C.; Lane, J.A.; van Sinderen, D.; Hickey, R.M. Human Milk Oligosaccharides: Shaping the Infant Gut Microbiota and Supporting Health. J. Funct. Foods 2020, 72, 104074. [Google Scholar] [CrossRef]
- Yang, Y.; Palm, N.W. Immunoglobulin A and the Microbiome. Curr. Opin. Microbiol. 2020, 56, 89–96. [Google Scholar] [CrossRef]
- Janzon, A.; Goodrich, J.K.; Koren, O.; the TEDDY Study Group; Waters, J.L.; Ley, R.E. Interactions between the Gut Microbiome and Mucosal Immunoglobulins A, M, and G in the Developing Infant Gut. mSystems 2019, 4, e00612-19. [Google Scholar] [CrossRef]
- Silva, E.; Oliveira, J.; Silva, Y.; Urbano, S.; Sales, D.; Moraes, E.; Rangel, A.; Anaya, K. Lactoperoxidase System in the Dairy Industry: Challenges and Opportunities. Czech J. Food Sci. 2020, 38, 337–346. [Google Scholar] [CrossRef]
- Köksal, Z.; Alim, Z. Lactoperoxidase, an Antimicrobial Enzyme, Is Inhibited by Some Indazoles. Drug Chem. Toxicol. 2020, 43, 22–26. [Google Scholar] [CrossRef]
- Lara-Aguilar, S.; Alcaine, S.D. Lactose Oxidase: A Novel Activator of the Lactoperoxidase System in Milk for Improved Shelf Life. J. Dairy Sci. 2019, 102, 1933–1942. [Google Scholar] [CrossRef]
- Bafort, F.; Parisi, O.; Perraudin, J.-P.; Jijakli, M.H. Mode of Action of Lactoperoxidase as Related to Its Antimicrobial Activity: A Review. Enzym. Res. 2014, 2014, 517164. [Google Scholar] [CrossRef] [PubMed]
- Al-Baarri, A.N.; Damayanti, N.T.; Legowo, A.M.; Tekiner, İ.H.; Hayakawa, S. Enhanced Antibacterial Activity of Lactoperoxidase–Thiocyanate–Hydrogen Peroxide System in Reduced-Lactose Milk Whey. Int. J. Food Sci. 2019, 8013402. [Google Scholar] [CrossRef] [PubMed]
- EL-Fakharany, E.M.; Abd-Elhamid, A.I.; El-Deeb, N.M. Preparation and Characterization of Novel Nanocombination of Bovine Lactoperoxidase with Dye Decolorizing and Anti-Bacterial Activity. Sci. Rep. 2019, 9, 8530. [Google Scholar] [CrossRef]
- Mahdi, L.; Mahdi, N.; Al-kakei, S.; Musafer, H.; Al-Joofy, I.; Essa, R.; Zwain, L.; Salman, I.; Mater, H.; Al-Alak, S.; et al. Treatment Strategy by Lactoperoxidase and Lactoferrin Combination: Immunomodulatory and Antibacterial Activity against Multidrug-Resistant Acinetobacter Baumannii. Microb. Pathog. 2018, 114, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Jo, R.; Yama, K.; Aita, Y.; Tsutsumi, K.; Ishihara, C.; Maruyama, M.; Takeda, K.; Nishinaga, E.; Shibasaki, K.; Morishima, S. Comparison of Oral Microbiome Profiles in 18-Month-Old Infants and Their Parents. Sci. Rep. 2021, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Cieslak, M.; Ferreira, C.H.F.; Shifrin, Y.; Pan, J.; Belik, J. Human Milk H2O2 Content: Does It Benefit Preterm Infants? Pediatr. Res. 2018, 83, 687–692. [Google Scholar] [CrossRef]
- Ulfman, L.H.; Leusen, J.H.W.; Savelkoul, H.F.J.; Warner, J.O.; Van Neerven, R.J.J. Effects of Bovine Immunoglobulins on Immune Function, Allergy, and Infection. Front. Nutr. 2018, 5, 52. [Google Scholar] [CrossRef]
- Hurley, W.L.; Theil, P.K. Perspectives on Immunoglobulins in Colostrum and Milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef]
- Sawa, T.; Kinoshita, M.; Inoue, K.; Ohara, J.; Moriyama, K. Immunoglobulin for Treating Bacterial Infections: One More Mechanism of Action. Antibodies 2019, 8, 52. [Google Scholar] [CrossRef]
- Rio-Aige, K.; Azagra-Boronat, I.; Castell, M.; Selma-Royo, M.; Collado, M.C.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J. The Breast Milk Immunoglobulinome. Nutrients 2021, 13, 1810. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, A.J.; Cakebread, J.; Callaghan, M.; Harris, P.; Brunt, R.; Anderson, R.C.; Armstrong, K.M.; Haigh, B. Comparative Innate Immune Interactions of Human and Bovine Secretory IgA with Pathogenic and Non-Pathogenic Bacteria. Dev. Comp. Immunol. 2017, 68, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Mathias, A.; Corthésy, B. N-Glycans on Secretory Component: Mediators of the Interaction between Secretory IgA and Gram-Positive Commensals Sustaining Intestinal Homeostasis. Gut Microbes 2011, 2, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Cakebread, J.A.; Humphrey, R.; Hodgkinson, A.J. Immunoglobulin A in Bovine Milk: A Potential Functional Food? J. Agric. Food Chem. 2015, 63, 7311–7316. [Google Scholar] [CrossRef] [PubMed]
- Palm, N.W.; de Zoete, M.R.; Cullen, T.W.; Barry, N.A.; Stefanowski, J.; Hao, L.; Degnan, P.H.; Hu, J.; Peter, I.; Zhang, W.; et al. Immunoglobulin A Coating Identifies Colitogenic Bacteria in Inflammatory Bowel Disease. Cell 2014, 158, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.Y.; Cisalpino, D.; Varadarajan, S.; Hellman, J.; Warren, H.S.; Cascalho, M.; Inohara, N.; Núñez, G. Gut Microbiota-Induced Immunoglobulin G Controls Systemic Infection by Symbiotic Bacteria and Pathogens. Immunity 2016, 44, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Petrechen, L.N.; Zago, F.H.; Sesso, M.L.T.; Bertoldo, B.B.; Silva, C.B.; Azevedo, K.P.; De Lima Pereira, S.A.; Geraldo-Martins, V.R.; Ferriani, V.P.L.; Nogueira, R.D. Levels and Complexity of IgA Antibody against Oral Bacteria in Samples of Human Colostrum. Immunobiology 2015, 220, 142–146. [Google Scholar] [CrossRef]
- Van Dijk, A.; Hedegaard, C.J.; Haagsman, H.P.; Heegaard, P.M.H. The Potential for Immunoglobulins and Host Defense Peptides (HDPs) to Reduce the Use of Antibiotics in Animal Production. Vet. Res. 2018, 49, 68. [Google Scholar] [CrossRef]
- Oliver, W.T.; Wells, J.E. Lysozyme as an Alternative to Antibiotics Improves Growth Performance and Small Intestinal Morphology in Nursery Pigs1. J. Anim. Sci. 2013, 91, 3129–3136. [Google Scholar] [CrossRef]
- Atyeo, C.; Alter, G. The Multifaceted Roles of Breast Milk Antibodies. Cell 2021, 184, 1486–1499. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, D.; Di Trana, A.; Salvatore, C. Oligosaccharides, Polyamines and Sphingolipids in Ruminant Milk. Small Rumin. Res. 2018, 160, 6. [Google Scholar] [CrossRef]
- Bode, L.; Contractor, N.; Barile, D.; Pohl, N.; Prudden, A.R.; Boons, G.-J.; Jin, Y.-S.; Jennewein, S. Overcoming the Limited Availability of Human Milk Oligosaccharides: Challenges and Opportunities for Research and Application. Nutr. Rev. 2016, 74, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, G.A.; Baumgärtner, F.; Albermann, C. Production of Human Milk Oligosaccharides by Enzymatic and Whole-Cell Microbial Biotransformations. J. Biotechnol. 2017, 258, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Urashima, T.; Hirabayashi, J.; Sato, S.; Kobata, A. Human Milk Oligosaccharides as Essential Tools for Basic and Application Studies on Galectins. Trends Glycosci. Glycotechnol. 2018, 30, SE51–SE65. [Google Scholar] [CrossRef]
- Ninonuevo, M.R.; Park, Y.; Yin, H.; Zhang, J.; Ward, R.E.; Clowers, B.H.; German, J.B.; Freeman, S.L.; Killeen, K.; Grimm, R.; et al. A Strategy for Annotating the Human Milk Glycome. J. Agric. Food Chem. 2006, 54, 7471–7480. [Google Scholar] [CrossRef]
- Wu, S.; Tao, N.; German, J.B.; Grimm, R.; Lebrilla, C.B. Development of an Annotated Library of Neutral Human Milk Oligosaccharides. J. Proteome Res. 2010, 9, 4138–4151. [Google Scholar] [CrossRef]
- Robinson, R.C. Structures and Metabolic Properties of Bovine Milk Oligosaccharides and Their Potential in the Development of Novel Therapeutics. Front. Nutr. 2019, 6, 50. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Mancilha, I.M. Non-Digestible Oligosaccharides: A Review. Carbohydr. Polym. 2007, 68, 587–597. [Google Scholar] [CrossRef]
- Sánchez, C.; Franco, L.; Regal, P.; Lamas, A.; Cepeda, A.; Fente, C. Breast Milk: A Source of Functional Compounds with Potential Application in Nutrition and Therapy. Nutrients 2021, 13, 1026. [Google Scholar] [CrossRef] [PubMed]
- Qiang, X.; YongLie, C.; QianBing, W. Health Benefit Application of Functional Oligosaccharides. Carbohydr. Polym. 2009, 77, 435–441. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Bai, Y.; Zhou, X.; Chen, L.; Qiu, C.; Lu, C.; Jin, Z.; Long, J.; Xie, Z. Natural Antimicrobial Oligosaccharides in the Food Industry. Int. J. Food Microbiol. 2023, 386, 110021. [Google Scholar] [CrossRef] [PubMed]
- Asadpoor, M.; Peeters, C.; Henricks, P.A.J.; Varasteh, S.; Pieters, R.; Folkerts, G.; Braber, S. Anti-Pathogenic Functions of Non-Digestible Oligosaccharides In Vitro. Nutrients 2020, 12, 1789. [Google Scholar] [CrossRef]
- Zokaityte, E.; Cernauskas, D.; Klupsaite, D.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Ruzauskas, M.; Gruzauskas, R.; Juodeikiene, G.; Rocha, J.M.; et al. Bioconversion of Milk Permeate with Selected Lactic Acid Bacteria Strains and Apple By-Products into Beverages with Antimicrobial Properties and Enriched with Galactooligosaccharides. Microorganisms 2020, 8, 1182. [Google Scholar] [CrossRef]
- Ruiz-Palacios, G.M.; Cervantes, L.E.; Ramos, P.; Chavez-Munguia, B.; Newburg, D.S. Campylobacter Jejuni Binds Intestinal H(O) Antigen (Fuc Alpha 1, 2Gal Beta 1, 4GlcNAc), and Fucosyloligosaccharides of Human Milk Inhibit Its Binding and Infection. J. Biol. Chem. 2003, 278, 14112–14120. [Google Scholar] [CrossRef]
- Yu, Z.-T.; Nanthakumar, N.N.; Newburg, D.S. The Human Milk Oligosaccharide 2′-Fucosyllactose Quenches Campylobacter Jejuni-Induced Inflammation in Human Epithelial Cells HEp-2 and HT-29 and in Mouse Intestinal Mucosa. J. Nutr. 2016, 146, 1980–1990. [Google Scholar] [CrossRef]
- Morrow, A.L.; Ruiz-Palacios, G.M.; Altaye, M.; Jiang, X.; Guerrero, M.L.; Meinzen-Derr, J.K.; Farkas, T.; Chaturvedi, P.; Pickering, L.K.; Newburg, D.S. Human Milk Oligosaccharides Are Associated with Protection against Diarrhea in Breast-Fed Infants. J. Pediatr. 2004, 145, 297–303. [Google Scholar] [CrossRef]
- Blaser, M.J. Antibiotic Use and Its Consequences for the Normal Microbiome. Science 2016, 352, 544–545. [Google Scholar] [CrossRef] [PubMed]
- Talbert, A.W.A.; Mwaniki, M.; Mwarumba, S.; Newton, C.R.J.C.; Berkley, J.A. Invasive Bacterial Infections in Neonates and Young Infants Born Outside Hospital Admitted to a Rural Hospital in Kenya. Pediatr. Infect. Dis. J. 2010, 29, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Henrick, B.M.; Chew, S.; Casaburi, G.; Brown, H.K.; Frese, S.A.; Zhou, Y.; Underwood, M.A.; Smilowitz, J.T. Colonization by B. Infantis EVC001 Modulates Enteric Inflammation in Exclusively Breastfed Infants. Pediatr. Res. 2019, 86, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium Longum Subspecies Infantis: Champion Colonizer of the Infant Gut. Pediatr. Res. 2015, 77, 229–235. [Google Scholar] [CrossRef]
- Nadimpalli, M.L.; Bourke, C.D.; Robertson, R.C.; Delarocque-Astagneau, E.; Manges, A.R.; Pickering, A.J. Can Breastfeeding Protect against Antimicrobial Resistance? BMC Med. 2020, 18, 392. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st Century: Epidemiology, Mechanisms, and Lifelong Effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Manthey, C.F.; Autran, C.A.; Eckmann, L.; Bode, L. Human Milk Oligosaccharides Protect against Enteropathogenic Escherichia Coli Attachment in Vitro and EPEC Colonization in Suckling Mice. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 165–168. [Google Scholar] [CrossRef]
- Coppa, G.V.; Zampini, L.; Galeazzi, T.; Facinelli, B.; Ferrante, L.; Capretti, R.; Orazio, G. Human Milk Oligosaccharides Inhibit the Adhesion to Caco-2 Cells of Diarrheal Pathogens: Escherichia Coli, Vibrio Cholerae, and Salmonella Fyris. Pediatr. Res. 2006, 59, 377–382. [Google Scholar] [CrossRef]
- Simon, P.M.; Goode, P.L.; Mobasseri, A.; Zopf, D. Inhibition of Helicobacter Pylori Binding to Gastrointestinal Epithelial Cells by Sialic Acid-Containing Oligosaccharides. Infect. Immun. 1997, 65, 750–757. [Google Scholar] [CrossRef]
- Weichert, S.; Jennewein, S.; Hüfner, E.; Weiss, C.; Borkowski, J.; Putze, J.; Schroten, H. Bioengineered 2′-Fucosyllactose and 3-Fucosyllactose Inhibit the Adhesion of Pseudomonas Aeruginosa and Enteric Pathogens to Human Intestinal and Respiratory Cell Lines. Nutr. Res. 2013, 33, 831–838. [Google Scholar] [CrossRef]
- Maldonado-Gomez, M.X.; Lee, H.; Barile, D.; Lu, M.; Hutkins, R.W. Adherence Inhibition of Enteric Pathogens to Epithelial Cells by Bovine Colostrum Fractions. Int. Dairy J. 2015, 40, 24–32. [Google Scholar] [CrossRef]
- Urakami, H.; Saeki, M.; Watanabe, Y.; Kawamura, R.; Nishizawa, S.; Suzuki, Y.; Watanabe, A.; Ajisaka, K. Isolation and Assessment of Acidic and Neutral Oligosaccharides from Goat Milk and Bovine Colostrum for Use as Ingredients of Infant Formulae. Int. Dairy J. 2018, 83, 4. [Google Scholar] [CrossRef]
- Ackerman, D.L.; Craft, K.M.; Doster, R.S.; Weitkamp, J.-H.; Aronoff, D.M.; Gaddy, J.A.; Townsend, S.D. Antimicrobial and Antibiofilm Activity of Human Milk Oligosaccharides against Streptococcus agalactiae, Staphylococcus aureus, and Acinetobacter baumannii. ACS Infect. Dis. 2018, 4, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Spicer, S.K.; Gaddy, J.A.; Townsend, S.D. Recent Advances on Human Milk Oligosaccharide Antimicrobial Activity. Curr. Opin. Chem. Biol. 2022, 71, 102202. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.E.; Autran, C.A.; Szyszka, A.; Escajadillo, T.; Huang, M.; Godula, K.; Prudden, A.R.; Boons, G.-J.; Lewis, A.L.; Doran, K.S.; et al. Human Milk Oligosaccharides Inhibit Growth of Group B Streptococcus. J. Biol. Chem. 2017, 292, 11243–11249. [Google Scholar] [CrossRef] [PubMed]
- Weinborn, V.; Li, Y.; Shah, I.M.; Yu, H.; Dallas, D.C.; German, J.B.; Mills, D.A.; Chen, X.; Barile, D. Production of Functional Mimics of Human Milk Oligosaccharides by Enzymatic Glycosylation of Bovine Milk Oligosaccharides. Int. Dairy J. 2020, 102, 104583. [Google Scholar] [CrossRef] [PubMed]
- Craft, K.M.; Thomas, H.C.; Townsend, S.D. Interrogation of Human Milk Oligosaccharide Fucosylation Patterns for Antimicrobial and Antibiofilm Trends in Group B Streptococcus. ACS Infect. Dis. 2018, 4, 1755–1765. [Google Scholar] [CrossRef]
- Permyakov, E.A. α-Lactalbumin, Amazing Calcium-Binding Protein. Biomolecules 2020, 10, 1210. [Google Scholar] [CrossRef]
- Layman, D.K.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-Lactalbumin in Human Nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef]
- Lajnaf, R.; Gharsallah, H.; Jridi, M.; Attia, H.; Ayadi, M.A. Antioxidant and Antibacterial Activities, Interfacial and Emulsifying Properties of the Apo and Holo Forms of Purified Camel and Bovine α-Lactalbumin. Int. J. Biol. Macromol. 2020, 165, 205–213. [Google Scholar] [CrossRef]
- Diao, M.; Yan, M.; Wang, Y.; Yan, X.; Dong, S.; Lu, Y.; Zhang, T. Characterization and Antibacterial Activity Study of α-Lactalbumin-Carvacrol Complex. Food Chem. 2022, 397, 133820. [Google Scholar] [CrossRef]
- Wong, R.W.C.; Guillaud, L. The Role of Epidermal Growth Factor and Its Receptors in Mammalian CNS. Cytokine Growth Factor Rev. 2004, 15, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Harris, R.C. Epidermal Growth Factor, from Gene Organization to Bedside. Semin. Cell Dev. Biol. 2014, 28, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Khodabakhshi, A.; Ghayour-Mobarhan, M.; Rooki, H.; Vakili, R.; Hashemy, S.-I.; Mirhafez, S.R.; Shakeri, M.-T.; Kashanifar, R.; Pourbafarani, R.; Mirzaei, H.; et al. Comparative Measurement of Ghrelin, Leptin, Adiponectin, EGF and IGF-1 in Breast Milk of Mothers with Overweight/Obese and Normal-Weight Infants. Eur. J. Clin. Nutr. 2015, 69, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, B. Milk Epidermal Growth Factor and Gut Protection. J. Pediatr. 2010, 156, S31–S35. [Google Scholar] [CrossRef] [PubMed]
- Knoop, K.A.; Coughlin, P.E.; Floyd, A.N.; Ndao, I.M.; Hall-Moore, C.; Shaikh, N.; Gasparrini, A.J.; Rusconi, B.; Escobedo, M.; Good, M.; et al. Maternal Activation of the EGFR Prevents Translocation of Gut-Residing Pathogenic Escherichia Coli in a Model of Late-Onset Neonatal Sepsis. Proc. Natl. Acad. Sci. USA 2020, 117, 7941–7949. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, L.; Ramy, N.; Saied, D.; Akmal, D.; Salama, N.; Abdel Haleim, M.M.; Aly, H. Emerging Antimicrobial Resistance in Early and Late-Onset Neonatal Sepsis. Antimicrob. Resist. Infect. Control 2017, 6, 63. [Google Scholar] [CrossRef]
- Tang, M.; Mei, J.; Sun, M.; Ma, K.; Zhao, A.; Fu, X. An Optimized Method to Visualize the Goblet Cell-Associated Antigen Passages and Identify Goblet Cells in the Intestine, Conjunctiva, and Airway. Immunobiology 2022, 227, 152260. [Google Scholar] [CrossRef]
- Eigel, W.N.; Butler, J.E.; Ernstrom, C.A.; Farrell, H.M., Jr.; Harwalkar, V.R.; Jenness, R.; Whitney, R.M. Nomenclature of Proteins of Cow’s Milk: Fifth Revision1. J. Dairy Sci. 1984, 67, 1599–1631. [Google Scholar] [CrossRef]
- Yvon, M.; Beucher, S.; Guilloteau, P.; Le Huerou-Luron, I.; Corring, T. Effects of Caseinomacropeptide (CMP) on Digestion Regulation. Reprod. Nutr. Dev. 1994, 34, 527–537. [Google Scholar] [CrossRef]
- Córdova-Dávalos, L.E.; Jiménez, M.; Salinas, E. Glycomacropeptide Bioactivity and Health: A Review Highlighting Action Mechanisms and Signaling Pathways. Nutrients 2019, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Brand-Miller, J.; McVeagh, P.; Petocz, P. Concentration and Distribution of Sialic Acid in Human Milk and Infant Formulas. Am. J. Clin. Nutr. 2001, 74, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Tamura, N.; Kobayashi-Hattori, K.; Yoshida, T.; Hara-Kudo, Y.; Ikedo, M.; Sugita-Konishi, Y.; Hattori, M. Prevention of Intestinal Infection by Glycomacropeptide. Biosci. Biotechnol. Biochem. 2005, 69, 2294–2301. [Google Scholar] [CrossRef]
- Rhoades, J.R.; Gibson, G.R.; Formentin, K.; Beer, M.; Greenberg, N.; Rastall, R.A. Caseinoglycomacropeptide Inhibits Adhesion of Pathogenic Escherichia Coli Strains to Human Cells in Culture. J. Dairy Sci. 2005, 88, 3455–3459. [Google Scholar] [CrossRef] [PubMed]
- Brück, W.M.; Kelleher, S.L.; Gibson, G.R.; Graverholt, G.; Lönnerdal, B.L. The Effects of Alpha-Lactalbumin and Glycomacropeptide on the Association of CaCo-2 Cells by Enteropathogenic Escherichia coli, Salmonella typhimurium and Shigella flexneri. FEMS Microbiol. Lett. 2006, 259, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Lauc, G.; Trbojević-Akmačić, I. (Eds.) The Role of Glycosylation in Health and Disease. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2021; Volume 1325, ISBN 978-3-030-70114-7. [Google Scholar]
- Prydz, K. Determinants of Glycosaminoglycan (GAG) Structure. Biomolecules 2015, 5, 2003–2022. [Google Scholar] [CrossRef] [PubMed]
- Linhardt, R.J.; Toida, T. Role of Glycosaminoglycans in Cellular Communication. Acc. Chem. Res. 2004, 37, 431–438. [Google Scholar] [CrossRef]
- Coppa, G.V.; Gabrielli, O.; Bertino, E.; Zampini, L.; Galeazzi, T.; Padella, L.; Santoro, L.; Marchesiello, R.L.; Galeotti, F.; Maccari, F.; et al. Human Milk Glycosaminoglycans: The State of the Art and Future Perspectives. Ital. J. Pediatr. 2013, 39, 2. [Google Scholar] [CrossRef]
- Coppa, G.V.; Facinelli, B.; Magi, G.; Marini, E.; Zampini, L.; Mantovani, V.; Galeazzi, T.; Padella, L.; Marchesiello, R.L.; Santoro, L.; et al. Human Milk Glycosaminoglycans Inhibit in Vitro the Adhesion of Escherichia Coli and Salmonella Fyris to Human Intestinal Cells. Pediatr. Res. 2016, 79, 603–607. [Google Scholar] [CrossRef]
- Seychell, B.C.; Vella, M.; Hunter, G.J.; Hunter, T.; Seychell, B.C.; Vella, M.; Hunter, G.J.; Hunter, T. The Good and the Bad: The Bifunctional Enzyme Xanthine Oxidoreductase in the Production of Reactive Oxygen Species. In Reactive Oxygen Species—Advances and Developments; IntechOpen: London, UK, 2023; ISBN 978-1-83768-253-9. [Google Scholar]
- Wiciński, M.; Sawicka, E.; Gębalski, J.; Kubiak, K.; Malinowski, B. Human Milk Oligosaccharides: Health Benefits, Potential Applications in Infant Formulas, and Pharmacology. Nutrients 2020, 12, 266. [Google Scholar] [CrossRef]
- Li, M.; Monaco, M.H.; Wang, M.; Comstock, S.S.; Kuhlenschmidt, T.B.; Fahey, G.C.; Miller, M.J.; Kuhlenschmidt, M.S.; Donovan, S.M. Human Milk Oligosaccharides Shorten Rotavirus-Induced Diarrhea and Modulate Piglet Mucosal Immunity and Colonic Microbiota. ISME J. 2014, 8, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Megha, K.B.; Mohanan, P.V. Role of Immunoglobulin and Antibodies in Disease Management. Int. J. Biol. Macromol. 2021, 169, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Azizi, G.; Abolhassani, H.; Asgardoon, M.H.; Shaghaghi, S.; Negahdari, B.; Mohammadi, J.; Rezaei, N.; Aghamohammadi, A. Managing Patients with Side Effects and Adverse Events to Immunoglobulin Therapy. Expert Rev. Clin. Pharmacol. 2016, 9, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Farkye, N.Y.; Bansal, N. Enzymes. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 327–334. ISBN 978-0-12-374407-4. [Google Scholar]
- Weinberg, E.D. The development of awareness of iron-withholding defense. Perspect. Biol. Med. 1993, 36, 215–221. [Google Scholar] [CrossRef]
- Weinberg, E.D. Human lactoferrin: A novel therapeutic with broad spectrum potential. J. Pharm. Pharmacol. 2001, 53, 1303–1310. [Google Scholar] [CrossRef]
- Onishi, H. Lactoferrin Delivery Systems: Approaches for Its More Effective Use. Expert Opin. Drug Deliv. 2011, 8, 1469–1479. [Google Scholar] [CrossRef]
| Component | Human | Bovine | Properties | ||
|---|---|---|---|---|---|
| Milk | Colostrum | Milk | Colostrum | ||
| Lactoferrin [6,9,12,13] | 1.5 g/L | 29.85 g/L | 0.02–0.75 g/L | 1.5–5 g/L | Antimicrobial activity, anti-inflammatory action, regulation of cell growth, immune regulation, ROS scavenging activity |
| Immunoglobulins [6,10,12,14] | 1.3 g/L (IgA, IgM, IgE, IgG) | IgG 27.9 ± 23.2 g/L SIgA 16.4 ± 6.1 mg/L | IgG1 0.31.0.40 g/L | IgG1 34–87 g/L | Immune booster, regulation, protection |
| IgG2 0.03–0.08 g/L | IgG2 1.6–6 g/L | Antimicrobial activity, pathogen recognition | |||
| IgA 0.04–0.06 g/L | IgA 3.2–6.2 g/L | ||||
| IgM 0.03–0.06 g/L | IgM 3.7–6.1 g/L | ||||
| Lactoperoxidase [6,10,15] | 0.89 mU/mL | 3.28 mU/mL | 13–30 mg/L | 11–45 mg/L | Antibacterial activity with systematic composition, combined activity with XO, Lf, and Ig |
| Lysozyme [6,9,16,17] | 200–400 µg/mL | 0.37 mg/mL | 0.05–0.22 µg/mL | 0.14–0.7 mg/L | Antimicrobial activity, complementary interaction with Lf and Igs, neuroprotection |
| Xanthine oxidase [15] | 0.52–0.91 mU/mL | 8 mU/mL | 35 mg/L | - | Antibacterial activity with ROS synthesis, synergic interaction with LPO |
| Oligosaccharides [9,18,19] | 12–13 g/L | 22–24 g/L | 0.1–0.2 g/L | 0.7–1.2 g/L | Antimicrobial, prebiotic activity, support immune/intestinal system, and brain development |
| Antibacterial Molecule | Result | Reference |
|---|---|---|
| CAMP211-225 peptide | Antibacterial activity against antibiotic-resistant S. aureus, E. coli, and Yersinia enterocolitica. | [36] |
| Lactalbumin | Antagonistic effects against E. coli O127 and reduction in diarrhea incidences. | [36] |
| Lysozyme | An increase in beneficial gut microbial diversity has been observed. | [37] |
| Lactoperoxidase | LPO-generated hypothiocyanite exhibited antibacterial activity against various Gram-positive and Gram-negative bacteria, and its effectiveness increased in reduced-lactose milk whey. | [38] |
| Lactoperoxidase | LPO synergically showed antibacterial activity with Lf against drug-resistant Acinetobacter baumanniii in mice models. | [39] |
| Lysozyme | Levels of Bacteroidetes, Bifidobacteriaceae, and Lactobacillaceae had been increased. Reduction in Firmicutes, Mycobacteriaceae, Streptococcaceae, and Campylobacter was observed. | [40] |
| Lysozyme | Increased levels of Lactobacillus and mucosal IgA responses had been observed. Faster recovery, lower morbidity, and less mortality from ETEC infection were also noted. | [41] |
| Lysozyme | Improvement in weaning weight, intestinal health, and levels of Lactobacillus had been observed in the group fed with 1.0 g/kg LZ for 14 days. | [42] |
| Lactoferrin | Lf exhibits antimicrobial properties against both Gram-positive and Gram-negative bacteria, including E. coli O157:H7. Its antimicrobial mechanisms comprise bacteriostatic, bactericidal, and anti-adhesion effects. | [43] |
| Lactoferrin | After four injections, complete eradication of S. aureus had not yet been achieved; however, viable bacterial counts demonstrated a two-log decrease following treatments with Lf and/or penicillin G. | [44] |
| Lactoferricin | Bactericidal activity against S. aureus and Pseudomonas aeruginosa strains was observed with lactoferricin, showing a minimum inhibitory concentration of 1.0–2.0 μg/mL for S. aureus and 4.0–8.0 μg/mL for Pseudomonas aeruginosa. | [45] |
| Lactoferrampin | Lactoferrampin displayed a wide-ranging antibacterial efficacy against various bacterial strains; however, Porphyromonas gingivalis, Actinomyces naeslundii, Streptococcus mutans, and Streptococcus sanguis exhibited resistance to this peptide. | [46] |
| Lactoferricin | Bactericidal activity against E. coli and E. faecalis strains was observed with lactoferricin, exhibiting a minimum inhibitory concentration of 0.5–1.0 μg/mL for E. coli and 2.0–4.0 μg/mL for E. faecalis. | [47] |
| Lactoperoxidase | Decreases in both Gram-positive and Gram-negative bacteria levels, notably E. coli and Pseudomonas species, occur with the addition of external hydrogen peroxide supplementation. | [48] |
| Immunoglobulin | The IgY protected fully inhibited diarrhea induced by enterotoxigenic E. coli in challenged piglets. | [49] |
| β-lactoglobulin | Bovine β-lactoglobulin displayed growth inhibition against S. aureus; however, it did not exhibit effectiveness against E. coli. Moreover, it demonstrated inhibitory activity against Streptococcus uberis. | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eker, F.; Akdaşçi, E.; Duman, H.; Yalçıntaş, Y.M.; Canbolat, A.A.; Kalkan, A.E.; Karav, S.; Šamec, D. Antimicrobial Properties of Colostrum and Milk. Antibiotics 2024, 13, 251. https://doi.org/10.3390/antibiotics13030251
Eker F, Akdaşçi E, Duman H, Yalçıntaş YM, Canbolat AA, Kalkan AE, Karav S, Šamec D. Antimicrobial Properties of Colostrum and Milk. Antibiotics. 2024; 13(3):251. https://doi.org/10.3390/antibiotics13030251
Chicago/Turabian StyleEker, Furkan, Emir Akdaşçi, Hatice Duman, Yalçın Mert Yalçıntaş, Ahmet Alperen Canbolat, Arda Erkan Kalkan, Sercan Karav, and Dunja Šamec. 2024. "Antimicrobial Properties of Colostrum and Milk" Antibiotics 13, no. 3: 251. https://doi.org/10.3390/antibiotics13030251
APA StyleEker, F., Akdaşçi, E., Duman, H., Yalçıntaş, Y. M., Canbolat, A. A., Kalkan, A. E., Karav, S., & Šamec, D. (2024). Antimicrobial Properties of Colostrum and Milk. Antibiotics, 13(3), 251. https://doi.org/10.3390/antibiotics13030251








