An Overview of Antibiotic Therapy for Early- and Late-Onset Neonatal Sepsis: Current Strategies and Future Prospects
Abstract
1. Introduction
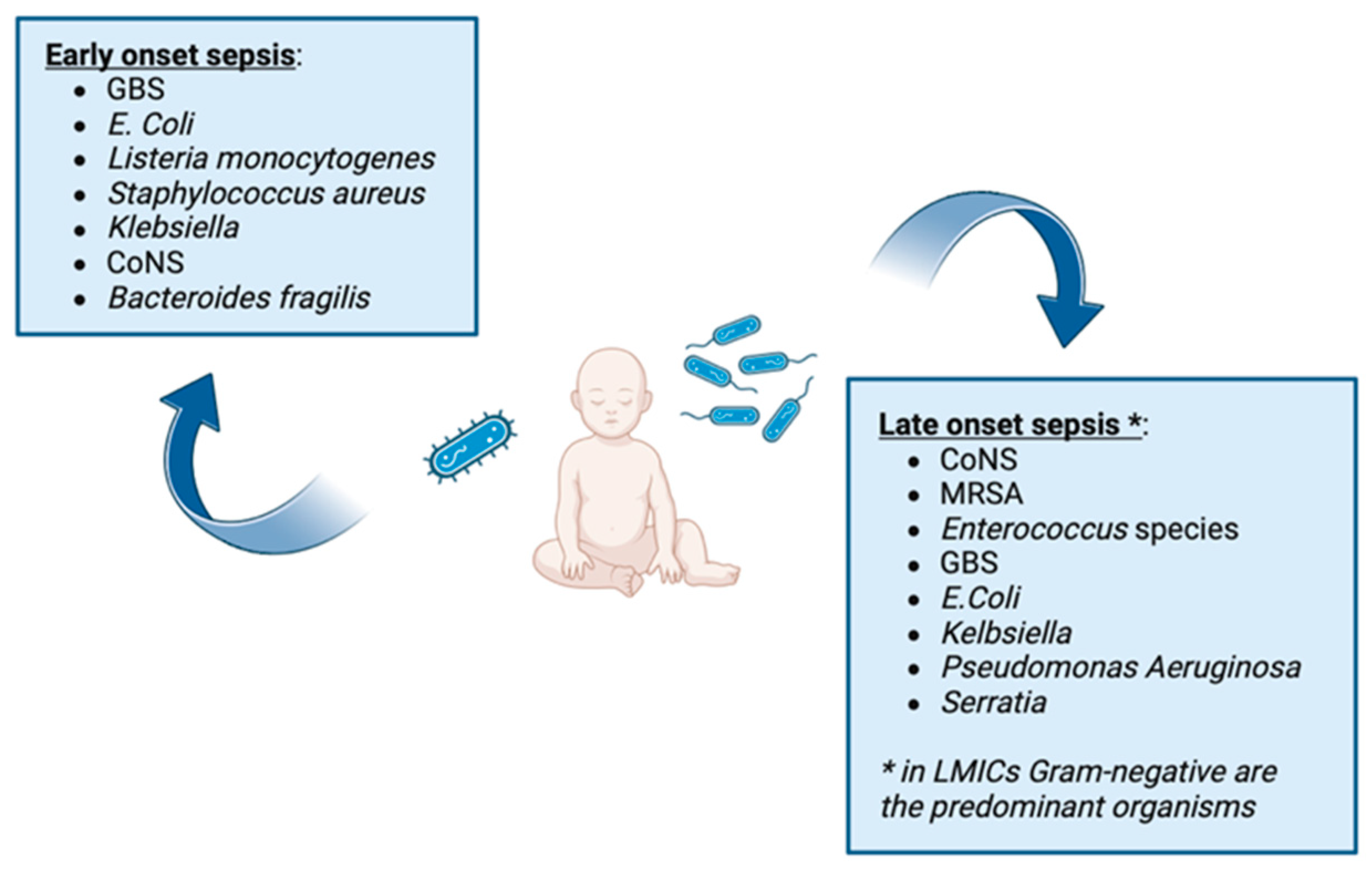
2. Early-Onset Sepsis
3. Late-Onset Sepsis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oza, S.; Lawn, J.E.; Hogan, D.R.; Mathers, C.; Cousens, S.N. Neonatal Cause-of-Death Estimates for the Early and Late Neonatal Periods for 194 Countries: 2000–2013. Bull. World Health Organ. 2015, 93, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Weston, E.J.; Pondo, T.; Lewis, M.M.; Martell-Cleary, P.; Morin, C.; Jewell, B.; Daily, P.; Apostol, M.; Petit, S.; Farley, M.; et al. The Burden of Invasive Early-Onset Neonatal Sepsis in the United States, 2005–2008. Pediatr. Infect. Dis. J. 2011, 30, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Hansen, N.I.; Adams-Chapman, I.; Fanaroff, A.A.; Hintz, S.R.; Vohr, B.; Higgins, R.D.; National Institute of Child Health and Human Development. Neonatal Research Network Neurodevelopmental and Growth Impairment among Extremely Low-Birth-Weight Infants with Neonatal Infection. JAMA 2004, 292, 2357–2365. [Google Scholar] [CrossRef]
- Fleischmann, C.; Reichert, F.; Cassini, A.; Homer, R.; Harder, T.; Markwart, R.; Tröndle, M.; Savova, Y.; Kissoon, N.; Schlattmann, P.; et al. Global incidence and mortality of neonatal sepsis: A systematic review and meta-analysis. Arch. Dis. Child. 2021, 106, 745–752. [Google Scholar] [CrossRef]
- Wright, N.; Francis, L.; Bonney, D.; Wang, Z.; Francis, J. Epidemiology of early and late-onset neonatal sepsis in an Australian regional special care nursery with a high proportion of Aboriginal and Torres Strait Islander births. J. Paediatr. Child Health 2022, 58, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Abu Nofal, M.; Massalha, M.; Diab, M.; Abboud, M.; Asla Jamhour, A.; Said, W.; Talmon, G.; Mresat, S.; Mattar, K.; Garmi, G.; et al. Perinatal Outcomes of Late Preterm Rupture of Membranes with or without Latency Antibiotics. Am. J. Perinatol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Terrin, G.; Passariello, A.; De Curtis, M.; Manguso, F.; Salvia, G.; Lega, L.; Messina, F.; Paludetto, R.; Canani, R.B. Ranitidine Is Associated with Infections, Necrotizing Enterocolitis, and Fatal Outcome in Newborns. Pediatrics 2012, 129, e40–e45. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.; Fanaroff, A.A.; Wright, L.L.; Carlo, W.A.; Ehrenkranz, R.A.; Lemons, J.A.; Donovan, E.F.; Stark, A.R.; Tyson, J.E.; et al. Late-Onset Sepsis in Very Low Birth Weight Neonates: The Experience of the NICHD Neonatal Research Network. Pediatrics 2002, 110, 285–291. [Google Scholar] [CrossRef]
- Kuppala, V.S.; Meinzen-Derr, J.; Morrow, A.L.; Schibler, K.R. Prolonged Initial Empirical Antibiotic Treatment Is Associated with Adverse Outcomes in Premature Infants. J. Pediatr. 2011, 159, 720–725. [Google Scholar] [CrossRef]
- Pandit, B.R.; Vyas, A. Clinical Symptoms, Pathogen Spectrum, Risk Factors and Antibiogram of Suspected Neonatal Sepsis Cases in Tertiary Care Hospital of Southern Part of Nepal: A Descriptive Cross-Sectional Study. J. Nepal Med. Assoc. 2020, 58, 976–982. [Google Scholar] [CrossRef]
- Gandra, S.; Alvarez-uria, G.; Murki, S.; Singh, S.K.; Kanithi, R.; Jinka, D.R.; Chikkappa, A.K.; Subramanian, S.; Sharma, A.; Dharmapalan, D.; et al. International Journal of Infectious Diseases Point Prevalence Surveys of Antimicrobial Use among Eight Neonatal Intensive Care Units in India: 2016. Int. J. Infect. Dis. 2018, 71, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.; Harahap, A.; Husada, D.; Faramarisa, F.N. Risk factors of multidrug-resistant organisms neonatal sepsis in Surabaya tertiary referral hospital: A single-center study. BMC Pediatr. 2024, 24, 153. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, J.A.; Ferrentino, F.L.; Goodstein, M.H.; Liss, J.; Shapiro, S.L.; Bankert, D.A. Frequency of Low Level Bacteremia in Infants from Birth to Two Months of Age. Pediatr. Infect. Dis. J. 1997, 16, 381–385. [Google Scholar] [CrossRef]
- Celik, I.H.; Hanna, M.; Canpolat, F.E.; Mohan, P. Diagnosis of neonatal sepsis: The past, present and future. Pediatr. Res. 2022, 91, 337–350. [Google Scholar] [CrossRef]
- Boscarino, G.; Migliorino, R.; Carbone, G.; Davino, G.; Dell’Orto, V.G.; Perrone, S.; Principi, N.; Esposito, S. Biomarkers of Neonatal Sepsis: Where We Are and Where We Are Going. Antibiotics 2023, 12, 1233. [Google Scholar] [CrossRef]
- Chakkarapani, A.A.; Russell, A.B. Antibiotic Stewardship in the Neonatal Intensive Care Unit. Paediatr. Child Health 2019, 29, 269–273. [Google Scholar] [CrossRef]
- Lee, K.R.; Bagga, B.; Arnold, S.R. Reduction of Broad-Spectrum Antimicrobial Use in a Tertiary Children’s Hospital Post Antimicrobial Stewardship Program Guideline Implementation. Pediatr. Crit. Care Med. 2016, 17, 187–193. [Google Scholar] [CrossRef]
- Beerlage-de Jong, N.; van Gemert-Pijnen, L.; Wentzel, J.; Hendrix, R.; Siemons, L. Technology to Support Integrated Antimicrobial Stewardship Programs: A User Centered and Stakeholder Driven Development Approach. Infect. Dis. Rep. 2017, 9, 6829. [Google Scholar] [CrossRef]
- Schulman, J.; Dimand, R.J.; Lee, H.C.; Duenas, G.V.; Bennett, M.V.; Gould, J.B. Neonatal Intensive Care Unit Antibiotic Use. Pediatrics 2015, 135, 826–833. [Google Scholar] [CrossRef]
- Stocker, M.; Klingenberg, C.; Navér, L.; Nordberg, V.; Berardi, A.; el Helou, S.; Fusch, G.; Bliss, J.M.; Lehnick, D.; Dimopoulou, V.; et al. Less Is More: Antibiotics at the Beginning of Life. Nat. Commun. 2023, 14, 2423. [Google Scholar] [CrossRef]
- Silva, A.C.B.; Anchieta, L.M.; de Paula Lopes, M.F.; de Castro Romanelli, R.M. Inadequate Use of Antibiotics and Increase in Neonatal Sepsis Caused by Resistant Bacteria Related to Health Care Assistance: A Systematic Review. Braz. J. Infect. Dis. 2018, 22, 328–337. [Google Scholar] [CrossRef]
- Esposito, S.; Principi, N. Adjunctive Therapy to Treat Neonatal Sepsis. Expert Rev. Clin. Pharmacol. 2020, 13, 65–73. [Google Scholar] [CrossRef]
- Manzoni, P.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Stolfi, I.; Decembrino, L.; Laforgia, N.; Vagnarelli, F.; et al. Bovine Lactoferrin Supplementation for Prevention of Late-Onset Sepsis in Very Low-Birth-Weight Neonates: A Randomized Trial. JAMA 2009, 302, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Fowler, T.; Walker, D.; Davies, S.C. The Risk/Benefit of Predicting a Post-Antibiotic Era: Is the Alarm Working? Ann. N. Y. Acad. Sci. 2014, 1323, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Campanini-Salinas, J.; Andrades-Lagos, J.; Mella-Raipan, J.; Vasquez-Velasquez, D. Novel Classes of Antibacterial Drugs in Clinical Development, a Hope in a Post-Antibiotic Era. Curr. Top. Med. Chem. 2018, 18, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, U.; Chiné, V.; Pappalardo, M.; Gismondi, P.; Esposito, S. Improving the Quality of Hospital Antibiotic Use: Impact on Multidrug-Resistant Bacterial Infections in Children. Front. Pharmacol. 2020, 11, 745. [Google Scholar] [CrossRef] [PubMed]
- Polin, R.A.; Papile, L.A.; Baley, J.E.; Benitz, W.; Carlo, W.A.; Cummings, J.; Kumar, P.; Tan, R.C.; Wang, K.S.; Watterberg, K.L.; et al. Management of Neonates with Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics 2012, 129, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.G.; Angelidou, A.; Diray-Arce, J.; Smolen, K.K.; Lasky-Su, J.; De Curtis, M.; Levy, O. Immunometabolic Approaches to Prevent, Detect, and Treat Neonatal Sepsis. Pediatr. Res. 2020, 87, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Puopolo, K.M.; Hansen, N.I.; Sánchez, P.J.; Bell, E.F.; Carlo, W.A.; Cotten, C.M.; D’Angio, C.T.; Kazzi, S.N.J.; Poindexter, B.B.; et al. Early-Onset Neonatal Sepsis 2015 to 2017, the Rise of Escherichia Coli, and the Need for Novel Prevention Strategies. JAMA Pediatr. 2020, 174, e200593. [Google Scholar] [CrossRef] [PubMed]
- Puopolo, K.M.; Benitz, W.E.; Zaoutis, T.E. Management of Neonates Born at ≥35 0/7 Weeks’ Gestation with Suspected or Proven Early-Onset Bacterial Sepsis Committee on Fetus and Newborn, Committee on Infectious Diseases from the American Academy of Pediatrics Guidance for the Clinician in Rendering Pediatric Care. Pediatrics 2018, 142, 20182894. [Google Scholar]
- Puopolo, K.M.; Benitz, W.E.; Zaoutis, T.E. Management of Neonates Born at ≤34 6/7 Weeks’ Gestation with Suspected or Proven Early-Onset Bacterial Sepsis Committee on Fetus and Newborn, Committee on Infectious Diseases. Pediatrics 2018, 142, e20182896. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.P.; Khattak, H.; Kini, P.K.; Heaton, P.A.; Goel, N. NICE Guideline Review: Neonatal Infection: Antibiotics for Prevention and Treatment (NG195). Arch. Dis. Child.-Educ. Pract. Ed. 2022, 107, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Oliver, E.; Reagan, P.; Slaughter, J.; Buhimschi, C.; Buhimschi, I. Patterns of Empiric Antibiotic Administration for Presumed Early-Onset Neonatal Sepsis in Neonatal Intensive Care Units in the United States. Am. J. Perinatol. 2016, 34, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Dretvik, T.; Solevåg, A.L.; Finvåg, A.; Størdal, E.H.; Størdal, K.; Klingenberg, C. Active Antibiotic Discontinuation in Suspected but Not Confirmed Early-onset Neonatal Sepsis—A Quality Improvement Initiative. Acta Paediatr. 2020, 109, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Farina, D.; Leonessa, M.; d’Oulx, E.A.; Galletto, P.; Mostert, M.; Miniero, R.; Gomirato, G. Risk Factors for Progression to Invasive Fungal Infection in Preterm Neonates with Fungal Colonization. Pediatrics 2006, 118, 2359–2364. [Google Scholar] [CrossRef] [PubMed]
- Cantey, J.B.; Huffman, L.W.; Subramanian, A.; Marshall, A.S.; Ballard, A.R.; Lefevre, C.; Sagar, M.; Pruszynski, J.E.; Mallett, L.H. Antibiotic Exposure and Risk for Death or Bronchopulmonary Dysplasia in Very Low Birth Weight Infants. J. Pediatr. 2017, 181, 289–293.e1. [Google Scholar] [CrossRef] [PubMed]
- Cotten, C.M. Adverse Consequences of Neonatal Antibiotic Exposure. Curr. Opin. Pediatr. 2016, 28, 141–149. [Google Scholar] [CrossRef]
- Patel, S.J.; Saiman, L. Antibiotic Resistance in Neonatal Intensive Care Unit Pathogens: Mechanisms, Clinical Impact, and Prevention Including Antibiotic Stewardship. Clin. Perinatol. 2010, 37, 547–563. [Google Scholar] [CrossRef]
- Vatne, A.; Hapnes, N.; Stensvold, H.J.; Dalen, I.; Guthe, H.J.; Støen, R.; Brigtsen, A.K.; Rønnestad, A.E.; Klingenberg, C. Early Empirical Antibiotics and Adverse Clinical Outcomes in Infants Born Very Preterm: A Population-Based Cohort. J. Pediatr. 2023, 253, 107–114.e5. [Google Scholar] [CrossRef]
- Vangay, P.; Ward, T.; Gerber, J.S.; Knights, D. Antibiotics, Pediatric Dysbiosis, and Disease. Cell Host Microbe 2015, 17, 553–564. [Google Scholar] [CrossRef]
- Pagano, F.; Conti, M.G.; Boscarino, G.; Pannucci, C.; Dito, L.; Regoli, D.; Di Chiara, M.; Battaglia, G.; Prota, R.; Cinicola, B.; et al. Atopic Manifestations in Children Born Preterm: A Long-Term Observational Study. Children 2021, 8, 843. [Google Scholar] [CrossRef]
- Morreale, C.; Giaroni, C.; Baj, A.; Folgori, L.; Barcellini, L.; Dhami, A.; Agosti, M.; Bresesti, I. Effects of Perinatal Antibiotic Exposure and Neonatal Gut Microbiota. Antibiotics 2023, 12, 258. [Google Scholar] [CrossRef]
- Bailey, L.C.; Forrest, C.B.; Zhang, P.; Richards, T.M.; Livshits, A.; DeRusso, P.A. Association of Antibiotics in Infancy with Early Childhood Obesity. JAMA Pediatr. 2014, 168, 1063. [Google Scholar] [CrossRef] [PubMed]
- Canova, C.; Ludvigsson, J.F.; Di Domenicantonio, R.; Zanier, L.; Barbiellini Amidei, C.; Zingone, F. Perinatal and Antibiotic Exposures and the Risk of Developing Childhood-Onset Inflammatory Bowel Disease: A Nested Case-Control Study Based on a Population-Based Birth Cohort. Int. J. Environ. Res. Public Health 2020, 17, 2409. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, G.M. Pharmacokinetics of Cephalosporins in the Neonate: A Review. Clinics 2011, 66, 1267–1274. [Google Scholar] [CrossRef]
- Bryan, C.S.; John, J.F.; Pai, M.S.; Austin, T.L. Gentamicin vs. Cefotaxime for Therapy of Neonatal Sepsis. Relationship to Drug Resistance. Am. J. Dis. Child. 1985, 139, 1086–1089. [Google Scholar] [CrossRef]
- Martin, E.; Fanconi, A.; Kälin, P.; Zwingelstein, C.; Crevoisier, C.; Ruch, W.; Brodersen, R. Ceftriaxone-Bilirubin-Albumin Interactions in the Neonate: An In Vivo Study. Eur. J. Pediatr. 1993, 152, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Steadman, E.; Raisch, D.W.; Bennett, C.L.; Esterly, J.S.; Becker, T.; Postelnick, M.; McKoy, J.M.; Trifilio, S.; Yarnold, P.R.; Scheetz, M.H. Evaluation of a Potential Clinical Interaction between Ceftriaxone and Calcium. Antimicrob. Agents Chemother. 2010, 54, 1534–1540. [Google Scholar] [CrossRef]
- Lona Reyes, J.C.; Verdugo Robles, M.Á.; Pérez Ramírez, R.O.; Pérez Molina, J.J.; Ascencio Esparza, E.P.; Benítez Vázquez, E.A. Etiology and Antimicrobial Resistance Patterns in Early and Late Neonatal Sepsis in a Neonatal Intensive Care Unit. Arch. Argent Pediatr. 2015, 113, 317–323. [Google Scholar] [CrossRef]
- Gastine, S.; Obiero, C.; Kane, Z.; Williams, P.; Readman, J.; Murunga, S.; Thitiri, J.; Ellis, S.; Correia, E.; Nyaoke, B.; et al. Simultaneous Pharmacokinetic/Pharmacodynamic (PKPD) Assessment of Ampicillin and Gentamicin in the Treatment of Neonatal Sepsis. J. Antimicrob. Chemother. 2022, 77, 448–456. [Google Scholar] [CrossRef]
- Ekman, B.; Paudel, P.; Basnet, O.; KC, A.; Wrammert, J. Adherence to World Health Organisation Guidelines for Treatment of Early Onset Neonatal Sepsis in Low-Income Settings; a Cohort Study in Nepal. BMC Infect. Dis. 2020, 20, 666. [Google Scholar] [CrossRef]
- Langer, B.I.; Johansson, A.B.; Mathé, K.; Jourdain, S.; Smeesters, P.R. Use of the “Sepsis Risk Calculator” in Belgian Newborns: A Retrospective Cohort Study. Pediatr. Infect. Dis. J. 2024. [Google Scholar] [CrossRef] [PubMed]
- Salsabila, K.; Toha, N.M.A.; Rundjan, L.; Pattanittum, P.; Sirikarn, P.; Rohsiswatmo, R.; Wandita, S.; Hakimi, M.; Lumbiganon, P.; Green, S.; et al. Early-Onset Neonatal Sepsis and Antibiotic Use in Indonesia: A Descriptive, Cross-Sectional Study. BMC Public Health 2022, 22, 992. [Google Scholar] [CrossRef] [PubMed]
- Flannery, D.D.; Puopolo, K.M.; Hansen, N.I.; Gerber, J.S.; Sánchez, P.J.; Stoll, B.J. Antimicrobial Susceptibility Profiles Among Neonatal Early-Onset Sepsis Pathogens. Pediatr. Infect. Dis. J. 2022, 41, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Snydman, D.R.; Jacobus, N.V.; McDermott, L.A.; Golan, Y.; Hecht, D.W.; Goldstein, E.J.C.; Harrell, L.; Jenkins, S.; Newton, D.; Pierson, C.; et al. Lessons Learned from the Anaerobe Survey: Historical Perspective and Review of the Most Recent Data (2005–2007). Clin. Infect. Dis. 2010, 50, S26–S33. [Google Scholar] [CrossRef] [PubMed]
- Berardi, A.; Baroni, L.; Bacchi Reggiani, M.L.; Ambretti, S.; Biasucci, G.; Bolognesi, S.; Capretti, M.G.; Carretto, E.; Ciccia, M.; Fiorini, V.; et al. The Burden of Early-Onset Sepsis in Emilia-Romagna (Italy): A 4-Year, Population-Based Study. J. Matern.-Fetal Neonatal Med. 2016, 29, 3126–3131. [Google Scholar] [CrossRef] [PubMed]
- Thomson, K.M.; Dyer, C.; Liu, F.; Sands, K.; Portal, E.; Carvalho, M.J.; Barrell, M.; Boostrom, I.; Dunachie, S.; Farzana, R.; et al. Effects of Antibiotic Resistance, Drug Target Attainment, Bacterial Pathogenicity and Virulence, and Antibiotic Access and Affordability on Outcomes in Neonatal Sepsis: An International Microbiology and Drug Evaluation Prospective Substudy (BARNARDS). Lancet Infect. Dis. 2021, 21, 1677–1688. [Google Scholar] [CrossRef] [PubMed]
- Mabena, F.C.; Olwagen, C.P.; Phosa, M.; Ngwenya, I.K.; Van der Merwe, L.; Khan, A.; Mwamba, T.M.; Mpembe, R.; Magobo, R.E.; Govender, N.P.; et al. Bacterial and Candida Colonization of Neonates in a Regional Hospital in South Africa. Pediatr. Infect. Dis. J. 2024, 43, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Dudeja, S. Neonatal Sepsis: Treatment of Neonatal Sepsis in Multidrug-Resistant (MDR) Infections: Part 2. Indian J. Pediatr. 2020, 87, 122–124. [Google Scholar] [CrossRef]
- Wen, S.C.H.; Ezure, Y.; Rolley, L.; Spurling, G.; Lau, C.L.; Riaz, S.; Paterson, D.L.; Irwin, A.D. Gram-Negative Neonatal Sepsis in Low- and Lower-Middle-Income Countries and WHO Empirical Antibiotic Recommendations: A Systematic Review and Meta-Analysis. PLoS Med. 2021, 18, e1003787. [Google Scholar] [CrossRef]
- Kontou, A.; Kourti, M.; Iosifidis, E.; Sarafidis, K.; Roilides, E. Use of Newer and Repurposed Antibiotics against Gram-Negative Bacteria in Neonates. Antibiotics 2023, 12, 1072. [Google Scholar] [CrossRef]
- Darlow, C.A.; Docobo-Perez, F.; Farrington, N.; Johnson, A.; McEntee, L.; Unsworth, J.; Jimenez-Valverde, A.; Gastine, S.; Kolamunnage-Dona, R.; de Costa, R.M.A.; et al. Amikacin Combined with Fosfomycin for Treatment of Neonatal Sepsis in the Setting of Highly Prevalent Antimicrobial Resistance. Antimicrob. Agents Chemother. 2021, 65, 10–1128. [Google Scholar] [CrossRef]
- Darlow, C.A.; McEntee, L.; Johnson, A.; Farrington, N.; Unsworth, J.; Jimenez-Valverde, A.; Jagota, B.; Kolamunnage-Dona, R.; Da Costa, R.M.A.; Ellis, S.; et al. Assessment of Flomoxef Combined with Amikacin in a Hollow-Fibre Infection Model for the Treatment of Neonatal Sepsis in Low- and Middle-Income Healthcare Settings. J. Antimicrob. Chemother. 2022, 77, 3349–3357. [Google Scholar] [CrossRef]
- Cortese, F.; Scicchitano, P.; Gesualdo, M.; Filaninno, A.; De Giorgi, E.; Schettini, F.; Laforgia, N.; Ciccone, M.M. Early and Late Infections in Newborns: Where Do We Stand? A Review. Pediatr. Neonatol. 2016, 57, 265–273. [Google Scholar] [CrossRef]
- Glaser, M.A.; Hughes, L.M.; Jnah, A.; Newberry, D.; Harris-Haman, P.A. Neonatal Sepsis: A Review of Pathophysiology and Current Management Strategies. Adv. Neonatal Care 2021, 21, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Shane, A.L.; Sánchez, P.J.; Stoll, B.J. Seminar Neonatal Sepsis. Lancet 2017, 390, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.P.; Burgner, D.P.; Hardikar, R.S.; Buttery, J.P. Empiric Antibiotic Regimens for Neonatal Sepsis in Australian and New Zealand Neonatal Intensive Care Units. J. Paediatr. Child Health 2017, 53, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.; Polin, R.A.; Hooven, T.A. Easily Missed? Neonatal Sepsis. BMJ 2020, 371, m3672. [Google Scholar] [CrossRef] [PubMed]
- Guideline Development Group of Clinical Practice Guidelines for Meropenem Therapy in Neonatal Sepsis; PekingUniversity Third Hospital; Editorial Department of Chinese Journal of Contemporary Pediatrics. Clinical practice guidelines for meropenem therapy in neonatal sepsis (2024). Zhongguo Dang Dai Er Ke Za Zhi 2024, 26, 107–117. [Google Scholar]
- Giannoni, E.; Agyeman, P.K.A.; Stocker, M.; Posfay-barbe, K.M.; Heininger, U.; Spycher, B.D.; Bernhard-stirnemann, S.; Niederer-loher, A.; Kahlert, C.R.; Donas, A.; et al. Neonatal Sepsis of Early Onset, and Hospital-Acquired and Community-Acquired Late Onset: A Prospective Population-Based Cohort Study. J. Pediatr. 2018, 201, 106–114.e4. [Google Scholar] [CrossRef] [PubMed]
- El Manouni El Hassani, S.; Berkhout, D.J.C.; Niemarkt, H.J.; Mann, S.; de Boode, W.P.; Cossey, V.; Hulzebos, C.V.; van Kaam, A.H.; Kramer, B.W.; van Lingen, R.A.; et al. Risk Factors for Late-Onset Sepsis in Preterm Infants: A Multicenter Case-Control Study. Neonatology 2019, 116, 42–51. [Google Scholar] [CrossRef]
- Stylianou-Riga, P.; Boutsikou, T.; Kouis, P.; Michailidou, K.; Kinni, P.; Sokou, R.; Iliodromiti, Z.; Pitsios, C.; Yiallouros, P.K.; Iacovidou, N. Epidemiology, Risk Factors, Clinical Presentation and Complications of Late-Onset Neonatal Sepsis among Preterm Neonates in Cyprus: A Prospective Case-Control Study. BMC Pediatr. 2024, 24, 50. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, L.; Yan, W.; Li, S.; Han, J.; Yang, Y.; Lee, S.K.; Cao, Y.; Ji, Y.; Han, S.; et al. Antibiotic Use in Neonatal Intensive Care Units in China: A Multicenter Cohort Study. J. Pediatr. 2021, 239, 136–142.e4. [Google Scholar] [CrossRef] [PubMed]
- Ballot, D.E.; Bandini, R.; Nana, T.; Bosman, N.; Thomas, T.; Davies, V.A.; Cooper, P.A.; Mer, M.; Lipman, J. A Review of -Multidrug-Resistant Enterobacteriaceae in a Neonatal Unit in Johannesburg, South Africa. BMC Pediatr. 2019, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Id, M.A.; Zia, R.; Id, I.M.; Ahmad, N.; Sarwar, S. Treatment Outcomes, Antibiotic Use and Its Resistance Pattern among Neonatal Sepsis Patients Attending Bahawal Victoria Hospital, Pakistan. PLoS ONE 2021, 16, e0244866. [Google Scholar] [CrossRef]
- Husada, D.; Chanthavanich, P.; Chotigeat, U.; Sunttarattiwong, P.; Sirivichayakul, C.; Pengsaa, K.; Chokejindachai, W.; Kaewkungwal, J. Predictive Model for Bacterial Late-Onset Neonatal Sepsis in a Tertiary Care Hospital in Thailand. BMC Infect. Dis. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Ghaith, D.M.; Zafer, M.M.; Said, H.M.; Elanwary, S.; Elsaban, S.; Al-agamy, M.H.; Bohol, M.F.F.; Bendary, M.M.; Al-qahtani, A. Genetic Diversity of Carbapenem-Resistant Klebsiella Pneumoniae Causing Neonatal Sepsis in Intensive Care Unit, Cairo, Egypt. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Jinka, D.R.; Gandra, S.; Alvarez-Uria, G.; Torre, N.; Tadepalli, D.; Nayakanti, R.P.R. Impact of Antibiotic Policy on Antibiotic Consumption in a Neonatal Intensive Care Unit in India. Indian Pediatr. 2017, 54, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Maia, R.; Romanelli, D.C.; Márcia, L.; Chaves, J.; Fernandes, A.; Antunes, M.; Lima, F.; Marina, T.; Souza, D.; Rosado, V.; et al. Original Article Serum Levels of Vancomycin: Is There a Prediction Using Doses in Mg/Kg/Day or m2/Day for Neonates? Braz. J. Infect. Dis. 2016, 20, 451–456. [Google Scholar] [CrossRef]
- Korang, S.K.; Safi, S.; Gluud, C.; Lausten-Thomsen, U.; Jakobsen, J.C. Antibiotic Regimens for Neonatal Sepsis—A Protocol for a Systematic Review with Meta-Analysis. Syst. Rev. 2019, 8, 306. [Google Scholar] [CrossRef]
- Cailes, B.; Kortsalioudaki, C.; Buttery, J.; Pattnayak, S.; Greenough, A.; Matthes, J.; Russell, A.B.; Kennea, N.; Heath, P.T. Antimicrobial Resistance in UK Neonatal Units: NeonIN Infection Surveillance Network. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 103, F474–F478. [Google Scholar] [CrossRef] [PubMed]
- Lutsar, I.; Chazallon, C.; Trafojer, U.; de Cabre, V.M.; Auriti, C.; Bertaina, C.; Calo Carducci, F.I.; Canpolat, F.E.; Esposito, S.; Fournier, I.; et al. Meropenem vs. standard of care for treatment of neonatal late onset sepsis (NeoMero1): A randomised controlled trial. PLoS ONE 2020, 15, e0229380. [Google Scholar] [CrossRef] [PubMed]
- Germovsek, E.; Lutsar, I.; Kipper, K.; Karlsson, M.O.; Planche, T.; Chazallon, C.; Meyer, L.; Trafojer, U.M.T.; Metsvaht, T.; Fournier, I.; et al. Plasma and CSF Pharmacokinetics of Meropenem in Neonates and Young Infants: Results from the NeoMero Studies * on Behalf of the NeoMero Consortium †. J. Antimicrob. Chemother. 2018, 73, 1908–1916. [Google Scholar] [CrossRef]
- Ahmer, M.; Yao, R.B.; Van Den Anker, G.H.J.; Zhao, W.; Xu, H. Optimal Dose of Meropenem for the Treatment of Neonatal Sepsis: Dosing Guideline Variations and Clinical Practice Deviations. Br. J. Clin. Pharmacol. 2022, 88, 3483–3489. [Google Scholar] [CrossRef]
- Ambreen, G.; Salat, M.S.; Hussain, K.; Raza, S.S.; Ali, U.; Azam, I.; Iqbal, J.; Fatmi, Z. Efficacy of Colistin in Multidrug-Resistant Neonatal Sepsis: Experience from a Tertiary Care Center in Karachi, Pakistan. Arch. Dis. Child. 2020, 105, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Folgori, L.; Bielicki, J.; Heath, P.T.; Sharland, M. Antimicrobial-Resistant Gram-Negative Infections in Neonates: Burden of Disease and Challenges in Treatment. Curr. Opin. Infect. Dis. 2017, 30, 281–288. [Google Scholar] [CrossRef]
- Pietrasanta, C.; Pugni, L.; Ronchi, A.; Bottino, I.; Ghirardi, B.; Sanchez-Schmitz, G.; Borriello, F.; Mosca, F.; Levy, O. Vascular Endothelium in Neonatal Sepsis: Basic Mechanisms and Translational Opportunities. Front. Pediatr. 2019, 7, 340. [Google Scholar] [CrossRef]
- Cinicola, B.; Conti, M.G.; Terrin, G.; Sgrulletti, M.; Elfeky, R.; Carsetti, R.; Fernandez Salinas, A.; Piano Mortari, E.; Brindisi, G.; De Curtis, M.; et al. The Protective Role of Maternal Immunization in Early Life. Front. Pediatr. 2021, 9, 638871. [Google Scholar] [CrossRef]
- WHO. Group B Streptococcus Vaccine Development Technology Roadmap. Priority Activities for Develop-Ment, Testing, Licensure and Global Availability of Group B Streptococcus Vaccines; World Health Organization: Geneve, Switzerland, 2017. [Google Scholar]
- Heath, P.T. Status of Vaccine Research and Development of Vaccines for GBS. Vaccine 2016, 34, 2876–2879. [Google Scholar] [CrossRef]
- Leroux-Roels, G.; Maes, C.; Willekens, J.; De Boever, F.; de Rooij, R.; Martell, L.; Bedell, L.; Wittke, F.; Slobod, K.; Dull, P. A Randomized, Observer-Blind Phase Ib Study to Identify Formulations and Vaccine Schedules of a Trivalent Group B Streptococcus Vaccine for Use in Non-Pregnant and Pregnant Women. Vaccine 2016, 34, 1786–1791. [Google Scholar] [CrossRef]
- Madhi, S.A.; Cutland, C.L.; Jose, L.; Koen, A.; Govender, N.; Wittke, F.; Olugbosi, M.; Meulen, A.S.-T.; Baker, S.; Dull, P.M.; et al. Safety and Immunogenicity of an Investigational Maternal Trivalent Group B Streptococcus Vaccine in Healthy Women and Their Infants: A Randomised Phase 1b/2 Trial. Lancet Infect. Dis. 2016, 16, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Swamy, G.K.; Metz, T.D.; Edwards, K.M.; Soper, D.E.; Beigi, R.H.; Campbell, J.D.; Grassano, L.; Buffi, G.; Dreisbach, A.; Margarit, I.; et al. Safety and Immunogenicity of an Investigational Maternal Trivalent Group B Streptococcus Vaccine in Pregnant Women and Their Infants: Results from a Randomized Placebo-Controlled Phase II Trial. Vaccine 2020, 38, 6930–6940. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G.G.; Halperin, S.A.; Devlieger, R.; Baker, S.; Forte, P.; Wittke, F.; Slobod, K.S.; Dull, P.M. Maternal Immunization with an Investigational Trivalent Group B Streptococcal Vaccine: A Randomized Controlled Trial. Obstet. Gynecol. 2016, 127, 213–221. [Google Scholar] [CrossRef] [PubMed]
| Red Flags | Other Indicators (Non-Red Flags) | ||
|---|---|---|---|
| Risk Factors | Clinical Findings | Risk Factors | Clinical Findings |
|
|
|
|
| Empirical Antimicrobial Policies | Indication |
|---|---|
| β-lactam antibiotic + Aminoglycoside (Gentamicin) | Gram-positive and Gram-negative agents; this should be used in neonates non-colonized with MRSA to offer anti-staphylococcal coverage |
| β-lactam antibiotic + Aminoglycoside (Amikacin) | More resistant Gram-negative and some Gram-positive bacteria (i.e., Staphylococcus aureus); this could replace Gentamicin in selected cases (higher-risk preterm neonates or neonates with severe disease) |
| Glycopeptide + Aminoglycoside | Empiric Gram-positive and Gram-negative coverage; confirmed CoNS and MRSA |
| Piperacillin + Tazobactam or Ampicillin + Sulbactam | In combination or in alternative to aminoglycoside; Gram-positive and Gram-negative beta-lactamase-producing bacteria |
| Third- or fourth-generation Cephalosporin | In addition to empiric regimen; for severe penicillin-resistant Gram-negative sepsis or Gram-negative meningitis (no Ceftriaxone) |
| Carbapenems | ESBL and AmpC chromosomal beta-lactamase-producing Gram-negative; bacterial meningitis |
| Colistin | CRO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boscarino, G.; Romano, R.; Iotti, C.; Tegoni, F.; Perrone, S.; Esposito, S. An Overview of Antibiotic Therapy for Early- and Late-Onset Neonatal Sepsis: Current Strategies and Future Prospects. Antibiotics 2024, 13, 250. https://doi.org/10.3390/antibiotics13030250
Boscarino G, Romano R, Iotti C, Tegoni F, Perrone S, Esposito S. An Overview of Antibiotic Therapy for Early- and Late-Onset Neonatal Sepsis: Current Strategies and Future Prospects. Antibiotics. 2024; 13(3):250. https://doi.org/10.3390/antibiotics13030250
Chicago/Turabian StyleBoscarino, Giovanni, Rossana Romano, Carlotta Iotti, Francesca Tegoni, Serafina Perrone, and Susanna Esposito. 2024. "An Overview of Antibiotic Therapy for Early- and Late-Onset Neonatal Sepsis: Current Strategies and Future Prospects" Antibiotics 13, no. 3: 250. https://doi.org/10.3390/antibiotics13030250
APA StyleBoscarino, G., Romano, R., Iotti, C., Tegoni, F., Perrone, S., & Esposito, S. (2024). An Overview of Antibiotic Therapy for Early- and Late-Onset Neonatal Sepsis: Current Strategies and Future Prospects. Antibiotics, 13(3), 250. https://doi.org/10.3390/antibiotics13030250









