Abstract
Antimicrobial resistance is an increasingly widespread phenomenon that is of particular concern because of the possible consequences in the years to come. The dynamics leading to the resistance of microbial strains are diverse, but certainly include the incorrect use of veterinary drugs both in terms of dosage and timing of administration. Moreover, the drug is often administered in the absence of a diagnosis. Many active ingredients in pharmaceutical formulations are, therefore, losing their efficacy. In this situation, it is imperative to seek alternative treatment solutions. Essential oils are mixtures of compounds with different pharmacological properties. They have been shown to possess the antibacterial, anti-parasitic, antiviral, and regulatory properties of numerous metabolic processes. The abundance of molecules they contain makes it difficult for treated microbial species to develop pharmacological resistance. Given their natural origin, they are environmentally friendly and show little or no toxicity to higher animals. There are several published studies on the use of essential oils as antimicrobials, but the present literature has not been adequately summarized in a manuscript. This review aims to shed light on the results achieved by the scientific community regarding the use of essential oils to treat the main agents of bacterial infection of veterinary interest in livestock. The Google Scholar, PubMed, SciELO, and SCOPUS databases were used for the search and selection of studies. The manuscript aims to lay the foundations for a new strategy of veterinary drug use that is more environmentally friendly and less prone to the emergence of drug resistance phenomena.
1. Introduction
One of the biggest issues facing livestock farming across the globe is the fast-growing demand for food of animal origin, particularly in low-income countries. Animal breeding methods are evolving as a result of this expanding tendency, and intensive livestock systems are quickly replacing rural livestock farms [1]. In today’s livestock system, antibiotics play a key role. Particularly, antibiotics are becoming an essential part of animal husbandry in order to fulfill the world population’s demand for food [2,3].
Sick animals may spread disease to other flock members either directly or indirectly via contaminated feed, water, soil, and plants. This is particularly true in intensive livestock, where space for each animal is limited [4]. Furthermore, at any point during the processing of animals in slaughterhouses, germs can be transferred from carcass to carcass [5,6]. Livestock infections have a significant impact on public health as well, and great efforts are made to control them because outbreaks of zoonotic diseases in humans are linked to direct or indirect contact with an infected animal, the animal’s environment, or, more commonly, the consumption of contaminated food of animal origin [7]. Furthermore, antibiotics are widely utilized not just for medicinal purposes but also as growth-promoting, and disease-prevention measures. However, their overuse has increased selection pressure, which has led to the current emergence of antibiotic resistance.
In 2014, the World Health Organization (WHO) stated that the global public health catastrophe posed by antibiotic-resistant bacteria is rapidly approaching [8]. The issue of antimicrobial resistance affects both human and veterinary medicine. Antimicrobial-resistant bacteria (ARBs) infections are on the rise today; it is predicted that by 2050, these infections will be responsible for 10 million deaths per year [9]. ARB infections in livestock are particularly dangerous as they are linked to higher veterinary care costs, higher death rates, declining populations, and decreased production [10].
When treated with antibiotics, animals eliminate the active ingredients or their metabolites into the environment to some extent [11]. The biggest source of environmental pollution is animal dung. After administration, antibiotics are absorbed by the body and processed. The main routes of elimination of the drug and/or its metabolites are urine and/or feces [12]. The animal excretes antibiotics through feces if oral absorption is inadequate.
The presence of antibiotics at levels below those used for therapy accelerates the spread of ARBs. The World Organization for Animal Health (OIE) advised the prudent and cautious use of antimicrobial drugs in animals to reduce this worldwide problem and guarantee the safety of animal-derived food [13]. Antibiotic usage in animal farms has already been reduced in several countries; the European Union (EU) banned the use of antibiotics as growth promoters in animal diets in 2006 [14]. Furthermore, consumer “feeling” is changing. When choosing food, they pay more attention to food labels and often choose products from animal farms where the use of drugs is limited [15,16].
In this scenario, in order to lower ARBs and the frequency of the most significant infections in livestock farming, the search for effective antibiotic alternatives is becoming mandatory [17,18]. Antimicrobial peptides, bacteriophage, bacteriocins, prebiotics, and probiotics have garnered interest as possible replacements or supplements to currently used antimicrobial chemicals [19,20]. To these promising alternatives are added the resources provided by the plant world.
For millennia, traditional medicine has been based on medicinal plants. Through observation and experience, humans have developed a vast traditional culture around the appropriate use of plants. Over the past hundred years or so, advancements in science and technology, particularly in the field of plant pharmacognosy, have led to the identification of several novel compounds with unusual modes of action [21]. The scientific use of drugs obtained from plants for the treatment and prevention of diseases is known as phytotherapy.
The use of phytotherapy in veterinary medicine is also known as ethnoveterinary medicine; the latter is practiced wherever people have a strong connection with animals. It is particularly important in communities where breeding animals are the main source of food. Numerous botanical species have historically been used—and in some cases still are—to cure horses, pigs, sheep, cows, and poultry [22]. In recent decades, ethnoveterinary treatment has become more popular in highly industrialized countries due to public health concerns (such as antibiotic/anthelmintic resistance) and the ongoing expansion of organic livestock farming [23,24]. The primary basis for phytotherapy is derived from natural complexes present in plants, which are mostly composed of low molecular weight chemical compounds. The components of fruits, vegetables, spices, and other plant parts (such as pulp, bark, peel, leaf, berry, or bloom) are sources of phyto-complexes. Today, essential oils (EOs) are among the most widely used natural substances [25]. The culinary, cosmetic, pharmaceutical, and agricultural sectors have all found many uses for EOs, which has led to a current wave of studies on secondary plant metabolites. Thus, studies on the medicinal properties of EOs have increased in recent years [26,27,28,29,30,31].
The investigation of EOs’ antibacterial qualities is becoming more and more important as antibiotic-resistant forms of bacteria pose a serious danger to both human and animal health, making alternative pharmacological treatments useful in the fight against diseases brought on by these pathogens. Numerous studies have examined the effectiveness of EOs both in vivo and in vitro [32,33]. Some of them have assessed the impact of EOs on animals by adding them to feed or sprinkling them on the animals’ quarters to lower bacterial contamination and raise farm cleanliness standards [34]. Other studies have been conducted on reference strains, bacterial strains isolated from farm animals, and food of animal origin to reduce bacterial contamination by extending the shelf life of goods meant for human consumption [35]. Several veterinary studies conducted in vivo and in vitro on the use of EOs as substitutes for available antibiotics were considered in this review. Literature reviews summarizing the various scientific publications in this field are lacking. This manuscript therefore aims to take stock of the situation and infer the suitability of certain compounds as promising candidates for the treatment of microbial diseases.
2. Methodology
Several databases were used to gather the papers for this review, including PubMed, Google Scholar, PubMed, SciELO, and SCOPUS. A variety of keywords pertaining to the review’s topic were included in the search box, such as “antibacterial activity”, “phytochemical” or “antimicrobial resistance”, and “essential oil and antibacterial activity.” Depending on the combination of phrases, the operators “AND” or “OR” were employed. Using “antibacterial activity” AND “plant extract” OR “essential oil” as examples. To be eligible for the evaluation process, the studies had to be published in English. Research on particular antimicrobial substances was limited to those with detailed descriptions of their mechanisms of action, accurate quantitative assessments of their antibacterial efficacy, and a well-established origin.
3. Essential Oils
The European Pharmacopoeia defines EO as: “Odorant product, generally of a complex composition, obtained from a botanically defined plant raw material, either by driving by steam of water, either by dry distillation, or by a suitable mechanical method without heating” [36]. According to the European Pharmacopoeia, an EO is typically extracted from the aqueous phase using a physical process that does not significantly alter its chemical composition. Due to their strong flavor, which decreases the appetite of herbivores, EOs have a significant protective effect on plants. When in the liquid phase, EOs are volatile, limpid, and seldom colored substances. Chemically, they are soluble in organic solvents that have lower densities than water [37]. All plant parts, including buds, flowers, leaves, stems, twigs, seeds, fruits, roots, wood, and bark, are useful to synthesize. Once generated, EOs are stored in glandular trichomes, secretory cells, cavities, and canals [38]. Only 10% of plant species—more than 17,000 plant species—produce and contain EOs, consequently, they are referred to as aromatic plants. The Lamiaceae, Lauraceae, Asteraceae, Rutaceae, Myrtaceae, Poaceae, Cupressaceae, and Piperaceae families make up the majority of their global distribution [39].
EOs are made up of a naturally occurring complex combination produced by plants’ secondary metabolism. This mixture typically contains 20–60 organic volatile components with molecular weights below 300. They are partially found in the vapor form because of their high enough vapor pressure at ambient temperature and atmospheric pressure [40]. Two or three primary constituents, present in high concentrations (20–70%), make up each EO. Terpenes (including monoterpenes and sesquerpenes), aromatic chemicals (such as alcohol, phenol, methoxy derivatives, and aldehydes), and terpenoids (isoprenoids) are generally the principal active ingredients. Terpene hydrocarbons and oxygenated molecules are the two main categories into which EO components and aroma can be separated [38,41]. The most prevalent class of chemical components included in EOs are terpenes and terpenoids. When several isoprene units (C5H8) are combined, terpenes, which are hydrocarbons, are produced. The production of terpenes takes place in the cytoplasm of plant cells, is initiated by acetyl-CoA, and progresses via the mevalonic acid pathway. Cyclases, which have a hydrocarbon backbone, are able to reorganize terpenes into cyclic structures, resulting in the formation of either monocyclic or bicyclic structures [42,43]. Terpene-specific synthetase modifies the allylic prenyldiphosphate to form the terpene skeleton. After synthesizing the isopentenyl diphosphate (IPP) precursor, IPPs are repeatedly added to form the prenyldiphosphate precursor of the various classes of terpenes. Finally, secondary enzymatic modification (redox reaction) of the skeleton confers functional properties to the various terpenes [42,43]. The two primary types of terpenes are monoterpenes (C10H16) and sesquiterpenes (C15H24), however, there are also longer chains like diterpenes (C20H32), triterpenes (C30H40), etc. Some examples of EO components having antimicrobial action include p-cymene, limonene, menthol, eugenol, anethole, estragole, geraniol, thymol, γ-terpinene, and cinnamonyl alcohol [44]. Plants that contain some of these chemicals include angelica, bergamot, lemongrass, mandarin, mint, caraway, celery, citronella, coriander, eucalyptus, geranium, petitgrain, pine, juniper, lavandin, lavander, lemon, orange, peppermint, rosemary, sage, and thyme [45].
Terpenoids are produced by biochemically modifying terpenes using enzymes that transfer or remove methyl groups and add oxygen molecules [42]. Alcohols, phenols, esters, aldehydes, ethers, ketones, and epoxides are the different subgroups of terpenoids. Terpenoids include thymol, carvacrol, linalool, linalyl acetate, citronellal, piperitone, menthol, and geraniol.
Phenylpropanoids (derived from phenyl-propane) are aromatic compounds produced via the shikimic acid route that leads to phenylalanine. The aromatic chemicals include phenols (eugenol and chavicol), aldehydes (cinnamaldehyde), alcohols (cinnamic alcohol), methylenedioxy compounds (apiole, myristicin, and safrole), and methoxy derivatives (anethole, estragole, and methyleugenols).
EOs exhibit a relatively high degree of variety in terms of qualitative and quantitative composition.
This variability is caused by a number of variables, which fall into two main categories: (1) intrinsic factors, which include the plant itself, its maturity, and its interactions with the environment (such as the type of soil and climate); and (2) extrinsic factors, which are associated with the extraction technology.
Seasonal fluctuations, plant organs, plant maturity, regional origin, and genetics are other aspects to consider. Due to their strong interdependence and mutual effect, these aspects can sometimes be challenging to separate from one another [40].
Antimicrobial Mechanism of Action
The composition of EOs, the presence of functional groups in their active components, and their synergistic interactions are factors that determine their activity [46]. The kind of EO or strain of the microbe utilized determines the antibacterial mode of action. It is well recognized that Gram-positive bacteria are more vulnerable to EOs than Gram-negative bacteria [47,48]. This is explained by the fact that Gram-negative bacteria have an outer membrane that is more complex, rigid, and rich in lipopolysaccharides (LPS), which limits the diffusion of hydrophobic compounds through it. In contrast, Gram-positive bacteria lack this extra complex membrane and are instead surrounded by a thick peptidoglycan wall that is not dense enough to withstand small antimicrobial molecules, which facilitates the entry of antimicrobial compounds through the cell membrane. Moreover, since lipophilic ends of lipoteichoic acid are present in cell membranes, the membrane composition of Gram-positive bacteria may facilitate the penetration of hydrophobic components of EOs [49].
Numerous studies have shown that the bioactive ingredients found in EOs can adhere to the cell’s surface before penetrating the phospholipid bilayer of the cell membrane. Their buildup compromises the structural integrity of the cell membrane, which can negatively impact cell metabolism and ultimately result in cell death [50,51]. Additionally, it has been reported that the action of EOs on the integrity of the cell membrane alters its permeability, causing the loss of essential intracellular components such as proteins, reducing sugars, ATP, and DNA, while also inhibiting the generation of energy (ATP) and related enzymes, resulting in cell breakdown and electrolyte leakage [52]. Therefore, a series of events encompassing the whole bacterial cell is thought to be responsible for the antimicrobial activity of EOs [53]. Figure 1 summarizes the mechanisms of toxic action of EOs against the bacterial cell.
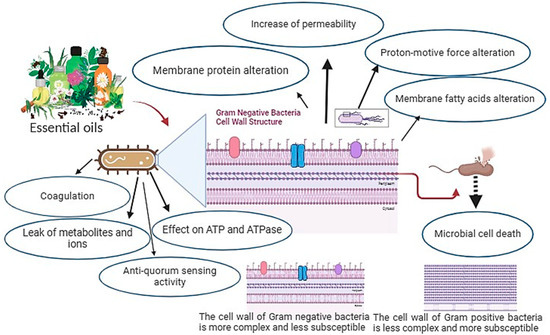
Figure 1.
Mechanisms of EOs action that lead to microbial cell death.
Table 1 shows some EOs and their components that have demonstrated antimicrobial action. The mechanisms by which they exert this action on the bacterial cell are also mentioned.

Table 1.
Essential oils and essential oil components with antimicrobial activity.
4. Control of Bacterial Disease via Essential Oils
Etiological agents of infection that can affect a large number of animals are common in intensive livestock farms; the most frequently affected animals are the young ones [61,62]. Equally frequent are infectious diseases that can affect individual animals. It is often opportunistic bacteria that sustain these pathological states, often conditioned by alterations in normal physiological conditions, as occurs during pregnancy. Opportunistic bacteria have become particularly important because the evolution of animal husbandry technologies often leads to a drop in the host’s protective reactivity, such that it is vulnerable even to bacteria proper to the normoergic host [63]. In general, several bacterial strains are developing resistance to synthetic drugs. Table 2 shows some important infectious agents and the main classes of antibiotics against which resistance has been recorded. In this situation, natural products, in particular Eos, could be a viable alternative. The results obtained by the scientific community in controlling the etiological agents of important bacterial diseases using EOs isolated from different botanical species are examined in the next paragraphs.

Table 2.
Important bacteria causing microbial infections and classes of antibiotics to which resistance has been recorded.
4.1. Gram-Negative Bacteria
4.1.1. Escherichia coli
Escherichia coli is a member of the Enterobacterales family responsible for numerous diseases in both humans and animals. It poses a serious risk to veterinary care as it can spread disease to all animal species, often resulting in financial losses. In calves, it is responsible for colibacillosis. This is an infection that can occur from the first days of life until 2–3 months of age. E. coli infects the calf soon after birth through the food route or the umbilical cord and colonizes the intestine. Depending on the age of the subjects, the serotypes involved, and their ability to settle in the various intestinal tracts, septicemic, enterotoxemic, and enteric forms of colibacillosis can occur [75]. In cattle, E. coli, Klebsiella, Enterobacter, and other microorganisms are responsible for mastitis [76]. Furthermore, infection by E. coli can result in avian colibacillosis, a systemic disease affecting birds. While contaminated dust is typically inhaled by animals, E. coli mostly affects poultry via an oral–fecal cycle. It produces a wide range of clinical symptoms in chickens, including enteritis, cellulitis, enlarged head syndrome, septicemia, polyserositis, coligranuloma, omphalitis, and salpingitis. Poultry colibacillosis is characterized by subacute symptoms such as pericarditis, airsacculitis, and perihepatitis, and acute symptoms such as septicemia that can be fatal [77].
Septicemia is often the first sign of the infection, followed by localized inflammation in many organs or abrupt death [78]. Avian colibacillosis is characterized by variable lesions, the most common of which are polyserositis and airsacculitis. Furthermore, E. coli can penetrate the egg shell if the eggs are contaminated with feces. Contamination of eggs by feces can cause yolk sac infection or allow E. coli to enter through the shell, infecting the chicks during hatching and frequently leading to significant mortality rates. When infected at an early stage, birds that survive clinical disease may not exhibit many symptoms of infection but may become carriers [79]. Economic losses due to necrotic cellulitis may occur at slaughter. Given the importance of this disease, several studies were conducted to confirm the anti-E. coli properties of some EOs derived from various botanical species. The goal of these studies was to identify natural products that could be used to improve environmental hygiene on chicken farms. Several EOs showed anti-E. coli action in vitro.
Rhayour et al. (2003) discovered that clove EO had an impact on E. coli, causing holes in the membrane and cell wall [80].
A study was carried out by Burt & Reinders (2003) [81] to measure the bactericidal qualities of five different EOs on a non-toxic strain of E. coli O157:H7 at three different temperatures and with and without an emulsifier or stabilizer. The disc diffusion test was used to screen for antibacterial qualities, and the most active ones were chosen for additional investigation using microdilution colorimetric assays. The greatest bacteriostatic and bactericidal effects were exhibited by Origanum vulgare and Thymus vulgaris (light and red variants) EOs, followed by Syzygium aromaticum and Pimenta racemosa EOs. Sixty-five μL L−1 of Origanum vulgare EO was colicidal at 10, 20, and 37 °C. While 0.25% (w/v) lecithin decreased bacterial activity, the inclusion of 0.05% (w/v) agar as a stabilizer strengthened the antibacterial capabilities, especially at 10 °C [81].
Applied alone or in combination, Cinnamomum verum and Syzygium aromaticum EOs have demonstrated strong antibacterial action against E. coli strains obtained from chickens with colibacillosis.
The primary components of the two oils—eugenol and its acetate form—have been linked to this action [82]. According to Zhang et al. (2016) [83], Cinnamomum verum EO alters the E. coli membrane’s permeability and integrity, resulting in the loss of inner cell components [83].
EOs extracted from the leaves of 29 medicinal plants in Brazil were tested in a study by Duarte et al. (2007) [84] against 13 distinct E. coli serotypes. The EO derived from Cymbopogon martini demonstrated a wide range of inhibition and high action (MIC between 100 and 500 μg/mL) against ten of the thirteen E. coli serotypes, including two enteropathogenics, three enteroinvasives, three enterotoxigenics, and two shiga-toxin-producing serotypes. Two enterotoxigenic, one enteropathogenic, one enteroinvasive, and one sigatoxin-producing serotype were all significantly suppressed by Cymbopogon winterianus. E. coli can be effectively killed by Aloysia citrodora with moderate to severe inhibition. Other EOs exhibited antibacterial qualities as well, although their effectiveness against the investigated serotypes was more constrained [84].
Raho and Benali (2012) [85] investigated the antibacterial properties of the EO derived from Eucalyptus globulus leaves in vitro. The EO’s inhibitory properties were evaluated using dilution broth and agar disc diffusion techniques against E. coli. The findings demonstrated that, depending on the inoculum size and the quantity of EO, the EO exhibited antibacterial activity to differing degrees. The EO of Eucalyptus globulus leaves has an inhibitory zone ranging in diameter from 8 to 26 mm. The largest zone of inhibition was obtained for E. coli (10−3 dilution) with a 100% concentration of Eucalyptus globulus EO.
Romero et al. (2015) [86] investigated the antibacterial impact and mechanism of action of some components of EOs (carveol, carvone, citronellol, and citronellal) against S. aureus and E. coli. Dimethyl sulfoxide (DMSO, 100%) at different concentrations ranging from 0.066 to 3000 μg/mL was used to test each component. The compounds were weighed to obtain μg/mL units. The microdilution broth technique was used to calculate the MICs of the EO components. When tested against E. coli, every chemical showed bactericidal and inhibitory properties. Citronellol (5 μg/mL) proved highly inhibitory against E. coli, with carveol/carvone (200 μg/mL) and citronellal (300 μg/mL) following [86].
The study by Thielmann et al. (2019) [87] is one of the most significant in terms of the quantity of plant species tested against E. coli. Using a single-method approach, the authors determined the minimum inhibitory concentrations (MICs) of 179 EO samples from 86 plant species. Using a dispersion-based method to integrate EOs in a concentration range between 6400 and 50 μg/mL, MICs were obtained in a broth microdilution experiment. Twenty-two samples from 12 plant genera inhibited E. coli at doses below 400 μg/mL.
At 50 μg/mL, only four EOs—Neolitsea cassia, Cinnamomum verum, Origanum vulgare, and Thymus zygis—showed efficacy against E. coli. Despite this, certain additional EOs from Cupressus sempervirens, Backhousia citriodora, Cymbopogon citratus, Cymbopogon martini, Cymbopogon nardus, Litsea cubeba, Origanum majorana, Origanum vulgare, and Syzygium aromaticum nevertheless showed encouraging action against E. coli, with MICs ranging from 100 to 400 μg/mL. Some EOs did not show antibacterial properties. In particular, Cananga odorata, Cupressus sempervirens, Daucus carota, Foeniculum vulgare, Juniperus communis, and Pimpinella anisum had no efficacy against E. coli [87].
Minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) were used in the study by Galgano et al. (2022) [88] to test the bacteriostatic–bactericidal activity of Citrus limon, Pinus sylvestris, Foeniculum vulgaris, Ocimum basilicum, Melissa officinalis, Thymus vulgaris, and Zingiber officinale against various E. coli strains in vitro. Except for T. vulgaris, all the studied EOs showed little antibacterial action against Escherichia coli. The antibacterial action of T. vulgaris was independent of oil content, and this EO completely inhibited the growth of E. coli [88].
It has also been shown that Litsea cubeba EO works well against E. coli [89]. When E. coli cells were treated with this EO, Li et al. (2014) [90] noticed holes and gaps on the outer and inner membranes. These were mostly ascribed to the existence of aldehydes like geranial and neral.
The strong action against E. coli isolates that the EO of Cymbopogon citratus showed in vitro can be traced back to aldehydes [89,91].
High action against E. coli was also shown by Mentha piperita EO [89,92]. The main ingredients of Mentha piperita EO, menthol, and its oxidative product, menthone, have been shown to possess antibacterial activities [93,94].
Eos from Pelargonium graveolens and Ocimum basilicum showed anti-E. coli action [89,95,96]. The significant quantity of linalool in basil EO has been linked to its activity, but to a varied degree depending on the strain tested, whereas the high concentration of oxygenated monoterpenes in Geranium robertianum EO seems to influence its antibacterial function [89].
4.1.2. Salmonella Species
Pathogenic to humans and animals, Salmonella spp. Causes gastroenteritis, septicaemia, pneumonia, and abortion. Some serovars, referred to as “host-restricted”, are closely adapted to the host; for example, S. abortusovis is adapted to sheep and is the main cause of abortion in this animal, while S. typhi suis, S. gallinarum, and S. pullorum are adapted to pigs and chickens, respectively. Other serovars possess a broad host spectrum and are known as “host-adapted” serovars. Diseases in pets and livestock animals result in financial losses and increase the possibility of transmission to people. In people, infection can occur through the ingestion of contaminated food and drink. The field of food microbiology has mostly studied the application of EOs in combating Salmonella infections. Indeed, foodborne diseases caused by Salmonella spp. pose a growing risk to human health; salmonellosis outbreaks are the result of recent changes in food processing and marketing practices [97]. Relatively few serovars are responsible for the majority of cases of salmonellosis in people and domestic animals; these can be divided into three categories based on host predominance.
The first group of serovars is host-specific. They usually cause systemic diseases in a few animals that are related by phylogeny. For instance, systemic disease in sheep, poultry, and humans is nearly solely linked to S. enterica serovar abortusovis, paratyphi, and pullorum. The second type of strain is host-restricted. Although they are mostly associated with one or two closely related host species, they can typically lead to disease in different hosts. For example, serovar Dublin and serovar Choleraesuis of S. enterica are often linked to severe systemic diseases in ruminants and pigs, respectively [98]. However, it’s possible for these serovars to infect humans and other animal species. The third category is S. enterica serovars, including infantis and enteritidis, which often cause gastroenteritis in a significant number of unrelated host species. Of course, there are wide variations in the type and severity of Salmonella infections in various animal species. These variations are caused by a variety of factors, such as the Salmonella serovar, dosage, age, virulence of the strain, host animal species, host immunological state, and geographic location [99]. Worldwide, S. enterica continues to be a major source of infection and disease in both humans and animals.
The R (+)-limonene, orange terpenes, trans-cinnamaldehyde, carvacrol, and cold compressed orange oil were evaluated against fifteen distinct strains of S. enterica subsp. serovar Heidelberg, recovered from a variety of sources, including cattle, pigs, turkeys, poultry, and poultry egg houses. The growth of all microorganisms was entirely suppressed by carvacrol and trans-cinnamaldehyde. However, cold compressed orange oil only hindered the development of two isolates, while R (+)-limonene and orange terpenes exhibited no inhibitory effect against the same strains [100].
EOs extracted from Aloysia triphylla, Cinnamomum verum, Cymbopogon citratus, Litsea cubeba, Mentha piperita, and Syzygium aromaticum have been put to the test with S. enterica Serovar Enteritidis isolated from chickens. The anti-Salmonella activity of Cinnamomum verum was greatest, followed by Syzygium aromaticum extract. It has been proposed that the inclusion of these EOs into chicken diets, together with Saccharomyces cerevisiae administration, is an integrated strategy to prevent intestinal colonization by Salmonella. Additionally, EOs isolated from Cinnamomum verum and Syzygium aromaticum could be used either alone or in combination for disinfection [101].
The primary ingredients in these EOs, cinnamonaldehyde and eugenol, have been linked to their antibacterial properties. Because of their capacity to donate hydrogen, these compounds react with lipid and hydroxyl radicals to transform them into stable products [102]. Furthermore, since the two substances include a carbonyl group that binds to the enzymes, rendering them inactive and/or damaging the bacterium’s cell wall, they can prevent bacteria from producing vital enzymes [103].
Research against S. typhimurium ATCC 14028 revealed notable seasonal variations in the EO components of Aristolochia fontanesii roots and their antibacterial action. The main components of Aristolochia fontanesii EO were oxygenated monoterpenes (5.9–28.0%) and oxygenated sesquiterpenes (50.2–81.1%). Because of the higher concentrations of alcohols and esters (23.0% of total oil) and the synergistic effect of other minor compounds such as linalool, terpinene-4-ol, camphor, and t-pinocarveol, the EO (15 μL) of Aristolochia fontanesii exhibits potent antibacterial (zone of inhibition: 8.2–12.0 mm) properties [104]. Nearly all tested Salmonella strains were shown to be significantly susceptible to the EOs of Thymus vulgaris, Syzygium aromaticum, and Cinnamomum verum. The clove, cinnamon, and thyme Eos’ MBC/MIC ratios for most bacteria were equal to 1, indicating that these oils had bactericidal effects at concentrations of at least 20 μL/mL. Microbes’ ability to form biofilms was seen to be suppressed in the presence of subinhibitory concentrations of Thymus vulgaris, Cinnamomum verum, and Syzygium aromaticum EOs [105]. The oxygenated monoterpene concentration of EOs isolated from Thymus mastichina and Calamintha nepeta was greater (86.0–91.0%), with 1,8-cineole (28.0–71.0%) as the most important component. On the other hand, Origanum virens EO exhibited a greater concentration of hydrocarbon monoterpenes (45.3%) and oxygenated monoterpenes (45.5%), with notable components including γ-terpinene (20.2%) and thymol (19.4%). When compared to other tested Gram-negative strains in the same investigation, S. enteritidis LFG 1005 and S. typhimurium LFG 1006 were more vulnerable to the strong antibacterial activity of the EOs of all three of these plants. The high content of 1,8-cineole in the EOs of Calamintha nepeta and Thymus mastichina was shown to exhibit strong antibacterial activity, with MICs ranging from 0.8 to >2 mg/mL [106].
In the EO isolated from the leaves of Taxodium distichum, 37 chemicals were found. Of all 37 compounds, α-pinene was identified as the main constituent with 83.1% [107]. With an inhibition zone of 17 mm and a minimum inhibitory concentration (MIC) of 78.1 g/mL, S. typhimurium was shown to be sensitive to Taxodium distichum leaf EO (ATCC 14028). According to Al-Sayed (2018) [107], the presence of terpene components in the EOs is responsible for the reported antibacterial action. In another investigation, it was found that Spondias pinnata leaves and flowers contain high levels of β-caryophyllene, a sesquiterpene hydrocarbon, with 49.9% in the leaves and 53.3% in the flowers, while nonacosane (25.0%) was a common ingredient in the EO extracted from the fruit. While the EO from flowers showed poor activity, the EO from leaves demonstrates considerable antibacterial action against S. typhymurium (ATCC 14028). Against S. typhimurium, leaf EO had a minimum inhibitory concentration (MIC) of 125.0 μg/mL, while gentamycin had a MIC of 0.5 μg/mL. The compounds shown to have antibacterial action against S. typhimurium were β-caryophyllene, α-terpineol, α-pinene, β-pinene, terpinolene, selinene, and limonene.
A further investigation revealed that the EO isolated from the leaves of Artemisia dracunculus var. qinghaiensis had moderate inhibitory effects at a concentration of 10 μL/mL against S. paratyphi (CMCCB 50094), with inhibition zones spanning from 10.03 to 13.03 mm [108]. Among the substances found in Artemisia dracunculus EO were α-pinene, β-phellandrene, linalool, terpinen-4-ol, α-terpineol, β-caryophyllene, germacrene D, and caryophyllene oxide, which were also identified as antibacterial substances from other plants. Overall, Artemisia dracunculus’s high content (19.2%) of sabinene has been linked to its antibacterial action against S. paratyphi [108]. Carvacrol (825.0–950.0 μg/mg) was found to be the main constituent of the EO isolated from Satureja montana (Lamiaceae) in another study. Satureja montana was found to have an in vitro minimum inhibitory concentration (MIC) of 250 μg/mL against Salmonella enterica sv Anatum SF2 [109]. According to this study, hydrophilic lipopolysaccharides (LPS), which function as a barrier to macromolecules and hydrophobic chemicals, preventing them from penetrating the target cell membrane, are thought to be responsible for Gram-negative bacteria’s lower susceptibility to EOs. Additionally, in another study, 28 distinct EOs, isolated from different parts of plants, were tested for their antibacterial potential against L. innocua ATCC 33090 and S. enteritidis ATCC 13076. The most effective EOs against S. enteritidis were found in the leaves of Eucalyptus globulus, Eucalyptus exserta, and Pimenta pseudocaryophyllus, as well as two EOs isolated from orange juice by-products (Citrus sinensis): orange oil phase essence and citrus terpenes [110].
4.1.3. Klebsiella Species
Environmental sources of Klebsiella bacteria include soil and water [111]. K. pneumoniae is regarded as one of the most hazardous multidrug-resistant bacteria [112], and is more common in insects, domestic animals, and wild animals than in the environment [113]. It can cause septicemia in foals, pneumonia, metritis, mastitis, and cervicitis in mares [114]. It can lead to lower milk production, worse milk quality, and increased death rates in cows. It can also be the causative agent of mastitis and pneumonia in cows [115]. Strains of K. pneumoniae that produce carbapenemase have been described in several papers during the past 20 years. The genesis of multidrug-resistant K. pneumoniae phenotypes is caused by the synthesis of these extended-spectrum β-lactamases and modification of the outer membrane permeability (decreased expression of porins and overexpression of efflux pumps) [116]. Since carbapenemases are able to hydrolyze practically all β-lactam antibiotics and even β-lactamase inhibitors, only a small number of second-line antibiotics, either alone or in combination, such as colistin, tigecycline, fosfomycin, polymyxin B, and gentamicin, can still be effective against carbapenem-resistant K. pneumoniae [117,118]. Cases of colistin-resistant K. pneumoniae have been reported [119]. When choosing second-line anti-K. pneumoniae drugs, specific toxicity aspects should be taken into account (gentamicin can potentially cause nephrotoxicity, and colistin is known to have nephrotoxic and neurotoxic effects). For these reasons, the search for alternatives is mandatory. Several plant species have shown antibacterial activity against K. pneumonia: Arbutus unedo [120]; Cistus spp. [121]; Cytinus hypocistis [122]; Myrtus communis [123]; Pistacia lentiscus [124]; Teucrium spp. [125]; Cytinus [126]; Thymus vulgaris L. [127]; Pistacia terebinthus [128]; Rapa catozza [129]; Crinum angustum [130]; Tinospora cordifolia, Alstonia scholaris [131]; Rhus coriaria [132]; Calicotome villosa [133]; Melaleuca alternifolia [134]; Mentha spp. [135]. Among these, Pistacia lentiscus [124], Myrtus communis [123], and Arbutus unedo [120] are undoubtedly common and readily available plants that could be utilized to enhance animal welfare and help fight this infection [136].
It has been shown that several EOs and volatile chemicals make clinical isolates or reference strains of K. pneumoniae more susceptible to conventional antibiotics. Research examining the synergistic effects of two native Moroccan thymes (Thymus maroccanus and Thymus broussonetii) EOs in conjunction with conventional antibiotics against a clinical isolate of K. pneumoniae was conducted. While Thymus broussonetii EO only exhibited synergistic effects with pristinamycin, Thymus maroccanus EO demonstrated synergistic effects with ciprofloxacin, gentamicin, and pristinamycin. The different chemical compositions of these two EOs may be the cause of their differing behaviors: carvacrol content was greater in Thymus maroccanus EO (76.35%) than in Thymus broussonetii EO (39.77%) [137]. Thymus saturejoides Coss. EOs, which have carvacrol as their primary ingredient (25.3–45.3%), worked in concert with cefixime to counteract a clinical isolate of K. pneumoniae [138]. The various targets on which the combinations act, including the bacterial membrane (cefixime, carvacrol), proteins (gentamicin, pristinamycin), enzymes (carvacrol), ATP (carvacrol), and DNA (carvacrol, ciprofloxacin), explain these synergistic effects [139,140,141]. Satureja kitaibelii EO has been shown to effectively work in concert with two conventional antibiotics, tetracycline and chloramphenicol, to combat K. pneumoniae ATCC 700603 (10-fold decrease in the MIC values of both drugs). Savory EO’s main ingredient, geraniol (50.4%), showed synergistic benefits only when combined with chloramphenicol (10-fold reduction in the MIC value), demonstrating antibacterial activity comparable to that of savory EO. By acting on the efflux pumps responsible for antibiotic resistance, geraniol modulates bacterial resistance [142,143]. The combination of geraniol and tetracycline has shown additive benefits; it appears that other chemicals in savory EO (limonene, ocimene, linalool, nerol, β-caryophyllene, and germacrene D) may also work in concert with tetracycline [142]. Against the same reference strain, K. pneumoniae ATCC 700603, Thymus pulegioides EO, which belongs to the geraniol/geranyl acetate chemotype, has shown synergistic action with tetracycline, streptomycin, and chloramphenicol [144].
4.1.4. Pseudomonas Species
Pseudomonas aeruginosa is a frequent source of infections in people, animals, and hospitalized patients. In animals, the morbid forms resulting from infection take the form of generalized septicemic-type phenomena or inflammatory processes of a predominantly purulent nature, circumscribed to various organs and apparatuses. It can cause skin, genital tract, and urinary tract infections, as well as bacteremia and pneumonia. Although less common, other species of the Pseudomonas genus can infect various body districts. The disease causes widespread deaths of embryos and significant mortality rates in newborn chickens. Fish with P. aeruginosa infection exhibit severe symptoms such as hemorrhagic septicemia, gill necrosis, distended abdomen, splenomegaly, friable liver, and enlarged kidney.
P. aeruginosa has become resistant to several drug classes. The pathogen’s outer membrane, which shields it from harmful substances and enables it to build a biofilm, is specifically responsible for the bacterium’s innate resistance to a broad spectrum of antibiotics. The inefficacy of some antibiotics against P. aeruginosa can also be attributed to the presence of extended-spectrum β-lactamases in this microbe [145,146]. To fight this infection, more than 23 distinct plants can be employed.
Among these plants are Citrus spp., which are widely cultivated in the southern region of the Italian peninsula.
EOs extracted from Cinnamomum spp. bark have been shown to have antibacterial action against several microorganisms, including P. aeruginosa [32,147].
According to research by Kavanaugh et al. [148], cinnamon EO can prevent P. aeruginosa from growing and forming biofilm. P. aeruginosa was effectively counteracted in vitro by the oils of Cinnamomum aromaticum, Syzygium aromaticum, Myroxylon balsamum, Thymus vulgaris, and Melaleuca alternifolia. Additionally, the EOs of Cinnamomum aromaticum, Thymus vulgaris, and Myroxylon balsamum were found to be effective in inhibiting biofilm development.
In previous investigations, T. vulgaris EO proved effective against P. aeruginosa, as it impeded the in vitro proliferation of human clinical multidrug-resistant isolates [149].
But thyme EOs weren’t always effective against this infection. When a P. aeruginosa strain was isolated from a dog that had externa otitis, for instance, tests revealed that it was resistant to many antibiotics, just as it was resistant to T. vulgaris oil. The canine strain was further tested using oils extracted from the following plants: Origanum basilicum, Origanum vulgare subsp. hirtum, Litsea cubeba, Lavandula latifolia, Illicium verum, Rosmarinus officinalis, Salvia sclarea, Ocimum basilicum, and Rosmarinum officinalis. Only the last three oils had little efficacy [150].
Kacaniova et al. (2017) [151] conducted an investigation to explore the EOs qualities towards Pseudomonas spp. isolated from fish. A variety of EOs were tested for their antibacterial efficacy against ten distinct strains of Pseudomonas isolated from recently captured freshwater fish. The following 21 EOs were used to test each isolate: Lavandula angustifolia, Cinnamomum verum, Pinus cembra, Mentha piperita, Foeniculum vulgare, Pinus sylvestris, Satureja hortensis, Origanum vulgare, Pimpinella anisum, Rosmarinus officinalis, Salvia officinalis, Abies alba, Citrus aurantium var. dulce, Citrus sinensis, Cymbopogon nardus, Mentha spicata var. crispa, Thymus vulgaris, Carum carvi, Thymus serpyllum, Ocimum basilicum, and Coriandrum sativum. Every tested EO showed antibacterial activity. The most successful EO against Pseudomonas species was Cinnamomum verum, both in accordance with the MIC and disc diffusion techniques. Hosseiny et al. verified the eventual synergism of EOs and antibiotics. The authors used the disc diffusion assay to assess the antibacterial activity of three EOs (Thymus vulgaris, Origanum majorana, and Salvia officinalis) against P. aeruginosa (ATCC 9027), either alone or in combination with conventional antibiotics (piperacillin, cefepime, meropenem, gentamicin, and norfloxacin). The examined EOs demonstrated a range of actions against the tested microorganisms, according to the results. Sage showed little effect against the tested microorganisms, whereas thyme and marjoram oils were the most effective. The antibacterial activity of antibiotics that target the cell wall, such as cefepime, meropenem, and piperacillin, was more than doubled when thyme oil was added. The action of every antibiotic studied, with the exception of norfloxacin, was enhanced by marjoram oil. Sage was used with meropenem, gentamicin, and piperacillin to increase their combined action, even though it was ineffective against Pseudomonas.
4.1.5. Campylobacter Species
Pathogens belonging to the genus Campylobacter cause illnesses in humans and several animal species. The Campylobacter genus therefore includes micro-organisms responsible for contagious infectious diseases. Domestic animals are sensitive, with symptoms affecting the genital system (abortion, metritis, and sterility), the digestive system (enteritis and hepatitis), and the mammary system (mastitis). The animal infection is transmissible to humans. It is regarded as one of the main causes of gastroenteritis in people globally. Campylobacter is the etiological agent of outbreaks linked to chicken products and can cause foodborne disease through the contamination of poultry carcasses.
Campylobacter was the cause of 1087 of the 4598 hospitalizations due to foodborne illness in 2015 (Centers for Disease Control and Prevention [CDC], 2017) [152]. Among bacterial infections, this impact on public health was surpassed only by that of Salmonella [153,154]. Averaging the number of deaths and illnesses that resulted in health loss, Scallan et al. (2015) [154] calculated that Campylobacter caused 22,500 disability-adjusted life years (DALY) every year. In terms of the most significant impact on public health resulting from foodborne illnesses, Campylobacter ranked third, behind Toxoplasma (32,700 DALY) and non-typhoidal Salmonella (32,900 DALY). After rotavirus, typhoid fever, and cryptosporidiosis, a comprehensive review conducted between 1990 and 2013 revealed Campylobacter as the fourth most common cause of diarrheal illness [155].
It has been repeatedly shown that broiler and layer flocks have very high Campylobacter prevalence. Since EOs seem to strengthen poultry’s immune systems, there is a chance to lower Campylobacter concentrations via immune system regulation [156]. Moreover, EOs may directly interact with populations of Campylobacter.
Several studies have shown the in vitro activity of various EOs against Campylobacter strains.
The effectiveness of EOs against Campylobacter isolates is connected to the EO, since each has a distinct chemical makeup and mode of action.
Friedman et al. (2002) [157] evaluated the antibacterial efficacy of many Eos using isolates from clinical and food sources. The most effective Eos against C. jejuni were Citrus aurantium, Daucus carota, Apium graveolens seed, Artemisia vulgare, Nardostachys jatamansi, Gardenia jasminoides, Jasminum abyssinicum, Pogostemon cablin, and Calendula officinalis.
It was shown that the EOs of Melaleuca alternifolia, Backhousia citriodora, and Leptospermum scoparium were effective against C. jejuni and C. coli isolated from chicken [158], while Origanum syriacum was effective against a strain of C. jejuni [159].
Also, when applied as a feed supplement, the EOs demonstrate good antibacterial activity. When added to diet in quantities less than 1%, caprylic acid, a component of coconut oil and palm kernel oil, greatly decreased the amounts of Campylobacter [160,161]. This was noted in 10-day-old and market-age broilers; it had no effect on body weight or feed conversion ratio.
To counteract the C. jejuni infection, Arsi et al. (2014) [162] investigated the use of thymol, carvacrol, or a combination thereof as a dietary supplement. In order to perform this, four separate studies were conducted with day-old broiler chicks (n = 10 chicks/dose) given thymol, carvacrol, or mixtures of these compounds in feed. On day three, the birds were orally challenged with C. jejuni, and on day ten, cecal samples were taken in order to count the number of Campylobacter. The study found that Campylobacter levels decreased for thymol treatments at 0.25% (trial 1), 1% carvacrol or 2% thymol (trial 2), or a combination of both thymol and carvacrol at 0.5% (trial 3). These findings back up the addition of these substances to feed in an effort to lessen hens’ colonization of Campylobacter. Thymol at a 0.25% concentration decreased Campylobacter by 0.6 log CFU/mL cecal contents. With 2% thymol, a 2 log CFU decrease was seen. Furthermore, a single attempt employing 0.5% thymol and carvacrol resulted in 2 log CFU/mL cecal contents.
Campylobacter species can also infect mammals. Specifically, dogs and cats may show asymptomatic or develop clinical signs related to the gastrointestinal tract, but in any case, they pose a risk of infection to their owners. Although there are no studies comparing the effectiveness of EOs to pet strains of Campylobacter in the literature, it is plausible that isolates from dogs and cats may be susceptible to EOs that have been shown to be effective against strains of Campylobacter that originate from other sources.
4.2. Gram-Positive Bacteria
4.2.1. Staphylococcus Species
Staphylococcus species are well-known to cause opportunistic infections in humans, but they also pose a serious threat in veterinary medicine. These pathogens infect cold-blooded animals, birds, and mammals in many anatomical areas.
Both coagulase-positive and coagulase-negative staphylococci, which are implicated in infections of varying degrees of severity, belong to this genus. Among coagulase-positive species, the most well-known is S. aureus. Staphylococci are the most common saprophytes of human and animal skin. Specific sites of colonization are, in cattle, the skin of the teats and udder. They are found in the nostrils and tonsils of cattle and sheep, on the skin of the head and neck, and in the external auditory meatus of pigs. In the presence of predisposing factors, they pass through the skin, causing mild infections. They are also responsible for specific diseases: mastitis, pyaemia in lambs, botryomycosis, endometritis, exudative epidermitis, pyoderma, and otitis. Numerous infections in humans, including moderate superficial skin infections, osteomyelitis, native valve endocarditis, heart valve implant-associated infections, severe sepsis, and bacteremia, are caused by staphylococci. S. aureus diseases are typically linked to mastitis in animals raised for dairy products. S. pneumoniae commonly infects companion animals. S. pseudointermedius is a component of the mucosal, cutaneous, and upper respiratory tract microflora, but it has the potential to develop into an invasive pathogen that may identify several illnesses. Still, some species, such as S. chromogenes, S. xylosus, and S. hyicus, have the ability to infect pets. Staphylococcal infections in agricultural animals may pose a serious financial risk.
Numerous investigations have confirmed EO’s anti-staphylococcal activity. Good anti-staphylococcal action was shown by Satureja montana EO [150]. Prior observations of S. montana EO’s antibacterial efficacy against a variety of Gram-positive and Gram-negative bacteria established a connection with the compound’s main ingredients, including carvacrol. Vitanza et al. (2019) [163] pointed out that S. aureus exposed to the EO of Satureja montana underwent cell wall disruption.
Ebani et al. (2020) [164] discovered that whereas EOs from C. zeylanicum and C. myrrha showed no action against S. xylosus, they exhibited modest activity against many canine isolates of S. aureus, S. pseudointermedius, S. chromogenes, and S. hyicus. In a similar vein, S. aureus was found to be somewhat sensitive to myrrh oil by Adam and Selim (2013) [165]. Furthermore, Mahboubi and Kazempour (2016) [166] found that Commiphora myrrha has relevant efficacy against S. aureus.
Studies have demonstrated that S. aureus is susceptible to the EOs of Cymbopogon citratus and Melissa officinalis. Aloysia citrodora EO also reduced the development of S. aureus reference strains by compromising the plasmic membrane’s structural integrity and causing a loss of cytoplasmic contents, which results in cellular death.
Most generally, S. aureus is regarded as the most significant microbe connected to mastitis. Because of their propensity for chronicity and recurrence, intramammary infections brought on by this pathogen are extremely difficult to treat [167]. The substantial economic effect of S. aureus-caused bovine mastitis is a major problem in veterinary practice. In an in vivo investigation, Abboud et al. (2015) [168] found that the EOs of Thymus vulgaris and Lavandula angustifolia exhibited potent antibacterial activity against strains of Streptococcus and Staphylococcus. After four consecutive days of therapy, an intramammary application of these oils, or a combination of them, resulted in a significant reduction in the number of bacteria in the various milk samples. In the same study, the external use of Thymus essential oil in Vaseline produced greater antibacterial action, leading to a 100% recovery rate. In a different trial conducted by Cho et al. in 2015 [169], single and double dosages of Origanum EO ointment (0.9 mL of EO in a 10 mL tube) were infused into the affected quarters twice a day for three days. The circumstances of the udders improved. Somatic cell counts considerably decreased in the groups as compared to pre-treatment levels. Additionally, on the fourth day following the therapy, no S. aureus or E. coli were found in the milk for three days. Comparable effectiveness was noted for white blood cell counts, which sharply dropped in comparison to pre-treatment levels [169].
4.2.2. Streptococcus Species
Streptococci are widespread in nature as commensals and parasites of the oral cavity, intestinal, and genito-urinary tracts of humans and animals. Pathogenic species are responsible for specific diseases. For example, S. agalactiae causes mastitis in ruminants, while S. equi is responsible for adenitis in horses. The majority of the information found in the literature relates to the effectiveness of EOs against strains of human-originating Streptococcus spp. While some streptococcal species—like S. pyogenes—can infect humans as well as animals, other streptococcal species primarily cause infections and illnesses in a variety of companion and farm animals.
Testing cinnamon oil extracts from Cinnamomum verum and Neolitsea cassia revealed antimicrobial efficacy against a number of diseases, including certain Streptococcus species [170,171,172].
Cinnamaldehyde, the primary constituent of Cinnamomum essential oil, has been linked to the antistreptococcal action of the plant against other bacteria.
S. iniae may affect a wide variety of freshwater, estuarine, and marine fish species. Among the most vulnerable species are Oncorhynchus mykiss, Seriola quinqueradiata, Lates calcarifer, and Oreochromis niloticus [173,174,175,176,177]. A fish infection that is spreading over the world and is devastating many freshwater and saltwater species financially is sustained by Streptococcus agalactiae [178]. The bacterium has been found in a variety of fish, including rainbow trout, seabream, tilapia, yellowtail, croaker (Micropogonius undulatus), killfish (Menhaden spp.), and silver pomfret (Pampus argenteus).
Serious zoonotic pathogens include some streptococci species. For example, S. iniae can cause bacteremia, cellulitis, meningitis, and osteomyelitis [179], S. agalactiae can cause neonatal meningitis, sepsis, and pneumonia [180,181]. Both S. dysgalactiae subsp. equisimilus and S. dysgalactiae subsp. dysgalactiae can cause bacteremia, lower limb cellulitis, and meningitis in infants.
In vitro, the ethanolic extract of Thymus daenensis was less effective in controlling S. iniae than its EO [182]. With the lowest minimum inhibitory concentrations (MICs) of 39 μg/mL and 31.2 μg/mL, respectively, the EO of Bakhtiari savory (Satureja bachtiarica) showed strong action against both S. iniae and S. agalactiae strains [166]. Aloe vera EO showed no inhibitory action against S. iniae strains that were first isolated from sick rainbow trout [183], but its ethanolic extract had an anti-S. iniae impact that was similar to that of erythromycin.
Because of its bacteriostatic action, Lavandula angustifolia EO has been demonstrated to be a potential inhibitor against isolates of S. iniae and S. parauberis [184]. With a MIC of 0.063% and a MIC:MBC ratio of 1:8, one strain of S. parauberis was the most sensitive. This suggests that, while it cannot survive at extremely low concentrations of lavender EO, there may be a chance for bacterial survival at high concentrations.
The fish pathogen S. iniae has been shown to be effectively inhibited by Cinnamomum verum both in vitro and in vivo. Five days before S. iniae infection, tilapia given fish diets supplemented with 0.4% (w/w) of cinnamon oil and 0.1% (w/w) of oxytetracycline showed no signs of death during an in vivo study [172].
In an in vivo investigation, the modifying impact of clove Syzygium aromaticum EO on the antioxidant and immunological state of Nile tilapia after S. iniae infection was assessed. Fish immunity was boosted by clove EO against bacterial assault. Furthermore, it was shown that this oil’s antibacterial activity was partially mediated via hepatic hepcidin expression [185].
De Aguiar et al. (2019) [186] demonstrated the in vitro efficacy of oregano and Thymus spp. EOs against S. suis isolates.
4.2.3. Mycobacterium Species
Although some research has been conducted to confirm the efficacy of EOs against Mycobacterium tuberculosis and M. bovis, the species that cause tuberculosis in both humans and animals [187], the use of alternative treatments for tuberculosis is discouraged given the seriousness of this infectious disease.
Conversely, EOs may serve as substitute natural treatments for diseases brought on by non-tuberculous mycobacteria (NTM).
These are opportunistic bacteria that are found in soil and water and are widely distributed in the environment. They are often spread via skin infection, eating or drinking infected food or water, and inhaling aerosol particles. A number of NTM strains generate biofilm, which increases their resistance to antimicrobial drugs, disinfectants, and environmental stressors [188]. Human forms caused by NTM include disseminated forms, pulmonary mycobacteriosis, skin infections, and lymphadenitis. These mycobacteria produce identical infections in a number of animal species, which makes them significant in veterinary medicine as well. Dogs and cats’ cutaneous forms are often linked to NTM species.
Conventional medication therapy is employed, although it’s not always effective. Because of this, using EOs topically may be a viable substitute; however, more research is required to determine the EOs’ additional in vivo functions in addition to their lack of toxicity. Even though M. avium is linked to TB in birds, it is still considered an NTM agent.
In vitro experiments were conducted to show the antibacterial and antibiofilm properties of Juniperus communis, Helichrysum italicum, and L. hybrida EOs against NTM, namely M. avium, M. intracellulare, and M. gordonae [188,189].
EO from Zingiber officinale, which is distinguished by a high concentration of monoterpenes and sesquiterpenes, has shown efficacy against a few NTM species, namely M. abscessus subsp. massiliense and M. chelonae [187].
Research has been conducted to confirm the in vitro efficacy of a few EOs against M. avium subsp. paratuberculosis, an NTM pathogen that causes paratuberculosis, a serious illness that causes significant economic losses in ruminants. Myrtus communis EO had poor efficacy [91], whereas oils of cinnamon and oregano showed strong action [190,191].
The infection known as M. avium subsp. paratuberculosis is found in the udders and the gastrointestinal tract. While it is unlikely that EOs might be utilized as a medication to treat ruminants afflicted with paratuberculosis, natural products, which have shown efficacy in vitro, may be used to lessen the pathogen’s ambient farm contamination.
The botanical species effective against the bacterial species considered in this manuscript are summarized in Table 3.

Table 3.
Essential oils used for the control of the main bacterial etiological agents causing diseases in zootechnical species.
5. Limitations and Future Perspective
Although there appears to be a strong link between medicinal plant extracts and antibacterial activity, the majority of current research is based on in vitro investigations; therefore, it is unclear how applicable this information would be in a clinical context. In vivo, compounds with antibacterial activity in vitro could not have much of an impact. The primary impediment to the application of medicinal plants as antimicrobials is the absence of treatment standardization. This is one of the reasons why the effectiveness of medicinal herbs is not widely believed. The link between the structure and activity of bioactive chemicals, as well as their methods of action, has largely remained unknown until recently. Standardizing treatment protocols will result from learning more about the pharmacology of substances obtained from medicinal plants [192]. There are few clinical studies assessing the efficacy of medicinal plant components for treating infectious disorders and identifying their side effects [193,194]. Standardized testing is necessary to evaluate the antimicrobial activity of pure bioactive chemicals; however, more sensitive bioassay approaches are required to test plant extracts or EOs [195]. The repeatability of plant extract composition is another significant constraint. It is well recognized that, depending on the suppliers, an extract might have various qualities. To create quality control methods, bioactive substances must be accurately characterized and authenticated [196,197]. In order to demonstrate a reliable association between in vitro effectiveness results and the therapeutic relevance of these drugs, it will be necessary to conduct in vivo research on animal models in the future [198]. For both skin and mucous membranes, topical application is currently the most promising approach [199,200]. The ability of EOs to increase the penetration of antiseptics might be used to both restore antibacterial action against resistant species and prevent surgical and medical device-related infections [201,202]. Although clinical testing is required, there is some potential for inhalation applications [203].
It is necessary to learn more about the pharmacokinetics, pharmacodynamics, and possible toxicity of EOs taken orally because there is currently little evidence about the safety of this method of administration. The acceptability of EOs or EO component supplements in animal feed has not been thoroughly studied [204]. This is typically seen when strong-tasting and odorous ingredients are used, including allicin and allyl isothiocyanate [205]. Inevitably, feed supplementation techniques (such as encapsulation) could be used to prevent these side effects.
With the application of EOs and substances like carvacrol and cinnamonaldehyde that have more agreeable sensory qualities. Due to their greater number of taste receptors, some animals, like pigs, are actually more sensitive to changes in flavor characteristics [206]. However, certain methods that make it easier to add EOs to animal feed may raise manufacturing costs, making industrial applications less feasible. Actually, for economic reasons primarily, certain manufacturers exhibit opposition to the use of EOC and other technologies. The widespread usage of antibiotics is a result of their affordability and convenience of use [204].
Compound structural alterations to enhance pharmacokinetics and pharmacodynamics, as well as evaluations of the structure-activity connection, should be included in future research. In order to elucidate the mechanism behind these compounds’ antimicrobial activity and identify other pathways that may be targeted, further research should be performed on the synergistic interactions seen in medicinal plant extracts and between substances and antibiotics. Antimicrobial drugs and medicinal plant extracts can interact in ways that are beneficial (synergism) or detrimental (antagonistic) [207]. To qualify these chemicals as biomedical agents, more study is needed, particularly on their toxicity and in vivo investigations.
6. Discussion
In the 1940s, researchers found that some antibiotics, such as oxytetracycline, promoted animal development when they were introduced for the treatment of bacterial infections in both people and animals [208]. Following research showing that the use of both oxytetracycline and chlortetracycline enhanced feed utilization, decreased mortality and morbidity from clinical and subclinical infections in animals, and increased growth rate, the Food and Drug Administration approved their use as additives in animal feed in 1951–1953 [208,209].
Sub-therapeutic tetracycline and other antibiotic administrations to farm animals have contributed to the development of antibiotic resistance in humans, according to the Swann report from 1969, which raised concerns about the use of antibiotics as growth promoters [208]. Because it is frequently linked to the animals involved in acquiring resistant enteric flora, which in turn adds to the human reservoir of antimicrobial-resistant coliforms and Salmonella, the practice has been under constant scrutiny since then because of the possible risks involved [209]. Since antibiotic resistance was discovered to be transferable more than 30 years ago, the use as growth promoters of those antibiotics for which cross-resistance has been demonstrated has been prohibited [210].
Antibiotic resistance in animal microorganisms and the establishment of antibiotic resistance in human pathogens are believed to be influenced by the use of antibiotics in veterinary medicine. There are several instances when bacteria or their genes are transferred from animals to humans via the food chain [211]. Resistance gene transfer across bacterial populations occurs via both straightforward and intricate pathways within the ecology of bacteria. Furthermore, there is evidence that resistance genes from humans have returned to the animal population, supporting the idea that resistant microorganisms may spread from animals to people. The possibility of these genes being amplified in animal populations makes this a serious problem. New antimicrobial agents need to be developed.
The pharmaceutical industry is said to generate two or three novel antibiotics from microbes annually on average [212]. As the number of novel antimicrobial medications in the research and development (R&D) pipeline has started to decline over the past 20 years, the pharmaceutical industry has grown more accepting of the potential applications of plant-derived drugs [212]. In addition, the general public is now very interested in complementary therapies like “medicinal plants” due to growing awareness of the abuse and overuse of antibiotics. An estimated 20–80% of people use and believe that botanical products are safe and beneficial in many different places around the globe. Therefore, “traditional knowledge,” or the variety of information gathered by indigenous peoples on the plant and animal items they have utilized to cure illnesses and preserve health, is of enormous interest to people all over the world [212].
In addition to boosting the biosafety of breeding stock, the advantageous impacts of Eos may also be used to substitute performance-enhancing additives, as shown by the zootechnical indices. For example, when EO extracted from Poliomintha longiflora (400 mg L−1) was added to chicken feed, Hernández-Coronado et al. (2019) [213] demonstrated improved sensory evaluation of chicken meat. When broiler feed was supplemented with 200 mg kg−1 of nanoencapsulated cumin EO with chitosan, it produced better body weight gain (BWG) and feed conversion ratio than 650 mg kg−1 of flavomycin [214]. Eucalyptus globulus was able to lower the E. coli population and encourage the formation of beneficial bacteria. Additionally, it boosted the digestibility of organic matter, which can enhance nutritional absorption, lower blood cholesterol, and boost superoxide dismutase activity. While cholesterol reduction improved the meat’s nutritional profile and increased BWG and feed conversion, superoxide dismutase’s inhibition of free radicals increased antioxidant activity [215].
A combination of 300 mg/day of Eos from Thymus kotschyanus, Lavandula angustifolia, Salvia officinalis, and Capparis spinosa was used in another experiment on calves. The results demonstrated an improvement in animal performance (59.1 kg final body weight for the control group and 62.3 kg final body weight for the EO group) because of the antioxidant and bactericidal properties of the mixture. The information provided suggests that it is quite likely that EOs will be used in the creation of biotechnological substitutes for traditional therapies. In addition to having possible antibacterial properties, the use of EOs in chicken diets is of interest as it seems to enhance egg quality [216].
Moreover, EOs are incredibly adaptable substances that have the ability to function as immunomodulators, detoxifiers, and performance boosters. Research should be done to confirm the adverse effects of administering EOs in vivo. Cytotoxicity and therapeutic index assessments must come first. Dušan et al. (2006) [217] proved the toxicology of several EOs towards intestinal cell viability. Dosage dependence was seen in the antimicrobial activity of certain plant extracts against enteroinvasive E. coli. In vitro cultures of intestinal-like cells revealed comparatively substantial cytotoxicity to EO concentrations that could totally block bacterial growth (0.05%). Reduced EO concentrations (0.01%) only partially inhibited microbes and had a negligible negative impact on Caco-2 cells. The essential oils of clove and cinnamon, as well as their main component, eugenol, had almost no cytotoxic impact at lower concentrations, according to an assessment of cell deathbased on morphological and viability staining followed by fluorescence microscopy. Despite a small increase in the incidence of apoptotic cell death, oregano essential oil and its constituent carvacrol demonstrated broad antibacterial action even at lower doses. Thyme oil showed a relatively significant level of cytotoxicity, increasing the incidence of both necrotic and apoptotic cell death. On the other hand, the constituent thymol has shown neither cytotoxic impact nor significantly diminished capacity to impede the pathogen’s apparent development at the employed levels [217].
7. Conclusions
There is a wealth of scientific data supporting traditional knowledge on the antibacterial properties of plant extracts. A simple search using the PubMed database reveals hundreds of publications in the scientific and medical literature describing the antibacterial properties of various plant species and their chemical components. Furthermore, 6345 plant species show experimental antibacterial activity, according to a search of the Napralert database, which is a database of natural products maintained by the University of Illinois at Chicago and contains 58,725 plant species. It is very likely that plant-based antimicrobial agents can replace traditional antibiotics. In this respect, EOs can also be used to reduce drug resistance. For example, some attempts have been made to improve or restore antibacterial efficacy against multidrug-resistant germs. Antimicrobial MICs can be reduced by adding essential oils to antibiotics; aminoglycosides, including amikacin, have shown the greatest reduction in MICs [218]. Their use has also been shown to restore drug sensitivity in strains that overexpress efflux pumps [143]. The way Eos modify drug resistance is particularly evident when it comes to drugs such as fluoroquinolones, β-lactams, and chloramphenicol. This manuscript, therefore, is particularly interesting as a catalyst for a new green pharmacology that triggers virtuous practices useful for what is often referred to as “One-health”.
Author Contributions
Conceptualization, C.L., F.C. and R.B.; methodology, C.L., F.C., R.B., G.S., D.B. and E.P.; writing—original draft preparation C.L., F.C., R.B., M.D.N., L.Z., M.M., G.S., B.T., P.R., D.B. and E.P.; writing—review and editing, C.L., F.C., R.B., M.D.N., L.Z., M.M., B.T., P.R., G.S., D.B. and E.P.; supervision, G.S., P.R., D.B. and E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bruinsma, J. World Agriculture: Towards 2015/2030: An FAO Study; Routledge: London, UK, 2017; ISBN 131508385X. [Google Scholar]
- Kousar, S.; Rehman, N.; Javed, A.; Hussain, A.; Naeem, M.; Masood, S.; Ali, H.A.; Manzoor, A.; Khan, A.A.; Akrem, A. Intensive poultry farming practices influence antibiotic resistance profiles in Pseudomonas aeruginosa inhabiting nearby soils. Infect. Drug Resist. 2021, 14, 4511–4516. [Google Scholar] [CrossRef] [PubMed]
- Fontana, C.; Patrone, V.; Lopez, C.M.; Morelli, L.; Rebecchi, A. Incidence of tetracycline and erythromycin resistance in meat-associated bacteria: Impact of different livestock management strategies. Microorganisms 2021, 9, 2111. [Google Scholar] [CrossRef] [PubMed]
- Sivaramalingam, T.; McEwen, S.A.; Pearl, D.L.; Ojkic, D.; Guerin, M.T. A temporal study of Salmonella serovars from environmental samples from poultry breeder flocks in Ontario between 1998 and 2008. Can. J. Vet. Res. 2013, 77, 1–11. [Google Scholar] [PubMed]
- Kore, K.; Asrade, B.; Demissie, K.; Aragaw, K. Characterization of Salmonella isolated from apparently healthy slaughtered cattle and retail beef in Hawassa, southern Ethiopia. Prev. Vet. Med. 2017, 147, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Nidaullah, H.; Abirami, N.; Shamila-Syuhada, A.K.; Chuah, L.-O.; Nurul, H.; Tan, T.P.; Abidin, F.W.Z.; Rusul, G. Prevalence of Salmonella in poultry processing environments in wet markets in Penang and Perlis, Malaysia. Vet. World 2017, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014; ISBN 9241564741. [Google Scholar]
- McAllister, T.A.; Wang, Y.; Diarra, M.S.; Alexander, T.; Stanford, K. Challenges of a one-health approach to the development of alternatives to antibiotics. Anim. Front. 2018, 8, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, W.; Thomas, L.F.; Coyne, L.; Rushton, J. Mitigating the risks posed by intensification in livestock production: The examples of antimicrobial resistance and zoonoses. Animal 2021, 15, 100123. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Li, W.; Lu, X.; Liu, B.; Wang, J. The residues and environmental risks of multiple veterinary antibiotics in animal faeces. Environ. Monit. Assess. 2013, 185, 2211–2220. [Google Scholar] [CrossRef]
- Klatte, S.; Schaefer, H.-C.; Hempel, M. Pharmaceuticals in the environment—A short review on options to minimize the exposure of humans, animals and ecosystems. Sustain. Chem. Pharm. 2017, 5, 61–66. [Google Scholar] [CrossRef]
- Pinto Ferreira, J.; Gochez, D.; Jeannin, M.; Magongo, M.W.; Loi, C.; Bucher, K.; Moulin, G.; Erlacher-Vindel, E. From OIE standards to responsible and prudent use of antimicrobials: Supporting stewardship for the use of antimicrobial agents in animals. JAC-Antimicrob. Resist. 2022, 4, dlac017. [Google Scholar] [CrossRef]
- Kools, S.A.E.; Moltmann, J.F.; Knacker, T. Estimating the use of veterinary medicines in the European Union. Regul. Toxicol. Pharmacol. 2008, 50, 59–65. [Google Scholar] [CrossRef]
- Castagna, F.; Palma, E.; Cringoli, G.; Bosco, A.; Nisticò, N.; Caligiuri, G.; Britti, D.; Musella, V. Use of complementary natural feed for gastrointestinal nematodes control in sheep: Effectiveness and benefits for animals. Animals 2019, 9, 1037. [Google Scholar] [CrossRef]
- Jahanabadi, E.A.; Mousavi, S.N.; Moosavihaghighi, M.H.; Eslami, M.R. Consumers’ willingness to pay for antibiotic-free chicken meat: Application of contingent valuation method. Environ. Dev. Sustain. 2023, 1–22. [Google Scholar] [CrossRef]
- Rello, J.; Parisella, F.R.; Perez, A. Alternatives to antibiotics in an era of difficult-to-treat resistance: New insights. Expert Rev. Clin. Pharmacol. 2019, 12, 635–642. [Google Scholar] [CrossRef]
- Allen, H.K.; Trachsel, J.; Looft, T.; Casey, T.A. Finding alternatives to antibiotics. Ann. N. Y. Acad. Sci. 2014, 1323, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Joerger, R.D. Alternatives to antibiotics: Bacteriocins, antimicrobial peptides and bacteriophages. Poult. Sci. 2003, 82, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Hume, M.E. Historic perspective: Prebiotics, probiotics, and other alternatives to antibiotics. Poult. Sci. 2011, 90, 2663–2669. [Google Scholar] [CrossRef] [PubMed]
- Colalto, C. What phytotherapy needs: Evidence-based guidelines for better clinical practice. Phyther. Res. 2018, 32, 413–425. [Google Scholar] [CrossRef]
- Viegi, L.; Pieroni, A.; Guarrera, P.M.; Vangelisti, R. A review of plants used in folk veterinary medicine in Italy as basis for a databank. J. Ethnopharmacol. 2003, 89, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Huffman, M.A. Animal self-medication and ethno-medicine: Exploration and exploitation of the medicinal properties of plants. Proc. Nutr. Soc. 2003, 62, 371–381. [Google Scholar] [CrossRef]
- Mayer, M.; Vogl, C.R.; Amorena, M.; Hamburger, M.; Walkenhorst, M. Treatment of organic livestock with medicinal plants: A systematic review of European ethnoveterinary research. Forsch. Komplementärmedizin/Res. Complement. Med. 2014, 21, 375–386. [Google Scholar] [CrossRef]
- Smith, R.L.; Cohen, S.M.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Portoghese, P.S.; Waddell, W.J.; Wagner, B.M.; Hall, R.L. A procedure for the safety evaluation of natural flavor complexes used as ingredients in food: Essential oils. Food Chem. Toxicol. 2005, 43, 345–363. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Piras, C.; Palma, E.; Cringoli, G.; Musolino, V.; Lupia, C.; Perri, M.R.; Statti, G.; Britti, D.; et al. In vitro evaluation of acute toxicity of five Citrus spp. Essential oils towards the parasitic mite Varroa destructor. Pathogens 2021, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Castagna, F.; Bava, R.; Piras, C.; Carresi, C.; Musolino, V.; Lupia, C.; Marrelli, M.; Conforti, F.; Palma, E.; Britti, D. Green Veterinary Pharmacology for Honey Bee Welfare and Health: Origanum heracleoticum L. (Lamiaceae) Essential Oil for the Control of the Apis mellifera Varroatosis. Vet. Sci. 2022, 9, 124. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Palma, E.; Musolino, V.; Carresi, C.; Cardamone, A.; Lupia, C.; Marrelli, M.; Conforti, F.; Roncada, P. Phytochemical Profile of Foeniculum vulgare Subsp. piperitum Essential Oils and Evaluation of Acaricidal Efficacy against Varroa destructor in Apis mellifera by In Vitro and Semi-Field Fumigation Tests. Vet. Sci. 2022, 9, 684. [Google Scholar] [PubMed]
- Bava, R.; Castagna, F.; Palma, E.; Marrelli, M.; Conforti, F.; Musolino, V.; Carresi, C.; Lupia, C.; Ceniti, C.; Tilocca, B. Essential Oils for a Sustainable Control of Honeybee Varroosis. Vet. Sci. 2023, 10, 308. [Google Scholar] [CrossRef]
- Štrbac, F.; Bosco, A.; Maurelli, M.P.; Ratajac, R.; Stojanović, D.; Simin, N.; Orčić, D.; Pušić, I.; Krnjajić, S.; Sotiraki, S.; et al. Anthelmintic Properties of Essential Oils to Control Gastrointestinal Nematodes in Sheep—In Vitro and In Vivo Studies. Vet. Sci. 2022, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Štrbac, F.; Bosco, A.; Pušić, I.; Stojanović, D.; Simin, N.; Cringoli, G.; Rinaldi, L.; Ratajac, R. The use of essential oils against sheep gastrointestinal nematodes. Anim. Health Perspect. 2022, 1, 86–94. [Google Scholar] [CrossRef]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Fazly Bazzaz, B.S.; Haghi, M.M. Antibacterial activity of total extracts and essential oil of Nigella sativa L. seeds in mice. Pharmacologyonline 2007, 2, 429–435. [Google Scholar]
- Franz, C.; Baser, K.H.C.; Windisch, W. Essential oils and aromatic plants in animal feeding—A European perspective. A review. Flavour Fragr. J. 2010, 25, 327–340. [Google Scholar] [CrossRef]
- Mucha, W.; Witkowska, D. The applicability of essential oils in different stages of production of animal-based foods. Molecules 2021, 26, 3798. [Google Scholar] [CrossRef]
- Council of Europe; European Pharmacopoeia Commission; European Directorate for the Quality of Medicines & Healthcare. European Pharmacopoeia; Council of Europe: London, UK, 2010; Volume 1, ISBN 9287167001. [Google Scholar]
- Srivastava, A.K.; Singh, V.K. Biological action of essential oils (terpenes). Int. J. Biol. Med. Res. 2019, 10, 6854–6859. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Tongnuanchan, P.; Benjakul, S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Chang, H.Y.; Cheng, T.H.; Wang, A.H.J. Structure, catalysis, and inhibition mechanism of prenyltransferase. IUBMB Life 2020, 73, 40–63. [Google Scholar] [CrossRef]
- Oldfield, E.; Lin, F.Y. Terpene biosynthesis: Modularity rules. Angew. Chem. Int. Ed. 2012, 51, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential oils and their major components: An updated review on antimicrobial activities, mechanism of action and their potential application in the food industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Gayán, E.; Torres, J.A.; Paredes-Sabja, D. Hurdle Approach to Increase the Microbial Inactivation by High Pressure Processing: Effect of Essential Oils. Food Eng. Rev. 2012, 4, 141–148. [Google Scholar] [CrossRef]
- Dorman, H.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Azhdarzadeh, F.; Hojjati, M. Chemical composition and antimicrobial activity of leaf, ripe and unripe peel of bitter orange (Citrus aurantium) essential oils. Nutr. Food Sci. Res. 2016, 3, 43–50. [Google Scholar] [CrossRef]
- Huang, D.F.; Xu, J.-G.; Liu, J.-X.; Zhang, H.; Hu, Q.P. Chemical constituents, antibacterial activity and mechanism of action of the essential oil from Cinnamomum cassia bark against four food-related bacteria. Microbiology 2014, 83, 357–365. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (Tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Sharma, A.; Baek, K.H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Lv, F.; Liang, H.; Yuan, Q.; Li, C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res. Int. 2011, 44, 3057–3064. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Zhou, H.; Zhao, C.; Lin, L. Antimicrobial activity and mechanisms of Salvia sclarea essential oil. Bot. Stud. 2015, 56, 16. [Google Scholar] [CrossRef]
- Macwan, S.R.; Dabhi, B.K.; Aparnathi, K.D.; Prajapati, J.B. Essential Oils of Herbs and Spices: Their Antimicrobial Activity and Application in Preservation of Food. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 885–901. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, H.; Lee, J.C.; Lee, Y.C.; Woo, E.-R.; Lee, D.G. Antibacterial activity of hibicuslide C on multidrug-resistant Pseudomonas aeruginosa isolates. Curr. Microbiol. 2016, 73, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Geethalakshmi, R.; Sundaramurthi, J.C.; Sarada, D.V.L. Antibacterial activity of flavonoid isolated from Trianthema decandra against Pseudomonas aeruginosa and molecular docking study of FabZ. Microb. Pathog. 2018, 121, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.; White, A.W.; Laird, K. Characterisation and screening of antimicrobial essential oil components against clinically important antibiotic-resistant bacteria using thin layer chromatography-direct bioautography hyphenated with GC-MS, LC-MS and NMR. Phytochem. Anal. 2019, 30, 121–131. [Google Scholar] [CrossRef]
- Tardugno, R.; Pellati, F.; Iseppi, R.; Bondi, M.; Bruzzesi, G.; Benvenuti, S. Phytochemical composition and in vitro screening of the antimicrobial activity of essential oils on oral pathogenic bacteria. Nat. Prod. Res. 2018, 32, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Leal, A.C.; Palou, E.; López-Malo, A.; Bach, H. Antimicrobial, cytotoxic, and anti-inflammatory activities of Pimenta dioica and Rosmarinus officinalis essential oils. Biomed Res. Int. 2019, 2019, 1639726. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Gruľová, D.; Baranová, B.; Caputo, L.; De Martino, L.; Sedlák, V.; Camele, I.; De Feo, V. Antimicrobial activity and chemical composition of essential oil extracted from Solidago canadensis L. growing wild in Slovakia. Molecules 2019, 24, 1206. [Google Scholar] [CrossRef] [PubMed]
- Holland, R.E. Some infectious causes of diarrhea in young farm animals. Clin. Microbiol. Rev. 1990, 3, 345–375. [Google Scholar] [CrossRef]
- Johnson, K.; Burn, C.C.; Wathes, D.C. Rates and risk factors for contagious disease and mortality in young dairy heifers. CABI Rev. 2011, 6, 059. [Google Scholar] [CrossRef]
- Hurst, C.J. Opportunistic bacteria associated with mammalian livestock disease. In The Connections between Ecology and Infectious Disease; Springer: Cham, Switzerland, 2018; pp. 185–238. [Google Scholar]
- Poirel, L.; Madec, J.-Y.; Lupo, A.; Schink, A.-K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 4–6. [Google Scholar] [CrossRef]
- Castro-Vargas, R.E.; Herrera-Sánchez, M.P.; Rodríguez-Hernández, R.; Rondón-Barragán, I.S. Antibiotic resistance in Salmonella spp. isolated from poultry: A global overview. Vet. World 2020, 13, 2070. [Google Scholar] [CrossRef]
- Bouza, E.; Cercenado, E. Klebsiella and enterobacter: Antibiotic resistance and treatment implications. Semin. Respir. Infect. 2002, 17, 215–230. [Google Scholar] [CrossRef]
- Botelho, J.; Grosso, F.; Peixe, L. Antibiotic resistance in Pseudomonas aeruginosa–Mechanisms, epidemiology and evolution. Drug Resist. Updat. 2019, 44, 100640. [Google Scholar] [CrossRef]
- Luangtongkum, T.; Jeon, B.; Han, J.; Plummer, P.; Logue, C.M.; Zhang, Q. Antibiotic Resistance in Campylobacter: Emergence, Transmission and Persistence; Future Medicine: London, UK, 2009. [Google Scholar]
- Moore, J.E.; Barton, M.D.; Blair, I.S.; Corcoran, D.; Dooley, J.S.G.; Fanning, S.; Kempf, I.; Lastovica, A.J.; Lowery, C.J.; Matsuda, M. The epidemiology of antibiotic resistance in Campylobacter. Microbes Infect. 2006, 8, 1955–1966. [Google Scholar] [CrossRef]
- Foster, T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef]
- Hayes, K.; O’Halloran, F.; Cotter, L. A review of antibiotic resistance in Group B Streptococcus: The story so far. Crit. Rev. Microbiol. 2020, 46, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Haenni, M.; Lupo, A.; Madec, J.-Y. Antimicrobial resistance in Streptococcus spp. Microbiol. Spectr. 2018, 6, 10–1128. [Google Scholar] [CrossRef]
- Gillespie, S.H. Evolution of drug resistance in Mycobacterium tuberculosis: Clinical and molecular perspective. Antimicrob. Agents Chemother. 2002, 46, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Sekyere, J.O.; Reta, M.A.; Maningi, N.E.; Fourie, P.B. Antibiotic resistance of Mycobacterium tuberculosis complex in Africa: A systematic review of current reports of molecular epidemiology, mechanisms and diagnostics. J. Infect. 2019, 79, 550–571. [Google Scholar] [CrossRef] [PubMed]
- Bashahun, G.M.; Amina, A. Colibacillosis in calves: A review of literature. J. Anim. Sci. Vet. Med. 2017, 2, 62–71. [Google Scholar] [CrossRef]
- Blum, S.E.; Heller, E.D.; Leitner, G. Long term effects of Escherichia coli mastitis. Vet. J. 2014, 201, 72–77. [Google Scholar] [CrossRef]
- Saif, Y.M. Diseases of Poultry; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 0813807239. [Google Scholar]
- Dziva, F.; Stevens, M.P. Colibacillosis in poultry: Unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathol. 2008, 37, 355–366. [Google Scholar] [CrossRef]
- Berchieri, A.J.; Murphy, C.K.; Marston, K.; Barrow, P.A. Observations on the persistence and vertical transmission of Salmonella enterica serovars Pullorum and Gallinarum in chickens: Effect of bacterial and host genetic background. Avian Pathol. 2001, 30, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Rhayour, K.; Bouchikhi, T.; Tantaoui-Elaraki, A.; Sendide, K.; Remmal, A. The mechanism of bactericidal action of oregano and clove essential oils and of their phenolic major components on Escherichia coli and Bacillus subtilis. J. Essent. Oil Res. JEOR 2003, 15, 356. [Google Scholar] [CrossRef]
- Burt, S.A.; Reinders, R.D. Antibacterial activity of selected plant essential oils against Escherichia coil O157:H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S.Y. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Duarte, M.C.T.; Leme, E.E.; Delarmelina, C.; Soares, A.A.; Figueira, G.M.; Sartoratto, A. Activity of essential oils from Brazilian medicinal plants on Escherichia coli. J. Ethnopharmacol. 2007, 111, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Bachir, R.G.; Benali, M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac. J. Trop. Biomed. 2012, 2, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Romero, J.C.; González-Ríos, H.; Borges, A.; Simões, M. Antibacterial Effects and Mode of Action of Selected Essential Oils Components against Escherichia coli and Staphylococcus aureus. Evid.-Based Complement. Altern. Med. 2015, 2015, 795435. [Google Scholar] [CrossRef]
- Thielmann, J.; Muranyi, P.; Kazman, P. Screening essential oils for their antimicrobial activities against the foodborne pathogenic bacteria Escherichia coli and Staphylococcus aureus. Heliyon 2019, 5, e01860. [Google Scholar] [CrossRef]
- Galgano, M.; Capozza, P.; Pellegrini, F.; Cordisco, M.; Sposato, A.; Sblano, S.; Camero, M.; Lanave, G.; Fracchiolla, G.; Corrente, M.; et al. Antimicrobial Activity of Essential Oils Evaluated In Vitro against Escherichia coli and Staphylococcus aureus. Antibiotics 2022, 11, 979. [Google Scholar] [CrossRef]
- Ebani, V.V.; Najar, B.; Bertelloni, F.; Pistelli, L.; Mancianti, F.; Nardoni, S. Chemical composition and in vitro antimicrobial efficacy of sixteen essential oils against Escherichia coli and Aspergillus fumigatus isolated from poultry. Vet. Sci. 2018, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Li, W.R.; Shi, Q.S.; Liang, Q.; Xie, X.B.; Huang, X.M.; Chen, Y. Ben Antibacterial activity and kinetics of Litsea cubeba oil on Escherichia coli. PLoS ONE 2014, 9, e110983. [Google Scholar] [CrossRef]
- Shah, G.; Shri, R.; Panchal, V.; Sharma, N.; Singh, B.; Mann, A.S. Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (Lemon grass). J. Adv. Pharm. Technol. Res. 2011, 2, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Goudjil, M.B.; Ladjel, S.; Bencheikh, S.E.; Zighmi, S.; Hamada, D. Chemical composition, antibacterial and antioxidant activities of the essential oil extracted from the Mentha piperita of southern Algeria. Res. J. Phytochem. 2015, 9, 79–87. [Google Scholar] [CrossRef]
- Işcan, G.; Kirimer, N.; Kürkcüoǧlu, M.; Başer, K.H.C.; Demirci, F. Antimicrobial screening of Mentha piperita essential oils. J. Agric. Food Chem. 2002, 50, 3943–3946. [Google Scholar] [CrossRef] [PubMed]
- Rasooli, I.; Gachkar, L.; Yadegarinia, D.; Rezaei, M.B.; Astaneh, S.D.A. Antibacterial and antioxidative characterisation of essential oils from Mentha piperita and Mentha spicata grown in Iran. Acta Aliment. 2008, 37, 41–52. [Google Scholar] [CrossRef]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Marshall, V.; Hamidpour, R. Pelargonium graveolens (Rose geranium)—A Novel Therapeutic Agent for Antibacterial, Antioxidant, Antifungal and Diabetics. Arch. Cancer Res. 2017, 5, 134. [Google Scholar] [CrossRef]
- Stefan, M.; Zamfirache, M.M.; Padurariu, C.; Trutǎ, E.; Gostin, I. The composition and antibacterial activity of essential oils in three Ocimum species growing in Romania. Cent. Eur. J. Biol. 2013, 8, 600–608. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Baek, K.H.; Kang, S.C. Control of Salmonella in foods by using essential oils: A review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- Sojka, W.J.; Wray, C.; Shreeve, J.; Benson, A.J. Incidence of salmonella infection in animals in England and Wales, 1968–1974. J. Hyg. 1977, 78, 43–56. [Google Scholar] [CrossRef]
- Parry, C.M. Salmonella Infections: Clinical, Immunological and Molecular Aspects; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Calo, J.R.; Baker, C.A.; Park, S.H.; Ricke, S.C. Salmonella Heidelberg Strain Responses to Essential Oil Components. J. Food Res. 2015, 4, 73. [Google Scholar] [CrossRef]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Tosi, G.; Massi, P.; Pistelli, L.; Mancianti, F. In vitro antimicrobial activity of essential oils against Salmonella enterica serotypes enteritidis and typhimurium strains isolated from poultry. Molecules 2019, 24, 900. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Negi, P.S.; Jena, B.S.; Jagan Mohan Rao, L. Antioxidant and antimutagenic activities of Cinnamomum zeylanicum fruit extracts. J. Food Compos. Anal. 2007, 20, 330–336. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Betts, G.; Hoskins, N.; Edwards, M.; Ercolini, D.; Mauriello, G. Membrane toxicity of antimicrobial compounds from essential oils. J. Agric. Food Chem. 2007, 55, 4863–4870. [Google Scholar] [CrossRef]
- Dhouioui, M.; Boulila, A.; Chaabane, H.; Zina, M.S.; Casabianca, H. Seasonal changes in essential oil composition of Aristolochia longa L. ssp. paucinervis Batt. (Aristolochiaceae) roots and its antimicrobial activity. Ind. Crops Prod. 2016, 83, 301–306. [Google Scholar] [CrossRef]
- Alibi, S.; Ben Selma, W.; Ramos-Vivas, J.; Smach, M.A.; Touati, R.; Boukadida, J.; Navas, J.; Ben Mansour, H. Anti-oxidant, antibacterial, anti-biofilm, and anti-quorum sensing activities of four essential oils against multidrug-resistant bacterial clinical isolates. Curr. Res. Transl. Med. 2020, 68, 59–66. [Google Scholar] [CrossRef]
- Arantes, S.M.; Piçarra, A.; Guerreiro, M.; Salvador, C.; Candeias, F.; Caldeira, A.T.; Martins, M.R. Toxicological and pharmacological properties of essential oils of Calamintha nepeta, Origanum virens and Thymus mastichina of Alentejo (Portugal). Food Chem. Toxicol. 2019, 133, 110747. [Google Scholar] [CrossRef]
- Al-Sayed, E. Unearthing the chemical composition of Taxodium distichum (L.) Rich. leaf essential oil and its antimicrobial activity. Ind. Crops Prod. 2018, 126, 76–82. [Google Scholar] [CrossRef]
- Liu, T.; Lin, P.; Bao, T.; Ding, Y.; Lha, Q.; Nan, P.; Huang, Y.; Gu, Z.; Zhong, Y. Essential oil composition and antimicrobial activity of Artemisia dracunculus L. var. qinghaiensis Y. R. Ling (Asteraceae) from Qinghai-Tibet Plateau. Ind. Crops Prod. 2018, 125, 1–4. [Google Scholar] [CrossRef]
- Santos, J.D.C.; Coelho, E.; Silva, R.; Passos, C.P.; Teixeira, P.; Henriques, I.; Coimbra, M.A. Chemical composition and antimicrobial activity of Satureja montana byproducts essential oils. Ind. Crops Prod. 2019, 137, 541–548. [Google Scholar] [CrossRef]
- Meenu, M.; Padhan, B.; Patel, M.; Patel, R.; Xu, B. Antibacterial activity of essential oils from different parts of plants against Salmonella and Listeria spp. Food Chem. 2023, 404, 134723. [Google Scholar] [CrossRef]
- dos S. Melo-Nascimento, A.O.; Treumann, C.; Neves, C.; Andrade, E.; Andrade, A.C.; Edwards, R.; Dinsdale, E.; Bruce, T. Functional characterization of ligninolytic Klebsiella spp. strains associated with soil and freshwater. Arch. Microbiol. 2018, 200, 1267–1278. [Google Scholar]
- Santajit, S.; Indrawattana, N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, H.; Qin, L.; Pang, Z.; Qin, T.; Ren, H.; Pan, Z.; Zhou, J. Frequency, antimicrobial resistance and genetic diversity of Klebsiella pneumoniae in food samples. PLoS ONE 2016, 11, e0153561. [Google Scholar] [CrossRef]
- Kikuchi, N.; Kagota, C.; Nomura, T.; Hiramune, T.; Takahashi, T.; Yanagawa, R. Plasmid profiles of Klebsiella pneumoniae isolated from bovine mastitis. Vet. Microbiol. 1995, 47, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Saishu, N.; Ozaki, H.; Murase, T. CTX-M-type extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolated from cases of bovine mastitis in Japan. J. Vet. Med. Sci. 2014, 76, 1153–1156. [Google Scholar] [CrossRef]
- Nordmann, P.; Cuzon, G.; Naas, T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 2009, 9, 228–236. [Google Scholar] [CrossRef]
- Vuotto, C.; Longo, F.; Balice, M.P.; Donelli, G.; Varaldo, P.E. Antibiotic resistance related to biofilm formation in Klebsiella pneumoniae. Pathogens 2014, 3, 743–758. [Google Scholar] [CrossRef]
- Munoz-Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Parisi, S.G.; Bartolini, A.; Santacatterina, E.; Castellani, E.; Ghirardo, R.; Berto, A.; Franchin, E.; Menegotto, N.; De Canale, E.; Tommasini, T. Prevalence of Klebsiella pneumoniae strains producing carbapenemases and increase of resistance to colistin in an Italian teaching hospital from January 2012 to December 2014. BMC Infect. Dis. 2015, 15, 224. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G.; Faleiro, M.L.; Guerreiro, A.C.; Antunes, M.D. Arbutus unedo L.: Chemical and biological properties. Molecules 2014, 19, 15799–15823. [Google Scholar] [CrossRef] [PubMed]
- Carev, I.; Maravić, A.; Ilić, N.; Čikeš Čulić, V.; Politeo, O.; Zorić, Z.; Radan, M. UPLC-MS/MS phytochemical analysis of two Croatian Cistus species and their biological activity. Life 2020, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Zucca, P.; Pintus, M.; Manzo, G.; Nieddu, M.; Steri, D.; Rinaldi, A.C. Antimicrobial, antioxidant and anti-tyrosinase properties of extracts of the Mediterranean parasitic plant Cytinus hypocistis. BMC Res. Notes 2015, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Man, A.; Santacroce, L.; Iacob, R.; Mare, A.; Man, L. Antimicrobial activity of six essential oils against a group of human pathogens: A comparative study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Milia, E.; Bullitta, S.M.; Mastandrea, G.; Szotáková, B.; Schoubben, A.; Langhansová, L.; Quartu, M.; Bortone, A.; Eick, S. Leaves and fruits preparations of Pistacia lentiscus L.: A review on the ethnopharmacological uses and implications in inflammation and infection. Antibiotics 2021, 10, 425. [Google Scholar] [CrossRef]
- Sadeghi, Z.; Yang, J.-L.; Venditti, A.; Moridi Farimani, M. A review of the phytochemistry, ethnopharmacology and biological activities of Teucrium genus (Germander). Nat. Prod. Res. 2022, 36, 5647–5664. [Google Scholar] [CrossRef]
- Maisetta, G.; Batoni, G.; Caboni, P.; Esin, S.; Rinaldi, A.C.; Zucca, P. Tannin profile, antioxidant properties, and antimicrobial activity of extracts from two Mediterranean species of parasitic plant Cytinus. BMC Complement. Altern. Med. 2019, 19, 82. [Google Scholar] [CrossRef]
- Mancini, E.; Senatore, F.; Del Monte, D.; De Martino, L.; Grulova, D.; Scognamiglio, M.; Snoussi, M.; De Feo, V. Studies on chemical composition, antimicrobial and antioxidant activities of five Thymus vulgaris L. essential oils. Molecules 2015, 20, 12016–12028. [Google Scholar] [CrossRef] [PubMed]
- Çoban, E.P.; Biyik, H.; Törün, B.; Yaman, F. Evaluation the antimicrobial effects of Pistacia terebinthus L. and Papaver rhoeas L. extracts against some pathogen microorganisms. Indian J Pharm Educ. Res. 2017, 51, 377–380. [Google Scholar] [CrossRef]
- Carlo Tenore, G.; Troisi, J.; Di Fiore, R.; Basile, A.; Novellino, E. Chemical composition, antioxidant and antimicrobial properties of Rapa Catozza Napoletana (Brassica rapa L. var. rapa DC.) seed meal, a promising protein source of Campania region (southern Italy) horticultural germplasm. J. Sci. Food Agric. 2012, 92, 1716–1724. [Google Scholar] [CrossRef]
- Iannello, C.; Bastida, J.; Bonvicini, F.; Antognoni, F.; Gentilomi, G.A.; Poli, F. Chemical composition, and in vitro antibacterial and antifungal activity of an alkaloid extract from Crinum angustum Steud. Nat. Prod. Res. 2014, 28, 704–710. [Google Scholar] [CrossRef]
- Bonvicini, F.; Mandrone, M.; Antognoni, F.; Poli, F.; Angela Gentilomi, G. Ethanolic extracts of Tinospora cordifolia and Alstonia scholaris show antimicrobial activity towards clinical isolates of methicillin-resistant and carbapenemase-producing bacteria. Nat. Prod. Res. 2014, 28, 1438–1445. [Google Scholar] [CrossRef]
- Lo Vecchio, G.; Cicero, N.; Nava, V.; Macrì, A.; Gervasi, C.; Capparucci, F.; Sciortino, M.; Avellone, G.; Benameur, Q.; Santini, A. Chemical Characterization, Antibacterial Activity, and Embryo Acute Toxicity of Rhus coriaria L. Genotype from Sicily (Italy). Foods 2022, 11, 538. [Google Scholar] [CrossRef]
- Loy, G.; Cottiglia, F.; Garau, D.; Deidda, D.; Pompei, R.; Bonsignore, L. Chemical composition and cytotoxic and antimicrobial activity of Calycotome villosa (Poiret) link leaves. Farm. 2001, 56, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Di Cerbo, A.; Aloisi, P.; Manelli, M.; Pellesi, V.; Provenzano, C.; Camellini, S.; Messi, P.; Sabia, C. In vitro activity of essential oils against planktonic and biofilm cells of extended-spectrum β-lactamase (ESBL)/carbapenamase-producing gram-negative bacteria involved in human nosocomial infections. Antibiotics 2020, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Pl’uchtová, M.; Gervasi, T.; Benameur, Q.; Pellizzeri, V.; Grul’ová, D.; Campone, L.; Sedlák, V.; Cicero, N. Antimicrobial activity of two Mentha species essential oil and its dependence on different origin and chemical diversity. Nat. Prod. Commun. 2018, 13, 1934578X1801300832. [Google Scholar] [CrossRef]
- Piras, C.; Tilocca, B.; Castagna, F.; Roncada, P.; Britti, D.; Palma, E. Plants with Antimicrobial Activity Growing in Italy: A Pathogen-Driven Systematic Review for Green Veterinary Pharmacology Applications. Antibiotics 2022, 11, 919. [Google Scholar] [CrossRef] [PubMed]
- Fadli, M.; Saad, A.; Sayadi, S.; Chevalier, J.; Mezrioui, N.-E.; Pagès, J.-M.; Hassani, L. Antibacterial activity of Thymus maroccanus and Thymus broussonetii essential oils against nosocomial infection–bacteria and their synergistic potential with antibiotics. Phytomedicine 2012, 19, 464–471. [Google Scholar] [CrossRef]
- Kasrati, A.; Jamali, C.A.; Fadli, M.; Bekkouche, K.; Hassani, L.; Wohlmuth, H.; Leach, D.; Abbad, A. Antioxidative activity and synergistic effect of Thymus saturejoides Coss. essential oils with cefixime against selected food-borne bacteria. Ind. Crops Prod. 2014, 61, 338–344. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Ultee, A.; Kets, E.P.W.; Smid, E.J. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 1999, 65, 4606–4610. [Google Scholar] [CrossRef] [PubMed]
- Helander, I.M.; Alakomi, H.L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; Von Wright, A. Characterization of the Action of Selected Essential Oil Components on Gram-Negative Bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Miladinović, D.L.; Ilić, B.S.; Kocić, B.D.; Miladinović, M.D. An in vitro antibacterial study of savory essential oil and geraniol in combination with standard antimicrobials. Nat. Prod. Commun. 2014, 9, 1934578X1400901125. [Google Scholar] [CrossRef]
- Lorenzi, V.; Muselli, A.; Bernardini, A.F.; Berti, L.; Pages, J.-M.; Amaral, L.; Bolla, J.-M. Geraniol restores antibiotic activities against multidrug-resistant isolates from gram-negative species. Antimicrob. Agents Chemother. 2009, 53, 2209–2211. [Google Scholar] [CrossRef]
- Miladinović, D.L.; Ilić, B.S.; Miladinović, L.C.; Kocić, B.D.; Ćirić, V.M.; Stankov-Jovanović, V.P.; Cvetković, O.G.; Govil, J.N.; Bhattacharya, S. Antibacterial activity of Thymus pulegioides essential oil and its synergistic potential with antibiotics: A chemometric approach. Recent Prog. Med. plants 2013, 38, 101–136. [Google Scholar]
- Potron, A.; Poirel, L.; Nordmann, P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: Mechanisms and epidemiology. Int. J. Antimicrob. Agents 2015, 45, 568–585. [Google Scholar] [CrossRef]
- Abi-Ayad, M.; Abi-Ayad, F.Z.; Lazzouni, H.A.; Rebiahi, S.A. Antibacterial Activity of Pinus halepensis Essential Oil from Algeria (Tlemcen). J. Nat. Prod. Plant Resour. 2011, 1, 33–36. [Google Scholar]
- Bouhdid, S.; Abrini, J.; Amensour, M.; Zhiri, A.; Espuny, M.J.; Manresa, A. Functional and ultrastructural changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Cinnamomum verum essential oil. J. Appl. Microbiol. 2010, 109, 1139–1149. [Google Scholar] [CrossRef]
- Kavanaugh, N.L.; Ribbeck, K. Selected antimicrobial essential oils eradicate Pseudomonas spp. and Staphylococcus aureus biofilms. Appl. Environ. Microbiol. 2012, 78, 4057–4061. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Łysakowska, M.; Denys, P.; Kowalczyk, E. The antimicrobial activity of thyme essential oil against multidrug resistant clinical bacterial strains. Microb. Drug Resist. 2012, 18, 137–148. [Google Scholar] [CrossRef]
- Ebani, V.; Nardoni, S.; Bertelloni, F.; Najar, B.; Pistelli, L.; Mancianti, F. Antibacterial and Antifungal Activity of Essential Oils against Pathogens Responsible for Otitis Externa in Dogs and Cats. Medicines 2017, 4, 21. [Google Scholar] [CrossRef]
- Kačániová, M.; Terentjeva, M.; Vukovic, N.; Puchalski, C.; Roychoudhury, S.; Kunová, S.; Klūga, A.; Tokár, M.; Kluz, M.; Ivanišová, E. The antioxidant and antimicrobial activity of essential oils against Pseudomonas spp. isolated from fish. Saudi Pharm. J. 2017, 25, 1108–1116. [Google Scholar] [CrossRef]
- CDC. Foodborne Diseases Active Surveillance Network (FoodNet): FoodNet 2015 Surveillance Report (Final Data); U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2017. Available online: https://www.cdc.gov/foodnet/reports/annual-reports-2015.html (accessed on 30 December 2023).
- Batz, M.B.; Hoffmann, S.; Morris, J.G., Jr. Ranking the disease burden of 14 pathogens in food sources in the United States using attribution data from outbreak investigations and expert elicitation. J. Food Prot. 2012, 75, 1278–1291. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Mahon, B.E.; Jones, T.F.; Griffin, P.M. An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiol. Infect. 2015, 143, 2795–2804. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Barber, R.M.; Foreman, K.J.; Ozgoren, A.A.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Abraham, J.P.; Abubakar, I.; Abu-Raddad, L.J. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: Quantifying the epidemiological transition. Lancet 2015, 386, 2145–2191. [Google Scholar] [CrossRef]
- Connerton, P.L.; Richards, P.J.; Lafontaine, G.M.; O’Kane, P.M.; Ghaffar, N.; Cummings, N.J.; Smith, D.L.; Fish, N.M.; Connerton, I.F. The effect of the timing of exposure to Campylobacter jejuni on the gut microbiome and inflammatory responses of broiler chickens. Microbiome 2018, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002, 65, 1545–1560. [Google Scholar] [CrossRef] [PubMed]
- Kurekci, C.; Padmanabha, J.; Bishop-Hurley, S.L.; Hassan, E.; Al Jassim, R.A.M.; McSweeney, C.S. Antimicrobial activity of essential oils and five terpenoid compounds against Campylobacter jejuni in pure and mixed culture experiments. Int. J. Food Microbiol. 2013, 166, 450–457. [Google Scholar] [CrossRef] [PubMed]
- El Gendy, A.N.; Leonardi, M.; Mugnaini, L.; Bertelloni, F.; Ebani, V.V.; Nardoni, S.; Mancianti, F.; Hendawy, S.; Omer, E.; Pistelli, L. Chemical composition and antimicrobial activity of essential oil of wild and cultivated Origanum syriacum plants grown in Sinai, Egypt. Ind. Crops Prod. 2015, 67, 201–207. [Google Scholar] [CrossRef]
- de Los Santos, F.S.; Donoghue, A.M.; Venkitanarayanan, K.; Dirain, M.L.; Reyes-Herrera, I.; Blore, P.J.; Donoghue, D.J. Caprylic acid supplemented in feed reduces enteric Campylobacter jejuni colonization in ten-day-old broiler chickens. Poult. Sci. 2008, 87, 800–804. [Google Scholar] [CrossRef]
- De Los Santos, F.S.; Donoghue, A.M.; Venkitanarayanan, K.; Metcalf, J.H.; Reyes-Herrera, I.; Dirain, M.L.; Aguiar, V.F.; Blore, P.J.; Donoghue, D.J. The natural feed additive caprylic acid decreases Campylobacter jejuni colonization in market-aged broiler chickens. Poult. Sci. 2009, 88, 61–64. [Google Scholar] [CrossRef]
- Arsi, K.; Donoghue, A.M.; Venkitanarayanan, K.; Kollanoor-Johny, A.; Fanatico, A.C.; Blore, P.J.; Donoghue, D.J. The efficacy of the natural plant extracts, thymol and carvacrol against Campylobacter colonization in broiler chickens. J. Food Saf. 2014, 34, 321–325. [Google Scholar] [CrossRef]
- Vitanza, L.; Maccelli, A.; Marazzato, M.; Scazzocchio, F.; Comanducci, A.; Fornarini, S.; Crestoni, M.E.; Filippi, A.; Fraschetti, C.; Rinaldi, F. Satureja montana L. essential oil and its antimicrobial activity alone or in combination with gentamicin. Microb. Pathog. 2019, 126, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Bertelloni, F.; Najar, B.; Nardoni, S.; Pistelli, L.; Mancianti, F. Antimicrobial activity of essential oils against Staphylococcus and Malassezia strains isolated from canine dermatitis. Microorganisms 2020, 8, 252. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, E.A.; Samy, A.S. Antimicrobial activity of essential oil and methanol extract from Commiphora molmol (Engl.) resin. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 1–6. [Google Scholar]
- Mahboubi, M.; Kazempour, N. The antimicrobial and antioxidant activities of Commiphora molmol extracts. Biharean Biol. 2016, 10, 131–133. [Google Scholar]
- Peton, V.; Le Loir, Y. Staphylococcus aureus in veterinary medicine. Infect. Genet. Evol. 2014, 21, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Abboud, M.; El Rammouz, R.; Jammal, B.; Sleiman, M. In vitro and in vivo antimicrobial activity of two essential oils Thymus vulgaris and Lavandula angustifolia against bovine Staphylococcus and Streptococcus mastitis pathogen. Middle East J. Agric. Res 2015, 4, 975–983. [Google Scholar]
- Cho, B.-W.; Cha, C.-N.; Lee, S.-M.; Kim, M.-J.; Park, J.-Y.; Yoo, C.-Y.; Son, S.-E.; Kim, S.; Lee, H.-J. Therapeutic effect of oregano essential oil on subclinical bovine mastitis caused by Staphylococcus aureus and Escherichia coli. Korean J. Vet. Res. 2015, 55, 253–257. [Google Scholar] [CrossRef]
- Chaudhry, N.M.A.; Tariq, P. Anti-microbial activity of Cinnamomum cassia against diverse microbial flora with its nutritional and medicinal impacts. Pakistan J. Bot. 2006, 38, 169. [Google Scholar]
- Mayaud, L.; Carricajo, A.; Zhiri, A.; Aubert, G. Comparison of bacteriostatic and bactericidal activity of 13 essential oils against strains with varying sensitivity to antibiotics. Lett. Appl. Microbiol. 2008, 47, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Potential of cinnamon (Cinnamomum verum) oil to control Streptococcus iniae infection in tilapia (Oreochromis niloticus). Fish. Sci. 2010, 76, 287–293. [Google Scholar] [CrossRef]
- Soltani, M.; Jamshidi, S.; Sharifpour, I. Streptococcosis caused by Streptococcus iniae in farmed rainbow trout (Oncorhynchys mykiss) in Iran: Biophysical characteristics and pathogenesis. Bull. Eur. Assoc. Fish Pathol. 2005, 25, 95–106. [Google Scholar]
- Agnew, W.; Barnes, A.C. Streptococcus iniae: An aquatic pathogen of global veterinary significance and a challenging candidate for reliable vaccination. Vet. Microbiol. 2007, 122, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Eldar, A.; Bejerano, Y.; Livoff, A.; Horovitcz, A.; Bercovier, H. Experimental streptococcal meningo-encephalitis in cultured fish. Vet. Microbiol. 1995, 43, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Salazar, S.; Oliver, C.; Yáñez, A.J.; Avendaño-Herrera, R. Comparative analysis of innate immune responses to Streptococcus phocae strains in Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2016, 51, 97–103. [Google Scholar] [CrossRef]
- Bromage, E.S.; Owens, L. Infection of barramundi Lates calcarifer with Streptococcus iniae: Effects of different routes of exposure. Dis. Aquat. Organ. 2002, 52, 199–205. [Google Scholar] [CrossRef]
- Evans, J.J.; Klesius, P.H.; Gilbert, P.M.; Shoemaker, C.A.; Al Sarawi, M.A.; Landsberg, J.; Duremdez, R.; Al Marzouk, A.; Al Zenki, S. Characterization of β-haemolytic Group B Streptococcus agalactiae in cultured seabream, Sparus auratus L., and wild mullet, Liza klunzingeri (Day), in Kuwait. J. Fish Dis. 2002, 25, 505–513. [Google Scholar] [CrossRef]
- Facklam, R.; Elliott, J.; Shewmaker, L.; Reingold, A. Identification and characterization of sporadic isolates of Streptococcus iniae isolated from humans. J. Clin. Microbiol. 2005, 43, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Tenenbaum, T.; Spellerberg, B.; Adam, R.; Vogel, M.; Kim, K.S.; Schroten, H. Streptococcus agalactiae invasion of human brain microvascular endothelial cells is promoted by the laminin-binding protein Lmb. Microbes Infect. 2007, 9, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, Y.; Maayan, M.; Wajsman, C. Streptococcus group B isolates in a regional hospital area. Med. Microbiol. Immunol. 1980, 169, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Pirbalouti, A.; Nourafcan, H.; Solyamani-Babadi, E. Variation in chemical composition and antibacterial activity of essential oils from Bakhtiari Savory (Satureja bachtiarica Bunge.). J. Essent. Oil Bear. Plants 2017, 20, 474–484. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Soltani, M.; Mirzargar, S.S.; Ghodratnama, M. Effects of eucalyptus camaldulensis, Mentha pulegium, Aloe vera essences and Chloramine T on Growth Behavior of Streptococcus iniae and Lactococcus garvieae the Causes of streptococcosis/lactococcosis in Farmed Rainbow Trout (Onchorhynchus mykiss). J. Fish. 2013, 66, 105–118. [Google Scholar]
- Wimalasena, S.H.; Pathirana, H.N.; De Silva, B.C.; Hossain, S.; Heo, G.J. Antimicrobial activity of lavender (Lavendula rangustifolia) oil against fish pathogenic bacteria isolated from cultured olive flounder (Paralichthys olivaceus) in Korea. Indian J. Fish. 2018, 65, 52–56. [Google Scholar]
- Abdelkhalek, N.K.; Risha, E.; Mohamed, A.; Salama, M.F.; Dawood, M.A.O. Antibacterial and antioxidant activity of clove oil against Streptococcus iniae infection in Nile tilapia (Oreochromis niloticus) and its effect on hepatic hepcidin expression. Fish Shellfish Immunol. 2020, 104, 478–488. [Google Scholar] [CrossRef]
- de Aguiar, F.C.; Solarte, A.L.; Tarradas, C.; Gómez-Gascón, L.; Astorga, R.; Maldonado, A.; Huerta, B. Combined effect of conventional antimicrobials with essential oils and their main components against resistant Streptococcus suis strains. Lett. Appl. Microbiol. 2019, 68, 562–572. [Google Scholar] [CrossRef]
- Baldin, V.P.; Bertin de Lima Scodro, R.; Mariano Fernandez, C.M.; Ieque, A.L.; Caleffi-Ferracioli, K.R.; Dias Siqueira, V.L.; de Almeida, A.L.; Gonçalves, J.E.; Garcia Cortez, D.A.; Cardoso, R.F. Ginger essential oil and fractions against Mycobacterium spp. J. Ethnopharmacol. 2019, 244, 112095. [Google Scholar] [CrossRef]
- Peruč, D.; Tićac, B.; Abram, M.; Broznić, D.; Štifter, S.; Staver, M.M.; Gobin, I. Synergistic potential of Juniperus communis and Helichrysum italicum essential oils against nontuberculous mycobacteria. J. Med. Microbiol. 2019, 68, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Peruč, D.; Gobin, I.; Abram, M.; Broznić, D.; Svalina, T.; Štifter, S.; Staver, M.M.; Tićac, B. Antimycobacterial potential of the juniper berry essential oil in tap water. Arh. Hig. Rada Toksikol. 2018, 69, 46–54. [Google Scholar] [CrossRef]
- Wong, S.Y.Y.; Grant, I.R.; Friedman, M.; Elliott, C.T.; Situ, C. Antibacterial activities of naturally occurring compounds against Mycobacterium avium subsp. paratuberculosis. Appl. Environ. Microbiol. 2008, 74, 5986–5990. [Google Scholar] [CrossRef]
- Nowotarska, S.W.; Nowotarski, K.; Grant, I.R.; Elliott, C.T.; Friedman, M.; Situ, C. Mechanisms of antimicrobial action of cinnamon and oregano oils, cinnamaldehyde, carvacrol, 2,5-dihydroxybenzaldehyde, and 2-hydroxy-5-methoxybenzaldehyde against Mycobacterium avium subsp. Paratuberculosis (Map). Foods 2017, 6, 82. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, B.C.; Zhang, Y.X. Clinical and experimental study on treatment of infantile mycotic enteritis by jiechang mixture. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi = Chin. J. Integr. Tradit. West. Med. 2001, 21, 419–421. [Google Scholar]
- Solórzano-Santos, F.; Miranda-Novales, M.G. Essential oils from aromatic herbs as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 136–141. [Google Scholar] [CrossRef]
- Yang, X.; Chen, D.; Chai, L.; Duan, H.; Guo, H.; Li, S.; Xiao, M.; Chen, H. A case report of poisoning caused by incorrect use of Salvia. Am. J. Case Rep. 2016, 17, 580. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mann, C.M.; Markham, J.L. A new method for determining the minimum inhibitory concentration of essential oils. J. Appl. Microbiol. 1998, 84, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Raclariu, A.C.; Heinrich, M.; Ichim, M.C.; de Boer, H. Benefits and limitations of DNA barcoding and metabarcoding in herbal product authentication. Phytochem. Anal. 2018, 29, 123–128. [Google Scholar] [CrossRef] [PubMed]
- McGrady, B.; Ho, C.S. Identifying gaps in international food safety regulation. Food Drug LJ 2011, 66, 183. [Google Scholar]
- Huie, C.W. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal. Bioanal. Chem. 2002, 373, 23–30. [Google Scholar] [CrossRef]
- Van Vuuren, S.F.; du Toit, L.C.; Parry, A.; Pillay, V.; Choonara, Y.E. Encapsulation of essential oils within a polymeric liposomal formulation for enhancement of antimicrobial efficacy. Nat. Prod. Commun. 2010, 5, 1934578X1000500912. [Google Scholar] [CrossRef]
- Low, W.L.; Martin, C.; Hill, D.J.; Kenward, M.A. Antimicrobial efficacy of silver ions in combination with tea tree oil against Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans. Int. J. Antimicrob. Agents 2011, 37, 162–165. [Google Scholar] [CrossRef]
- Karpanen, T.J.; Conway, B.R.; Worthington, T.; Hilton, A.C.; Elliott, T.S.J.; Lambert, P.A. Enhanced chlorhexidine skin penetration with eucalyptus oil. BMC Infect. Dis. 2010, 10, 278. [Google Scholar] [CrossRef]
- Warnke, P.H.; Becker, S.T.; Podschun, R.; Sivananthan, S.; Springer, I.N.; Russo, P.A.J.; Wiltfang, J.; Fickenscher, H.; Sherry, E. The battle against multi-resistant strains: Renaissance of antimicrobial essential oils as a promising force to fight hospital-acquired infections. J. Cranio-Maxillofacial Surg. 2009, 37, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Rodrigures, F.F.G.; Coutinho, H.D.M. Enhancement of the antibiotic activity of gentamicin by volatile compounds of Zanthoxylum articulatum. Indian J. Med. Res. 2010, 131, 833–835. [Google Scholar]
- Evangelista, A.G.; Corrêa, J.A.F.; Pinto, A.C.S.M.; Luciano, F.B. The impact of essential oils on antibiotic use in animal production regarding antimicrobial resistance—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 5267–5283. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.N.; Walczak, M.; Skrzypczak-Zielińska, M.; Jeleń, H.H. Bitter taste of Brassica vegetables: The role of genetic factors, receptors, isothiocyanates, glucosinolates, and flavor context. Crit. Rev. Food Sci. Nutr. 2018, 58, 3130–3140. [Google Scholar] [CrossRef] [PubMed]
- Roura, E.; Fu, M. Taste, nutrient sensing and feed intake in pigs (130 years of research: Then, now and future). Anim. Feed Sci. Technol. 2017, 233, 3–12. [Google Scholar] [CrossRef]
- Chou, T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- DuPont, H.L.; Steele, J.H. The human health implication of the use of antimicrobial agents in animal feeds. Vet. Q. 1987, 9, 309–320. [Google Scholar] [CrossRef]
- Witte, W.; Klare, I.; Werner, G. Selective pressure by antibiotics as feed additives. Infection 1999, 27 (Suppl. S2), S35–S38. [Google Scholar] [CrossRef]
- Teale, C.J. Antimicrobial resistance and the food chain. J. Appl. Microbiol. 2002, 92, 85S–89S. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Hernández-Coronado, A.C.; Silva-Vázquez, R.; Rangel-Nava, Z.E.; Hernández-Martínez, C.A.; Kawas-Garza, J.R.; Hume, M.E.; Méndez-Zamora, G. Mexican oregano essential oils given in drinking water on performance, carcass traits, and meat quality of broilers. Poult. Sci. 2019, 98, 3050–3058. [Google Scholar] [CrossRef]
- Amiri, N.; Afsharmanesh, M.; Salarmoini, M.; Meimandipour, A.; Hosseini, S.A.; Ebrahimnejad, H. Nanoencapsulation (in vitro and in vivo) as an efficient technology to boost the potential of garlic essential oil as alternatives for antibiotics in broiler nutrition. Animal 2021, 15, 100022. [Google Scholar] [CrossRef]
- Mohebodini, H.; Jazi, V.; Ashayerizadeh, A.; Toghyani, M.; Tellez-Isaias, G. Productive parameters, cecal microflora, nutrient digestibility, antioxidant status, and thigh muscle fatty acid profile in broiler chickens fed with Eucalyptus globulus essential oil. Poult. Sci. 2021, 100, 100922. [Google Scholar] [CrossRef]
- Krishan, G.; Narang, A. Use of essential oils in poultry nutrition: A new approach. J. Adv. Vet. Anim. Res. 2014, 1, 156. [Google Scholar] [CrossRef]
- Dušan, F.; Marián, S.; Katarína, D.; Dobroslava, B. Essential oils-their antimicrobial activity against Escherichia coli and effect on intestinal cell viability. Toxicol. Vitr. 2006, 20, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Sousa, E.O.; Silva, N.F.; Rodrigues, F.F.G.; Campos, A.R.; Lima, S.G.; Costa, J.G.M. Chemical composition and resistance-modifying effect of the essential oil of Lantana camara Linn. Pharmacogn. Mag. 2010, 6, 79. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).