Abstract
Optimizing antibiotic therapy is imperative with rising bacterial resistance and high infection mortality. Extended infusion defined as a continuous infusion (COI) or prolonged infusion (PI) of beta-lactams and glycopeptides might improve efficacy and safety compared to their intermittent administration (IA). This study aimed to evaluate the efficacy and safety of extended infusion in pediatric patients. Adhering to Cochrane standards, we conducted a systematic review with meta-analysis investigating the efficacy and safety of COI (24 h/d) and PI (>1 h/dose) compared to IA (≤1 h/dose) of beta-lactams and glycopeptides in pediatrics. Primary outcomes included mortality, clinical success, and microbiological eradication. Five studies could be included for the outcome mortality, investigating meropenem, piperacillin/tazobactam, cefepime, or combinations of these. The pooled relative risk estimate was 0.48 (95% CI 0.26–0.89, p = 0.02). No significant differences between the administration modes were found for the outcomes of clinical success, microbiological eradication (beta-lactams; glycopeptides), and mortality (glycopeptides). No study reported additional safety issues, e.g., adverse drug reactions when using COI/PI vs. IA. Our findings suggest that the administration of beta-lactams by extended infusion leads to a reduction in mortality for pediatric patients.
1. Introduction
The global rise in antibiotic resistance due to misuse and overuse of antibiotics [1] complicates infection treatment, potentially leading to treatment failure [2,3,4,5,6], while the development of new antibiotics remains limited [7]. In pediatrics, restricted antibiotic options, with contraindications for tetracyclines and fluoroquinolones [6,8], contribute to the challenge. Approximately 37% to 61% of hospitalized pediatric patients receive antibiotics, making them the most prescribed drugs in pediatrics [9]. WHO reports that multidrug-resistant bacterial infections cause 700,000 global fatalities annually, including 200,000 newborns [6]. Optimizing antibiotic treatment is crucial for improving efficacy and safety, with rapid detection of bacterial infection and the selection of effective and safe treatments being essential for reducing mortality [10,11,12].
1.1. Antibiotic Resistance
The Swiss Strategy against Antibiotic Resistance (StAR) emphasizes the importance of proper antibacterial agent use to mitigate the development of antibiotic resistance and prevent infections with resistant pathogens [1].
Appropriate usage should align with pharmacokinetic (PK) and pharmacodynamic (PD) data for efficacious pathogen eradication [13]. Consequently, hospitals globally have implemented antimicrobial stewardship programs to advocate for judicious antibiotic usage [3].
1.2. PK/PD of Beta-Lactams and Glycopeptides
Beta-lactams and glycopeptides, the predominant antibiotics in pediatrics, are time-dependent bactericidal drugs that inhibit bacterial cell wall synthesis [14,15]. Their effectiveness depends on substantiating the free drug concentration above the minimum inhibitory concentration (MIC) for bacterial growth [15,16].
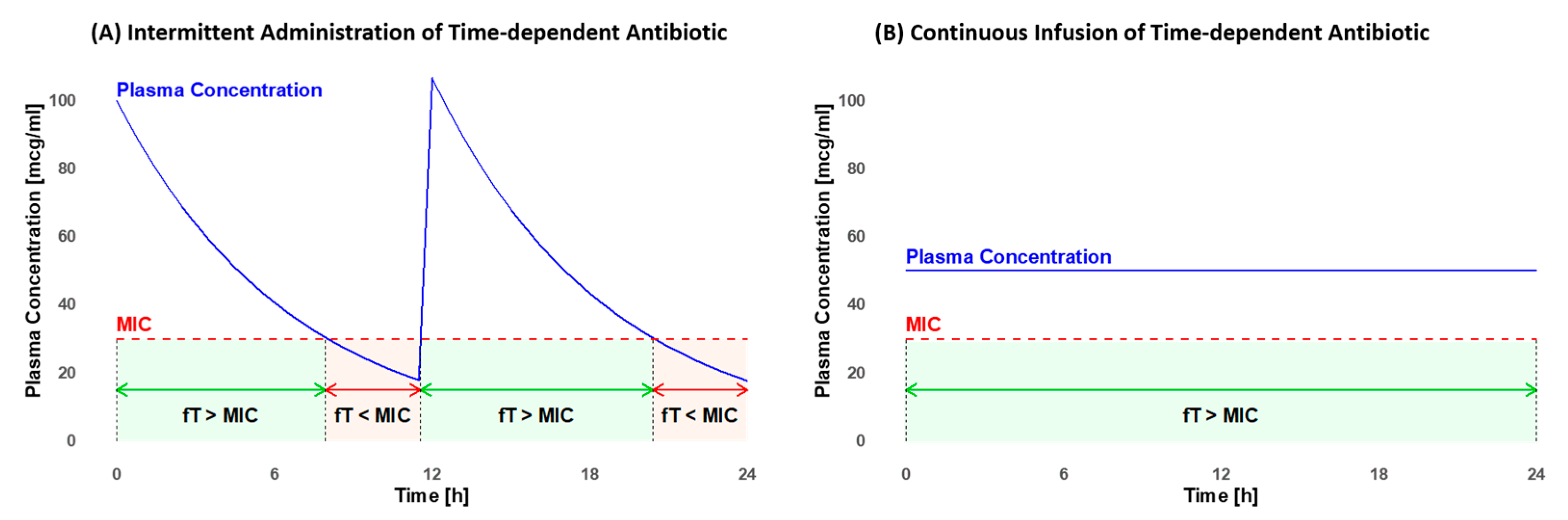
The MIC value varies depending on the pathogen and the antibiotic utilized [15]. It is crucial to maximize the fraction of time (fT) that the antibiotic concentration remains above the MIC. The minimum efficacy targets for pediatrics include fTs of >50% for penicillins, >60% for cephalosporins, and >40% for carbapenems [15]. The efficacy further increases when concentrations exceed the MIC, extending up to four times the MIC for beta-lactams [17]. For glycopeptides, the therapeutic target is an fT > MIC of 100% [15]. These objectives are frequently not achieved with the standard intravenous (i.v.) intermittent administration (IA), but they may be attainable with continuous infusion (COI) [16]. As illustrated in Figure 1, plasma concentration fluctuates with IA but remains constant with COI (own illustration). Furthermore, COI may diminish the emergence of antibiotic resistance [18], decrease the risk of inadequate antibiotic concentration, and enhance the efficacy of infection treatment [8,19].
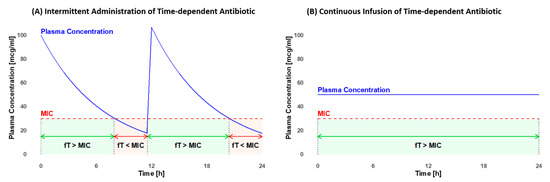
Figure 1.
Comparison of the fraction of time (fT) that the plasma concentration remains above the minimal inhibitory concentration (MIC) in intermittent administration (A) and continuous infusion (B). In intermittent administration, there are efficacy gaps where the fT < MIC (light red). With continuous infusion, a constant fT of 100% > MIC (light green) can be maintained throughout the entire administration period.
The time-dependent pharmacokinetic/pharmacodynamic (PK/PD) mode of action of beta-lactams and glycopeptides suggests that COI might be less toxic and more effective than IA [19,20]. By minimizing peak concentrations, both COI and prolonged infusion (PI) may result in fewer adverse drug reactions (ADRs), such as nephrotoxicity, hypersensitivity reactions, or neurological deterioration [21,22].
COI also facilitates therapeutic drug monitoring (TDM) by maintaining a constant drug concentration (Figure 1B). In contrast to IA, the timing of blood sampling and antibiotic dose administration is not critical. This reduces the risk of incorrect dose adjustments [12,14]. When employing COI, the area under the curve (AUC), which is in this case used for dose adjustments, can be easily calculated by multiplying the drug concentration with the duration of application. Through COI, the attainment of the target concentration is achieved more efficiently for both glycopeptides [19] and beta-lactams [23], potentially improving clinical outcomes [24].
Evidence increasingly supports the correlation between meeting PK/PD targets and achieving clinical success in adults [12,25,26]. Although PK studies in pediatric patients suggest potential advantages of COI/PI over IA [27,28,29,30], there is limited evidence establishing its superiority in clinical efficacy and safety to warrant its adoption as the standard method for pediatric patients [8,16,31].
1.3. Considerations for the Pediatric Population
Developmental stages significantly alter PK (see Table 1), causing variations in antibiotic plasma concentrations based on changes in distribution volume, blood clearance, and drug half-life [31]. Primary factors contributing to this variability include body weight and maturation effects [27]. Despite demonstrated clinical benefits of COI/PI for beta-lactams and glycopeptides in adults [7,11,32], we cannot extrapolate the same outcomes for pediatric patients. SwissPedDose guidelines currently recommend IA for almost all beta-lactams and glycopeptides, with PI limited to meropenem and ceftazidime specifically for cystic fibrosis [33]. Notably, there is no recommendation for COI/PI for glycopeptides in SwissPedDose as of now [33].

Table 1.
Pharmacokinetic differences between children and adults [25,34].
1.4. Objective
We hypothesized that COI of beta-lactams and glycopeptides would be effective and safe in pediatrics. Our objective was to perform a systematic review with a meta-analysis to evaluate the safety and efficacy of COI/PI of beta-lactams and glycopeptides compared to IA in pediatrics, consolidating existing knowledge for a robust conclusion [35].
2. Results
2.1. Study Selection
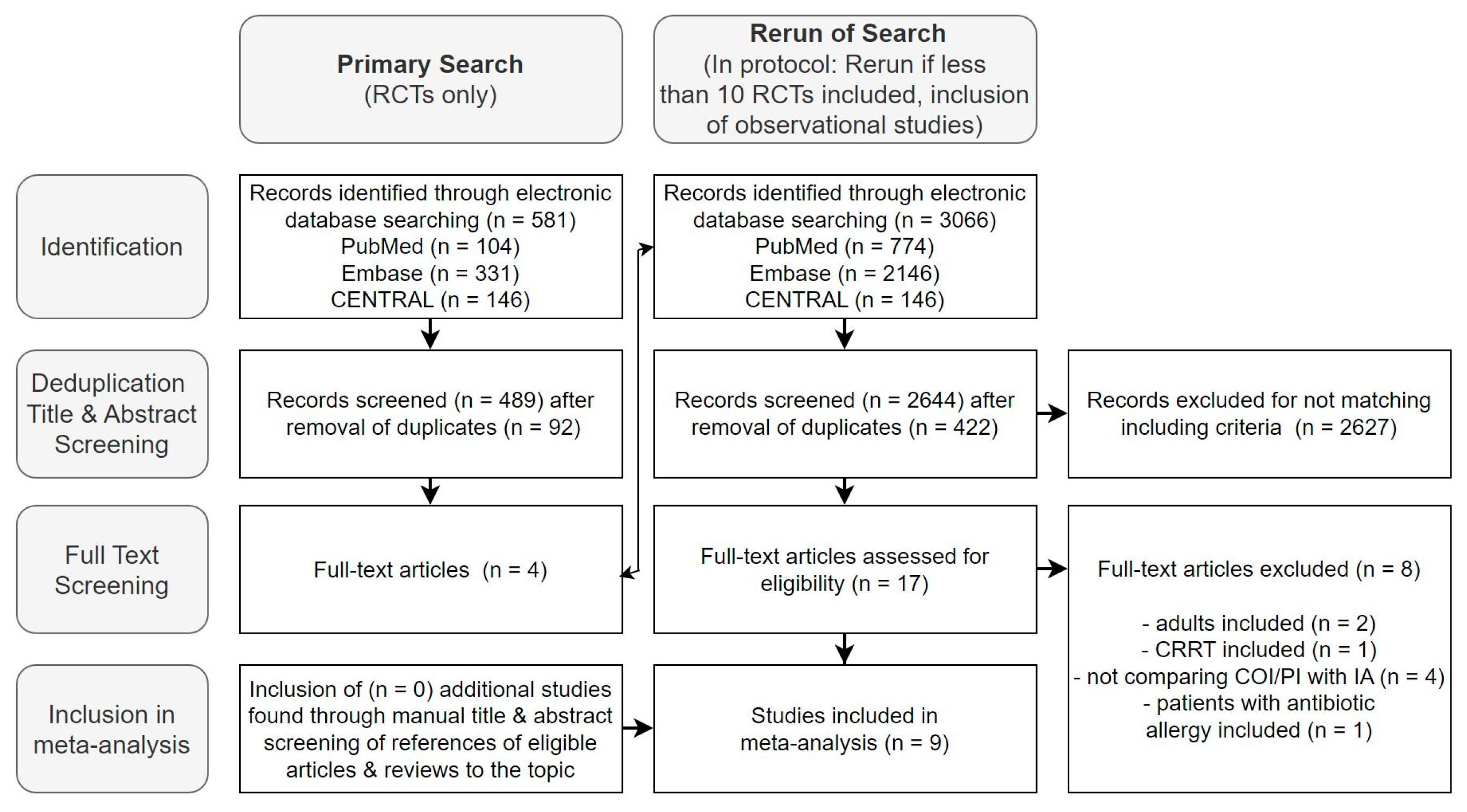
The initial search, limited to RCTs, yielded 581 studies, with four deemed relevant for full-text screening. Following the protocol, the search was expanded to include observational studies [36]. Across EMBASE, CENTRAL, and PUBMED, we identified 3066 studies. After duplicate removal (n = 422) and title and abstract selection, we found nine studies to be included in the meta-analysis during the full-text analysis—six on beta-lactams and three on glycopeptides (Figure 2).
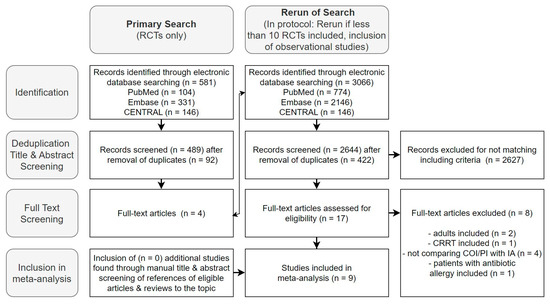
Figure 2.
Flow diagram of the selection process of studies for inclusion in the meta-analysis.
Figure 2 presents the results of the mentioned selection process in a flow diagram. Further exclusion details are available in Supplement E.
2.2. Risk of Bias Assessment
With the exception of one study, the overall risk of bias (ROB) assessment was moderate or high, as indicated in Table 2 and Table 3, with additional details provided in Supplement F.

Table 2.
Overall risk of bias of primary outcomes in studies investigating beta-lactams.

Table 3.
Overall risk of bias of primary outcomes in studies investigating glycopeptides.
The Wysocki study was excluded from data synthesis due to its study design, which could introduce significant bias to our outcome analysis (Supplement F). Sensitivity analysis showed no substantial modification of effect estimates after excluding studies classified as having a high ROB (Supplement G).
2.3. Study Characteristics
Study characteristics are outlined in Table 4 and Table 5, with additional details available in Supplement H. The administered drug doses in all studies were within the recommended range of SwissPedDose [33].

Table 4.
Characteristics of beta-lactam studies included in the systematic review.

Table 5.
Characteristics of glycopeptide studies included in the systematic review.
2.4. Data Synthesis
2.4.1. Beta-Lactams: Outcome Mortality
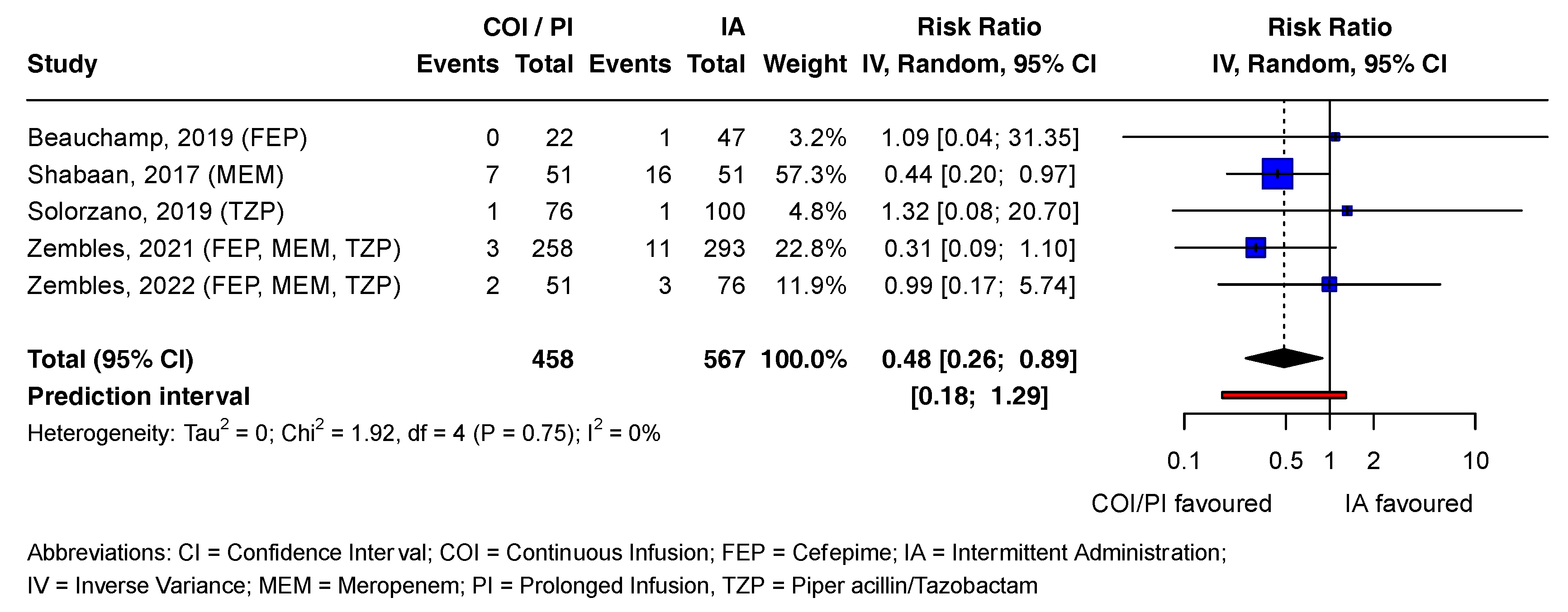
In the COI/PI group, 2.8% (13/458) of patients died, compared to 5.6% (32/567) in the IA group [21,37,38,39,40]. The pooled RR estimate was statistically significant (RR = 0.48; CI = 0.26 to 0.89; p = 0.02). Except for one study, all contributing studies included the no-effect value in the CI, consistent with the prediction interval. Tau2 and I2 statistics were 0 and 0%, respectively, as Chi2 was smaller than the degrees of freedom (df). Visual inspection of the forest plot suggested high heterogeneity between studies (Figure 3).
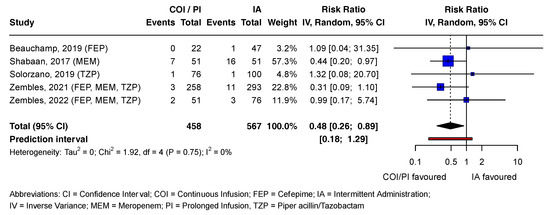
Figure 3.
Forest plot examining mortality outcomes associated with beta-lactam antibiotics. References [21,37,38,39,40] are cited in Figure 3.
Figure 3 presents the mortality outcomes associated with beta-lactam antibiotics in a forest plot. Statistically non-significant effect estimates of all outcomes and reported adverse drug reactions (ADRs) for both antibiotic groups are detailed in Supplement I.
2.4.2. Subgroup Analyses
Subgroup analyses, as planned in the protocol (Supplement J), could not be conducted due to the lack of studies [36].
2.4.3. Publication Bias
None of the outcomes had a sufficient number of studies (n 10) for a meaningful assessment of publication bias using funnel plots (Supplement K). As a result, no detectable publication bias was observed.
2.4.4. Certainty of Evidence
We assigned a “very low” certainty of evidence for primary outcomes in both drug groups (Table 6).

Table 6.
GRADE evidence profile: continuous infusion of beta-lactams and glycopeptides in pediatric patients.
3. Discussion
3.1. Overview of Findings
To our knowledge, this is the first meta-analysis comparing extended infusion with IA for glycopeptides in pediatric patients and one of the first ones for beta-lactams. Our results indicate a statistically significant lower pooled RR for mortality when administering beta-lactams via extended infusion instead of IA. This aligns with findings from adult studies, where optimal antimicrobial exposure is associated with better clinical outcomes and lower mortality [11,25]. Additionally, also very recently published data from the meta-analysis of Budai et al. align with our results [44]. Several Monte-Carlo simulation studies investigating the probability of PK/PD target attainment in pediatrics also support the use of COI for beta-lactams, further validating our findings [27,45]. Consistent with the findings of Grupper et al. [46], we did not observe COI/PI to be inferior to IA in terms of the safety and efficacy of glycopeptides. However, we did not find significant beneficial differences in mortality, clinical success, or microbiological eradication when using glycopeptides.
Potential reasons for this include the study setting, aiming for high target concentrations to combat even the most resistant pathogens. If patients had infections caused by pathogens requiring lower antibiotic concentrations, IA possibly remained sufficiently effective. Consequently, any potential superiority of COI/PI would not have been observable. Supporting this finding, clinical trials showed that clinical success was still achieved, event when most patients did not reach the target antibiotic blood concentrations [25,47]. Therefore, non-critically ill patients may not experience the same clinical benefits as critically ill patients, who typically require higher antibiotic efficacy to combat pathogens [48]. This aligns with research on COI in adults and echoes clinical suggestions applied in the clinic of Heidenheim [25,39,47,49,50]. Additionally, this assumption is supported by Shabaan et al., the only beta-lactam study included in the meta-analysis conducted on critically ill patients [21]. It was the only study to demonstrate statistically significant benefits for all three primary outcomes assessed. While direct evidence is lacking, the literature indirectly suggests that COI may reduce antibiotic resistance emergence by avoiding subtherapeutic concentrations [11,51]. However, this hypothesis requires further investigation.
3.2. Implications for Clinical Practice and Outlook
COI of beta-lactams and glycopeptides aligns better with the PK/PD profile than IA [49,52]. Our findings, demonstrating safety and efficacy benefits in pediatric patients, are supported by a review on COI use in the pediatric population [7,49,53,54]. Cheng et al. also reported that COI of meropenem was more effective in treating sepsis than IA [55]. COI offers additional potential benefits, such as reducing patients’ length of stay and enhancing cost-effectiveness, which can be attributed to increased therapy efficacy and align with the interests of hospitals [8,11].
We recommend conducting an RCT to further explore the comparative benefit of COI combined with therapeutic drug monitoring (TDM) versus IA for the specified beta-lactams and glycopeptides in our guideline.
3.3. Strengths and Limitations
Our systematic review employed well-documented methods outlined in a registered protocol, adhering to the Cochrane Handbook of Systematic Reviews, with a specific focus on the relatively unexplored pediatric population [36,56].
A primary limitation was the scarcity of studies available for inclusion in the meta-analysis. The identification of only six eligible beta-lactam studies (including three RCTs) and three glycopeptide studies (including one RCT) underscores the reported lack of evidence [49]. Ethical considerations and insufficient financial incentives for conducting such studies in pediatrics may contribute to the limited research. With over 50% of the studies being non-RCTs, uncontrolled confounding factors could have influenced our results. The shortage of studies constrained both publication bias assessment and subgroup analysis, necessitating the pooling of all pediatric age groups, beta-lactam drugs, COI with PI, and all disease severities. This, in turn, compromised the robustness of the pooled RR estimates. Exclusion of the Shabaan et al. study, which showed reduced mortality with COI for beta-lactams [21], would have prevented achieving statistical significance. This study had the highest weight (inverse variance), further reducing the overall robustness. However, it was the only study with a low risk of bias, enhancing the credibility of the effect estimate. To elevate the low GRADE of the findings, additional well-designed RCTs are imperative to further explore the benefits of COI.
A limitation is excluding patients on continuous renal replacement therapy (CRRT) to minimize confounding, despite our advocacy for COI for critically ill patients, many of whom require CRRT. Future research should explore CRRT’s impact on outcomes of COI of antimicrobials. Additionally, our study did not consider pharmacokinetic changes like fluid shifts or organ dysfunction associated with severe illness [57]. We were also unable to adjust for concomitant antibiotic use such as aminoglycosides, potentially confounding our findings.
3.4. Conclusions
Our research suggests that COI of beta-lactams and glycopeptides for pediatric patients is feasible, safe, and more efficacious. Existing PK simulation studies and those conducted with adults support the benefits of COI. However, further validation of our findings and paving the way for clinical implementation require more RCTs. Therefore, we propose conducting an RCT to investigate the comparative benefits of COI combined with TDM over IA of beta-lactams and glycopeptides, specifically in critically ill pediatric patients who are likely to derive the most benefit from COI.
4. Methods
4.1. Eligibility Criteria
Following the Cochrane Handbook of Systematic Reviews, we formulated a study protocol adhering to the PRISMA-P checklist to ensure transparency and comprehensiveness [56,58]. The protocol was registered in PROSPERO (CRD42023407772) before the literature search [36]. Deviations from the protocol were documented (Supplement A). We established inclusion and exclusion criteria for the publications (Table 7). For infusion durations, we defined 24 h/day as COI, 1 h as IA, and 1 h 24 h/day as PI.

Table 7.
Inclusion and exclusion criteria for studies for the systematic review.
4.2. Search Strategy and Information Sources
After refining the search strategy with a professional librarian [61], we systematically searched EMBASE, MEDLINE, and CENTRAL databases for relevant studies published between 1960 and 17 April 2023 (Supplements B and C). To filter for randomized controlled trials (RCTs), we utilized the Cochrane filter [62]. A second search, including observational studies, was conducted, as the initial search yielded fewer than 10 RCTs, as predefined in the protocol [36]. No other search filters were applied. We validated the search by checking the inclusion of defined key papers. Before data synthesis, we reran the search to include new publications. Duplicate removal was conducted manually using the deduplication tool in EndNote, comparing titles, years, and authors, followed by the digital object identifier (DOI) if available [63]. Additional relevant publications were manually sought in the references of reviews and studies included in the full-text review.
4.3. Study Selection
Two independent reviewers screened titles and abstracts for inclusion in the full-text assessment, with a third reviewer resolving discrepancies. Full texts of eligible studies were screened based on inclusion and exclusion criteria (Table 7). Results were compared after each step, with consensus decisions in cases of differences.
4.4. Data Collection and Analysis
Following the Cochrane Handbook for Systematic Reviews, two independent reviewers conducted the data extraction in an EXCEL table [56,64]. Applicability was tested using a sample study, and differences were resolved through consensus, involving a third reviewer when necessary. In cases of missing data, study authors were contacted via email for the required information. Relative risks (RRs) were chosen as the measure of effect for the outcomes (Supplement D). A statistically non-significant result on a p-level of 0.05 was indicated if the 95% confidence interval (CI) contained the value one. To prevent calculation errors due to division by zero in studies with no events in one arm, an event value of 0.5 was used, adhering to the Cochrane Handbook for Systematic Reviews of Interventions [56]. Studies with no events in either study arm for a specific outcome were excluded from the meta-analysis.
4.5. Risk of Bias Assessment
Two independent reviewers used the Cochrane tool for randomized trials ROB2 [65] to assess the risk of bias (ROB) for each outcome. Non-randomized trials were evaluated using the risk of bias in non-randomized studies of interventions (ROBINS-I) tool [66]. A sensitivity analysis was performed to gauge the robustness of the effect estimates by excluding studies with a high ROB. The reviewers reached a consensus to determine if the ROB for each study was too high for inclusion in the analysis.
4.6. Data Synthesis
Glycopeptides and beta-lactams data syntheses were conducted independently. A meta-analysis with a random effects model was performed for each outcome of each antibiotic group if at least two studies reported the outcome. Forest plots were generated using the meta package in R in RStudio [67,68], employing inverse variance to weight studies as per the Cochrane handbook [56]. If fewer than two studies reported the outcome, results were reported in prose.
4.6.1. Subgroup Analyses and Heterogeneity Assessment
Planned subgroup analyses for both drug groups encompassed different drugs, age, sex, treatment indication, infectious agent, severity of infection, concomitant diseases, and concurrent use of other antibiotics [36]. Forest plots were utilized for qualitative heterogeneity assessment, where a significant overlap of CI indicated high heterogeneity [56]. Qualitative and quantitative heterogeneity assessment employed Chi2 tests, the Higgins I2 statistic, τ2, and prediction intervals calculated in R using the meta and metafor packages [56,67,69,70]. To address heterogeneity resulting from pooling PI with COI and pooling different beta-lactam antibiotics, a random effects model was chosen.
4.6.2. Publication Bias
We planned to assess publication bias by visually inspecting funnel plots of primary outcomes, created using the metafor package in R [68,69]. A minimum of 10 studies is generally considered sufficient for adequate test power to assess funnel plot asymmetry [56,71]. If publication bias was detectable, we intended to use the trim and fill function to obtain an effect estimate of the true unbiased effect [56].
4.6.3. Grade Assessment
We used the grading of recommendations assessment, development, and evaluation (GRADE) approach to assess the certainty of the evidence for each primary outcome [56,72].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics13020164/s1, Supplement A: Differences Between the Protocol and the Final Review. Supplement B: Search Keys and Strategies. Supplement C: Search Strategies. Supplement D: Calculation of Variables. Supplement E: Reason for Exclusion of Articles in Full-Text Review. Supplement F: Risk of Bias (ROB) Judgement. Supplement G: Sensitivity Analyses. Supplement H: Summary of Findings Tables for all Outcomes. Supplement I: Forest Plots of Statistically Non-significant Outcomes. Supplement J: Subgroup Analysis Considerations and Results. Supplement K: Funnel Plots for Publication Bias Assessment. Supplement L: PRISMA checklist. References [73,74,75] are cited in supplementary materials.
Author Contributions
Conceptualization, A.R.B. and V.N.; methodology, L.v.A. and V.N.; formal analysis, L.v.A.; validation, A.R.B.; investigation, L.v.A. and V.N.; resources, A.R.B. and V.N.; data curation, L.v.A., A.R.B. and V.N.; writing—original draft preparation, L.v.A.; writing—review and editing, L.v.A., A.R.B. and B.H.; visualization, L.v.A.; supervision, A.R.B. and V.N.; project administration, A.R.B.; funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available upon request from the corresponding author.
Acknowledgments
The authors thank Andrea Burden from the Swiss Federal Institute of Technology (ETH) for her support and kind revision of the study protocol.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bundesrat. StAR Strategie Antibiotikaresistenzen Schweiz; Vertrieb Bundespublikationen: Bern, Switzerland, 2015; pp. 35–40. [Google Scholar]
- Gerber, J.S.; Newland, J.G.; Coffin, S.E.; Hall, M.; Thurm, C.; Prasad, P.A.; Feudtner, C.; Zaoutis, T.E. Variability in Antibiotic Use at Children’s Hospitals. Pediatrics 2010, 126, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Wathne, J.S.; Kleppe, L.K.S.; Harthug, S.; Blix, H.S.; Nilsen, R.M.; Charani, E.; Smith, I. The effect of antibiotic stewardship interventions with stakeholder involvement in hospital settings: A multicentre, cluster randomized controlled intervention study. Antimicrob. Resist. Infect. Control 2018, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Dhaese, S.; Van Vooren, S.; Boelens, J.; De Waele, J. Therapeutic drug monitoring of β-lactam antibiotics in the ICU. Expert Rev. Anti Infect. Ther. 2020, 18, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Dhaese, S.A.M.; Hoste, E.A.; De Waele, J.J. Why We May Need Higher Doses of Beta-Lactam Antibiotics: Introducing the ‘Maximum Tolerable Dose’. Antibiotics 2022, 11, 889. [Google Scholar] [CrossRef]
- Romandini, A.; Pani, A.; Schenardi, P.A.; Pattarino, G.A.C.; De Giacomo, C.; Scaglione, F. Antibiotic Resistance in Pediatric Infections: Global Emerging Threats, Predicting the Near Future. Antibiotics 2021, 10, 393. [Google Scholar] [CrossRef]
- Tamma, P.D.; Putcha, N.; Suh, Y.D.; Van Arendonk, K.J.; Rinke, M.L. Does prolonged β-lactam infusions improve clinical outcomes compared to intermittent infusions? A meta-analysis and systematic review of randomized, controlled trials. BMC Infect. Dis. 2011, 11, 181. [Google Scholar] [CrossRef]
- Costenaro, P.; Minotti, C.; Cuppini, E.; Barbieri, E.; Giaquinto, C.; Donà, D. Optimizing Antibiotic Treatment Strategies for Neonates and Children: Does Implementing Extended or Prolonged Infusion Provide any Advantage? Antibiotics 2020, 9, 329. [Google Scholar] [CrossRef]
- Donà, D.; Barbieri, E.; Daverio, M.; Lundin, R.; Giaquinto, C.; Zaoutis, T.; Sharland, M. Implementation and impact of pediatric antimicrobial stewardship programs: A systematic scoping review. Antimicrob. Resist. Infect. Control 2020, 9, 3. [Google Scholar] [CrossRef]
- Folgori, L.; Ellis, S.J.; Bielicki, J.A.; Heath, P.T.; Sharland, M.; Balasegaram, M. Tackling antimicrobial resistance in neonatal sepsis. Lancet Glob. Health 2017, 5, e1066–e1068. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Tansarli, G.S.; Ikawa, K.; Vardakas, K.Z. Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: A systematic review and meta-analysis. Clin. Infect. Dis. 2013, 56, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Röhr, A.; Köberer, A.; Fuchs, T.; von Freyberg, P.; Frey, O.; Brinkmann, A. SOP Individuelle Dosierung und Applikation von Antiinfektiva auf der Intensivstation. Intensivmedizin Up2date 2018, 14, 238–243. [Google Scholar] [CrossRef]
- Pichichero, M.E.; Casey, J.R. Acute otitis media disease management. Minerva Pediatr. 2003, 55, 415–438. [Google Scholar] [PubMed]
- Gwee, A.; Cranswick, N.; Donath, S.M.; Hunt, R.; Curtis, N. Protocol for a randomised controlled trial of continuous infusions of vancomycin to improve the attainment of target vancomycin levels in young infants: The VANC trial. BMJ Open 2018, 8, e022603. [Google Scholar] [CrossRef]
- De Hoog, M.; Mouton, J.W.; van den Anker, J.N. New dosing strategies for antibacterial agents in the neonate. Semin. Fetal Neonatal Med. 2005, 10, 185–194. [Google Scholar] [CrossRef]
- Walker, M.C.; Lam, W.M.; Manasco, K.B. Continuous and extended infusions of β-lactam antibiotics in the pediatric population. Ann. Pharmacother. 2012, 46, 1537–1546. [Google Scholar] [CrossRef]
- Chongcharoenyanon, T.; Wacharachaisurapol, N.; Anugulruengkitt, S.; Maimongkol, P.; Anunsittichai, O.; Sophonphan, J.; Chatsuwan, T.; Puthanakit, T. Comparison of piperacillin plasma concentrations in a prospective randomised trial of extended infusion versus intermittent bolus of piperacillin/tazobactam in paediatric patients. Int. J. Infect. Dis. 2021, 108, 102–108. [Google Scholar] [CrossRef]
- Fantin, B.; Farinotti, R.; Thabaut, A.; Carbon, C. Conditions for the emergence of resistance to cefpirome and ceftazidime in experimental endocarditis due to Pseudomonas aeruginosa. J. Antimicrob. Chemother. 1994, 33, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Gwee, A.; Cranswick, N.; McMullan, B.; Perkins, E.; Bolisetty, S.; Gardiner, K.; Daley, A.; Ward, M.; Chiletti, R.; Donath, S.; et al. Continuous versus intermittent vancomycin infusions in infants: A randomized controlled trial. Pediatrics 2019, 143, e20182179. [Google Scholar] [CrossRef]
- Downes, K.J.; Hahn, A.; Wiles, J.; Courter, J.D.; Vinks, A.A. Dose optimisation of antibiotics in children: Application of pharmacokinetics/pharmacodynamics in paediatrics. Int. J. Antimicrob. Agents 2014, 43, 223–230. [Google Scholar] [CrossRef]
- Shabaan, A.E.; Nour, I.; Elsayed Eldegla, H.; Nasef, N.; Shouman, B.; Abdel-Hady, H. Conventional Versus Prolonged Infusion of Meropenem in Neonates With Gram-negative Late-onset Sepsis: A Randomized Controlled Trial. Pediatr. Infect. Dis. J. 2017, 36, 358–363. [Google Scholar] [CrossRef]
- Hagel, S.; Fiedler, S.; Hohn, A.; Brinkmann, A.; Frey, O.R.; Hoyer, H.; Schlattmann, P.; Kiehntopf, M.; Roberts, J.A.; Pletz, M.W. Therapeutic drug monitoring-based dose optimisation of piperacillin/tazobactam to improve outcome in patients with sepsis (TARGET): A prospective, multi-centre, randomised controlled trial. Trials 2019, 20, 330. [Google Scholar] [CrossRef] [PubMed]
- Aardema, H.; Bult, W.; van Hateren, K.; Dieperink, W.; Touw, D.J.; Alffenaar, J.C.; Zijlstra, J.G. Continuous versus intermittent infusion of cefotaxime in critically ill patients: A randomized controlled trial comparing plasma concentrations. J. Antimicrob. Chemother. 2020, 75, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.V.; Mabasa, V.H.; Chow, I.; Ensom, M.H. Evaluating outcomes of alternative dosing strategies for cefepime: A qualitative systematic review. Ann. Pharmacother. 2015, 49, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Cotta, M.; Raman, S.; Graham, N.; Schlapbach, L.; Roberts, J.A. Individualized precision dosing approaches to optimize antimicrobial therapy in pediatric populations. Expert Rev. Clin. Pharmacol. 2021, 14, 1383–1399. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Joynt, G.M.; Lee, A.; Choi, G.; Bellomo, R.; Kanji, S.; Mudaliar, M.Y.; Peake, S.L.; Stephens, D.; Taccone, F.S.; et al. The Effect of Renal Replacement Therapy and Antibiotic Dose on Antibiotic Concentrations in Critically Ill Patients: Data from the Multinational Sampling Antibiotics in Renal Replacement Therapy Study. Clin. Infect. Dis. 2021, 72, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Béranger, A.; Oualha, M.; Urien, S.; Genuini, M.; Renolleau, S.; Aboura, R.; Hirt, D.; Heilbronner, C.; Toubiana, J.; Tréluyer, J.-M.; et al. Population Pharmacokinetic Model to Optimize Cefotaxime Dosing Regimen in Critically Ill Children. Clin. Pharmacokinet. 2018, 57, 867–875. [Google Scholar] [CrossRef]
- Krueger, W.A.; Bulitta, J.; Kinzig-Schippers, M.; Landersdorfer, C.; Holzgrabe, U.; Naber, K.G.; Drusano, G.L.; Sörgel, F. Evaluation by monte carlo simulation of the pharmacokinetics of two doses of meropenem administered intermittently or as a continuous infusion in healthy volunteers. Antimicrob. Agents Chemother. 2005, 49, 1881–1889. [Google Scholar] [CrossRef]
- De Cock, P.A.J.G.; van Dijkman, S.C.; de Jaeger, A.; Willems, J.; Carlier, M.; Verstraete, A.G.; Delanghe, J.R.; Robays, H.; Walle, J.V.; Della Pasqua, O.E.; et al. Dose optimization of piperacillin/tazobactam in critically ill children. J. Antimicrob. Chemother. 2017, 72, 2002–2011. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, D.; Fakhoury, M.; Fahd, M.; Duquesne, F.; Storme, T.; Baruchel, A.; Jacqz-Aigrain, E. Population pharmacokinetics and dosing optimization of vancomycin in children with malignant hematological disease. Antimicrob. Agents Chemother. 2014, 58, 3191–3199. [Google Scholar] [CrossRef]
- Agathe, D.; Delphine, C.; Deborah, H.; Emmanuelle, B.; Sylvain, R.; Laurent, C.; Jean-Marc, T.; Mehdi, O.; Agathe, B. Beta-lactam exposure and safety in intermittent or continuous infusion in critically ill children. Ann. Intensive Care 2022, 182, 965–973. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Zhou, Q.; Wang, Y.; Chen, L. Clinical Outcomes with Alternative Dosing Strategies for Piperacillin/Tazobactam: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0116769. [Google Scholar] [CrossRef] [PubMed]
- SwissPedDose. Nationale Datenbak zur Dosierung von Arzneimitteln bei Kindern. Available online: https://db.swisspeddose.ch/de/search/?_roa=iv (accessed on 19 May 2023).
- Testa, B.; Krämer, S.D. The biochemistry of drug metabolism—An introduction: Part 1. Principles and overview. Chem. Biodivers. 2006, 3, 1053–1101. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.; Kang, H. Introduction to systematic review and meta-analysis. Korean J. Anesthesiol. 2018, 71, 103–112. [Google Scholar] [CrossRef] [PubMed]
- von Arx, L.E.; Burch, A.R.; Neumeier, V.; Burden, A. Safety and Efficacy of Continuous and Prolonged Infusion of Beta-Lactams and Glycopeptides Compared to Intermittent Administration in Children: A Systematic Review and Meta-Analysis. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=407772 (accessed on 31 March 2023).
- Beauchamp, L.C.; Nichols, K.R.; Knoderer, C.A. Outcomes of Extended Infusion Cefepime in Pediatric Patients. Infect. Dis. Clin. Pract. 2019, 27, 283–287. [Google Scholar] [CrossRef]
- Solórzano-Santos, F.; Quezada-Herrera, A.; Fuentes-Pacheco, Y.; Rodríguez-Coello, G.; Aguirre-Morales, C.E.; Izelo-Flores, D.; Muñoz-Hernández, O.; Miranda-Novales, M.G.; Labra-Zamora, M.G. Piperacillin/Tazobactam in Continuous Infusion versus Intermittent Infusion in Children with Febrile Neutropenia. Rev. Investig. Clin. 2019, 71, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Zembles, T.N.; Schortemeyer, R.; Kuhn, E.M.; Bushee, G.; Thompson, N.E.; Mitchell, M.L. Extended infusion of beta-lactams is associated with improved outcomes in pediatric patients. J. Pediatr. Pharmacol. Ther. 2021, 26, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Zembles, T.N.; Kuhn, E.M.; Thompson, N.E.; Mitchell, M.L. Extended Infusion b-Lactams for the Treatment of Gram-Negative Bacteremia in Children. J. Pediatr. Pharmacol. Ther. 2022, 27, 677–681. [Google Scholar] [CrossRef]
- Demirel, B.; Imamoglu, E.; Gursoy, T.; Demirel, U.; Topçuoglu, S.; Karatekin, G.; Ovali, F. Comparison of intermittent versus continuous vancomycin infusion for the treatment of late-onset sepsis in preterm infants. J. Neonatal-Perinat. Med. 2015, 8, 149–155. [Google Scholar] [CrossRef]
- Gwee, A.; Cranswick, N. Anti-infective use in children and pregnancy: Current deficiencies and future challenges. Br. J. Clin. Pharmacol. 2015, 79, 216–221. [Google Scholar] [CrossRef]
- Wysocki, E.; Tansmore, J. When There Is No Trough: Use and Outcomes of Continuous-Infusion Vancomycin at a Free-Standing Children’s Hospital. J. Pediatr. Pharmacol. Ther. 2022, 27, 452–456. [Google Scholar] [CrossRef]
- Budai, K.A.; Timar, A.E.; Obeidat, M.; Mate, V.; Nagy, R.; Harnos, A.; Kiss-Dala, S.; Hegyi, P.; Garami, M.; Hanko, B.; et al. Extended infusion of beta-lactams significantly reduces mortality and enhances microbiological eradication in paediatric patients: A systematic review and meta-analysis. EClinicalMedicine 2023, 65, 102293. [Google Scholar] [CrossRef]
- Van Den Anker, J.N.; Pokorna, P.; Kinzig-Schippers, M.; Martinkova, J.; De Groot, R.; Drusano, G.L.; Sorgel, F. Meropenem pharmacokinetics in the newborn. Antimicrob. Agents Chemother. 2009, 53, 3871–3879. [Google Scholar] [CrossRef] [PubMed]
- Grupper, M.; Kuti, J.L.; Nicolau, D.P. Continuous and Prolonged Intravenous β-Lactam Dosing: Implications for the Clinical Laboratory. Clin. Microbiol. Rev. 2016, 29, 759–772. [Google Scholar] [CrossRef]
- Hagel, S.; Bach, F.; Brenner, T.; Bracht, H.; Brinkmann, A.; Annecke, T.; Hohn, A.; Weigand, M.; Michels, G.; Kluge, S.; et al. Effect of therapeutic drug monitoring-based dose optimization of piperacillin/tazobactam on sepsis-related organ dysfunction in patients with sepsis: A randomized controlled trial. Intensive Care Med. 2022, 48, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Dalle, J.H.; Gnansounou, M.; Husson, M.O.; Lambilliotte, A.; Mazingue, F.; Nelken, B. Continuous infusion of ceftazidime in the empiric treatment of febrile neutropenic children with cancer. J. Pediatr. Hematol. Oncol. 2002, 24, 714–716. [Google Scholar] [CrossRef]
- Richter, D.C.; Frey, O.; Röhr, A.; Roberts, J.A.; Köberer, A.; Fuchs, T.; Papadimas, N.; Heinzel-Gutenbrunner, M.; Brenner, T.; Lichtenstern, C.; et al. Therapeutic drug monitoring-guided continuous infusion of piperacillin/tazobactam significantly improves pharmacokinetic target attainment in critically ill patients: A retrospective analysis of four years of clinical experience. Infection 2019, 47, 1001–1011. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Sulaiman, H.; Mat-Nor, M.-B.; Rai, V.; Wong, K.K.; Hasan, M.S.; Abd Rahman, A.N.; Jamal, J.A.; Wallis, S.C.; Lipman, J.; et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): A prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016, 42, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Croom, K.; Adomakoh, N. Continuous infusion of beta-lactam antibiotics: Narrative review of systematic reviews, and implications for outpatient parenteral antibiotic therapy. Expert Rev. Anti-Infect. Ther. 2023, 21, 375–385. [Google Scholar] [CrossRef]
- Jamal, J.A.; Mat-Nor, M.B.; Mohamad-Nor, F.S.; Udy, A.A.; Wallis, S.C.; Lipman, J.; Roberts, J.A. Pharmacokinetics of meropenem in critically ill patients receiving continuous venovenous haemofiltration: A randomised controlled trial of continuous infusion versus intermittent bolus administration. Int. J. Antimicrob. Agents 2015, 45, 41–45. [Google Scholar] [CrossRef]
- Imburgia, T.A.; Kussin, M.L. A Review of Extended and Continuous Infusion Beta-Lactams in Pediatric Patients. J. Pediatr. Pharmacol. Ther. 2022, 27, 214–227. [Google Scholar] [CrossRef]
- Nichols, K.R.; Karmire, L.C.; Cox, E.G.; Kays, M.B.; Knoderer, C.A. Implementing Extended-Infusion Cefepime as Standard of Care in a Children’s Hospital: A Prospective Descriptive Study. Ann. Pharmacother. 2015, 49, 419–426. [Google Scholar] [CrossRef]
- Chen, P.; Chen, F.; Lei, J.; Zhou, B. Clinical outcomes of continuous vs intermittent meropenem infusion for the treatment of sepsis: A systematic review and meta-analysis. Adv. Clin. Exp. Med. 2020, 29, 993–1000. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Roberts, J.A.; Lipman, J. Antibacterial Dosing in Intensive Care. Clin. Pharmacokinet. 2006, 45, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Matusik, E.; Lemtiri, J.; Wabont, G.; Lambiotte, F. Beta-lactam dosing during continuous renal replacement therapy: A survey of practices in french intensive care units. BMC Nephrol. 2022, 23, 48. [Google Scholar] [CrossRef] [PubMed]
- Deldot, M.E.; Lipman, J.; Tett, S.E. Vancomycin pharmacokinetics in critically ill patients receiving continuous venovenous haemodiafiltration. Br. J. Clin. Pharmacol. 2004, 58, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; Blunt, H.; Brigham, T.; Chang, S.; et al. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Glanville, J.; Briscoe, S.; Featherstone, R.; Littlewood, A.; Marshall, C.; Metzendorf, M.-I.; Noel-Storr, A.; Paynter, R.; Rader, T.; et al. Technical Supplement to Chapter 4: Searching for and Selecting Studies. In Cochrane Handbook for Systematic Reviews of Interventions, version 6.3 (updated February 2022); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane; John Wiley & Sons: Chichester, UK, 2022. [Google Scholar]
- The EndNote Team. EndNote, Endnote 20; Clarivate: Philadelphia, PA, USA, 2020. [Google Scholar]
- Page, M.J.; Mckenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.; Savovic, J.; Page, M.J.; Sterne, J.A.C. Revised Cochrane Risk-of-Bias Tool for Randomized Trials (ROB 2); Cochrane; John Wiley & Sons: Chichester, UK, 2022. [Google Scholar]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid.-Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R; Version 2022.12.0+353 (2022.12.0+353); RStudio, PBC: Boston, MA, USA; Available online: http://www.rstudio.com/ (accessed on 1 April 2023).
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Schulz, A.; Schürmann, C.; Skipka, G.; Bender, R. Performing Meta-analyses with Very Few Studies. Methods Mol. Biol. 2022, 2345, 91–102. [Google Scholar] [PubMed]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rucker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Coello, P.A.; Guyatt, G.H.; Yepes-Nuñez, J.J.; Akl, E.A.; Hazlewood, G.; Pardo-Hernandez, H.; Etxeandia-Ikobaltzeta, I.; Qaseem, A.; Williams, J.W., Jr.; et al. GRADE guidelines: 20. Assessing the certainty of evidence in the importance of outcomes or values and preferences-inconsistency, imprecision, and other domains. J. Clin. Epidemiol. 2019, 111, 83–93. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. How to obtain the P value from a confidence interval. BMJ 2011, 343, d2304. [Google Scholar] [CrossRef]
- Aguinis, H.; Vassar, M.; Wayant, C. On reporting and interpreting statistical significance and p values in medical research. BMJ Evid.-Based Med. 2021, 26, 39–42. [Google Scholar] [CrossRef]
- Wysocki, E.; Tansmore, J. When there is no trough: Continuous infusion vancomycin utilization at a free-standing children’s hospital. J. Pediatr. Pharmacol. Ther. 2021, 26, 525–526. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).