A Real-World Study on the Clinical Characteristics, Outcomes, and Relationship between Antibiotic Exposure and Clostridioides difficile Infection
Abstract
1. Introduction
2. Results
2.1. Descriptive Analysis of Reports from Sibiu County Clinical Emergency Hospital (SCCEH)
2.1.1. Baseline Patients’ Characteristics
2.1.2. Hospital Length of Stay
2.1.3. Influence of Age on the Patients’ Outcome
2.1.4. Wards
2.1.5. Admission Diagnosis
2.1.6. Charlson Comorbidity Index
2.1.7. Antibiotic Exposure
2.2. Analysis of Spontaneous Reports from EudraVigilance
2.2.1. Descriptive Analysis of ICSRs Uploaded in 2022
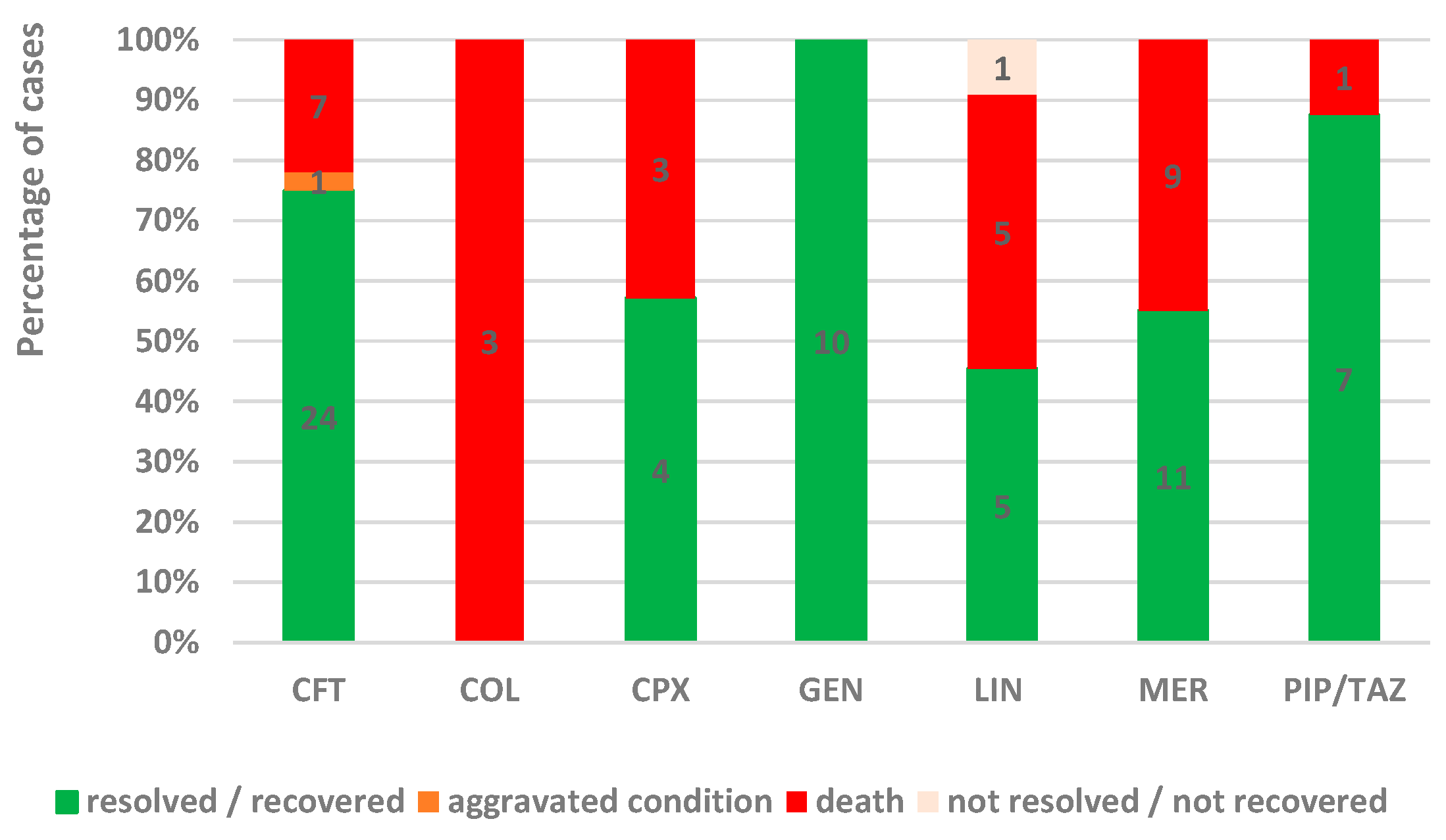
2.2.2. Outcomes
2.3. Comparison between the Reports from Sibiu County Clinical Emergency Hospital (SCCEH) and the Spontaneous Reports from EudraVigilance (EV)
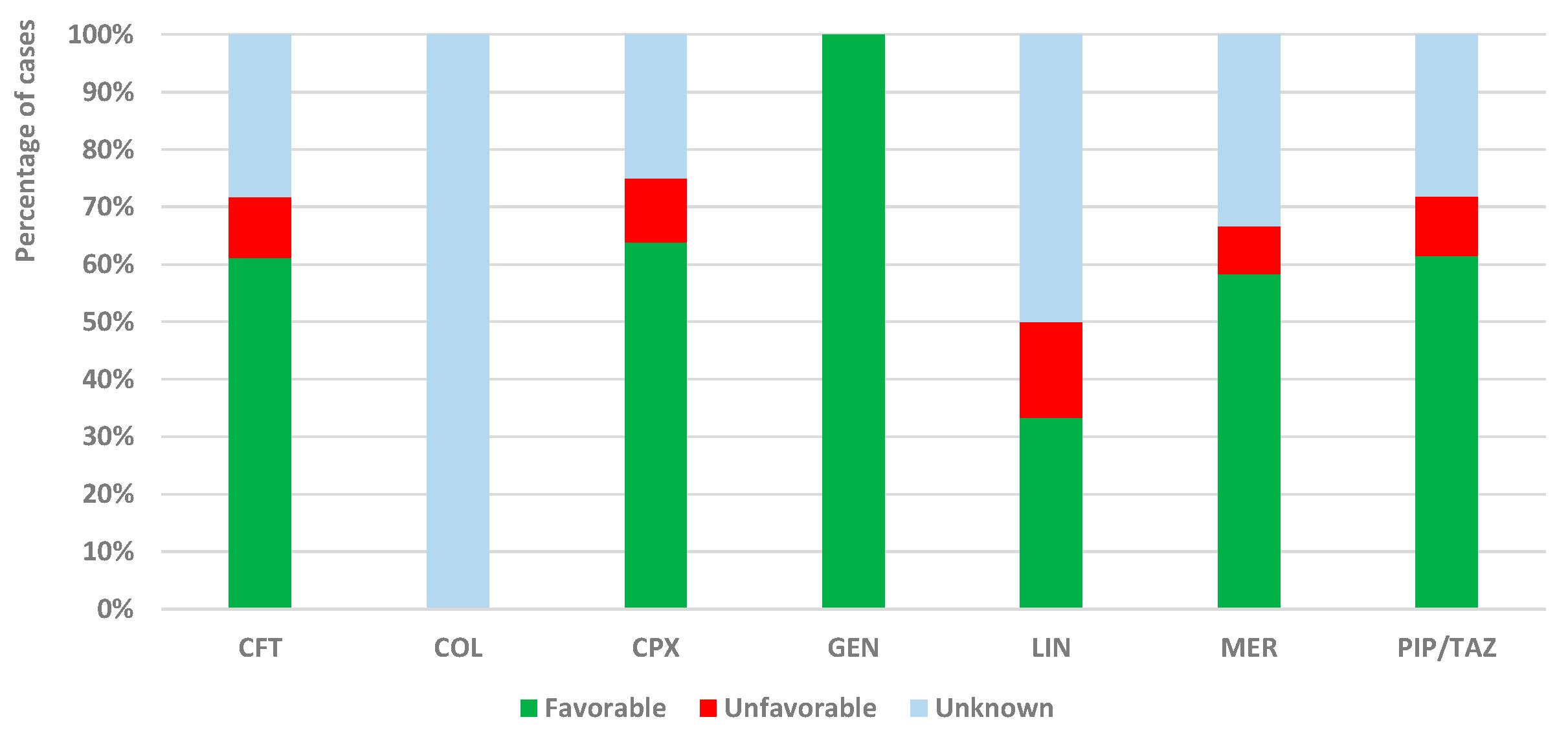
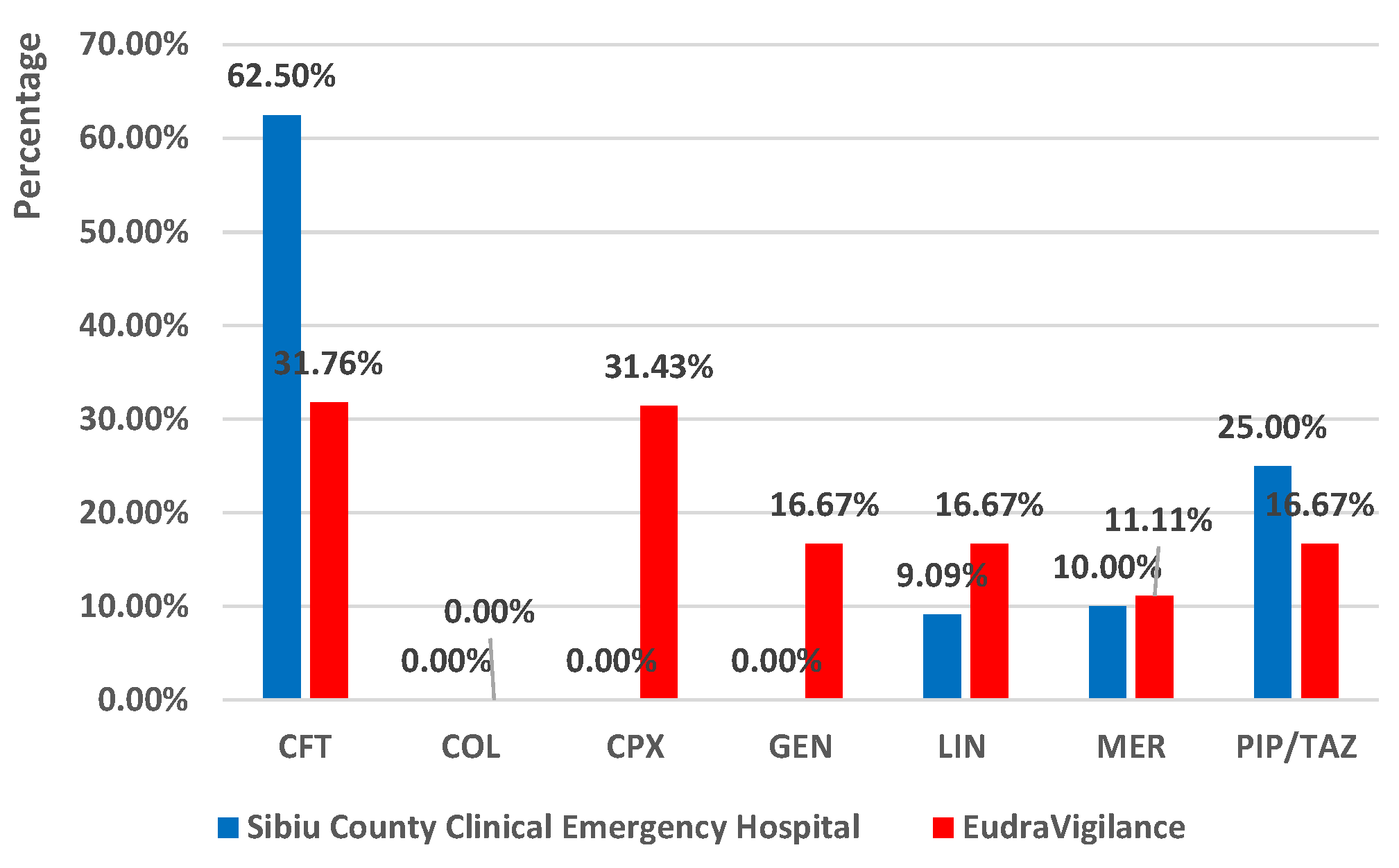
2.3.1. Exposure to Analyzed Drugs as a Single Suspected Antibiotic
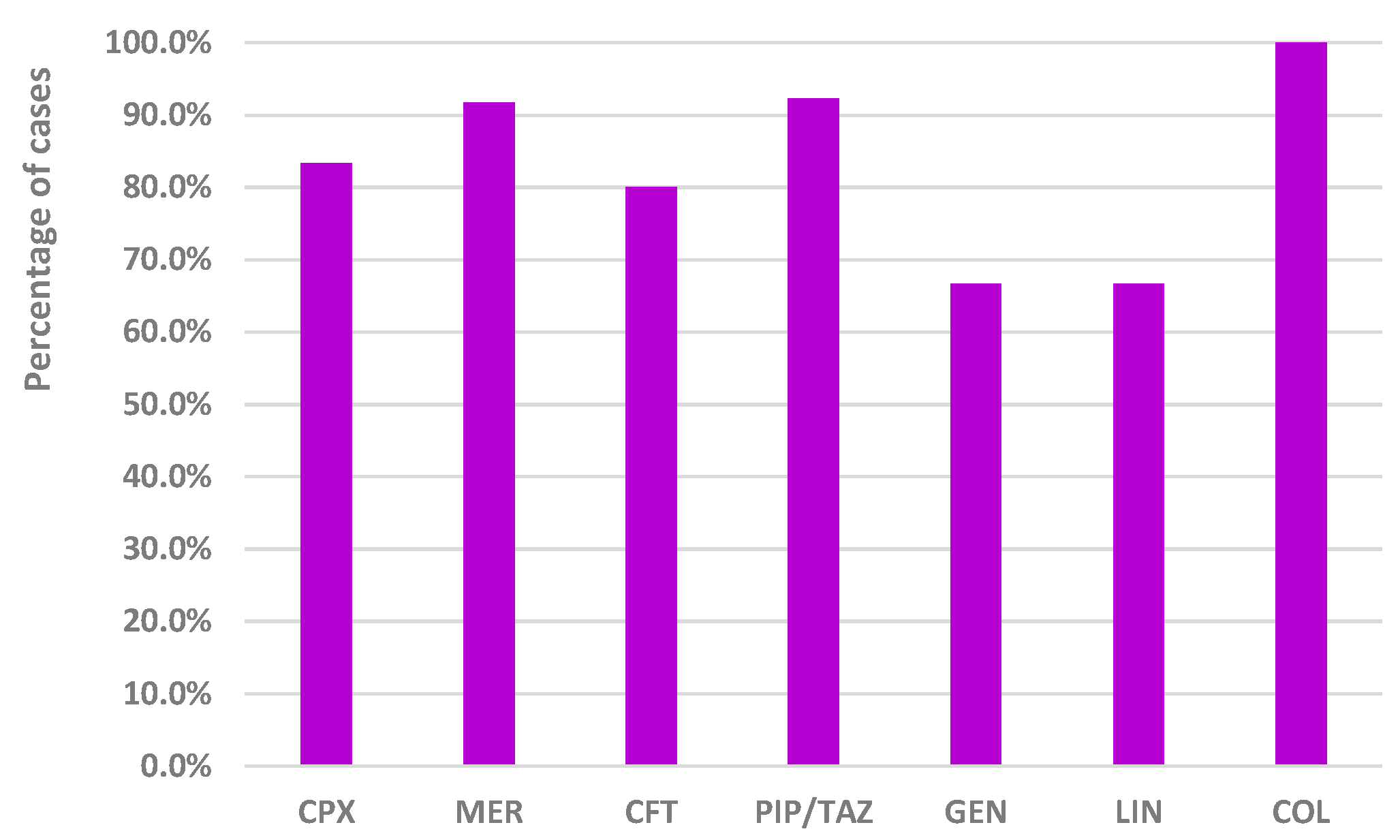
2.3.2. Frequency of Exposure to Other Antibiotics in Cases Where the Analyzed Drug Was Not the Only Suspected Antibiotic
3. Discussion
Limitations
4. Materials and Methods
4.1. Study Design
4.2. Materials
4.3. Data Analysis
4.3.1. Descriptive Analysis of Reports from Sibiu County Clinical Emergency Hospital
- Demographic data: age, gender, patient’s category (surgical or non-surgical patient). The data collected from SCCEH referred to adult patients, and the age categories were chosen according to European Medicines Agency regulations regarding pharmacovigilance activity. This is to enable better comparison between the two datasets.
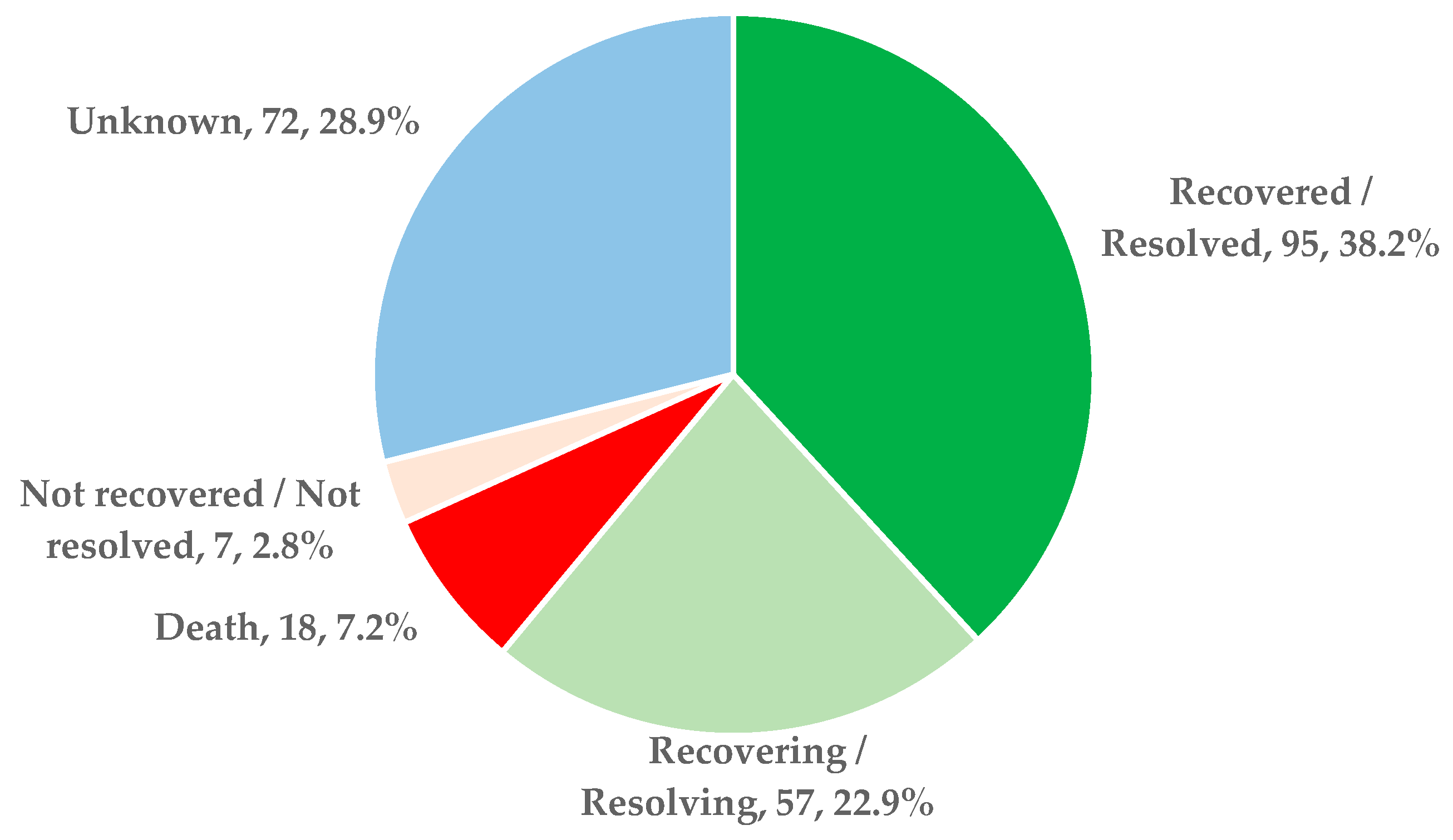
- Evolution: resolved/recovered, aggravated conditions, not resolved/not recovered (transfers), or death. The resolved/recovered cases represented the favorable evolution, and unfavorable evolution included aggravated conditions, not resolved/not recovered (transfers included), or death.
- Type of detection: active or passive. The active or passive detection mode indicates how the infection was reported to the infection surveillance department from SCCEH. Active detection means that the patient’s attending physician reported the infection, while passive detection means that the infection surveillance department detected the infection.
4.3.2. Analysis of Spontaneous Reports from EudraVigilance
4.3.3. Comparison between the Reports from Sibiu County Clinical Emergency Hospital (SCCEH) and the Spontaneous Reports from EudraVigilance (EV)
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Popa, D.; Neamtu, B.; Mihalache, M.; Boicean, A.; Banciu, A.; Banciu, D.D.; Moga, D.F.C.; Birlutiu, V. Fecal Microbiota Transplant in Severe and Non-Severe Clostridioides Difficile Infection. Is There a Role of FMT in Primary Severe CDI? J. Clin. Med. 2021, 10, 5822. [Google Scholar] [CrossRef] [PubMed]
- Chiș, A.A.; Rus, L.L.; Morgovan, C.; Arseniu, A.M.; Frum, A.; Vonica-țincu, A.L.; Gligor, F.G.; Mureșan, M.L.; Dobrea, C.M. Microbial Resistance to Antibiotics and Effective Antibiotherapy. Biomedicines 2022, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- Czepiel, J.; Dróżdż, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultańska, D.; Garlicki, A.; Biesiada, G. Clostridium Difficile Infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1211. [Google Scholar] [CrossRef] [PubMed]
- Birlutiu, V.; Dobritoiu, E.S.; Lupu, C.D.; Herteliu, C.; Birlutiu, R.M.; Dragomirescu, D.; Vorovenci, A. Our Experience with 80 Cases of SARS-CoV-2-Clostridioides Difficile Co-Infection: An Observational Study. Medicine 2022, 101, E29823. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013. [Google Scholar]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate Point-Prevalence Survey of Health Care-Associated Infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Zhu, N.A. Community-Acquired Clostridium Difficile Infection. Can. Fam. Physician 2017, 63, 131. [Google Scholar]
- Boicean, A.; Neamtu, B.; Birsan, S.; Batar, F.; Tanasescu, C.; Dura, H.; Roman, M.D.; Hașegan, A.; Bratu, D.; Mihetiu, A.; et al. Fecal Microbiota Transplantation in Patients Co-Infected with SARS-CoV2 and Clostridioides Difficile. Biomedicines 2022, 11, 7. [Google Scholar] [CrossRef]
- Slimings, C.; Riley, T.V. Antibiotics and Healthcare Facility-Associated Clostridioides Difficile Infection: Systematic Review and Meta-Analysis 2020 Update. J. Antimicrob. Chemother. 2021, 76, 1676–1688. [Google Scholar] [CrossRef]
- Perić, A.; Rančić, N.; Dragojević-Simić, V.; Milenković, B.; Ljubenović, N.; Rakonjac, B.; Begović-Kuprešanin, V.; Šuljagić, V. Association between Antibiotic Use and Hospital-Onset Clostridioides Difficile Infection in University Tertiary Hospital in Serbia, 2011–2021: An Ecological Analysis. Antibiotics 2022, 11, 1178. [Google Scholar] [CrossRef]
- Armstrong, T.; Fenn, S.J.; Hardie, K.R. JMM Profile: Carbapenems: A Broad-Spectrum Antibiotic. J. Med. Microbiol. 2021, 70, 1462. [Google Scholar] [CrossRef]
- Janssen, J.; Kinkade, A.; Man, D. CARBapenem UtilizatiON Evaluation in a Large Community Hospital (CARBON): A Quality Improvement Study. Can. J. Hosp. Pharm. 2015, 68, 327. [Google Scholar] [CrossRef]
- Hashemian, S.M.R.; Farhadi, T.; Ganjparvar, M. Linezolid: A Review of Its Properties, Function, and Use in Critical Care. Drug Des. Devel. Ther. 2018, 12, 1759–1767. [Google Scholar] [CrossRef]
- Moubareck, C.A. Polymyxins and Bacterial Membranes: A Review of Antibacterial Activity and Mechanisms of Resistance. Membranes 2020, 10, 181. [Google Scholar] [CrossRef]
- Khilnani, G.C.; Zirpe, K.; Hadda, V.; Mehta, Y.; Madan, K.; Kulkarni, A.; Mohan, A.; Dixit, S.; Guleria, R.; Bhattacharya, P. Guidelines for Antibiotic Prescription in Intensive Care Unit. Indian J. Crit. Care Med. 2019, 23, S1. [Google Scholar] [CrossRef]
- El-Haffaf, I.; Caissy, J.A.; Marsot, A. Piperacillin-Tazobactam in Intensive Care Units: A Review of Population Pharmacokinetic Analyses. Clin. Pharmacokinet. 2021, 60, 855–875. [Google Scholar] [CrossRef]
- Kayambankadzanja, R.K.; Lihaka, M.; Barratt-Due, A.; Kachingwe, M.; Kumwenda, W.; Lester, R.; Bilima, S.; Eriksen, J.; Baker, T. The Use of Antibiotics in the Intensive Care Unit of a Tertiary Hospital in Malawi. BMC Infect. Dis. 2020, 20, 776. [Google Scholar] [CrossRef]
- Grigore, N.; Totan, M.; Pirvut, V.; Mitariu, S.I.C.; Chicea, R.; Sava, M.; Hasegan, A. A Risk Assessment of Clostridium Difficile Infection after Antibiotherapy for Urinary Tract Infections in the Urology Department for Hospitalized Patients. Rev. Chim. 2017, 68, 1453–1456. [Google Scholar] [CrossRef]
- Gieling, E.M.; Wallenburg, E.; Frenzel, T.; de Lange, D.W.; Schouten, J.A.; ten Oever, J.; Kolwijck, E.; Burger, D.M.; Pickkers, P.; ter Heine, R.; et al. Higher Dosage of Ciprofloxacin Necessary in Critically Ill Patients: A New Dosing Algorithm Based on Renal Function and Pathogen Susceptibility. Clin. Pharmacol. Ther. 2020, 108, 770–774. [Google Scholar] [CrossRef]
- Codru, I.R.; Sava, M.; Vintilă, B.I.; Bereanu, A.S.; Bîrluțiu, V. A Study on the Contributions of Sonication to the Identification of Bacteria Associated with Intubation Cannula Biofilm and the Risk of Ventilator-Associated Pneumonia. Medicina 2023, 59, 1058. [Google Scholar] [CrossRef]
- Vintila, B.I.; Arseniu, A.M.; Morgovan, C.; Butuca, A.; Sava, M.; Bîrluțiu, V.; Rus, L.L.; Ghibu, S.; Bereanu, A.S.; Codru, I.R.; et al. A Pharmacovigilance Study Regarding the Risk of Antibiotic-Associated Clostridioides Difficile Infection Based on Reports from the EudraVigilance Database: Analysis of Some of the Most Used Antibiotics in Intensive Care Units. Pharmaceuticals 2023, 16, 1585. [Google Scholar] [CrossRef]
- Vintila, B.I.; Arseniu, A.M.; Butuca, A.; Sava, M.; Bîrluțiu, V.; Rus, L.L.; Axente, D.D.; Morgovan, C.; Gligor, F.G. Adverse Drug Reactions Relevant to Drug Resistance and Ineffectiveness Associated with Meropenem, Linezolid, and Colistin: An Analysis Based on Spontaneous Reports from the European Pharmacovigilance Database. Antibiotics 2023, 12, 918. [Google Scholar] [CrossRef]
- Mullish, B.H.; Williams, H.R.T. Clostridium Difficile Infection and Antibiotic-Associated Diarrhoea. Clin. Med. (Northfield. Il) 2018, 18, 237. [Google Scholar] [CrossRef]
- Laza, R.; Jurac, R.; Crişan, A.; Lăzureanu, V.; Licker, M.; Popovici, E.D.; Bădiţoiu, L.M. Clostridium Difficile in Western Romania: Unfavourable Outcome Predictors in a Hospital for Infectious Diseases. BMC Infect. Dis. 2015, 15, 1–9. [Google Scholar] [CrossRef][Green Version]
- Balsells, E.; Shi, T.; Leese, C.; Lyell, I.; Burrows, J.; Wiuff, C.; Campbell, H.; Kyaw, M.H.; Nair, H. Global Burden of Clostridium Difficile Infections: A Systematic Review and Meta-Analysis. J. Glob. Health 2019, 9, 10407. [Google Scholar] [CrossRef]
- Turner, N.A.; Grambow, S.C.; Woods, C.W.; Fowler, V.G.; Moehring, R.W.; Anderson, D.J.; Lewis, S.S. Epidemiologic Trends in Clostridioides Difficile Infections in a Regional Community Hospital Network. JAMA Netw. Open 2019, 2, e1914149. [Google Scholar] [CrossRef]
- Kuntz, J.L.; Smith, D.H.; Petrik, A.F.; Yang, X.; Thorp, M.L.; Barton, T.; Barton, K.; Labreche, M.; Spindel, S.J.; Johnson, E.S. Predicting the Risk of Clostridium Difficile Infection upon Admission: A Score to Identify Patients for Antimicrobial Stewardship Efforts. Perm. J. 2016, 20, 20–25. [Google Scholar] [CrossRef]
- Shiode, J.; Fujii, M.; Nasu, J.; Itoh, M.; Ishiyama, S.; Fujiwara, A.; Yoshioka, M. Correlation between Hospital-Onset and Community-Onset Clostridioides Difficile Infection Incidence: Ward-Level Analysis Following Hospital Relocation. Am. J. Infect. Control 2022, 50, 1240–1245. [Google Scholar] [CrossRef]
- Fonseca, F.; Forrester, M.; Advinha, A.M.; Coutinho, A.; Landeira, N.; Pereira, M. Clostridioides Difficile Infection in Hospitalized Patients—A Retrospective Epidemiological Study. Healthcare 2024, 12, 76. [Google Scholar] [CrossRef]
- Wiegand, P.N.; Nathwani, D.; Wilcox, M.H.; Stephens, J.; Shelbaya, A.; Haider, S. Clinical and Economic Burden of Clostridium Difficile Infection in Europe: A Systematic Review of Healthcare-Facility-Acquired Infection. J. Hosp. Infect. 2012, 81, 1–14. [Google Scholar] [CrossRef]
- Chu, Y.; Lee, C.; Chen, H.; Hung, C. Predictors of Mortality in Patients with Clostridium Difficile Infection. Adv. Dig. Med. 2020, 7, 77–82. [Google Scholar] [CrossRef]
- Hensgens, M.P.M.; Goorhuis, A.; Dekkers, O.M.; Van Benthem, B.H.B.; Kuijper, E.J. All-Cause and Disease-Specific Mortality in Hospitalized Patients with Clostridium Difficile Infection: A Multicenter Cohort Study. Clin. Infect. Dis. 2013, 56, 1108–1116. [Google Scholar] [CrossRef]
- Czepiel, J.; Krutova, M.; Mizrahi, A.; Khanafer, N.; Enoch, D.A.; Patyi, M.; Deptuła, A.; Agodi, A.; Nuvials, X.; Pituch, H.; et al. Mortality Following Clostridioides Difficile Infection in Europe: A Retrospective Multicenter Case-Control Study. Antibiotics 2021, 10, 299. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Yu, K.C.; Wei, R.; Tseng, H.F.; Jacobsen, S.J.; Rieg, G.K. Incidence of Polymerase Chain Reaction-Diagnosed Clostridium Difficile in a Large High-Risk Cohort, 2011–2012. Mayo Clin. Proc. 2014, 89, 1229–1238. [Google Scholar] [CrossRef]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through Ageing, and beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Lee, G.C.; Reveles, K.R.; Attridge, R.T.; Lawson, K.A.; Mansi, I.A.; Lewis, J.S.; Frei, C.R. Outpatient Antibiotic Prescribing in the United States: 2000 to 2010. BMC Med. 2014, 12, 1–8. [Google Scholar] [CrossRef]
- Seto, C.T.; Jeraldo, P.; Orenstein, R.; Chia, N.; DiBaise, J.K. Prolonged Use of a Proton Pump Inhibitor Reduces Microbial Diversity: Implications for Clostridium Difficile Susceptibility. Microbiome 2014, 2, 42. [Google Scholar] [CrossRef]
- Owens, R.C.; Donskey, C.J.; Gaynes, R.P.; Loo, V.G.; Muto, C.A. Antimicrobial-Associated Risk Factors for Clostridium Difficile Infection. Clin. Infect. Dis. 2008, 46 (Suppl. S1), S19–S31. [Google Scholar] [CrossRef]
- Shin, J.H.; High, K.P.; Warren, C.A. Older Is Not Wiser, Immunologically Speaking: Effect of Aging on Host Response to Clostridium Difficile Infections. J. Gerontol. A. Biol. Sci. Med. Sci. 2016, 71, 916–922. [Google Scholar] [CrossRef]
- Rogers, M.A.M.; Greene, M.T.; Young, V.B.; Saint, S.; Langa, K.M.; Kao, J.Y.; Aronoff, D.M. Depression, Antidepressant Medications, and Risk of Clostridium Difficile Infection. BMC Med. 2013, 11, 121. [Google Scholar] [CrossRef]
- Rogers, M.A.M.; Greene, M.T.; Saint, S.; Chenoweth, C.E.; Malani, P.N.; Trivedi, I.; Aronoff, D.M. Higher Rates of Clostridium Difficile Infection among Smokers. PLoS ONE 2012, 7, e42091. [Google Scholar] [CrossRef]
- Yurkovetskiy, L.; Burrows, M.; Khan, A.A.; Graham, L.; Volchkov, P.; Becker, L.; Antonopoulos, D.; Umesaki, Y.; Chervonsky, A.V. Gender Bias in Autoimmunity Is Influenced by Microbiota. Immunity 2013, 39, 400–412. [Google Scholar] [CrossRef]
- Markle, J.G.M.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; Von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex Differences in the Gut Microbiome Drive Hormone-Dependent Regulation of Autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef]
- Sartelli, M.; Di Bella, S.; McFarland, L.V.; Khanna, S.; Furuya-Kanamori, L.; Abuzeid, N.; Abu-Zidan, F.M.; Ansaloni, L.; Augustin, G.; Bala, M.; et al. 2019 Update of the WSES Guidelines for Management of Clostridioides (Clostridium) Difficile Infection in Surgical Patients. World J. Emerg. Surg. 2019, 14, 8. [Google Scholar] [CrossRef]
- Herzog, T.; Deleites, C.; Belyaev, O.; Chromik, A.M.; Uhl, W. [Clostridium Difficile in Visceral Surgery]. Chirurg. 2015, 86, 781–786. [Google Scholar] [CrossRef]
- Silva-Velazco, J.; Hull, T.L.; Craig Messick, J.M.C. Medical versus Surgical Patients with Clostridium Difficile Infection: Is There Any Difference? Am Surg 2016, 82, 1155–1159. [Google Scholar] [CrossRef]
- Kimura, T.; Stanhope, S.; Sugitani, T. Excess Length of Hospital Stay, Mortality and Cost Attributable to Clostridioides (Clostridium) Difficile Infection and Recurrence: A Nationwide Analysis in Japan. Epidemiol. Infect. 2020, 148, e65. [Google Scholar] [CrossRef]
- van Kleef, E.; Green, N.; Goldenberg, S.; Robotham, J.; Cookson, B.; Jit, M.; Edmunds, W.; Deeny, S. Excess Length of Stay and Mortality Due to Clostridium Difficile Infection: A Multi-State Modelling Approach. J. Hosp. Infect. 2014, 88, 213–217. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Akram, A.R.; Singanayagam, A.; Wilcox, M.H.; Hill, A.T. Risk Factors for Clostridium Difficile Infection in Hospitalized Patients with Community-Acquired Pneumonia. J. Infect. 2016, 73, 45–53. [Google Scholar] [CrossRef]
- Ragusa, R.; Giorgianni, G.; Lupo, L.; Sciacca, A.; Rametta, S.; Verde, M.L.A.; Mulè, S.; Marranzano, M. Healthcare-Associated Clostridium Difficile Infection: Role of Correct Hand Hygiene in Cross-Infection Control. J. Prev. Med. Hyg. 2018, 59, E145. [Google Scholar]
- Huang, Y.J.; Chen, J.S.; Luo, S.F.; Kuo, C.F. Comparison of Indexes to Measure Comorbidity Burden and Predict All-Cause Mortality in Rheumatoid Arthritis. J. Clin. Med. 2021, 10, 5460. [Google Scholar] [CrossRef]
- Covino, M.; Gallo, A.; Pero, E.; Simeoni, B.; Macerola, N.; Murace, C.A.; Ibba, F.; Landi, F.; Franceschi, F.; Montalto, M. Early Prognostic Stratification of Clostridioides Difficile Infection in the Emergency Department: The Role of Age and Comorbidities. J. Pers. Med. 2022, 12, 1573. [Google Scholar] [CrossRef]
- Charlson Comorbidity Index (CCI): An Independent Predictor of Outcomes in Clostridium Difficile Infection (CDI). Available online: https://journals.lww.com/ajg/fulltext/2018/10001/charlson_comorbidity_index__cci___an_independent.2742.aspx (accessed on 10 December 2023).
- Study Details Risk of C Difficile with Clindamycin, Other Antibiotics. Available online: https://www.ajmc.com/view/study-details-risk-of-c-difficile-with-clindamycin-other-antibiotics (accessed on 18 January 2024).
- Your Risk of C. Diff | CDC. Available online: https://www.cdc.gov/cdiff/risk.html (accessed on 18 January 2024).
- Webb, B.J.; Subramanian, A.; Lopansri, B.; Goodman, B.; Jones, P.B.; Ferraro, J.; Stenehjem, E.; Brown, S.M. Antibiotic Exposure and Risk for Hospital-Associated Clostridioides Difficile Infection. Antimicrob. Agents Chemother. 2020, 64, e02169-19. [Google Scholar] [CrossRef]
- Stevens, V.; Dumyati, G.; Fine, L.S.; Fisher, S.G.; Van Wijngaarden, E. Cumulative Antibiotic Exposures over Time and the Risk of Clostridium Difficile Infection. Clin. Infect. Dis. 2011, 53, 42–48. [Google Scholar] [CrossRef]
- Hensgens, M.P.M.; Goorhuis, A.; Dekkers, O.M.; Kuijper, E.J. Time Interval of Increased Risk for Clostridium Difficile Infection after Exposure to Antibiotics. J. Antimicrob. Chemother. 2012, 67, 742–748. [Google Scholar] [CrossRef]
- Del Mar Aldrete, S.; Magee, M.J.; Friedman-Moraco, R.J.; Chan, A.W.; Banks, G.G.; Burd, E.M.; Kraft, C.S. Characteristics and Antibiotic Use Associated With Short-Term Risk of Clostridium Difficile Infection Among Hospitalized Patients. Am. J. Clin. Pathol. 2015, 143, 895–900. [Google Scholar] [CrossRef][Green Version]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive Statistics and Normality Tests for Statistical Data. Ann. Card. Anaesth. 2019, 22, 67. [Google Scholar] [CrossRef]
- The Application of Statistical Analysis in the Biomedical Sciences | Basicmedical Key. Available online: https://basicmedicalkey.com/the-application-of-statistical-analysis-in-the-biomedical-sciences/ (accessed on 2 December 2023).
- Miller, A.C.; Arakkal, A.T.; Sewell, D.K.; Segre, A.M.; Tholany, J.; Polgreen, P.M. Comparison of Different Antibiotics and the Risk for Community-Associated Clostridioides Difficile Infection: A Case–Control Study. Open Forum Infect. Dis. 2023, 10, 413. [Google Scholar] [CrossRef]
- Slimings, C.; Riley, T.V. Antibiotics and Hospital-Acquired Clostridium Difficile Infection: Update of Systematic Review and Meta-Analysis. J. Antimicrob. Chemother. 2014, 69, 881–891. [Google Scholar] [CrossRef]
- Schinas, G.; Polyzou, E.; Spernovasilis, N.; Gogos, C.; Dimopoulos, G.; Akinosoglou, K. Preventing Multidrug-Resistant Bacterial Transmission in the Intensive Care Unit with a Comprehensive Approach: A Policymaking Manual. Antibiotics 2023, 12, 1255. [Google Scholar] [CrossRef]
- Karaoui, W.R.; Rustom, L.B.O.; Bou Daher, H.; Rimmani, H.H.; Rasheed, S.S.; Matar, G.M.; Mahfouz, R.; Araj, G.F.; Zahreddine, N.; Kanj, S.S.; et al. Incidence, Outcome, and Risk Factors for Recurrence of Nosocomial Clostridioides Difficile Infection in Adults: A Prospective Cohort Study. J. Infect. Public Health 2020, 13, 485–490. [Google Scholar] [CrossRef]
- European Database of Suspected Adverse Drug Reaction Reports. Available online: https://www.adrreports.eu/en/ (accessed on 11 October 2023).
- Medicines Agency, E. Introductory Cover Note, Last Updated with Chapter P.IV on Pharmacovigilance for the Paediatric Population Finalised Post-Public Consultation. In Guidelines on Good Pharmacovigilance Practices (GVP); HMA: Brussels, Belgium, 2018. [Google Scholar]
- Postigo, R.; Brosch, S.; Slattery, J.; van Haren, A.; Dogné, J.M.; Kurz, X.; Candore, G.; Domergue, F.; Arlett, P. EudraVigilance Medicines Safety Database: Publicly Accessible Data for Research and Public Health Protection. Drug Saf. 2018, 41, 665–675. [Google Scholar] [CrossRef]
- Charlson Comorbidity Index (CCI). Available online: https://www.mdcalc.com/calc/3917/charlson-comorbidity-index-cci (accessed on 18 January 2024).
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]





| Patient Characteristic | Cases n (%) | |
|---|---|---|
| Age Mean (Minimum–Maximum) | 72.1 years (18.3–94.8 years) | 111 |
| Age category * Mean (Minimum–Maximum) | 18–64 years 54.7 years (18.3–63.9 years) | 25 (22.5) |
| 65–85 years 75.1 years (65.3–84.3 years) | 74 (66.7) | |
| >85 years 89.7 years (85.8–94.8 years) | 12 (10.8) | |
| Gender | Female | 61 (55.0) |
| Male | 50 (45.0) | |
| Patients’ category | Surgical | 38 (34.2) |
| favorable outcome | 33 (86.8) | |
| unfavorable outcome | 5 (13.2) | |
| Non-surgical | 73 (65.8) | |
| favorable outcome | 46 (63.0) | |
| unfavorable outcome | 27 (37.0) | |
| Evolution | Favorable | 79 (71.2) |
| resolved/recovered | 79 (100) | |
| Unfavorable | 32 (28.8) | |
| aggravated condition | 3 (9.0) | |
| Death | 28 (88.0) | |
| not resolved/not recovered | 1 (3.0) | |
| Detection mode | Active | 64 (57.7) |
| Passive | 47 (42.3) |
| Outcome | Average Duration of Hospitalization (Days) | p-Value | |
|---|---|---|---|
| HLS-UD | favorable | 9.78 | p > 0.05 |
| unfavorable | 7.75 | ||
| HLS-AD | favorable | 11.08 | p > 0.05 |
| unfavorable | 10.91 | ||
| HLS-ICU | favorable | 2.57 | p > 0.05 |
| unfavorable | 5.63 | ||
| T-HLS | favorable | 20.80 | p > 0.05 |
| unfavorable | 18.66 |
| Age Category | Favorable n (%) | Unfavorable n (%) |
|---|---|---|
| 18–64 years | 22 (88.0) | 3 (12.0) |
| 65–85 years | 52 (70.3) | 22 (29.7) |
| >85 years | 5 (41.7) | 7 (58.3) |
| Variables | Charlson Comorbidity Index | p-Value | ||
|---|---|---|---|---|
| Average | Range | |||
| All patients | 7.6 | (0–16 points) | ||
| Gender | Female | 8.04 | (4–15 points) | p > 0.05 |
| Male | 7.14 | (0–16 points) | ||
| Category of age | 18–64 years | 6 | (4–16 points) | p < 0.01 |
| 65–85 years | 8.1 | (0–15 points) | ||
| >85 years | 8.2 | (6–11 points) | ||
| Outcome | Favorable | 7.2 | (0–15 points) | p < 0.01 |
| resolved/recovered | 7.2 | (0–15 points) | ||
| Unfavorable | 8.8 | (4–16 points) | ||
| aggravated condition | 8.3 | (7–11 points) | ||
| death | 8.8 | (4–16 points) | ||
| not resolved/not recovered | 11 | (11 points) | ||
| All Group | Subgroup 1 | Subgroup 2 | Subgroup 3 | |
|---|---|---|---|---|
| 18–64 Years | 65–85 Years | >85 Years | ||
| Mean | 1.504505 | 1.68 | 1.5 | 1.166667 |
| Standard Error | 0.103084 | 0.243036 | 0.129434 | 0.112367 |
| Median | 1 | 2 | 1 | 1 |
| Mode | 1 | 3 | 1 | 1 |
| Standard Deviation | 1.086059 | 1.215182 | 1.11343 | 0.389249 |
| Sample Variance | 1.179525 | 1.476667 | 1.239726 | 0.151515 |
| Kurtosis | 0.202974 | −1.16656 | 0.517144 | 2.64 |
| Skewness | 0.66032 | 0.07172 | 0.734379 | 2.055237 |
| Range | 5 | 4 | 5 | 1 |
| Minimum | 0 | 0 | 0 | 1 |
| Maximum | 5 | 4 | 5 | 2 |
| Sum | 167 | 42 | 111 | 14 |
| Count | 111 | 25 | 74 | 12 |
| Favorable | Unfavorable | |
|---|---|---|
| Mean | 1.531646 | 1.4375 |
| Standard Error | 0.120633 | 0.200491 |
| Median | 1 | 1 |
| Mode | 1 | 1 |
| Standard Deviation | 1.072206 | 1.134147 |
| Sample Variance | 1.149627 | 1.28629 |
| Kurtosis | 0.439452 | −0.07617 |
| Skewness | 0.652101 | 0.730805 |
| Range | 5 | 4 |
| Minimum | 0 | 0 |
| Maximum | 5 | 4 |
| Sum | 121 | 46 |
| Count | 79 | 32 |
| CFT | COL | CPX | GEN | LIN | MER | PIP/TAZ | |||
|---|---|---|---|---|---|---|---|---|---|
| Total ICSRs, n | 85 | 2 | 36 | 6 | 6 | 36 | 78 | ||
| Reporter Group | HPs | n | 85 | 2 | 30 | 6 | 6 | 34 | 76 |
| (%) | (100) | (100) | (85.7) | (100) | (100) | (94.4) | (97.4) | ||
| N-HPs | n | 0 | 0 | 5 | 0 | 0 | 2 | 2 | |
| (%) | (0.0) | (0.0) | (14.3) | (0.0) | (0.0) | (5.6) | (2.6) | ||
| Countries | EEA | n | 27 | 2 | 7 | 6 | 5 | 10 | 14 |
| (%) | (31.8) | (100.0) | (20.0) | (100.0) | (83.3) | (27.8) | (17.9) | ||
| Non-EEA | n | 58 | 0 | 28 | 0 | 1 | 26 | 64 | |
| (%) | (68.2) | (0.0) | 80.0) | (0.0) | (16.7) | (72.2) | (82.1) | ||
| Age Category | 2 months–2 years | n | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| (%) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (2.6) | ||
| 3–11 years | n | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| (%) | (1.2) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (1.3) | ||
| 18–64 years | n | 17 | 2 | 13 | 1 | 2 | 13 | 13 | |
| (%) | (20.0) | (100.) | (36.1) | (16.7) | (33.3) | (36.1) | (16.7) | ||
| 65–85 years | n | 34 | 0 | 17 | 3 | 4 | 18 | 31 | |
| (%) | (40.0) | (0.0) | (47.2) | (50.0) | (66.7) | (50.0) | (39.7) | ||
| >85 years | n | 31 | 0 | 4 | 1 | 0 | 4 | 27 | |
| (%) | (36.5) | (0.0) | (11.1) | (16.7) | (0.0) | (11.1) | (34.6) | ||
| Not specified | n | 2 | 0 | 2 | 1 | 0 | 1 | 4 | |
| (%) | (2.4) | (0.0) | (5.6) | (16.7) | (0.0) | (2.8) | (5.1) | ||
| Gender | Male | n | 47 | 0 | 14 | 3 | 2 | 26 | 30 |
| (%) | (55.3) | (0.0) | (38.9) | (50.0) | (33.3) | (72.2) | (38.5) | ||
| Female | n | 38 | 2 | 22 | 3 | 4 | 9 | 46.00 | |
| (%) | (44.7) | (100.0) | (61.1) | (50.0) | (66.7) | (25.0) | (59.0) | ||
| Not specified | n | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 2.00 | |
| (%) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | (2.8) | (2.6) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vintila, B.I.; Arseniu, A.M.; Morgovan, C.; Butuca, A.; Bîrluțiu, V.; Dobrea, C.M.; Rus, L.L.; Ghibu, S.; Bereanu, A.S.; Arseniu, R.; et al. A Real-World Study on the Clinical Characteristics, Outcomes, and Relationship between Antibiotic Exposure and Clostridioides difficile Infection. Antibiotics 2024, 13, 144. https://doi.org/10.3390/antibiotics13020144
Vintila BI, Arseniu AM, Morgovan C, Butuca A, Bîrluțiu V, Dobrea CM, Rus LL, Ghibu S, Bereanu AS, Arseniu R, et al. A Real-World Study on the Clinical Characteristics, Outcomes, and Relationship between Antibiotic Exposure and Clostridioides difficile Infection. Antibiotics. 2024; 13(2):144. https://doi.org/10.3390/antibiotics13020144
Chicago/Turabian StyleVintila, Bogdan Ioan, Anca Maria Arseniu, Claudiu Morgovan, Anca Butuca, Victoria Bîrluțiu, Carmen Maximiliana Dobrea, Luca Liviu Rus, Steliana Ghibu, Alina Simona Bereanu, Rares Arseniu, and et al. 2024. "A Real-World Study on the Clinical Characteristics, Outcomes, and Relationship between Antibiotic Exposure and Clostridioides difficile Infection" Antibiotics 13, no. 2: 144. https://doi.org/10.3390/antibiotics13020144
APA StyleVintila, B. I., Arseniu, A. M., Morgovan, C., Butuca, A., Bîrluțiu, V., Dobrea, C. M., Rus, L. L., Ghibu, S., Bereanu, A. S., Arseniu, R., Roxana Codru, I., Sava, M., & Gabriela Gligor, F. (2024). A Real-World Study on the Clinical Characteristics, Outcomes, and Relationship between Antibiotic Exposure and Clostridioides difficile Infection. Antibiotics, 13(2), 144. https://doi.org/10.3390/antibiotics13020144










