Outpatient Antibiotic Prescribing Patterns in Children among Primary Healthcare Institutions in China: A Nationwide Retrospective Study, 2017–2019
Abstract
1. Introduction
2. Results
2.1. Characteristics of Antibiotic Prescriptions for Children
2.2. Antibiotic Prescribing at the National Level
2.3. Antibiotic Prescribing at Diagnostic Levels
2.4. Predictors Associated with Antibiotic Prescribing
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Data Sampling and Collection
4.3. Definition
4.4. Measurements
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Adjei, A.; Kukula, V.; Narh, C.T.; Odopey, S.; Arthur, E.; Odonkor, G.; Mensah, M.M.; Olliaro, P.; Horgan, P.; Dittrich, S.; et al. Impact of Point-of-Care Rapid Diagnostic Tests on Antibiotic Prescription Among Patients Aged < 18 Years in Primary Healthcare Settings in 2 Peri-Urban Districts in Ghana: Randomized Controlled Trial Results. Clin. Infect. Dis. 2023, 77 (Suppl. S2), S145–S155. [Google Scholar] [CrossRef]
- Global Action Plan on Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 3 February 2023).
- Sulis, G.; Adam, P.; Nafade, V.; Gore, G.; Daniels, B.; Daftary, A.; Das, J.; Gandra, S.; Pai, M. Antibiotic prescription practices in primary care in low- and middle-income countries: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003139. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Song, F.; Gong, Y.; Tu, X.; Wang, Y.; Cao, S.; Liu, J.; Lu, Z. A systematic review of antibiotic utilization in China. J. Antimicrob. Chemother. 2013, 68, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Notification on Establishing Surveillance Network for Antibiotic Use and Antimicrobial Resistance. Available online: https://www.gov.cn/govweb/zwgk/2006-07/18/content_337795.htm (accessed on 5 February 2023).
- Raut, S.; Adhikari, B. The need to focus China’s national plan to combat antimicrobial resistance. Lancet Infect. Dis. 2017, 17, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Duong, Q.A.; Pittet, L.F.; Curtis, N.; Zimmermann, P. Antibiotic exposure and adverse long-term health outcomes in children: A systematic review and meta-analysis. J. Infect. 2022, 85, 213–300. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Sun, R.; Yao, T.; Zhou, X.; Harbarth, S. Factors influencing inappropriate use of antibiotics in outpatient and community settings in China: A mixed-methods systematic review. BMJ Glob. Health 2020, 5, e003599. [Google Scholar] [CrossRef]
- Wushouer, H.; Zhou, Y.; Zhang, X.; Fu, M.; Fan, D.; Shi, L.; Guan, X. Secular trend analysis of antibiotic utilisation in China’s hospitals 2011-2018, a retrospective analysis of procurement data. Antimicrob. Resist. Infect. Control 2020, 9, 53. [Google Scholar] [CrossRef]
- Wushouer, H.; Zhou, Y.; Zhang, W.; Hu, L.; Du, K.; Yang, Y.; Yao, G.; Little, P.; Zheng, B.; Guan, X.; et al. Inpatient antibacterial use trends and patterns, China, 2013–2021. Bull. World Health Organ. 2023, 101, 248–261B. [Google Scholar] [CrossRef]
- Fu, M.; Wushouer, H.; Hu, L.; Li, N.; Guan, X.; Shi, L.; Ross-Degnan, D. Outpatient prescribing pattern for acute bronchitis in primary healthcare settings in China. NPJ Prim. Care Respir. Med. 2021, 31, 24. [Google Scholar] [CrossRef]
- Fu, M.; Gong, Z.; Zhu, Y.; Li, C.; Zhou, Y.; Hu, L.; Li, H.; Wushouer, H.; Guan, X.; Shi, L. Inappropriate antibiotic prescribing in primary healthcare facilities in China: A nationwide survey, 2017–2019. Clin. Microbiol. Infect. 2023, 29, 602–609. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; Wang, X.; Zheng, Y.; Xiao, Y. Use and prescription of antibiotics in primary health care settings in China. JAMA Intern. Med. 2014, 174, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Xu, B.; Shen, A.; Shen, K. Antibiotic prescriptions for children younger than 5 years with acute upper respiratory infections in China: A retrospective nationwide claims database study. BMC Infect. Dis. 2021, 21, 339. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Li, F.; Wang, J.; Zhuo, C.; Zou, G. Antibiotic prescription for children with acute respiratory tract infections in rural primary healthcare in Guangdong province, China: A cross-sectional study. BMJ Open 2023, 13, e068545. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lu, J.; Sun, C.; Wang, X.; Hu, Y.J.; Zhou, X. A cross-sectional study of antibiotic misuse among Chinese children in developed and less developed provinces. J. Infect. Dev. Ctries. 2020, 14, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Trinh, N.T.H.; Cohen, R.; Lemaitre, M.; Chahwakilian, P.; Coulthard, G.; Bruckner, T.A.; Milic, D.; Levy, C.; Chalumeau, M.; Cohen, J.F. Community antibiotic prescribing for children in France from 2015 to 2017: A cross-sectional national study. J. Antimicrob. Chemother. 2020, 75, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Howarth, T.; Brunette, R.; Davies, T.; Andrews, R.M.; Patel, B.K.; Tong, S.; Barzi, F.; Kearns, T.M. Antibiotic use for Australian Aboriginal children in three remote Northern Territory communities. PLoS ONE 2020, 15, e0231798. [Google Scholar] [CrossRef]
- Van Aerde, K.J.; de Haan, L.; van Leur, M.; Gerrits, G.P.; Schers, H.; Moll, H.A.; Hagedoorn, N.N.; Herberg, J.A.; Levin, M.; Rivero-Calle, I.; et al. Respiratory Tract Infection Management and Antibiotic Prescription in Children: A Unique Study Comparing Three Levels of Healthcare in The Netherlands. Pediatr. Infect. Dis. J. 2021, 40, e100–e105. [Google Scholar] [CrossRef]
- Dik, J.W.; Sinha, B.; Friedrich, A.W.; Lo-Ten-Foe, J.R.; Hendrix, R.; Köck, R.; Bijker, B.; Postma, M.J.; Freitag, M.H.; Glaeske, G.; et al. Cross-border comparison of antibiotic prescriptions among children and adolescents between the north of the Netherlands and the north-west of Germany. Antimicrob. Resist. Infect. Control 2016, 5, 14. [Google Scholar] [CrossRef]
- De Bie, S.; Kaguelidou, F.; Verhamme, K.M.; De Ridder, M.; Picelli, G.; Straus, S.M.; Giaquinto, C.; Stricker, B.H.; Bielicki, J.; Sharland, M.; et al. Using Prescription Patterns in Primary Care to Derive New Quality Indicators for Childhood Community Antibiotic Prescribing. Pediatr. Infect. Dis. J. 2016, 35, 1317–1323. [Google Scholar] [CrossRef]
- Di Mario, S.; Gagliotti, C.; Buttazzi, R.; Cisbani, L.; Di Girolamo, C.; Brambilla, A.; Moro, M.L.; Regional Working Group “Progetto ProBA-Progetto Bambini e Antibiotici-2014”. Observational pre-post study showed that a quality improvement project reduced paediatric antibiotic prescribing rates in primary care. Acta Paediatr. 2018, 107, 1805–1809. [Google Scholar] [CrossRef]
- Ekins-Daukes, S.; McLay, J.S.; Taylor, M.W.; Simpson, C.R.; Helms, P.J. Antibiotic prescribing for children. Too much and too little? Retrospective observational study in primary care. Br. J. Clin. Pharmacol. 2003, 56, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Wushouer, H.; Wang, Z.; Tian, Y.; Zhou, Y.; Zhu, D.; Vuillermin, D.; Shi, L.; Guan, X. The impact of physicians’ knowledge on outpatient antibiotic use: Evidence from China’s county hospitals. Medicine 2020, 99, e18852. [Google Scholar] [CrossRef]
- Currie, J.; Lin, W.; Meng, J. Addressing Antibiotic Abuse in China: An Experimental Audit Study. J. Dev. Econ. 2014, 110, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Pfoh, E.R.; Misra Hebert, A.D.; Chaitoff, A.; Shapiro, A.; Gupta, N.; Rothberg, M.B. Attitudes of High Versus Low Antibiotic Prescribers in the Management of Upper Respiratory Tract Infections: A Mixed Methods Study. J. Gen. Intern. Med. 2020, 35, 1182–1188. [Google Scholar] [CrossRef]
- Liu, C.; Wang, D.; Duan, L.; Zhang, X.; Liu, C. Coping With Diagnostic Uncertainty in Antibiotic Prescribing: A Latent Class Study of Primary Care Physicians in Hubei China. Front. Public Health 2021, 9, 741345. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Harbarth, S.; Hargreaves, J.R.; Zhou, X.; Li, L. Large-scale survey of parental antibiotic use for paediatric upper respiratory tract infections in China: Implications for stewardship programmes and national policy. Int. J. Antimicrob. Agents 2021, 57, 106302. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, X.; Sun, K.S.; Lin, L.; Zhou, X. Parental self-medication with antibiotics for children promotes antibiotic over-prescribing in clinical settings in China. Antimicrob. Resist. Infect. Control 2020, 9, 150. [Google Scholar] [CrossRef]
- Nowakowska, M.; van Staa, T.; Mölter, A.; Ashcroft, D.M.; Tsang, J.Y.; White, A.; Welfare, W.; Palin, V. Antibiotic choice in UK general practice: Rates and drivers of potentially inappropriate antibiotic prescribing. J. Antimicrob. Chemother. 2019, 74, 3371–3378. [Google Scholar] [CrossRef]
- Piovani, D.; Clavenna, A.; Cartabia, M.; Bortolotti, A.; Fortino, I.; Merlino, L.; Bonati, M. Assessing the quality of paediatric antibiotic prescribing by community paediatricians: A database analysis of prescribing in Lombardy. BMJ Paediatr. Open 2017, 1, e000169. [Google Scholar] [CrossRef]
- Xu, J.B.; Xu, C.; Zhang, R.B.; Wu, M.; Pan, C.K.; Li, X.J.; Wang, Q.; Zeng, F.F.; Zhu, S. Associations of procalcitonin, C-reaction protein and neutrophil-to-lymphocyte ratio with mortality in hospitalized COVID-19 patients in China. Sci. Rep. 2020, 10, 15058. [Google Scholar] [CrossRef] [PubMed]
- Gerber, J.S.; Ross, R.K.; Bryan, M.; Localio, A.R.; Szymczak, J.E.; Wasserman, R.; Barkman, D.; Odeniyi, F.; Conaboy, K.; Bell, L.; et al. Association of Broad- vs Narrow-Spectrum Antibiotics with Treatment Failure, Adverse Events, and Quality of Life in Children With Acute Respiratory Tract Infections. JAMA 2017, 318, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.; Greene, G.; Naik, G.; Hughes, K.; Butler, C.C.; Hay, A.D. Antibiotic prescribing quality for children in primary care: An observational study. Br. J. Gen. Pract. 2018, 68, e90–e96. [Google Scholar] [CrossRef] [PubMed]
- Group A Streptococcal Tonsilopharyngitis in Children and Adolescents: Clinical Features and Diagnosis. Available online: https://www.uptodate.com/contents/zh-Hans/group-a-streptococcal-tonsillopharyngitis-in-children-and-adolescents-clinical-features-and-diagnosis?topicRef=2875 (accessed on 1 November 2023).
- Cotten, C.M. Adverse consequences of neonatal antibiotic exposure. Curr. Opin. Pediatr. 2016, 28, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Broom, J.K.; Broom, A.F.; Kirby, E.R.; Gibson, A.F.; Post, J.J. Clinical and social barriers to antimicrobial stewardship in pulmonary medicine: A qualitative study. Am. J. Infect. Control 2017, 45, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Tarrant, C.; Krockow, E.M. Antibiotic overuse: Managing uncertainty and mitigating against overtreatment. BMJ Qual. Saf. 2022, 31, 163–167. [Google Scholar] [CrossRef]
- Guidelines for ATC Classification and DDD Assignment. Available online: https://www.whocc.no/atc_ddd_index_and_guidelines/guidelines/ (accessed on 5 February 2023).
- National Guiding Principles for Antimicrobial. Available online: https://www.gov.cn/xinwen/2015-08/27/content_2920799.htm (accessed on 10 February 2023).
- 2021 AWaRe Classification. Available online: https://www.who.int/publications/i/item/2021-aware-classification (accessed on 5 March 2023).
- China Health and Family Planning Statistical Yearbook in 2017. Available online: https://www.cpdrc.org.cn/en/publications/Yearbook/202006/t20200630_3038.html (accessed on 6 March 2023).
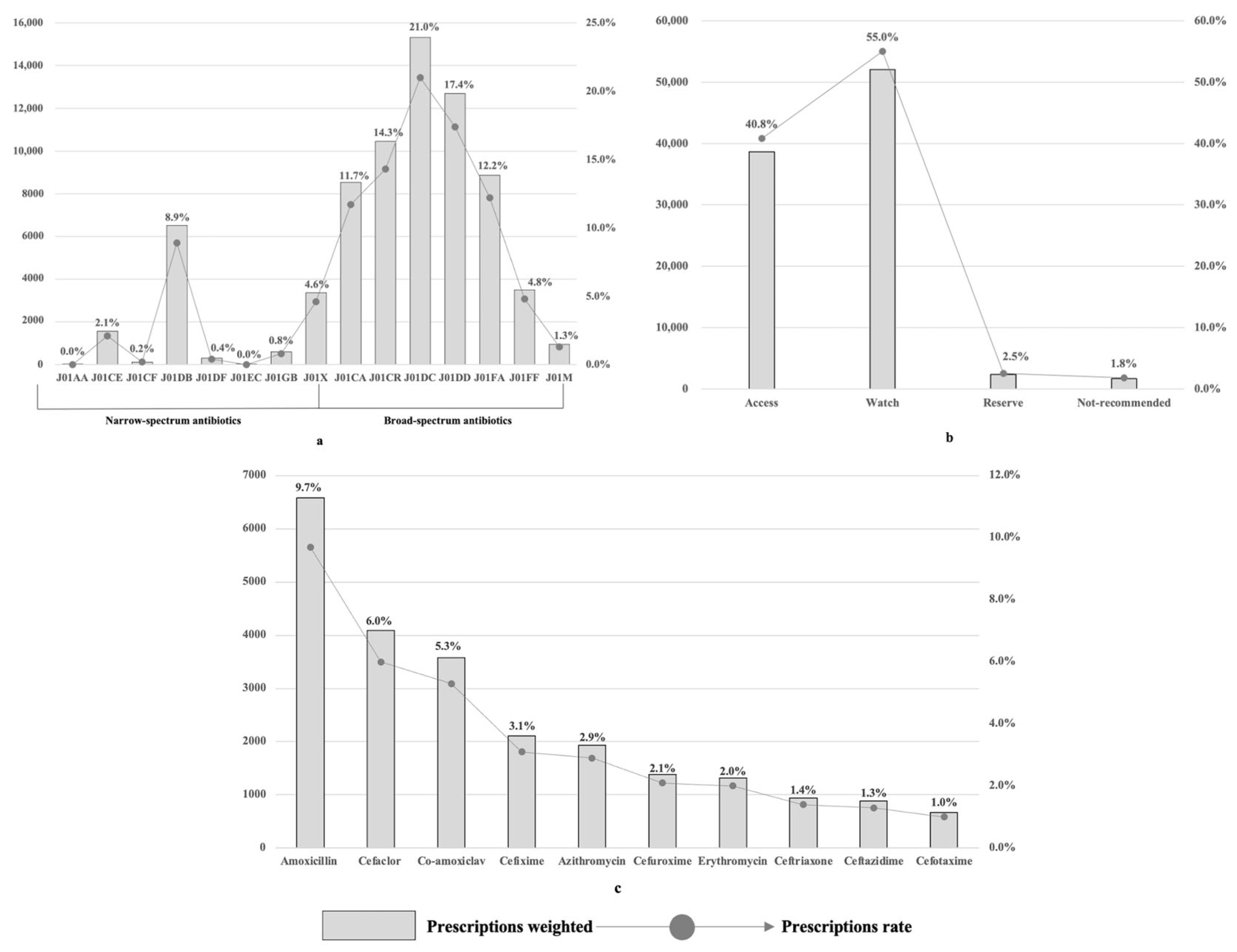
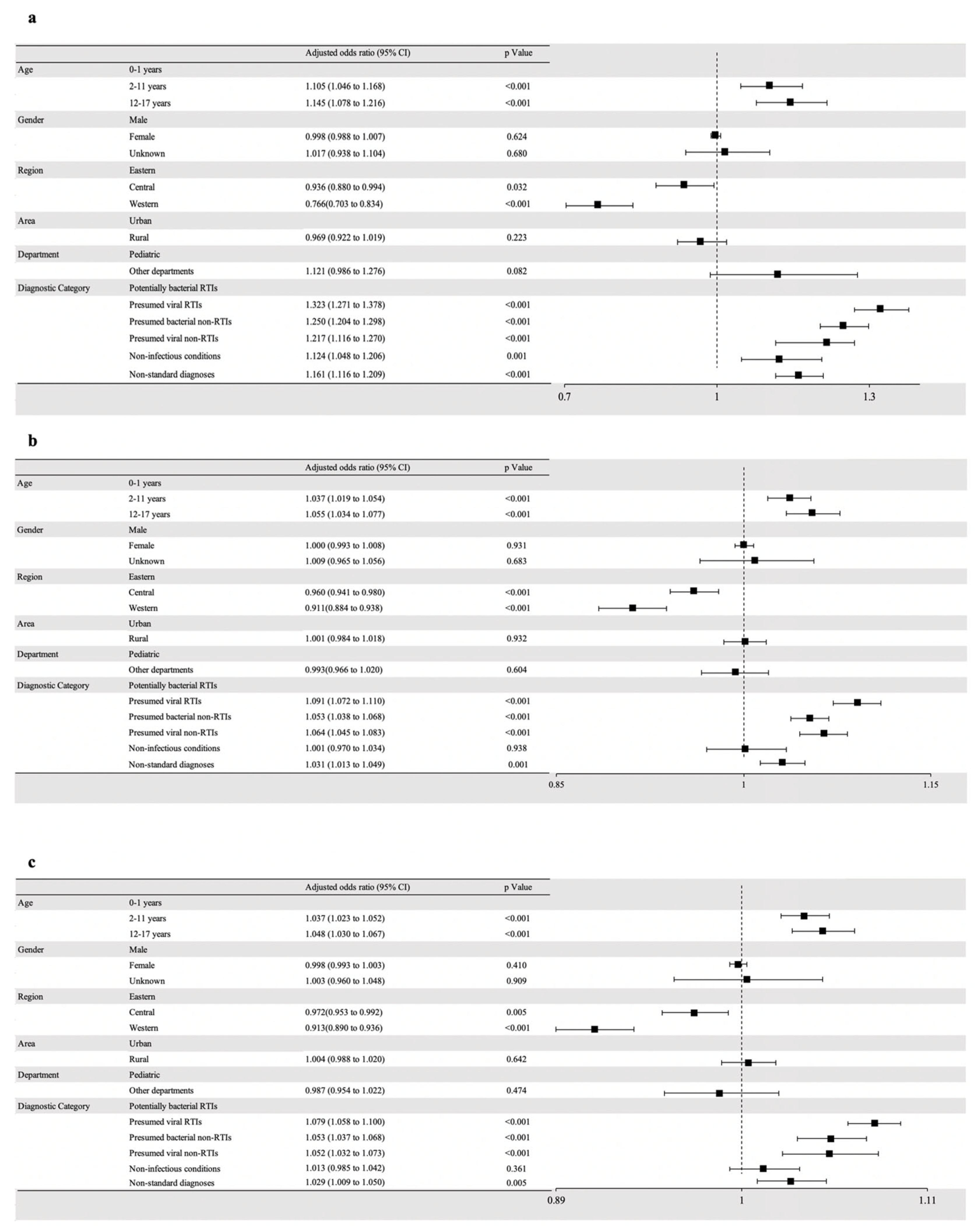
| Characteristics | Weighted Sample Size | Prescribed ATBs | Multi-Prescribing ATBs | Broad-Spectrum ATBs | ||||
|---|---|---|---|---|---|---|---|---|
| Prescriptions | % | Prescriptions | PR 1 | Prescriptions | PR 2 | Prescriptions | PR 3 | |
| N = 155,262.2 | 100.0 | N = 67,519.1 | 43.5% | N = 6663.6 | 9.9% | N = 57,222.5 | 84.8% | |
| Age | ||||||||
| 0–1 years | 6559.9 | 4.2 | 2257.2 | 34.4% | 308.1 | 13.6% | 1973.8 | 87.4% |
| 2–11 years | 114,698.9 | 73.9 | 50,168.7 | 43.7% | 4642.8 | 9.3% | 42,942.0 | 85.6% |
| 12–17 years | 34,003.5 | 21.9 | 15,093.2 | 44.4% | 1712.7 | 11.3% | 12,306.8 | 81.5% |
| Gender | ||||||||
| Male | 89,288.2 | 57.5 | 38,899.8 | 43.6% | 3904.3 | 10.0% | 33,064.6 | 85.0% |
| Female | 65,789.4 | 42.4 | 28,544.3 | 43.4% | 2757.0 | 9.7% | 24,105.1 | 84.4% |
| Unknown | 147.5 | 0.1 | 59.4 | 40.3% | 1.12 | 1.9% | 39.45 | 66.4% |
| Region | ||||||||
| Eastern | 57,929.4 | 37.3 | 29,238.2 | 50.5% | 2623.9 | 9.0% | 23,083.7 | 79.0% |
| Central | 79,351.5 | 51.1 | 33,031.9 | 41.6% | 3416.7 | 10.3% | 29,778.5 | 90.2% |
| Western | 17,981.4 | 11.6 | 5248.9 | 29.2% | 623.0 | 12.0% | 4360.3 | 83.1% |
| Area | ||||||||
| Urban | 79,202.4 | 51.0 | 36,576.4 | 46.2% | 3659.3 | 10.0% | 31,031.8 | 84.8% |
| Rural | 76,059.8 | 49.0 | 30,942.7 | 40.7% | 3004.2 | 9.7% | 26,190.7 | 84.6% |
| Department | ||||||||
| Pediatric | 131,551.1 | 84.7 | 55,177.9 | 41.9% | 5898.4 | 10.7% | 47,043.6 | 85.3% |
| Other departments | 23,711.2 | 15.3 | 12,341.2 | 52.0% | 765.1 | 6.2% | 10,178.9 | 82.5% |
| Diagnostic Category 1 | Conditions | Prescribed ATBs | Multi-Prescribing ATBs | Broad-Spectrum ATBs | |||
|---|---|---|---|---|---|---|---|
| Prescriptions | PR 2 | Prescriptions | PR 3 | Prescriptions | PR 4 | ||
| All conditions (n = 155,262.2) | N = 67,519.1 | 43.5% | N = 6663.6 | 9.9% | N = 57,222.5 | 84.8% | |
| Potentially bacterial RTIs (n = 9977.2) | n = 5487.1 | 55.0% | n = 687.4 | 12.5% | n = 4630.3 | 83.3% | |
| Otitis (n = 736.5) | 502.2 | 68.2% | 87.6 | 17.4% | 418.2 | 87.0% | |
| Pharyngitis (n = 5194.8) | 2640.5 | 50.8% | 202.1 | 7.7% | 2297.2 | 83.0% | |
| Sinusitis (n = 764.4) | 464.1 | 60.7% | 39.7 | 8.6% | 385.1 | 81.4% | |
| Pneumonia (n = 3281.5) | 1880.3 | 57.3% | 373.9 | 19.9% | 1529.8 | 84.4% | |
| Presumed viral RTIs (n = 84,514.7) | n = 39,859.8 | 47.2% | n = 3539.5 | 8.9% | n = 34,440.0 | 86.4% | |
| Bronchitis (n = 25,846.6) | 13,883.5 | 53.7% | 1357.0 | 9.8% | 11,974.1 | 86.2% | |
| Common cold (n = 3963.9) | 1331.3 | 33.6% | 134.6 | 10.1% | 1157.6 | 87.0% | |
| Bronchiolitis (n = 205.9) | 107.1 | 52.0% | 5.06 | 4.7% | 97.5 | 91.1% | |
| Other viral RTIs (n = 54,498.3) | 24,538.0 | 45.0% | 2073.9 | 8.5% | 21,210.8 | 86.4% | |
| Presumed bacterial non-RTIs (n = 26,911.9) | n = 12,223.5 | 45.4% | n = 1336.1 | 10.9% | n = 10,218.7 | 83.6% | |
| Skin and soft tissue infections (n = 2418.6) | 440.1 | 18.2% | 139.89 | 31.8% | 388.9 | 88.4% | |
| Urinary tract infections (n = 144.2) | 110.1 | 76.3% | 39.42 | 35.8% | 99.0 | 90.0% | |
| Gastrointestinal infections (n = 6335.7) | 2623.4 | 41.4% | 232.0 | 8.8% | 2081.9 | 79.4% | |
| Miscellaneous bacterial infections (n = 18,013.4) | 9050.0 | 50.2% | 979.5 | 10.8% | 7649.0 | 84.5% | |
| Presumed viral (Other pathogens) non-RTIs (n = 4144.9) | n = 1324.2 | 31.9% | n = 509.8 | 38.5% | n = 922.9 | 69.7% | |
| Miscellaneous viral illnesses (n = 1079.1) | 384.3 | 35.6% | 75.47 | 19.6% | 325.3 | 84.6% | |
| Miscellaneous illnesses caused by another micro-organism (n = 3065.8) | 939.9 | 30.7% | 68.4 | 7.3% | 597.7 | 63.6% | |
| Non-infectious conditions (n = 20,557.0) | n = 4826.1 | 23.5% | n = 483.4 | 10.0% | n = 3923.4 | 81.3% | |
| Non-standard diagnose (n = 9156.6) | n = 3798.4 | 41.5% | n = 107.4 | 2.8% | n = 3087.2 | 81.3% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wushouer, H.; Du, K.; Yu, J.; Zhang, W.; Hu, L.; Ko, W.; Fu, M.; Zheng, B.; Shi, L.; Guan, X. Outpatient Antibiotic Prescribing Patterns in Children among Primary Healthcare Institutions in China: A Nationwide Retrospective Study, 2017–2019. Antibiotics 2024, 13, 70. https://doi.org/10.3390/antibiotics13010070
Wushouer H, Du K, Yu J, Zhang W, Hu L, Ko W, Fu M, Zheng B, Shi L, Guan X. Outpatient Antibiotic Prescribing Patterns in Children among Primary Healthcare Institutions in China: A Nationwide Retrospective Study, 2017–2019. Antibiotics. 2024; 13(1):70. https://doi.org/10.3390/antibiotics13010070
Chicago/Turabian StyleWushouer, Haishaerjiang, Kexin Du, Junxuan Yu, Wanmeng Zhang, Lin Hu, Weihsin Ko, Mengyuan Fu, Bo Zheng, Luwen Shi, and Xiaodong Guan. 2024. "Outpatient Antibiotic Prescribing Patterns in Children among Primary Healthcare Institutions in China: A Nationwide Retrospective Study, 2017–2019" Antibiotics 13, no. 1: 70. https://doi.org/10.3390/antibiotics13010070
APA StyleWushouer, H., Du, K., Yu, J., Zhang, W., Hu, L., Ko, W., Fu, M., Zheng, B., Shi, L., & Guan, X. (2024). Outpatient Antibiotic Prescribing Patterns in Children among Primary Healthcare Institutions in China: A Nationwide Retrospective Study, 2017–2019. Antibiotics, 13(1), 70. https://doi.org/10.3390/antibiotics13010070









