Antibiotic-Coated Intramedullary Nailing Managing Long Bone Infected Non-Unions: A Meta-Analysis of Comparative Studies
Abstract
1. Introduction
2. Results
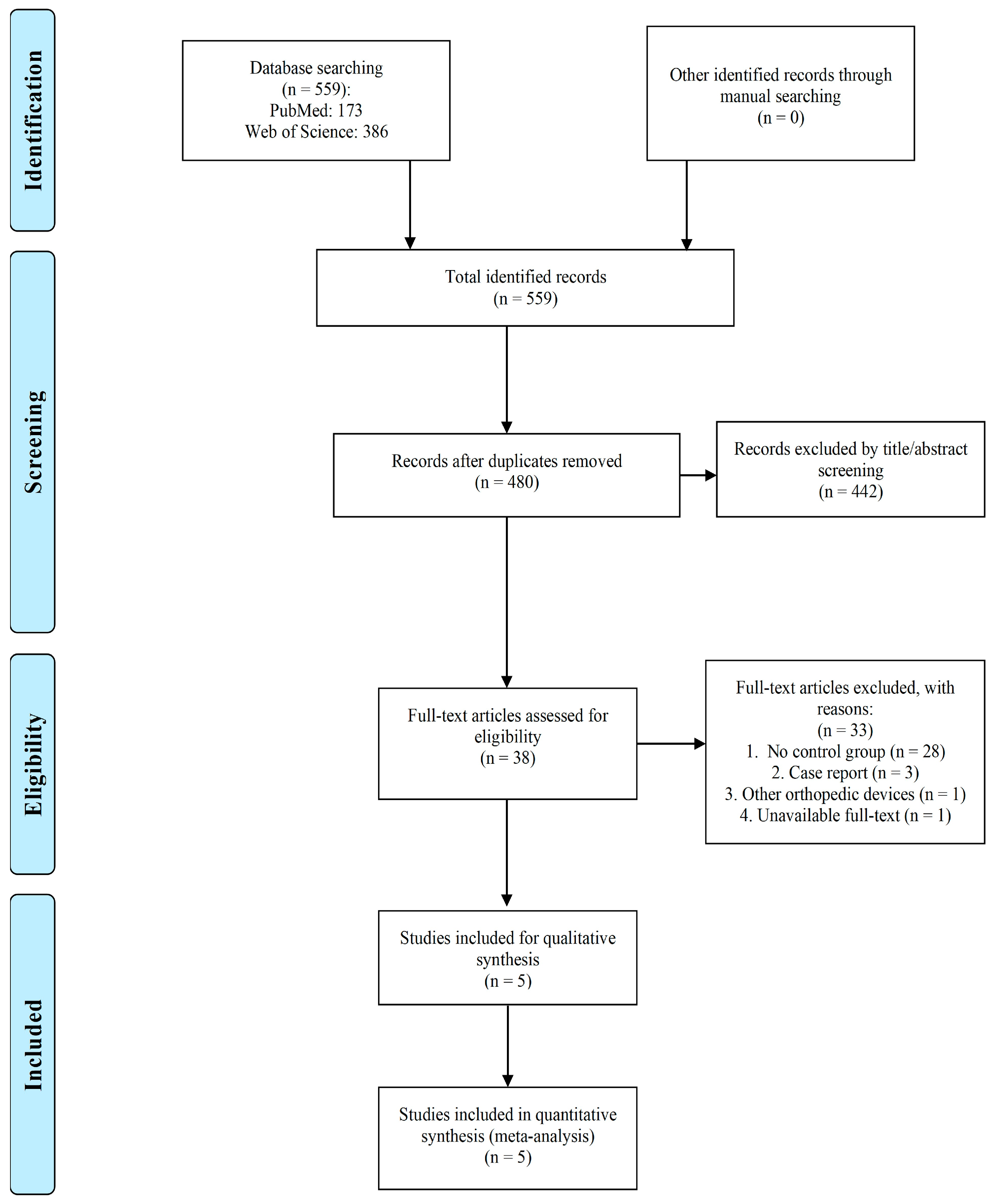
2.1. Study Selection
2.2. Risk of Bias
2.3. Baseline Characteristics
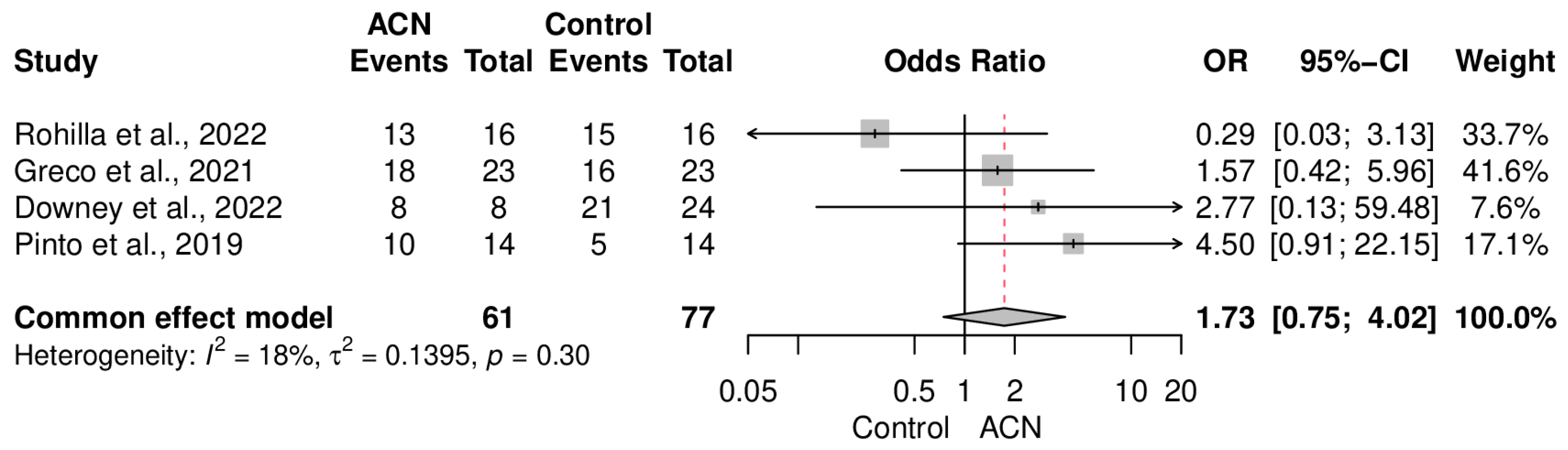
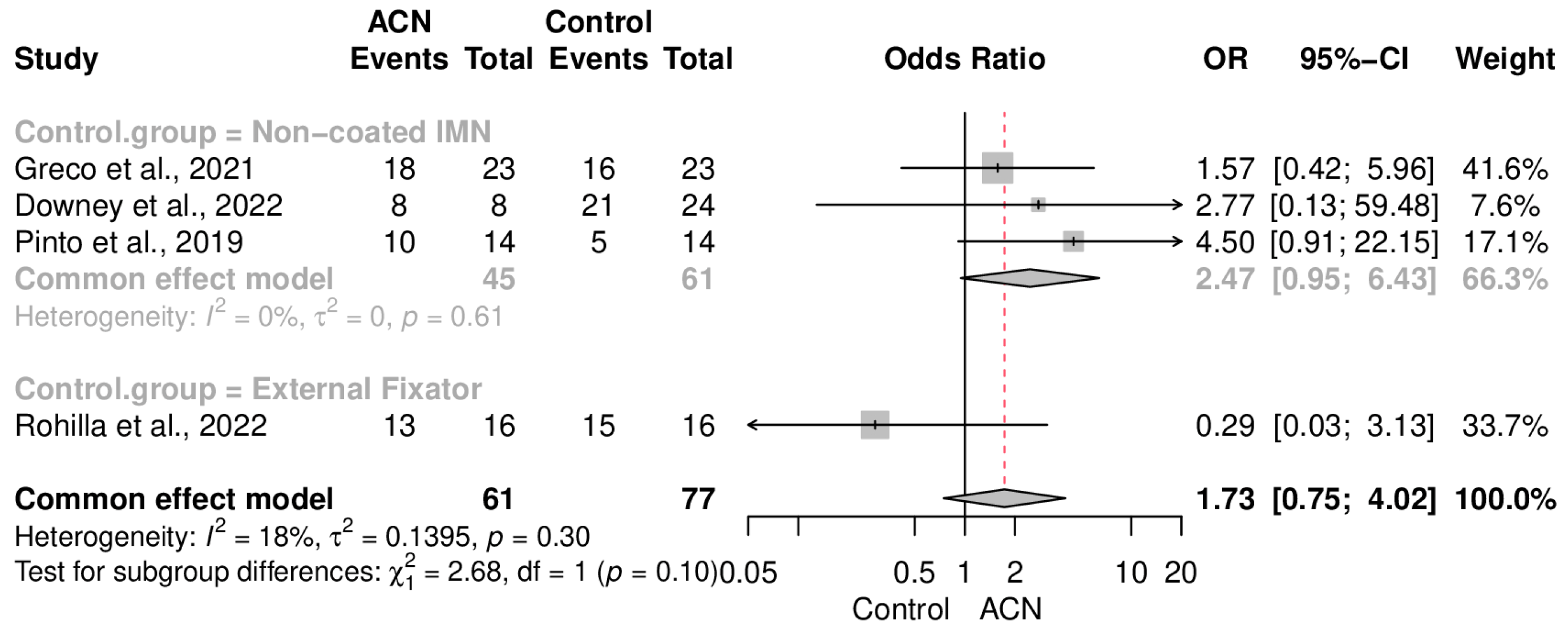
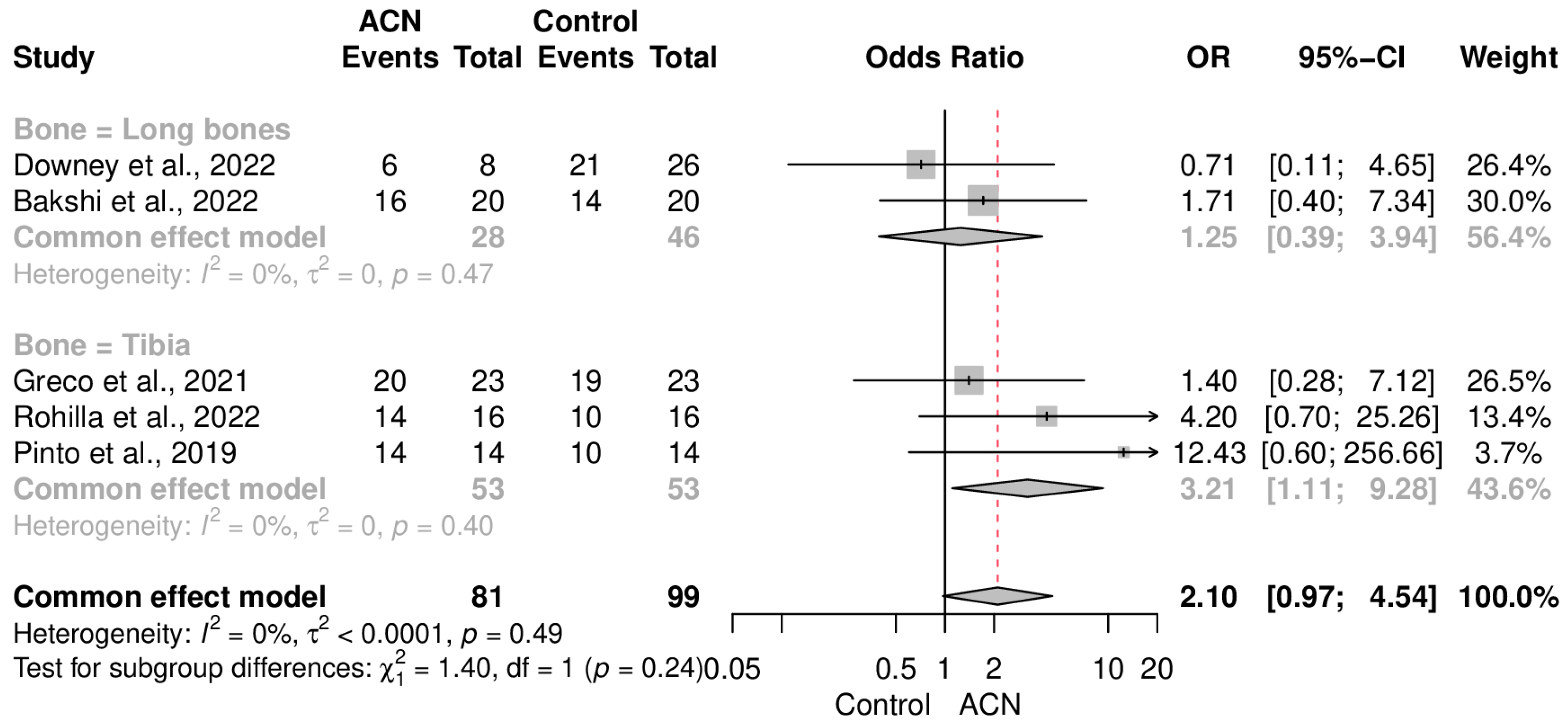
2.4. Infection Control
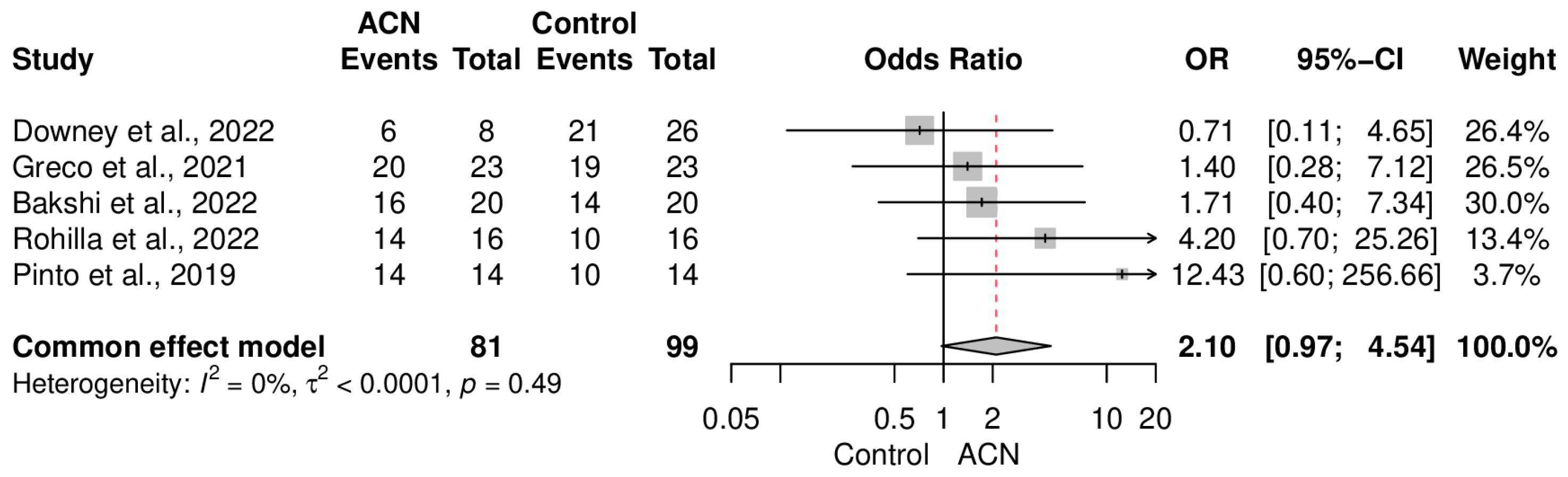
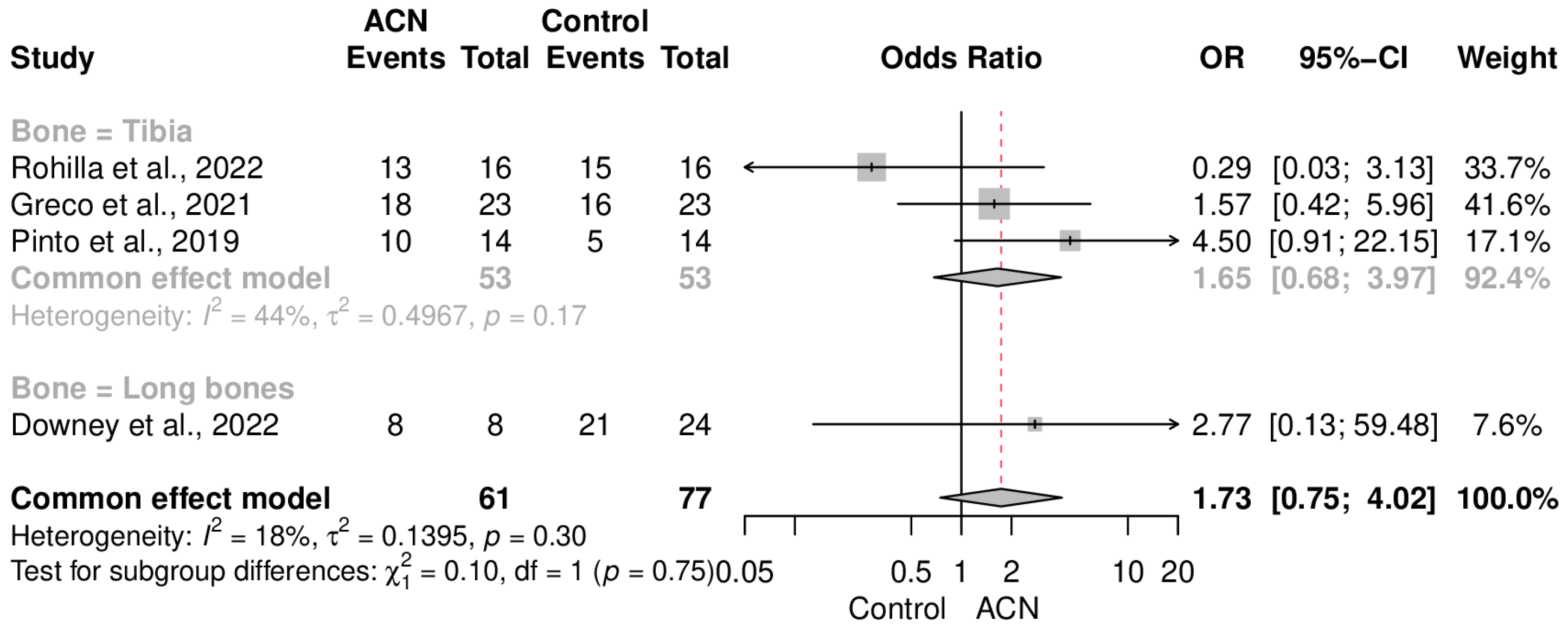
2.5. Union Rate
2.6. Secondary Endpoints
3. Discussion
4. Materials and Methods
4.1. Search Strategy and Screening
4.2. Inclusion and Exclusion Criteria
4.3. Data Extraction and Quality Assessment
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Search strategy |
| PubMed: |
| (“Antibacterial coating*”[Title/Abstract] OR “Antibacterial coated”[Title/Abstract] OR “Antimicrobial coated”[Title/Abstract] OR “Antibacterial-coated”[Title/Abstract] OR “Antimicrobial-coated”[Title/Abstract] OR “Antimicrobial coating*”[Title/Abstract] OR “Antibiotic-loaded”[Title/Abstract] OR “Antibiotic coating*”[Title/Abstract] OR “Gentamicin coating*”[Title/Abstract] OR “Antibiotic coated”[Title/Abstract] OR “Gentamicin coated”[Title/Abstract] OR “Antibiotic-coated”[Title/Abstract] OR “Gentamicin-coated”[Title/Abstract] OR “Antibiotic-impregnated”[Title/Abstract] OR “Antibiotic impregnated”[Title/Abstract]) AND (“Fracture Healing”[MeSH] OR “Fracture Healing*”[Title/Abstract] OR “Union”[Title/Abstract] OR “Malunion”[Title/Abstract] OR “Mal union”[Title/Abstract] OR “Mal-union”[Title/Abstract] OR “Nonunion”[Title/Abstract] OR “Non-union”[Title/Abstract] OR “Non union”[Title/Abstract] OR “delayed union”[Title/Abstract] OR “Ununited”[Title/Abstract] OR “United”[Title/Abstract]) |
| Result: 173 |
| Web of Science: |
| (ALL = (“Antibacterial coating” OR “Antibacterial coated” OR “Antimicrobial coated” OR “Antibacterial-coated” OR “Antimicrobial-coated” OR “Antimicrobial coating” OR “Antibiotic-loaded” OR “Antibiotic coating” OR “Gentamicin coating” OR “Antibiotic coated” OR “Gentamicin coated” OR “Antibiotic-coated” OR “Gentamicin-coated” OR “Antibiotic-impregnated” OR “Antibiotic impregnated”)) AND ALL = (“Fracture Healing” OR “Union” OR “Malunion” OR “Mal union” OR “Mal-union” OR “Nonunion” OR “Non-union” OR “Non union” OR “delayed union” OR “Ununited” OR “United”) |
| Results: 386 |
Appendix B
| Author, Year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rohilla et al., 2022 [22] | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | 10 |
| Bakshi et al., 2022 [18] | Y | Y | Y | N | N | Y | Y | Y | Y | N | Y | 8 |
| Downey et al., 2022 [19] | Y | Y | Y | N | N | Y | Y | Y | Unclear | N | Y | 7 |
| Pinto et al., 2019 [21] | Y | Y | Y | N | N | Y | Y | N | Y | N | Y | 7 |
| Greco et al., 2021 [20] | Y | Y | Y | Y | Y | Y | Y | Y | Unclear | N | Y | 9 |
References
- Calori, G.M.; Mazza, E.L.; Mazzola, S.; Colombo, A.; Giardina, F.; Romanò, F.; Colombo, M. Non-unions. Clin. Cases Miner. Bone Metab. 2017, 14, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Wittauer, M.; Burch, M.-A.; McNally, M.; Vandendriessche, T.; Clauss, M.; Della Rocca, G.J.; Giannoudis, P.V.; Metsemakers, W.-J.; Morgenstern, M. Definition of long-bone nonunion: A scoping review of prospective clinical trials to evaluate current practice. Injury 2021, 52, 3200–3205. [Google Scholar] [CrossRef]
- Nicholson, J.A.; Makaram, N.; Simpson, A.; Keating, J.F. Fracture nonunion in long bones: A literature review of risk factors and surgical management. Injury 2021, 52, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Zura, R.; Xiong, Z.; Einhorn, T.; Watson, J.T.; Ostrum, R.F.; Prayson, M.J.; Della Rocca, G.J.; Mehta, S.; McKinley, T.; Wang, Z.; et al. Epidemiology of Fracture Nonunion in 18 Human Bones. JAMA Surg. 2016, 151, e162775. [Google Scholar] [CrossRef] [PubMed]
- Zura, R.; Mehta, S.; Della Rocca, G.J.; Steen, R.G. Biological Risk Factors for Nonunion of Bone Fracture. JBJS Rev. 2016, 4, e5. [Google Scholar] [CrossRef] [PubMed]
- Quan, K.; Xu, Q.; Zhu, M.; Liu, X.; Dai, M. Analysis of Risk Factors for Non-union After Surgery for Limb Fractures: A Case-Control Study of 669 Subjects. Front. Surg. 2021, 8, 754150. [Google Scholar] [CrossRef] [PubMed]
- Struijs, P.A.; Poolman, R.W.; Bhandari, M. Infected nonunion of the long bones. J. Orthop. Trauma 2007, 21, 507–511. [Google Scholar] [CrossRef]
- Makridis, K.G.; Tosounidis, T.; Giannoudis, P.V. Management of infection after intramedullary nailing of long bone fractures: Treatment protocols and outcomes. Open Orthop. J. 2013, 7, 219–226. [Google Scholar] [CrossRef]
- Shyam, A.K.; Sancheti, P.K.; Patel, S.K.; Rocha, S.; Pradhan, C.; Patil, A. Use of antibiotic cement-impregnated intramedullary nail in treatment of infected non-union of long bones. Indian J. Orthop. 2009, 43, 396–402. [Google Scholar] [CrossRef]
- Chen, A.F.; Fleischman, A.; Austin, M.S. Use of Intrawound Antibiotics in Orthopaedic Surgery. J. Am. Acad. Orthop. Surg. 2018, 26, e371–e378. [Google Scholar] [CrossRef]
- Flores, M.J.; Brown, K.E.; Morshed, S.; Shearer, D.W. Evidence for Local Antibiotics in the Prevention of Infection in Orthopaedic Trauma. J. Clin. Med. 2022, 11, 7461. [Google Scholar] [CrossRef]
- Cancienne, J.M.; Burrus, M.T.; Weiss, D.B.; Yarboro, S.R. Applications of Local Antibiotics in Orthopedic Trauma. Orthop. Clin. 2015, 46, 495–510. [Google Scholar] [CrossRef]
- Keller, D.M.; Pizzo, R.A.; Patel, J.N.; Viola, A.; Yoon, R.S.; Liporace, F.A. Use of antibiotic-cement coated locking plates in the setting of periprosthetic infection and infected nonunion. Injury 2022, 53, 2567–2572. [Google Scholar] [CrossRef] [PubMed]
- Tarity, T.D.; Xiang, W.; Jones, C.W.; Gkiatas, I.; Nocon, A.; Selemon, N.A.; Carli, A.; Sculco, P.K. Do Antibiotic-Loaded Calcium Sulfate Beads Improve Outcomes After Debridement, Antibiotics, and Implant Retention? A Matched Cohort Study. Arthroplast. Today 2022, 14, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, K.; Xing, X.; Zhang, J.; Zhang, M.R.; Ma, X.; Shi, R.; Zhang, L. Smart Titanium Coating Composed of Antibiotic Conjugated Peptides as an Infection-Responsive Antibacterial Agent. Macromol. Biosci. 2021, 21, e2000194. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, C.; Tiwari, A.K.; Sharma, S.B.; Thalanki, S.; Rai, A. Role of Antibiotic Cement Coated Nailing in Infected Nonunion of Tibia. Malays. Orthop. J. 2017, 11, 6–11. [Google Scholar] [CrossRef]
- Walter, N.; Rupp, M.; Krückel, J.; Alt, V. Individual and commercially available antimicrobial coatings for intramedullary nails for the treatment of infected long bone non-unions: A systematic review. Injury 2022, 53, S74–S80. [Google Scholar] [CrossRef]
- Bakshi, A.S.; Singh, A.; Kaur, H.; Kaur, G.; Singh, J. A Comparative Study of Treatment with External Fixator Versus Antibiotic Coated Intramedullary Nail in Infected Non-union Long Bones. Cureus 2022, 14, e29659. [Google Scholar] [CrossRef]
- Downey, E.A.; Jaime, K.M.; Reif, T.J.; Makhdom, A.M.; Rozbruch, S.R.; Fragomen, A.T. Prophylaxis and treatment of infection in long bones using an antibiotic-loaded ceramic coating with interlocking intramedullary nails. J. Bone Jt. Infect. 2022, 7, 101–107. [Google Scholar] [CrossRef]
- Greco, T.; Cianni, L.; Polichetti, C.; Inverso, M.; Maccauro, G.; Perisano, C. Uncoated vs. Antibiotic-Coated Tibia Nail in Open Diaphyseal Tibial Fracture (42 according to AO Classification): A Single Center Experience. BioMed Res. Int. 2021, 2021, 7421582. [Google Scholar] [CrossRef]
- Pinto, D.; Manjunatha, K.; Savur, A.D.; Ahmed, N.R.; Mallya, S.; Ramya, V. Comparative study of the efficacy of gentamicin-coated intramedullary interlocking nail versus regular intramedullary interlocking nail in Gustilo type I and II open tibia fractures. Chin. J. Traumatol. 2019, 22, 270–273. [Google Scholar] [CrossRef]
- Rohilla, R.; Arora, S.; Kundu, A.; Singh, R.; Govil, V.; Khokhar, A. Functional and radiological outcomes of primary ring fixator versus antibiotic nail in open tibial diaphyseal fractures: A prospective study. Injury 2022, 53, 3464–3470. [Google Scholar] [CrossRef] [PubMed]
- Hoit, G.; Bonyun, M.; Nauth, A. Hardware considerations in infection and nonunion management: When and how to revise the fixation. OTA Int. Open Access J. Orthop. Trauma 2020, 3, e055. [Google Scholar] [CrossRef] [PubMed]
- Greco, T.; Vitiello, R.; Cazzato, G.; Cianni, L.; Malerba, G.; Maccauro, G.; Perisano, C. Intramedullary antibiotic coated nail in tibial fracture: A systematic review. J. Biol. Regul. Homeost. Agents 2020, 34 (Suppl. S2), 63–69. [Google Scholar] [PubMed]
- Savvidou, O.D.; Kaspiris, A.; Trikoupis, I.; Kakouratos, G.; Goumenos, S.; Melissaridou, D.; Papagelopoulos, P.J. Efficacy of antimicrobial coated orthopaedic implants on the prevention of periprosthetic infections: A systematic review and meta-analysis. J. Bone Jt. Infect. 2020, 5, 212–222. [Google Scholar] [CrossRef]
- Koury, K.L.; Hwang, J.S.; Sirkin, M. The Antibiotic Nail in the Treatment of Long Bone Infection: Technique and Results. Orthop. Clin. 2017, 48, 155–165. [Google Scholar] [CrossRef]
- Mills, L.; Tsang, J.; Hopper, G.; Keenan, G.; Simpson, A.H. The multifactorial aetiology of fracture nonunion and the importance of searching for latent infection. Bone Jt. Res. 2016, 5, 512–519. [Google Scholar] [CrossRef]
- Craig, J.; Fuchs, T.; Jenks, M.; Fleetwood, K.; Franz, D.; Iff, J.; Raschke, M. Systematic review and meta-analysis of the additional benefit of local prophylactic antibiotic therapy for infection rates in open tibia fractures treated with intramedullary nailing. Int. Orthop. 2014, 38, 1025–1030. [Google Scholar] [CrossRef]
- Thonse, R.; Conway, J.D. Antibiotic cement-coated nails for the treatment of infected nonunions and segmental bone defects. J. Bone Jt. Surg. 2008, 90, 163–174. [Google Scholar] [CrossRef]
- Simpson, A.H.; Tsang, J.S.T. Current treatment of infected non-union after intramedullary nailing. Injury 2017, 48, S82–S90. [Google Scholar] [CrossRef]
- Kang, N.W.W.; Tan, W.P.J.; Phua, Y.M.C.; Min, A.T.G.; Naidu, K.; Umapathysivam, K.; Smitham, P.J. Intramedullary nail: The past, present and the future—A review exploring where the future may lead us. Orthop. Rev. 2021, 13, 25546. [Google Scholar] [CrossRef]
- Kalbas, Y.; Klingebiel, F.; Pape, H.C. Antibiotic coated nails: Rationale, development, indications and outcomes. J. Orthop. Surg. 2022, 30, 10225536221118521. [Google Scholar] [CrossRef] [PubMed]
- Franz, D.; Raschke, M.; Giannoudis, P.; Leliveld, M.; Metsemakers, W.; Verhofstad, M.; Craig, J.; Shore, J.; Smith, A.; Muehlendyck, C.; et al. Use of antibiotic coated intramedullary nails in open tibia fractures: A European medical resource use and cost-effectiveness analysis. Injury 2021, 52, 1951–1958. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Aloe, A.M. Evaluation of various estimators for standardized mean difference in meta-analysis. Stat. Med. 2021, 40, 403–426. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef]
| Study ID | Country | Design | Sample Size | Age, Years ± SD [Range] | M/F | Bone | Control Group | Fracture Type, n | Antibiotic Type | Follow-up, Months |
|---|---|---|---|---|---|---|---|---|---|---|
| Rohilla et al., 2022 [22] | India | Prospective | ACN = 16 Control = 16 | ACN = 33.06 ± 11.23 Control = 31.06 ± 9.72 | Total = 26/6 | Tibia | External fixator | Gustilo type II: 18 patients Gustilo type IIIA: 14 patients | Gentamicin | ACN = 24.08 Control = 23.34 |
| Bakshi et al., 2022 [18] | India | Prospective | ACN = 20 Control = 20 | ACN = [41–60] Control = [21–40] | NM | Long bones (mostly tibia) | External fixator | NM | Gentamicin + Vancomycin | 12 months |
| Downey et al., 2022 [19] | USA | Retrospective | ACN = 9 Control = 28 | ACN = 45 [31–73] Control = 62 [22–28] | ACN = 7/2 Control = 16/12 | Long bones (mostly tibia) | Uncoated nail | NM | Vancomycin + Tobramycin | ACN = 28.3 (21.3–43.8) Control = 40 (28–84) |
| Pinto et al., 2019 [21] | India | Prospective | ACN = 14 Control = 14 | ACN = 35.07 Control = 32.35 | NM | Tibia | Uncoated nail | Gustilo type I: 14 patients Gustilo type II: 14 patients | Gentamicin | 6 months |
| Greco et al., 2021 [20] | Italy | Retrospective | ACN = 23 Control = 23 | ACN = 45.81 ± 19.13 Control = 41.09 ± 17.56 | ACN = 18/5 Control = 19/4 | Tibia | Uncoated nail | Gustilo type I: 9 patients Gustilo type II: 21 patients Gustilo type IIIA: 10 patients Gustilo type IIIB: 4 patients Gustilo type IIIC: 2 patients | Gentamicin | 18–30 months |
| Study ID | Outcome Measure | Antibiotic-Coated Nailing | Control Group | p-Value |
|---|---|---|---|---|
| Rohilla et al., 2022 [22] | Knee stiffness | 2 patients (12.5%) | 4 patients (25%) | 0.65 |
| Limping | 3 patients (18.8%) | 2 patients (12.5%) | 0.99 | |
| Knee deformity > 7° | 0 patients (0.0%) | 2 patients (12.5%) | 0.46 | |
| Significant pain | 2 patients (12.5%) | 1 patient (6.2%) | 0.99 | |
| SMFA score | 23.703 ± 8.02 | 24.41 ± 5.87 | 0.77 | |
| Bakshi et al., 2022 [18] | Knee stiffness | 2 patients (10%) | 5 patients (25%) | 0.049 |
| Ankle stiffness | 2 patients (10%) | 5 patients (25%) | 0.005 | |
| LLD more than before | 8 patients (40%) | 10 patients (50%) | 0.021 | |
| Fair or poor ASAMI score (bone results) | 6 patients (30%) | 6 patients (30%) | 0.79 | |
| Fair or poor ASAMI score (functional results) | 4 patients (20%) | 2 patients (10%) | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaseminejad-Raeini, A.; Azarboo, A.; Pirahesh, K.; Sharafi, A.; Hoveidaei, A.H.; Nwankwo, B.O.; Annasamudram, A.; Conway, J.D. Antibiotic-Coated Intramedullary Nailing Managing Long Bone Infected Non-Unions: A Meta-Analysis of Comparative Studies. Antibiotics 2024, 13, 69. https://doi.org/10.3390/antibiotics13010069
Ghaseminejad-Raeini A, Azarboo A, Pirahesh K, Sharafi A, Hoveidaei AH, Nwankwo BO, Annasamudram A, Conway JD. Antibiotic-Coated Intramedullary Nailing Managing Long Bone Infected Non-Unions: A Meta-Analysis of Comparative Studies. Antibiotics. 2024; 13(1):69. https://doi.org/10.3390/antibiotics13010069
Chicago/Turabian StyleGhaseminejad-Raeini, Amirhossein, Alireza Azarboo, Kasra Pirahesh, Amirmohammad Sharafi, Amir Human Hoveidaei, Basilia Onyinyechukwu Nwankwo, Abhijith Annasamudram, and Janet D. Conway. 2024. "Antibiotic-Coated Intramedullary Nailing Managing Long Bone Infected Non-Unions: A Meta-Analysis of Comparative Studies" Antibiotics 13, no. 1: 69. https://doi.org/10.3390/antibiotics13010069
APA StyleGhaseminejad-Raeini, A., Azarboo, A., Pirahesh, K., Sharafi, A., Hoveidaei, A. H., Nwankwo, B. O., Annasamudram, A., & Conway, J. D. (2024). Antibiotic-Coated Intramedullary Nailing Managing Long Bone Infected Non-Unions: A Meta-Analysis of Comparative Studies. Antibiotics, 13(1), 69. https://doi.org/10.3390/antibiotics13010069