Physicochemical and Biological Characterization of Encapsulated Olive Leaf Extracts for Food Preservation
Abstract
1. Introduction
2. Results and Discussion
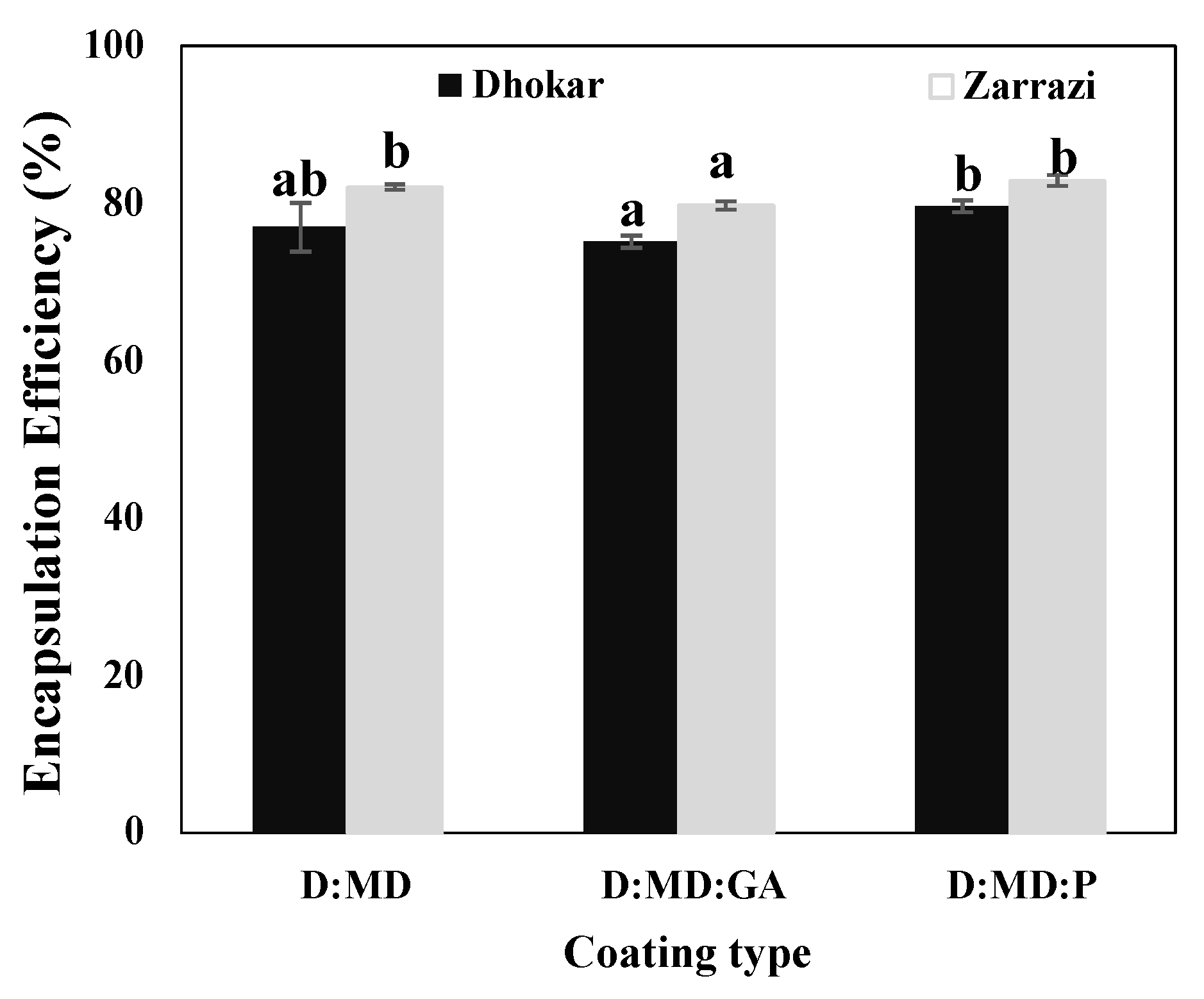
2.1. Encapsulation Efficiency (EE)
2.2. Drying Yields
2.3. Characterization of Microcapsules
2.3.1. Particle Size Distribution
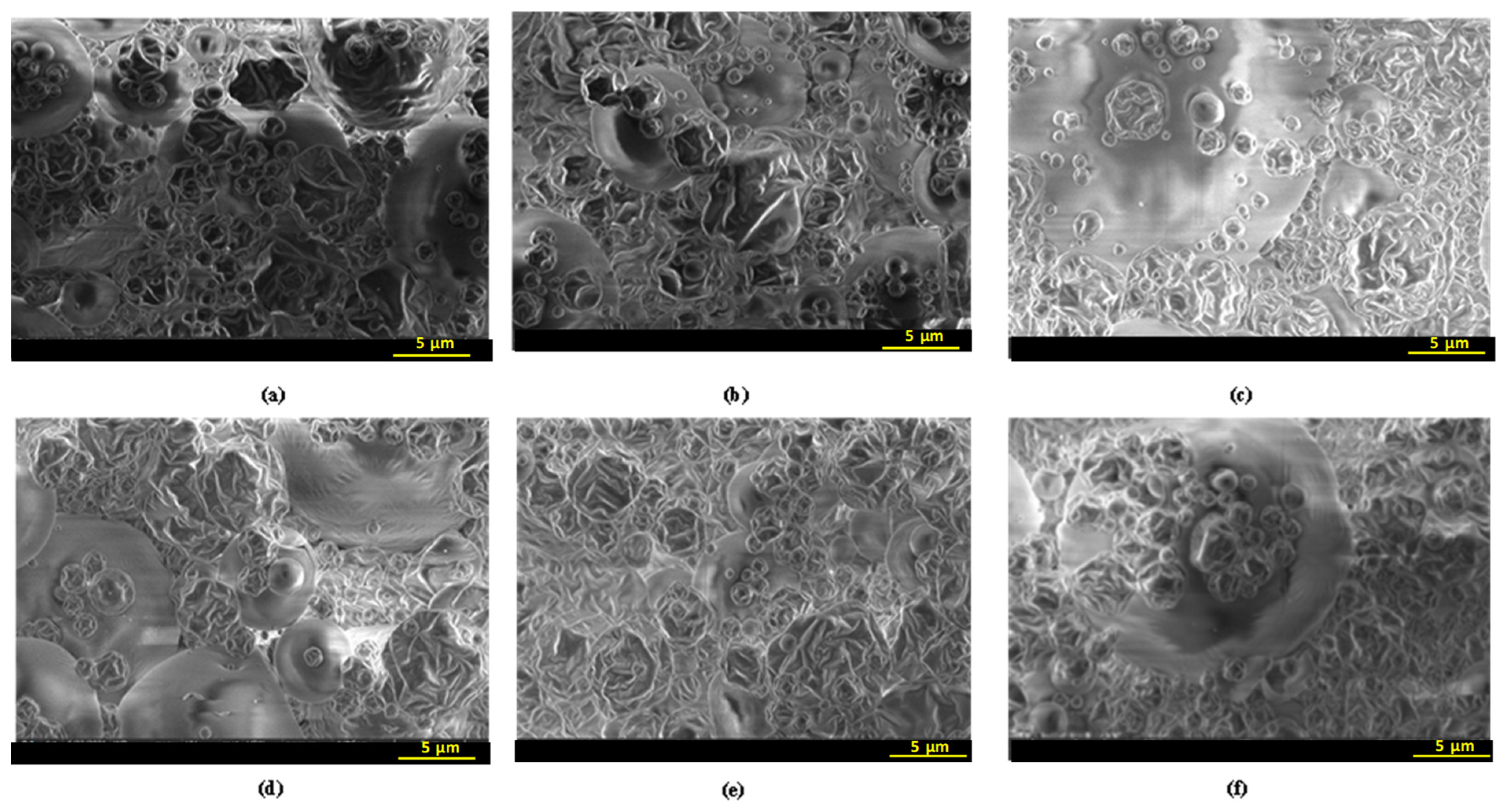
2.3.2. Scanning Electron Microscopy (SEM)
2.4. Antiradical Activity
2.5. Heat Stability of Microcapsules
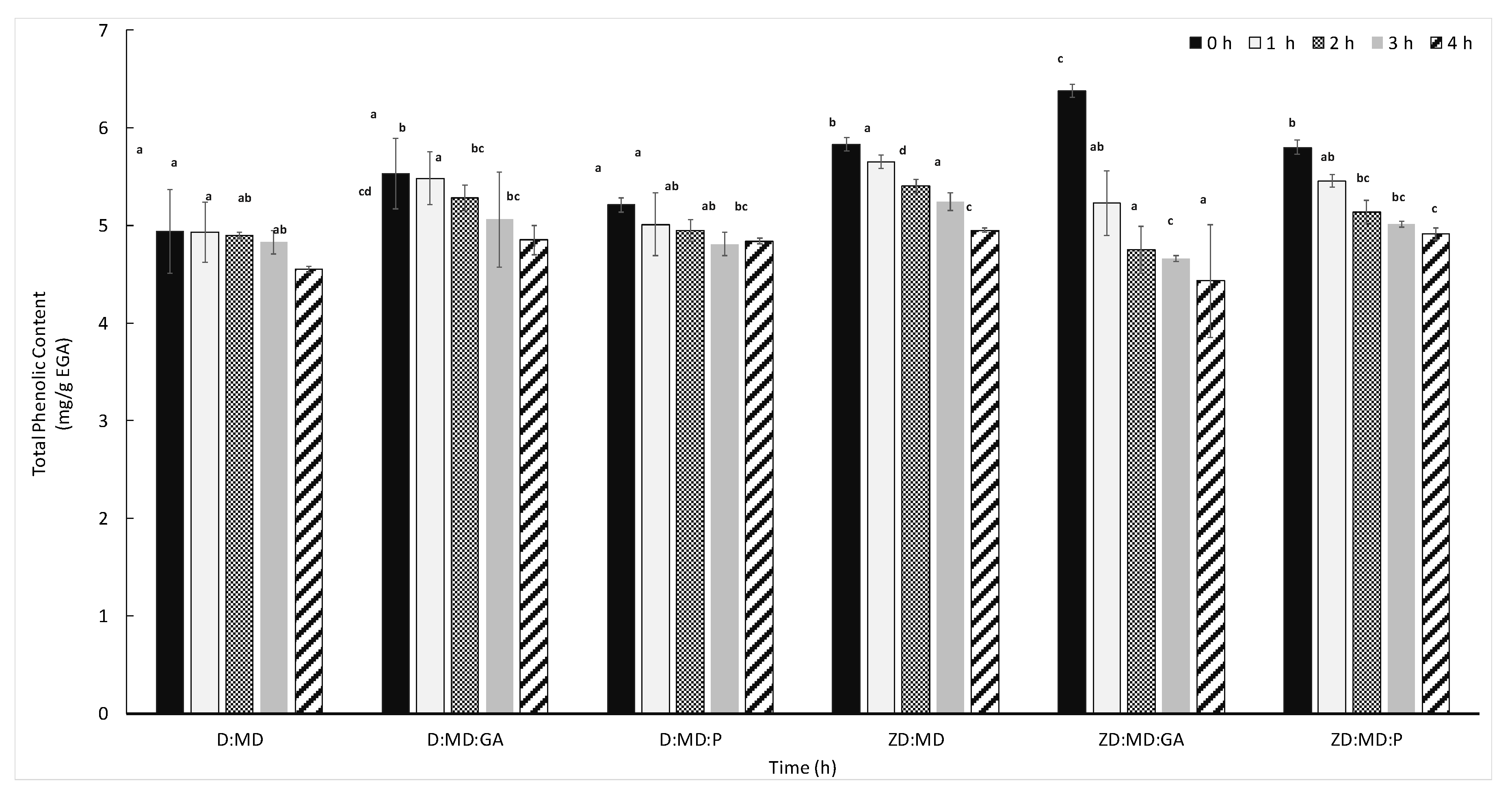
2.6. Storage Stability
2.7. Determination of Antimicrobial Activity of Microencapsulated OLE
3. Materials and Methods
3.1. Chemicals, Reagents, and Bacteria
3.2. Sampling
3.3. Preparation of Olive Leaf Extracts
3.4. Preparation of the Microcapsules
3.5. Encapsulation Efficiency (EE) and Load Yield (Y)
3.6. Total Phenolic Content (TPC)
3.7. Antioxidant Capacity (DPPH Radical Scavenging Method)
3.8. Physical Properties of Microcapsules
3.8.1. Particle Size Distribution
3.8.2. Particle Morphology
3.9. Storage Stability of Microcapsules
3.10. Heat Stability Test of Microcapsules
3.11. Determination of Antimicrobial Activity
3.12. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiritsakis, K.; Goula, A.M.; Adamopoulos, K.G.; Gerasopoulos, D. Valorization of Olive Leaves: Spray Drying of Olive Leaf Extract. Waste Biomass Valoriz. 2018, 9, 619–633. [Google Scholar] [CrossRef]
- Pereira, A.P.; Ferreira, I.C.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic Compounds and Antimicrobial Activity of Olive (Olea europaea L. Cv. Cobrançosa) Leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Pérez-Sánchez, A.; Herrero, M.; Ibañez, E.; Micol, V.; Zarrouk, M.; Segura-Carretero, A. Use of Advanced Techniques for the Extraction of Phenolic Compounds from Tunisian Olive Leaves: Phenolic Composition and Cytotoxicity against Human Breast Cancer Cells. Food Chem. Toxicol. 2012, 50, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Lama-Muñoz, A.; del Mar Contreras, M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Optimization of Oleuropein and Luteolin-7-o-Glucoside Extraction from Olive Leaves by Ultrasound-Assisted Technology. Energies 2019, 12, 2486. [Google Scholar] [CrossRef]
- da Fonseca Antunes, B.; Otero, D.M.; Oliveira, F.M.; Jacques, A.C.; Gandra, E.A.; Zambiazi, R.C. Antioxidant and Antimicrobial Activity of Olive Trees Cultivated in the Campanha Gaúcha Region. Braz. J. Dev. 2020, 6, 21791–21805. [Google Scholar] [CrossRef]
- Cavaca, L.A.; López-Coca, I.M.; Silvero, G.; Afonso, C.A. The Olive-Tree Leaves as a Source of High-Added Value Molecules: Oleuropein. Stud. Nat. Prod. Chem. 2020, 64, 131–180. [Google Scholar] [CrossRef]
- Guex, C.G.; Reginato, F.Z.; de Jesus, P.R.; Brondani, J.C.; Lopes, G.H.H.; de Freitas Bauermann, L. Antidiabetic Effects of Olea europaea L. Leaves in Diabetic Rats Induced by High-Fat Diet and Low-Dose Streptozotocin. J. Ethnopharmacol. 2019, 235, 1–7. [Google Scholar] [CrossRef]
- Wang, B.; Qu, J.; Luo, S.; Feng, S.; Li, T.; Yuan, M.; Huang, Y.; Liao, J.; Yang, R.; Ding, C. Optimization of Ultrasound-Assisted Extraction of Flavonoids from Olive (Olea europaea) Leaves, and Evaluation of Their Antioxidant and Anticancer Activities. Molecules 2018, 23, 2513. [Google Scholar] [CrossRef] [PubMed]
- Souilem, S.; Fki, I.; Kobayashi, I.; Khalid, N.; Neves, M.A.; Isoda, H.; Sayadi, S.; Nakajima, M. Emerging Technologies for Recovery of Value-Added Components from Olive Leaves and Their Applications in Food/Feed Industries. Food Bioprocess Technol. 2017, 10, 229–248. [Google Scholar] [CrossRef]
- Multisona, R.R.; Shirodkar, S.; Arnold, M.; Gramza-Michalowska, A. Clitoria Ternatea Flower and Its Bioactive Compounds: Potential Use as Microencapsulated Ingredient for Functional Foods. Appl. Sci. 2023, 13, 2134. [Google Scholar] [CrossRef]
- González-Ortega, R.; Faieta, M.; Di Mattia, C.D.; Valbonetti, L.; Pittia, P. Microencapsulation of Olive Leaf Extract by Freeze-Drying: Effect of Carrier Composition on Process Efficiency and Technological Properties of the Powders. J. Food Eng. 2020, 285, 110089. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of Spray-Drying in Microencapsulation of Food Ingredients: An Overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Yinbin, L.; Wu, L.; Weng, M.; Tang, B.; Lai, P.; Chen, J. Effect of Different Encapsulating Agent Combinations on Physicochemical Properties and Stability of Microcapsules Loaded with Phenolics of Plum (Prunus salicina Lindl.). Powder Technol. 2018, 340, 459–464. [Google Scholar] [CrossRef]
- Moser, P.; Souza, R.T.D.; Nicoletti Telis, V.R. Spray Drying of Grape Juice from Hybrid Cv. BRS Violeta: Microencapsulation of Anthocyanins Using Protein/Maltodextrin Blends as Drying Aids. J. Food Process. Preserv. 2017, 41, e12852. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Shahgol, M.; Estevinho, B.N.; Rocha, F. Microencapsulation of Vitamin A by Spray-Drying, Using Binary and Ternary Blends of Gum Arabic, Starch and Maltodextrin. Food Hydrocoll. 2020, 108, 106029. [Google Scholar] [CrossRef]
- Mahdavi, S.A.; Jafari, S.M.; Assadpoor, E.; Dehnad, D. Microencapsulation Optimization of Natural Anthocyanins with Maltodextrin, Gum Arabic and Gelatin. Int. J. Biol. Macromol. 2016, 85, 379–385. [Google Scholar] [CrossRef]
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of Grape Polyphenols Using Maltodextrin and Gum Arabic as Two Alternative Coating Materials: Development and Characterization. J. Biotechnol. 2016, 239, 23–33. [Google Scholar] [CrossRef]
- Tupuna, D.S.; Paese, K.; Guterres, S.S.; Jablonski, A.; Flôres, S.H.; de Oliveira Rios, A. Encapsulation Efficiency and Thermal Stability of Norbixin Microencapsulated by Spray-Drying Using Different Combinations of Wall Materials. Ind. Crops Prod. 2018, 111, 846–855. [Google Scholar] [CrossRef]
- Silva, V.M.; Kurozawa, L.E.; Park, K.J.; Hubinger, M.D. Influence of Carrier Agents on the Physicochemical Properties of Mussel Protein Hydrolysate Powder. Dry. Technol. 2012, 30, 653–663. [Google Scholar] [CrossRef]
- Ma, X.; Hou, F.; Zhao, H.; Wang, D.; Chen, W.; Miao, S.; Liu, D. Conjugation of Soy Protein Isolate (SPI) with Pectin by Ultrasound Treatment. Food Hydrocoll. 2020, 108, 106056. [Google Scholar] [CrossRef]
- Akdeniz, B.; Sumnu, G.; Sahin, S. Microencapsulation of Phenolic Compounds Extracted from Onion (Allium cepa) Skin. J. Food Process. Preserv. 2018, 42, e13648. [Google Scholar] [CrossRef]
- Corrêa-Filho, L.C.; Lourenço, S.C.; Duarte, D.F.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of Tomato (Solanum lycopersicum L.) Pomace Ethanolic Extract by Spray Drying: Optimization of Process Conditions. Appl. Sci. 2019, 9, 612. [Google Scholar] [CrossRef]
- Pieczykolan, E.; Kurek, M.A. Use of Guar Gum, Gum Arabic, Pectin, Beta-Glucan and Inulin for Microencapsulation of Anthocyanins from Chokeberry. Int. J. Biol. Macromol. 2019, 129, 665–671. [Google Scholar] [CrossRef]
- Sansone, F.; Mencherini, T.; Picerno, P.; d’Amore, M.; Aquino, R.P.; Lauro, M.R. Maltodextrin/Pectin Microparticles by Spray Drying as Carrier for Nutraceutical Extracts. J. Food Eng. 2011, 105, 468–476. [Google Scholar] [CrossRef]
- Navarro-Flores, M.J.; Ventura-Canseco, L.M.C.; Meza-Gordillo, R.; del Rosario Ayora-Talavera, T.; Abud-Archila, M. Spray Drying Encapsulation of a Native Plant Extract Rich in Phenolic Compounds with Combinations of Maltodextrin and Non-Conventional Wall Materials. J. Food Sci. Technol. 2020, 57, 4111–4122. [Google Scholar] [CrossRef] [PubMed]
- Purnamayati, L.; Dewi, E.N.; Kurniasih, R.A. Phycocyanin Stability in Microcapsules Processed by Spray Drying Method Using Different Inlet Temperature. IOP Conf. Ser. Earth Environ. Sci. 2018, 116, 012076. [Google Scholar] [CrossRef]
- Tan, S.P.; Kha, T.C.; Parks, S.; Stathopoulos, C.; Roach, P.D. Optimising the Encapsulation of an Aqueous Bitter Melon Extract by Spray-Drying. Foods 2015, 4, 400–419. [Google Scholar] [CrossRef]
- Şahin-Nadeem, H.; Dinçer, C.; Torun, M.; Topuz, A.; Özdemir, F. Influence of Inlet Air Temperature and Carrier Material on the Production of Instant Soluble Sage (Salvia fruticosa Miller) by Spray Drying. LWT-Food Sci. Technol. 2013, 52, 31–38. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. Microencapsulation of Morinda citrifolia L. Extract by Spray-Drying. Chem. Eng. Res. Des. 2012, 90, 622–632. [Google Scholar] [CrossRef]
- Nunes, I.L.; Mercadante, A.Z. Encapsulation of Lycopene Using Spray-Drying and Molecular Inclusion Processes. Braz. Arch. Biol. Technol. 2007, 50, 893–900. [Google Scholar] [CrossRef]
- Flamminii, F.; Di Mattia, C.D.; Nardella, M.; Chiarini, M.; Valbonetti, L.; Neri, L.; Difonzo, G.; Pittia, P. Structuring Alginate Beads with Different Biopolymers for the Development of Functional Ingredients Loaded with Olive Leaves Phenolic Extract. Food Hydrocoll. 2020, 108, 105849. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Biopolymer-Based Nanoparticles and Microparticles: Fabrication, Characterization, and Application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Santiago-Adame, R.; Calderas, F.; Gallegos-Infante, J.A.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Núñez-Ramírez, D.M.; Bernad-Bernad, M.J.; Manero, O. Microencapsulation by Spray Drying of Laurel Infusions (Litsea glaucescens) with Maltodextrin. Ind. Crops Prod. 2016, 90, 1–8. [Google Scholar] [CrossRef]
- Kosaraju, S.L.; D’ath, L.; Lawrence, A. Preparation and Characterisation of Chitosan Microspheres for Antioxidant Delivery. Carbohydr. Polym. 2006, 64, 163–167. [Google Scholar] [CrossRef]
- Rosenberg, M.; Kopelman, I.J.; Talmon, Y. A Scanning Electron Microscopy Study of Microencapsulation. J. Food Sci. 1985, 50, 139–144. [Google Scholar] [CrossRef]
- Sarabandi, K.; Peighambardoust, S.H.; Mahoonak, A.S.; Samaei, S.P. Effect of Carrier Types and Compositions on the Production Yield, Microstructure and Physical Characteristics of Spray Dried Sour Cherry Juice Concentrate. J. Food Meas. Charact. 2017, 11, 1602–1612. [Google Scholar] [CrossRef]
- Alamilla-Beltran, L.; Chanona-Perez, J.J.; Jimenez-Aparicio, A.R.; Gutierrez-Lopez, G.F. Description of Morphological Changes of Particles along Spray Drying. J. Food Eng. 2005, 67, 179–184. [Google Scholar] [CrossRef]
- Medina-Torres, L.; García-Cruz, E.E.; Calderas, F.; Laredo, R.G.; Sánchez-Olivares, G.; Gallegos-Infante, J.A.; Rocha-Guzmán, N.E.; Rodriguez-Ramirez, J. Microencapsulation by Spray Drying of Gallic Acid with Nopal Mucilage (Opuntia ficus indica). LWT-Food Sci. Technol. 2013, 50, 642–650. [Google Scholar] [CrossRef]
- Cheng, Z.; Moore, J.; Yu, L. High-Throughput Relative DPPH Radical Scavenging Capacity Assay. J. Agric. Food Chem. 2006, 54, 7429–7436. [Google Scholar] [CrossRef] [PubMed]
- Medfai, W.; del Mar Contreras, M.; Lama-Muñoz, A.; Mhamdi, R.; Oueslati, I.; Castro, E. How Cultivar and Extraction Conditions Affect Antioxidants Type and Extractability for Olive Leaves Valorization. ACS Sustain. Chem. Eng. 2020, 8, 5107–5118. [Google Scholar] [CrossRef]
- Zoidou, E.; Magiatis, P.; Melliou, E.; Constantinou, M.; Haroutounian, S.; Skaltsounis, A.-L. Oleuropein as a Bioactive Constituent Added in Milk and Yogurt. Food Chem. 2014, 158, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Bakowska-Barczak, A.M.; Kolodziejczyk, P.P. Black Currant Polyphenols: Their Storage Stability and Microencapsulation. Ind. Crops Prod. 2011, 34, 1301–1309. [Google Scholar] [CrossRef]
- Nunes, G.L.; Boaventura, B.C.B.; Pinto, S.S.; Verruck, S.; Murakami, F.S.; Prudêncio, E.S.; de Amboni, R.D.M.C. Microencapsulation of Freeze Concentrated Ilex paraguariensis Extract by Spray Drying. J. Food Eng. 2015, 151, 60–68. [Google Scholar] [CrossRef]
- Saénz, C.; Tapia, S.; Chávez, J.; Robert, P. Microencapsulation by Spray Drying of Bioactive Compounds from Cactus Pear (Opuntia ficus-indica). Food Chem. 2009, 114, 616–622. [Google Scholar] [CrossRef]
- Dadi, D.W.; Emire, S.A.; Hagos, A.D.; Eun, J.-B. Physical and Functional Properties, Digestibility, and Storage Stability of Spray-and Freeze-Dried Microencapsulated Bioactive Products from Moringa stenopetala Leaves Extract. Ind. Crops Prod. 2020, 156, 112891. [Google Scholar] [CrossRef]
- Bayraktar, O.; Köse, M.D.; Baspinar, Y. Development of Olive Leaf Extract Loaded Fibroin Microparticles by Spray Drying. Open Drug Discov. J. 2019, 13, 39–45. [Google Scholar]
- Overly Cottom, C.; Stephenson, R.; Wilson, L.; Noinaj, N. Targeting BAM for Novel Therapeutics against Pathogenic Gram-Negative Bacteria. Antibiotics 2023, 12, 679. [Google Scholar] [CrossRef]
- Oueslati, I.; Haddada, F.M.; Manai, H.; Zarrouk, W.; Taamalli, W.; Fernandez, X.; Lizzani-Cuvelier, L.; Zarrouk, M. Characterization of Volatiles in Virgin Olive Oil Produced in the Tunisian Area of Tataouine. J. Agric. Food Chem. 2008, 56, 7992–7998. [Google Scholar] [CrossRef]
- Laine, P.; Kylli, P.; Heinonen, M.; Jouppila, K. Storage Stability of Microencapsulated Cloudberry (Rubus chamaemorus) Phenolics. J. Agric. Food Chem. 2008, 56, 11251–11261. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Bersuder, P.; Hole, M.; Smith, G. Antioxidants from a Heated Histidine-Glucose Model System. I: Investigation of the Antioxidant Role of Histidine and Isolation of Antioxidants by High-Performance Liquid Chromatography. J. Am. Oil Chem. Soc. 1998, 75, 181–187. [Google Scholar] [CrossRef]



| Coating Type | Drying Yield (%) | Particle Size (µm) | Antiradical Activity (%) |
|---|---|---|---|
| Dhokar–Maltodextrins | 44.3 ± 1.72 a | 10.2 ± 0.37 c | 42.05 ± 0.01 d |
| Dhokar–Maltodextrins–gum Arabic | 74.2 ± 2.36 d | 10.3 ± 0.27 c | 39.95 ± 0.02 d |
| Dhokar–Maltodextrins–pectin | 68.5 ± 1.89 b | 10.8 ± 0.21 d | 31.07 ± 0.01 c |
| Zarrazi–Maltodextrins | 76.0 ± 2.02 d | 10.1 ± 0.23 b | 20.39 ± 0.01 a |
| Zarrazi–Maltodextrins–gum Arabic | 71.9 ± 1.58 c | 09.8 ± 0.29 b | 22.05 ± 0.02 a |
| Zarrazi–Maltodextrins–pectin | 70.0 ± 2.17 c | 09.1 ± 0.27 a | 25.37 ± 0.03 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medfai, W.; Oueslati, I.; Dumas, E.; Harzalli, Z.; Viton, C.; Mhamdi, R.; Gharsallaoui, A. Physicochemical and Biological Characterization of Encapsulated Olive Leaf Extracts for Food Preservation. Antibiotics 2023, 12, 987. https://doi.org/10.3390/antibiotics12060987
Medfai W, Oueslati I, Dumas E, Harzalli Z, Viton C, Mhamdi R, Gharsallaoui A. Physicochemical and Biological Characterization of Encapsulated Olive Leaf Extracts for Food Preservation. Antibiotics. 2023; 12(6):987. https://doi.org/10.3390/antibiotics12060987
Chicago/Turabian StyleMedfai, Wafa, Imen Oueslati, Emilie Dumas, Zina Harzalli, Christophe Viton, Ridha Mhamdi, and Adem Gharsallaoui. 2023. "Physicochemical and Biological Characterization of Encapsulated Olive Leaf Extracts for Food Preservation" Antibiotics 12, no. 6: 987. https://doi.org/10.3390/antibiotics12060987
APA StyleMedfai, W., Oueslati, I., Dumas, E., Harzalli, Z., Viton, C., Mhamdi, R., & Gharsallaoui, A. (2023). Physicochemical and Biological Characterization of Encapsulated Olive Leaf Extracts for Food Preservation. Antibiotics, 12(6), 987. https://doi.org/10.3390/antibiotics12060987








