Helicobacter pylori Infection: Antibiotic Resistance and Solutions for Effective Management in Africa
Abstract
1. Introduction
2. Recommended Therapies for H. pylori
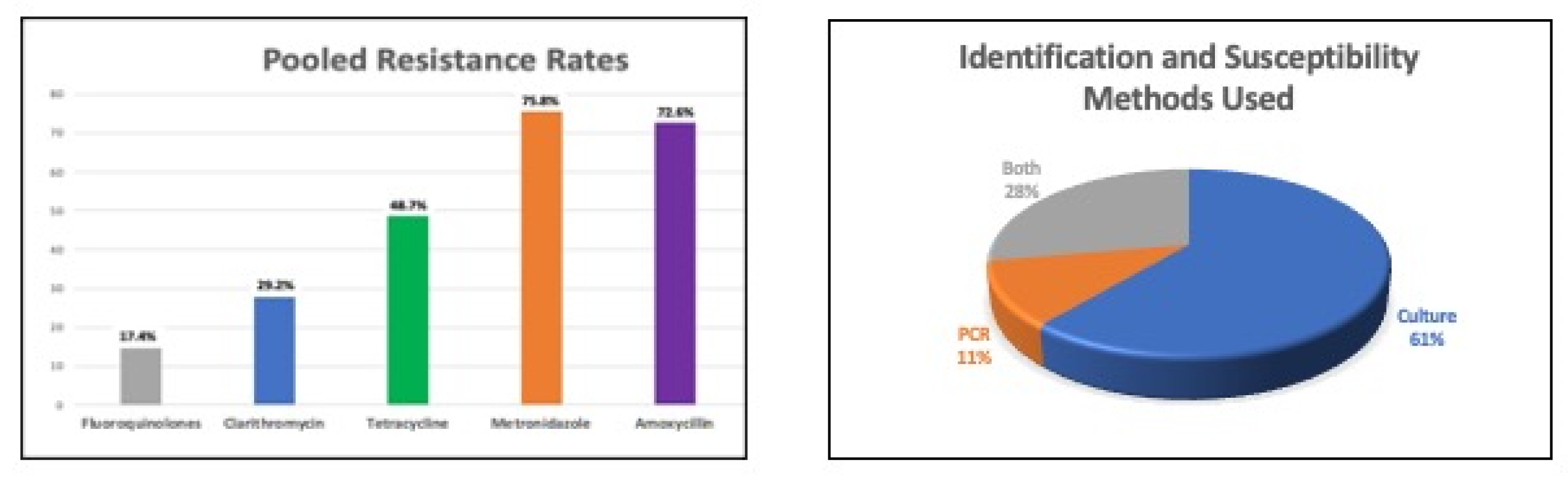
3. Antibiotic Resistance in Africa
4. Comprehensive Management Solutions for Africa
4.1. Treat Symptomatic Patients
4.2. Centralized Antimicrobial Susceptibility Testing Facilities
4.3. Screening of Symptomatic Family Members
4.4. Stakeholder Involvement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.H.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M.H. Systematic review with meta-analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.-M.; Malfertheiner, P.; Lee, Y.-C.; Sheu, B.-S.; Sugano, K.; Cheng, H.-C.; Yeoh, K.-G.; Hsu, P.-I.; Goh, K.-L.; Mahachai, V.; et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: The Taipei global consensus. Gut 2020, 69, 2093–2112. [Google Scholar] [CrossRef] [PubMed]
- Kotilea, K.; Bontems, P.; Touati, E. Epidemiology, Diagnosis and Risk Factors of Helicobacter pylori Infection. Gastroenterol. Clin. N. Am. 2019, 1149, 17–33. [Google Scholar] [CrossRef]
- Mégraud, F. Epidemiology of Helicobacter pylori infection. Gastroenterol. Clin. N. Am. 1993, 22, 73–88. [Google Scholar] [CrossRef]
- Denic, M.; Touati, E.; De Reuse, H. Review: Pathogenesis of Helicobacter pylori infection. Helicobacter 2020, 25, e12736. [Google Scholar] [CrossRef]
- Dorer, M.S.; Talarico, S.; Salama, N.R. Helicobacter pylori's unconventional role in health and disease. PLoS Pathog. 2009, 5, e1000544. [Google Scholar] [CrossRef]
- Gravina, A.G.; Priadko, K.; Ciamarra, P.; Granata, L.; Facchiano, A.; Miranda, A.; Dallio, M.; Federico, A.; Romano, M. Extra-Gastric Manifestations of Helicobacter pylori Infection. J. Clin. Med. 2020, 9, 3887. [Google Scholar] [CrossRef]
- Guevara, B.; Cogdill, A.G. Helicobacter pylori: A Review of Current Diagnostic and Management Strategies. Dig. Dis. Sci. 2020, 65, 1917–1931. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Sugano, K.; Tack, J.; Kuipers, E.J.; Graham, D.Y.; El-Omar, E.M.; Miura, S.; Malfertheiner, P. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015, 64, 1353–1367. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef] [PubMed]
- Nyssen, O.P.; Bordin, D.; Tepes, B.; Pérez-Aisa, Á.; Vaira, D.; Caldas, M.; Gisbert, J.P. European Registry on Helicobacter pylori management (Hp-EuReg): Patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut 2021, 70, 40–54. [Google Scholar] [CrossRef]
- Setshedi, M. Is the current Maastricht consensus report applicable for H. pylori management in Sub-Saharan Africa? Dig. Dis. 2023, 17, 1–2. [Google Scholar] [CrossRef]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology 2020, 155, 1372–1382.e17. [Google Scholar] [CrossRef]
- Megraud, F.; Bruyndonckx, R.; Coenen, S.; Wittkop, L.; Huang, T.-D.; Hoebeke, M.; Bénéjat, L.; Lehours, P.; Goossens, H.; Glupczynski, Y. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut 2021, 70, 1815–1822. [Google Scholar] [CrossRef]
- Vialle-Valentin, C.E.; LeCates, R.F.; Zhang, F.; Desta, A.T.; Ross-Degnan, D. Predictors of antibiotic use in African communities: Evidence from medicines household surveys in five countries. Trop. Med. Int. Health 2011, 17, 211–222. [Google Scholar] [CrossRef]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef]
- Fallone, C.A.; Chiba, N.; van Zanten, S.V.; Fischbach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P.; et al. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology 2016, 151, 51–69.e14. [Google Scholar] [CrossRef]
- Alboraie, M.; Elhossary, W.; Aly, O.A.; Abbas, B.; Abdelsalam, L.; Ghaith, D.; Shady, Z.; Gaber, Y.; Adel, E.; Peura, D.; et al. Egyptian recommendations for management of Helicobacter pylori infection: 2018 report. Arab. J. Gastroenterol. 2019, 20, 175–179. [Google Scholar] [CrossRef]
- Zamani, M.; Alizadeh-Tabari, S.; Zamani, V.; Shokri-Shirvani, J.; Derakhshan, M.H. Worldwide and Regional Efficacy Estimates of First-line Helicobacter pylori Treatments: A Systematic Review and Network Meta-Analysis. J. Clin. Gastroenterol. 2022, 56, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Fekadu, S.; Engiso, H.; Seyfe, S.; Iizasa, H.; Godebo, A.; Deyno, S.; Yoshiyama, H. Effectiveness of eradication therapy for Helicobacter pylori infection in Africa: A systematic review and meta-analysis. BMC Gastroenterol. 2023, 23, 55. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Jeon, S.R.; Kim, H.G.; Jin, S.Y.; Park, S. Cost-effectiveness of a tailored Helicobacter pylori eradication strategy based on the presence of a 23S ribosomal RNA point mutation that causes clarithromycin resistance in Korean patients. J. Gastroenterol. Hepatol. 2019, 34, 700–706. [Google Scholar] [CrossRef]
- Cosme, A.; Montes, M.; Ibarra, B.; Tamayo, E.; Alonso, H.; Mendarte, U.; Lizasoan, J.; Herreros-Villanueva, M.; Bujanda, L. Antimicrobial susceptibility testing before first-line treatment for Helicobacter pylori infection in patients with dual or triple antibiotic resistance. World J. Gastroenterol. 2017, 23, 3367–3373. [Google Scholar] [CrossRef] [PubMed]
- Bujanda, L.; Nyssen, O.P.; Vaira, D.; Saracino, I.M.; Fiorini, G.; Lerang, F.; Hp-EuReg, Investigators. Antibiotic Resistance Prevalence and Trends in Patients Infected with Helicobacter pylori in the Period 2013–2020: Results of the European Registry on H. pylori Management (Hp-EuReg). Antibiotics 2021, 10, 1058. [Google Scholar] [CrossRef] [PubMed]
- Sue, S.; Shibata, W.; Sasaki, T.; Kaneko, H.; Irie, K.; Kondo, M.; Maeda, S. Randomized trial of vonoprazan-based versus proton-pump inhibitor-based third-line triple therapy with sitafloxacin for Helicobacter pylori. J. Gastroenterol. Hepatol. 2019, 34, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Santamaría, D.; Nyssen, O.P.; Gasbarrini, A.; Vaira, D.; Pérez-Aisa, Á.; Rodrigo, L.; Gisbert, J.P. Empirical rescue treatment of Helicobacter pylori infection in third and subsequent lines: 8-year experience in 2144 patients from the European Registry on H. pylori management (Hp-EuReg). Gut 2023, 72, 1054–1072. [Google Scholar] [CrossRef]
- De Francesco, V.; Giorgio, F.; Hassan, C.; Manes, G.; Vannella, L.; Panella, C.; Ierardi, E.; Zullo, A. Worldwide H. pylori antibiotic resistance: A systematic review. J. Gastrointest. Liver Dis. 2010, 19, 409–414. [Google Scholar]
- Graham, D.Y.; Fischbach, L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010, 59, 1143–1153. [Google Scholar] [CrossRef]
- Thung, I.; Aramin, H.; Vavinskaya, V.; Gupta, S.; Park, J.Y.; Crowe, S.E.; Valasek, M.A. Review article: The global emergence of Helicobacter pylori antibiotic resistance. Aliment. Pharmacol. Ther. 2016, 43, 514–533. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Jaka, H.; Mushi, M.; Mirambo, M.M.; Wilson, L.; Seni, J.; Mtebe, M.; Mshana, S.E. Sero-prevalence and associated factors of Helicobacter pylori infection among adult patients with dyspepsia attending the gastroenterology unit in a tertiary hospital in Mwanza, Tanzania. Afr. Health Sci. 2016, 16, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Moss, S.F. Antimicrobial Susceptibility Testing for Helicobacter pylori Is Now Widely Available: When, How, Why. Am. J. Gastroenterol. 2022, 117, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Group, A.H.M.S. 2022 [cited 2022 July 30]. Available online: https://www.ahmsg-africa.org/#/ (accessed on 29 April 2023).
- Smith, S.I.; Ajayi, A.; Jolaiya, T.; Onyekwere, C.; Setshedi, M.; Schulz, C.; Otegbayo, J.A.; Ndip, R.; Dieye, Y.; Alboraie, M.; et al. Helicobacter pylori Infection in Africa: Update of the Current Situation and Challenges. Dig. Dis. 2021, 40, 535–544. [Google Scholar] [CrossRef] [PubMed]
| WHO Region | Clarithromycin, % (95% CI) | Metronidazole, % (95% CI) | Amoxycillin, % (95% CI) | Levofloxacin, % (95% CI) |
|---|---|---|---|---|
| Africa | 15 (0–30) | 91 (87–94) | 38 (32–45) | 14 (12–28) |
| Americas | 10 (4–16) | 23 (2–44) | 10 (2–19) | 15 (5–16) |
| Eastern Mediterranean | 33 (23–44) | 56 (46–66) | 14 (8–20) | 19 (0–29) |
| European | 18 (16–20) | 32 (27–36) | 0 (0–0) | 11 (9–13) |
| South-East Asia | 10 (5–16) | 51 (26–76) | 2 (0–5) | 30 (14–46) |
| Western Pacific | 34 (30–38) | 47 (37–57) | 1 (1–1) | 22 (17–28) |
| Overall | 34 (30–38) | 55 (51–59) | 1 (1–1) | 24 (21–26) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setshedi, M.; Smith, S.I. Helicobacter pylori Infection: Antibiotic Resistance and Solutions for Effective Management in Africa. Antibiotics 2023, 12, 969. https://doi.org/10.3390/antibiotics12060969
Setshedi M, Smith SI. Helicobacter pylori Infection: Antibiotic Resistance and Solutions for Effective Management in Africa. Antibiotics. 2023; 12(6):969. https://doi.org/10.3390/antibiotics12060969
Chicago/Turabian StyleSetshedi, Mashiko, and Stella I. Smith. 2023. "Helicobacter pylori Infection: Antibiotic Resistance and Solutions for Effective Management in Africa" Antibiotics 12, no. 6: 969. https://doi.org/10.3390/antibiotics12060969
APA StyleSetshedi, M., & Smith, S. I. (2023). Helicobacter pylori Infection: Antibiotic Resistance and Solutions for Effective Management in Africa. Antibiotics, 12(6), 969. https://doi.org/10.3390/antibiotics12060969






