Impact of the COVID-19 Pandemic on Ambulatory Care Antibiotic Use in Hungary: A Population-Based Observational Study
Abstract
1. Introduction
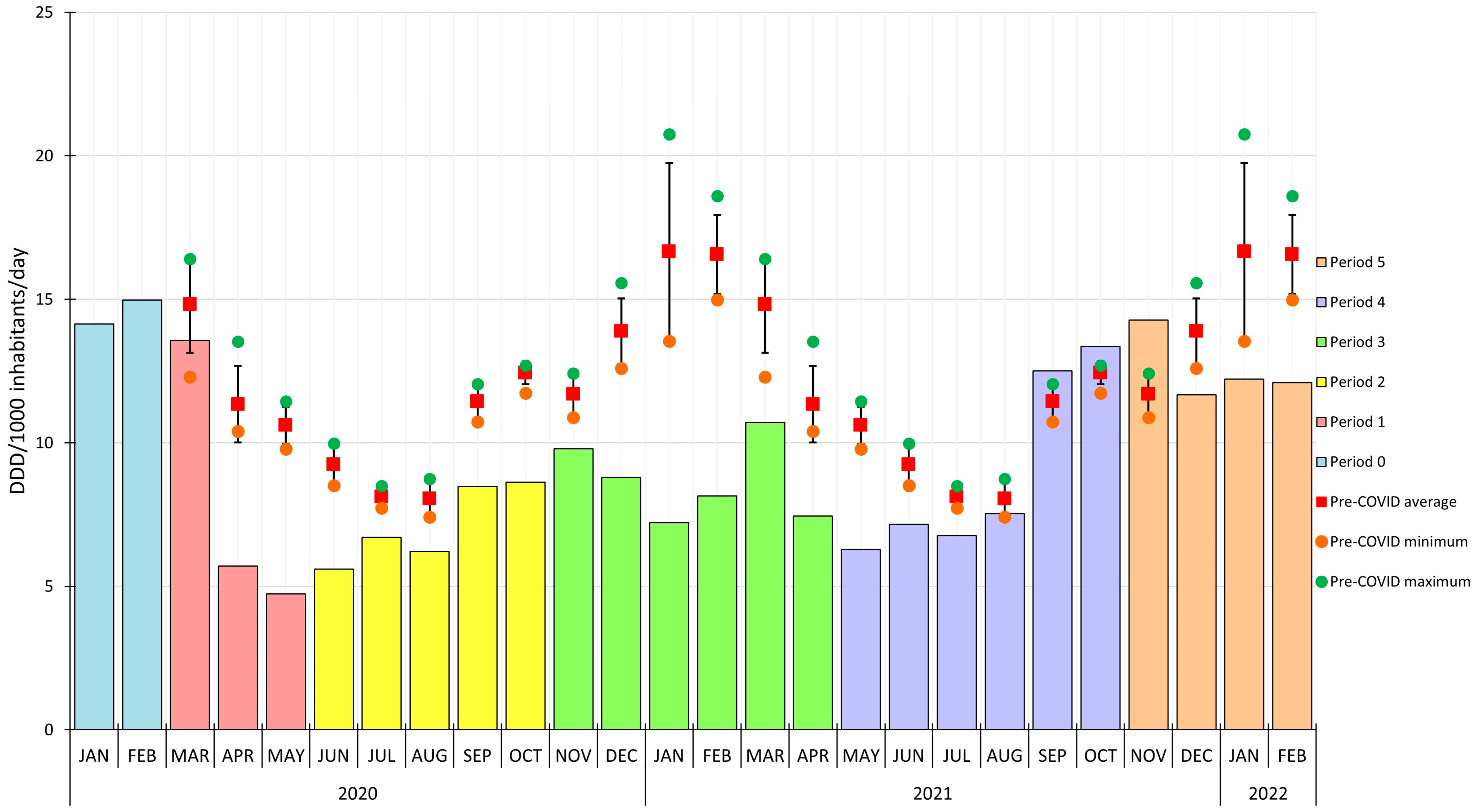
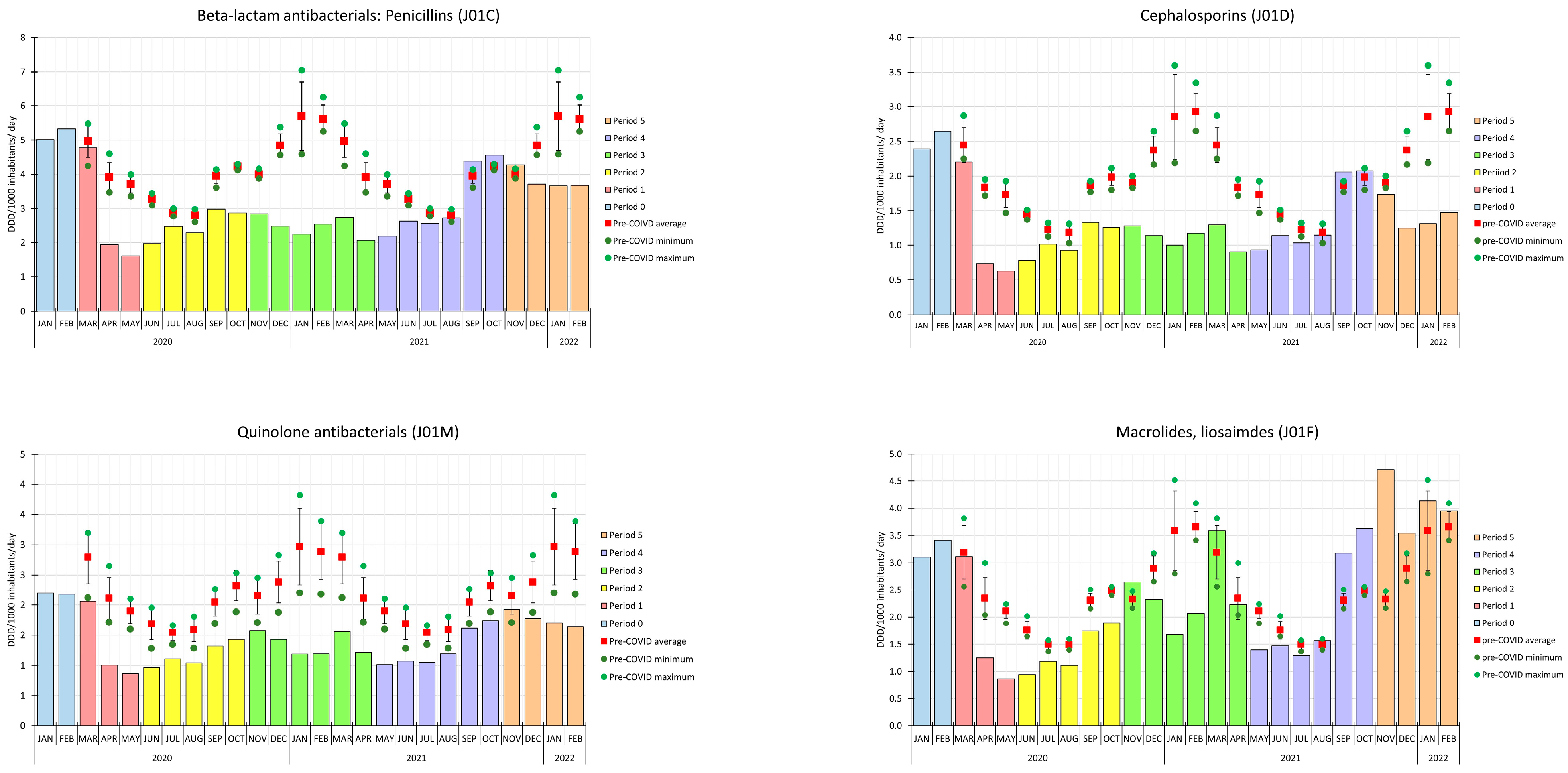
2. Results
3. Discussion
Strengths and Limitations
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Högberg, L.D.; Vlahović-Palčevski, V.; Pereira, C.; Weist, K.; Monnet, D.L.; ESAC-Net study group. Decrease in Community Antibiotic Consumption during the COVID-19 Pandemic, EU/EEA, 2020. Euro Surveill. 2021, 26, 2101020. [Google Scholar] [CrossRef] [PubMed]
- King, L.M.; Lovegrove, M.C.; Shehab, N.; Tsay, S.; Budnitz, D.S.; Geller, A.I.; Lind, J.N.; Roberts, R.; Hicks, L.A.; Kabbani, S. Trends in U.S. Outpatient Antibiotic Prescriptions during the COVID-19 Pandemic. Clin. Infect. Dis. 2020, 73, e652–e660. [Google Scholar] [CrossRef] [PubMed]
- Kitano, T.; Brown, K.A.; Daneman, N.; MacFadden, D.R.; Langford, B.J.; Leung, V.; So, M.; Leung, E.; Burrows, L.; Manuel, D.; et al. The Impact of COVID-19 on Outpatient Antibiotic Prescriptions in Ontario, Canada; An Interrupted Time Series Analysis. Open Forum Infect. Dis. 2021, 8, ofab533. [Google Scholar] [CrossRef] [PubMed]
- Knight, B.D.; Shurgold, J.; Smith, G.; MacFadden, D.R.; Schwartz, K.L.; Daneman, N.; Tropper, D.G.; Brooks, J. The Impact of COVID-19 on Community Antibiotic Use in Canada: An Ecological Study. Clin. Microbiol. Infect. 2022, 28, 426–432. [Google Scholar] [CrossRef]
- Malcolm, W.; Seaton, R.A.; Haddock, G.; Baxter, L.; Thirlwell, S.; Russell, P.; Cooper, L.; Thomson, A.; Sneddon, J. Impact of the COVID-19 Pandemic on Community Antibiotic Prescribing in Scotland. JAC Antimicrob. Resist. 2020, 2, dlaa105. [Google Scholar] [CrossRef]
- Silva, T.M.; Estrela, M.; Gomes, E.R.; Piñeiro-Lamas, M.; Figueiras, A.; Roque, F.; Herdeiro, M.T. The Impact of the COVID-19 Pandemic on Antibiotic Prescribing Trends in Outpatient Care: A Nationwide, Quasi-Experimental Approach. Antibiotics 2021, 10, 1040. [Google Scholar] [CrossRef]
- Colliers, A.; De Man, J.; Adriaenssens, N.; Verhoeven, V.; Anthierens, S.; De Loof, H.; Philips, H.; Coenen, S.; Morreel, S. Antibiotic Prescribing Trends in Belgian Out-of-Hours Primary Care during the COVID-19 Pandemic: Observational Study Using Routinely Collected Health Data. Antibiotics 2021, 10, 1488. [Google Scholar] [CrossRef] [PubMed]
- Hek, K.; Ramerman, L.; Weesie, Y.M.; Lambooij, A.C.; Lambert, M.; Heins, M.J.; Hendriksen, J.M.T.; Verheij, R.A.; Cals, J.W.L.; van Dijk, L. Antibiotic Prescribing in Dutch Daytime and Out-of-Hours General Practice during the COVID-19 Pandemic: A Retrospective Database Study. Antibiotics 2022, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- ECDC European Centre for Disease Prevention and Control (ECDC) Antimicrobial Consumption Database (ESAC-Net). Available online: https://www.ecdc.europa.eu/en/antimicrobial-consumption/surveillance-and-disease-data/database (accessed on 27 April 2023).
- Matuz, M.; Soós, G.; Hajdú, E.; Papfalvi, E.; Visnyovszki, Á.; Viola, R.; Benkő, R. The characteristics and trends of Hungarian outpatient antibiotic use (2010–2019). Orv. Hetil. 2022, 163, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, N.; Bruyndonckx, R.; Versporten, A.; Hens, N.; Monnet, D.L.; Molenberghs, G.; Goossens, H.; Weist, K.; Coenen, S. Quality Appraisal of Antibiotic Consumption in the Community, European Union/European Economic Area, 2009 and 2017. J. Antimicrob. Chemother. 2021, 76, ii60–ii67. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, N.; Coenen, S.; Versporten, A.; Muller, A.; Vankerckhoven, V.; Goossens, H. European Surveillance of Antimicrobial Consumption (ESAC): Quality Appraisal of Antibiotic Use in Europe. J. Antimicrob. Chemother. 2011, 66, vi71–vi77. [Google Scholar] [CrossRef] [PubMed]
- Hungarian Central Statistical Office. Available online: https://www.ksh.hu/stadat_eng?lang=en&theme=ege (accessed on 21 May 2023).
- Buehrle, D.J.; Wagener, M.M.; Nguyen, M.H.; Clancy, C.J. Trends in Outpatient Antibiotic Prescriptions in the United States During the COVID-19 Pandemic in 2020. JAMA Netw. Open 2021, 4, e2126114. [Google Scholar] [CrossRef] [PubMed]
- Bleyzac, N.; Goutelle, S.; Bourguignon, L.; Tod, M. Azithromycin for COVID-19: More Than Just an Antimicrobial? Clin. Drug Investig. 2020, 40, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Hinks, T.S.C.; Cureton, L.; Knight, R.; Wang, A.; Cane, J.L.; Barber, V.S.; Black, J.; Dutton, S.J.; Melhorn, J.; Jabeen, M.; et al. Azithromycin versus Standard Care in Patients with Mild-to-Moderate COVID-19 (ATOMIC2): An Open-Label, Randomised Trial. Lancet Respir. Med. 2021, 9, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Berezowski, I.; Abdelmonem, A.; Pourmand, A. Azithromycin for Mild-to-Moderate COVID-19—The Lancet Respiratory Medicine. Lancet Respir. Med. 2021, 9, e99. Available online: https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(21)00379-9/fulltext (accessed on 27 April 2023). [CrossRef] [PubMed]
- Sultana, J.; Cutroneo, P.M.; Crisafulli, S.; Puglisi, G.; Caramori, G.; Trifirò, G. Azithromycin in COVID-19 Patients: Pharmacological Mechanism, Clinical Evidence and Prescribing Guidelines. Drug Saf. 2020, 43, 691–698. [Google Scholar] [CrossRef] [PubMed]
- PRINCIPLE Trial Collaborative Group. Azithromycin for Community Treatment of Suspected COVID-19 in People at Increased Risk of an Adverse Clinical Course in the UK (PRINCIPLE): A Randomised, Controlled, Open-Label, Adaptive Platform Trial. Lancet 2021, 397, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Antibiotic Utilisation Data. Available online: http://neak.gov.hu/felso_menu/szakmai_oldalak/publikus_forgalmi_adatok/gyogyszer_forgalmi_adatok/gyogyszer_forgalmi_adatok (accessed on 21 May 2023).
- WHOCC—ATC/DDD Index. Available online: https://www.whocc.no/atc_ddd_index/?code=J01&showdescription=no (accessed on 27 April 2023).
- Chronological Overview—Council Action on Covid19. Available online: https://www.consilium.europa.eu/hu/policies/coronavirus/timeline/ (accessed on 27 April 2023).
- Coronavirus Factsheets. Available online: https://www.nnk.gov.hu/index.php/koronavirus-tajekoztatok (accessed on 27 April 2023).
- Precautionary Measures. Available online: https://www.nnk.gov.hu/index.php/koronavirus/ovintezkedesek (accessed on 27 April 2023).
- Vokó, Z.; Kiss, Z.; Surján, G.; Surján, O.; Barcza, Z.; Pályi, B.; Formanek-Balku, E.; Molnár, G.A.; Herczeg, R.; Gyenesei, A.; et al. Nationwide Effectiveness of Five SARS-CoV-2 Vaccines in Hungary-the HUN-VE Study. Clin. Microbiol. Infect. 2022, 28, 398–404. [Google Scholar] [CrossRef] [PubMed]
| Pre-COVID Period | COVID-19 Period | |||
|---|---|---|---|---|
| DID 1 | % | DID 1 | % | |
| J01A Tetracyclines | 0.77 | 6.34 | 0.78 | 8.35 |
| J01CA Penicillins with extended spectrum | 0.61 | 5.04 | 0.29 | 3.12 |
| J01CE Beta-lactamase sensitive penicillins | 0.17 | 1.41 | 0.06 | 0.65 |
| J01CR Combinations of penicillins, incl. beta-lactamase inhibitors | 3.37 | 27.85 | 2.71 | 29.17 |
| J01C Beta-lactam antibacterials, penicillins | 4.15 | 34.28 | 3.06 | 32.91 |
| J01DC Second-generation cephalosporins | 1.70 | 14.05 | 1.08 | 11.62 |
| J01DD Third-generations cephalosporins | 0.27 | 2.23 | 0.24 | 2.58 |
| J01D Cephalosporins | 1.97 | 16.25 | 1.32 | 14.24 |
| J01E Sulfonamides and trimethoprim | 0.43 | 3.57 | 0.36 | 3.88 |
| J01FA Macrolides | 2.00 | 16.53 | 1.89 | 20.34 |
| J01FF Lincosamides | 0.50 | 4.13 | 0.47 | 5.06 |
| J01F Macrolides, lincosamides | 2.49 | 20.59 | 2.35 | 25.34 |
| J01M Quinolone antibacterials | 2.22 | 18.35 | 1.41 | 15.15 |
| J01X Other antibacterials | 0.06 | 0.53 | 0.01 | 0.07 |
| J01 Antibacterials | 12.10 | 100.00 | 9.29 | 100.00 |
| Pre-COVID Period | COVID-19 Period | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | ATC Code | Substance | DID 1 | % | Cum% 2 | ATC Code | Substance | DID 1 | % | Cum% 2 |
| 1. | J01CR02 | AMC 3 | 3.36 | 27.80 | 27.80 | J01CR02 | AMC 3 | 2.70 | 29.10 | 29.10 |
| 2. | J01DC02 | cefuroxime | 1.34 | 11.12 | 38.92 | J01FA10 | azithromycin | 1.41 | 15.14 | 44.24 |
| 3. | J01FA10 | azithromycin | 1.22 | 10.05 | 48.97 | J01DC02 | cefuroxime | 0.84 | 9.07 | 53.31 |
| 4. | J01MA12 | levofloxacin | 1.10 | 9.07 | 58.04 | J01AA02 | doxycycline | 0.78 | 8.35 | 61.66 |
| 5. | J01AA02 | doxycycline | 0.77 | 6.34 | 64.38 | J01MA12 | levofloxacin | 0.64 | 6.93 | 68.58 |
| 6. | J01FA09 | clarithromycin | 0.73 | 6.05 | 70.44 | J01FF01 | clindamycin | 0.47 | 5.04 | 73.63 |
| 7. | J01MA02 | ciprofloxacin | 0.66 | 5.46 | 75.89 | J01MA02 | ciprofloxacin | 0.46 | 5.00 | 78.62 |
| 8. | J01CA04 | amoxicillin | 0.61 | 5.01 | 80.91 | J01FA09 | clarithromycin | 0.46 | 4.97 | 83.59 |
| 9. | J01FF01 | clindamycin | 0.50 | 4.09 | 85.00 | J01EE01 | SMX-TMP 4 | 0.36 | 3.88 | 87.47 |
| 10. | J01EE01 | SMX-TMP 4 | 0.43 | 3.57 | 88.57 | J01CA04 | amoxicillin | 0.29 | 3.08 | 90.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hambalek, H.; Matuz, M.; Ruzsa, R.; Engi, Z.; Visnyovszki, Á.; Papfalvi, E.; Hajdú, E.; Doró, P.; Viola, R.; Soós, G.; et al. Impact of the COVID-19 Pandemic on Ambulatory Care Antibiotic Use in Hungary: A Population-Based Observational Study. Antibiotics 2023, 12, 970. https://doi.org/10.3390/antibiotics12060970
Hambalek H, Matuz M, Ruzsa R, Engi Z, Visnyovszki Á, Papfalvi E, Hajdú E, Doró P, Viola R, Soós G, et al. Impact of the COVID-19 Pandemic on Ambulatory Care Antibiotic Use in Hungary: A Population-Based Observational Study. Antibiotics. 2023; 12(6):970. https://doi.org/10.3390/antibiotics12060970
Chicago/Turabian StyleHambalek, Helga, Mária Matuz, Roxána Ruzsa, Zsófia Engi, Ádám Visnyovszki, Erika Papfalvi, Edit Hajdú, Péter Doró, Réka Viola, Gyöngyvér Soós, and et al. 2023. "Impact of the COVID-19 Pandemic on Ambulatory Care Antibiotic Use in Hungary: A Population-Based Observational Study" Antibiotics 12, no. 6: 970. https://doi.org/10.3390/antibiotics12060970
APA StyleHambalek, H., Matuz, M., Ruzsa, R., Engi, Z., Visnyovszki, Á., Papfalvi, E., Hajdú, E., Doró, P., Viola, R., Soós, G., Csupor, D., & Benko, R. (2023). Impact of the COVID-19 Pandemic on Ambulatory Care Antibiotic Use in Hungary: A Population-Based Observational Study. Antibiotics, 12(6), 970. https://doi.org/10.3390/antibiotics12060970








