Abstract
Antibiotics are one of the most frequently dispensed classes of medicines. However, excessive misuse and abuse enhances antimicrobial resistance (AMR). Previous studies in Pakistan have documented extensive dispensing of ‘Watch’ and ‘Reserve’ antibiotics, which is a concern. In view of this, there is a need to assess current dispensing patterns following COVID-19 in Pakistan. A cross-sectional study was undertaken, collecting dispensing data from 39 pharmacies and 53 drug stores from November 2022 to February 2023. Outlets were principally in urban areas (60.9%), with pharmacists/pharmacy technicians present in 32.6% of outlets. In total, 11,092 prescriptions were analyzed; 67.1% of patients were supplied at least one antimicrobial, 74.3% antibiotics, 10.2% antifungals and 7.9% anthelmintics. A total of 33.2% of antimicrobials were supplied without a prescription. Common indications for dispensed antibiotics were respiratory (34.3%) and gastrointestinal (16.8%) infections, which can be self-limiting. In addition, 12% of antibiotics were dispensed for the prevention or treatment of COVID-19. The most frequent antibiotics dispensed were ceftriaxone (18.4%) and amoxicillin (15.4%). Overall, 59.2% antibiotics were ‘Watch’ antibiotics, followed by ‘Access’ (40.3%) and ‘Reserve’ (0.5%) antibiotics. Of the total antibiotics dispensed for treating COVID-19, 68.3% were ‘Watch’ and 31.7% ‘Access’. Overall, there appeared to be an appreciable number of antibiotics dispensed during the recent pandemic, including for patients with COVID-19, alongside generally extensive dispensing of ‘Watch’ antibiotics. This needs to be urgently addressed with appropriate programs among pharmacists/pharmacy technicians to reduce AMR.
1. Introduction
Since the emergence of the COVID-19 outbreak caused by the SARS-Co-V2 outbreak in December 2019, neighboring countries to China, including Pakistan, have been at considerable risk of the virus [1,2]. This is enhanced by a porous border, as well as extensive trade and travel ties with China through land, sea and air [2,3,4]. In Pakistan, the first positive case of COVID-19 was reported on 26 February 2020 [4]. This was followed by a substantial number of positive cases throughout the country in various waves of the disease; however, prevalence rates may be under-reported [4,5,6]. Similar to other countries, Pakistan implemented many precautionary measures to slow the spread of the virus and its impact, including country-wide lockdown measures [7,8,9,10]. However, despite these measures, COVID-19 still caused a considerable impact on the country, which included the healthcare system [4,8,9,10]. Up to mid-to-late March 2023, more than 1.57 million positive cases and 30,000 deaths were reported in Pakistan [11]. In late March, more than 3500 suspect patients were being tested for COVID-19 daily, with approximately 100 patients daily being reported to have COVID-19 [12]. In addition to the instigation of multiple preventive measures, certain public sector hospitals were designated for the treatment of patients with presumed moderate-to-severe COVID-19 [13,14]. Many medicines, including corticosteroids, antipyretics, antihistamines and anti-thrombotics, have been prescribed to patients admitted with COVID-19 to different secondary and tertiary hospitals in Pakistan [15,16,17,18]. Alongside this, there was appreciable prescribing of antibiotics, especially ‘Watch’ antibiotics, among patients admitted with COVID-19 to public sector facilities in Pakistan despite limited prevalence of bacterial co-infections and secondary bacterial infections [13,14,18,19,20]. During the first five waves, this averaged 1.14% of patients with bacterial co-infections being prescribed antibiotics and 3.14% with secondary infections, which is similar to other countries [14,21,22,23]. Alongside this, extensive purchasing of antibiotics in Pakistan without a prescription, including both ‘Watch’ and ‘Reserve’ antibiotics, also occurred before and during the current pandemic [24,25,26,27,28]. We have seen appreciable purchasing of antibiotics without a prescription across low- and middle-income countries (LMICs), which includes African and Asian countries, despite high rates of antimicrobial resistance [25,29,30,31,32,33,34]. This is driven by patient requests, previous experiences, limited governance of laws banning such behavior and limited knowledge regarding antibiotics and AMR among key stakeholder groups [34,35,36,37,38,39].
This overuse of antibiotics across sectors, particularly ‘Watch’ antibiotics, exacerbated by the recent COVID-19 pandemic, is a concern, as this will increase antimicrobial resistance (AMR) [14,40,41,42], which is already a key issue in Pakistan [43]. Globally in 2019, there were 1.27 million deaths directly attributable to AMR, with this figure expected to continue rising unless addressed [29,44]. There is currently a disproportionate burden of AMR among LMICs, exacerbated by numerous financial, political and sociological factors [29,45,46]. The lack of adequate sanitation and hygiene, as well as poor infection control and preventive practices, alongside excessive and irrational use of antimicrobial agents, have increased the prevalence of AMR in LMICs in recent years [45,47,48]. Pakistan is no exception.
Pakistan is a LMIC located in South Asia and is currently the third-largest consumer of antibiotics globally, increasing AMR [49,50]. Multi-drug-resistant (MDR) and extensively drug-resistant (XDR) organisms, as well as other resistant organisms, have been reported throughout Pakistan, adding to concerns regarding AMR [50,51]. In view of increasing rates of AMR globally, international organizations, including the World Health Organization (WHO), have instigated a number of international activities. These include the development of the ‘global action plan’ (GAP) against AMR [52,53,54,55]. In line with the recommendations, Pakistan developed its own ‘National Action Plan’ (NAP) against AMR in 2017; however, there are challenges in its implementation [43,56].
In both the GAP and the NAP of Pakistan, assessing current antibiotic utilization patterns, alongside issues of inappropriate use, are seen as necessary starting points to develop appropriate plans to reduce AMR. Potential NAP activities include developing pertinent strategies to reduce inappropriate prescribing and dispensing in patients with COVID-19. We are aware that a number of studies have documented inappropriate prescribing and dispensing of antibiotics among patients with COVID-19 in Pakistan [14,19,20,57]. However, we are currently unaware of any study to date that has evaluated dispensing practices for antimicrobials from drug sale outlets during the current COVID-19 pandemic. This is important given growing concerns with increased antibiotic utilization during the pandemic, especially ‘Watch’ antibiotics, and the implications for increasing AMR. Consequently, the aim of this study was to undertake a multicenter study to access current patterns of antimicrobials dispensed in Pakistan at drug sale outlets during the pandemic. The findings can be used to develop future strategies, if pertinent, to reduce rising AMR rates in Pakistan. This can include the instigation of appropriate antimicrobial stewardship programs (ASPs) in ambulatory care to improve future prescribing and dispensing [48,58,59].
2. Results
Ninety-two drug sale outlets were included in the study, which incorporated 53 medical stores and 39 pharmacies (Table 1). Most of the sales outlets were located in urban areas (60.9%), with only one-third (32.6%) of outlets having a qualified pharmacist or pharmacy technician dispensing medicines. Most of the patients who were dispensed antibiotics were male (55.2%) and aged 46–60 years (29.5%) out of a total of 11,092 encounters where patients were dispensed a medicine.

Table 1.
Information about the drug sale outlets and encounters.
More than two-thirds of the encounters (67.1%) resulted in antimicrobials being dispensed (Table 2). Among the dispensed antimicrobials, nearly three-quarters (74.3%) were antibiotics, followed by antifungals (10.2%) and anthelmintics (7.9%). In total, 48.7% of antimicrobials dispensed were oral preparations, with 33.2% of antimicrobials being dispensed without a prescription from registered medical practitioners.

Table 2.
Detail of dispensed antimicrobials.
The indications for the dispensed antimicrobials are given in Table 3. As can be seen, more than a quarter (34.3%) of the antibiotics dispensed were for respiratory tract infections, followed by gastrointestinal (16.8%), skin and soft-tissue infections (12.9%), as well as pre- or post-operative prophylaxis (12.6%). Out of the total number of antibiotics dispensed in the four divisions, 12% of antibiotics were dispensed for patients with COVID-19.

Table 3.
Indications for dispensed antibiotics.
Details of the top ten most frequently dispensed antibiotics are shown in Table 4. The most frequently dispensed antibiotics were ceftriaxone (18.4%—‘Watch’), amoxicillin (15.4%—‘Access’) and azithromycin (14.9%—‘Watch’).

Table 4.
Top ten most frequently dispensed antibiotics from drug sale outlets.
The most frequently dispensed antibiotics for patients with COVID-19 were ceftriaxone (28%—‘Watch’), followed by azithromycin (25%—‘Watch’) and amoxicillin (22.4%—‘Access’) (Table 5).

Table 5.
Dispensed antibiotics for COVID-19.
Antibiotic dispensing practices as per the WHO AWaRe classification are shown for each Division in Figure 1. As can be seen, most of the antibiotics dispensed were from the ‘Watch’ category (59.2%) followed by the ‘Access’ category (40.3%), with a few also from the ‘Reserve’ category (0.5%).
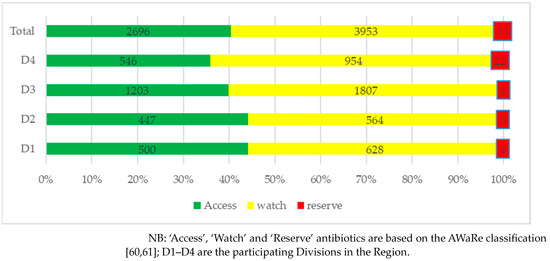
Figure 1.
Dispensing practices of antibiotics according to AWaRe classification distributed by Division.
Dispensing practices of antibiotics among patients with COVID-19 showed that slightly more than two-thirds of antibiotics dispensed (68.3%) were from the ‘Watch’ category, followed by the ‘Access’ category (31.7%). No antibiotic dispensed was from the ‘Reserve’ category (Figure 2).
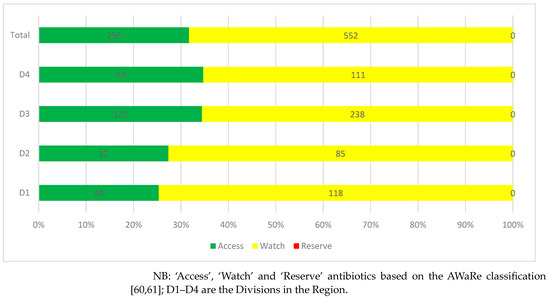
Figure 2.
Dispensing practices of antibiotics among patients with COVID-19 distributed by Division.
3. Discussion
We believe this is one of the first studies from Pakistan that has assessed antimicrobial dispensing practices during the current COVID-19 pandemic. This is important given concerns across countries with the over-prescribing of antibiotics in patients with COVID-19 despite limited evidence of bacterial infections or co-infections [21,22,23,62,63,64]. In addition, extensive prescribing of ‘Watch’ antibiotics has been seen in patients hospitalized with COVID-19 in Pakistan [13,14,20].
More than two-thirds of the total patient encounters in our study were supplied with at least one antimicrobial, with nearly three-quarters of these being antibiotics. This overuse of antibiotics in ambulatory care during the COVID-19 pandemic is in line with previous studies from other South Asian countries and beyond [62,63,64,65]. A recent study analyzing sales data from 71 countries also concluded similar findings, with an increase in antibiotic utilization during the COVID-19 pandemic [66].
This is a concern alongside the finding that respiratory tract infections and gastrointestinal infections were the most common complaints and where antibiotics are typically not necessary [67]. Others common indications were the prevention and treatment of COVID-19, where again antibiotics may not be necessary, with, as mentioned, little evidence of bacterial infections in these patients [14,22,23]. This is similar to a study from Russia where the majority of the antibiotics dispensed in ambulatory care were for respiratory and skin and soft-tissue infections [68].
The top three antibiotics dispensed at the surveyed drug sale outlets in Pakistan during the study period were ceftriaxone, amoxicillin and azithromycin. The appreciable use of ceftriaxone and azithromycin resulted in most of the antibiotics dispensed during the study period being from the ‘Watch’ category (59.2%), followed by the ‘Access’ category (40.3%), in addition to a minority from the ‘Reserve’ group of antibiotics (0.5%). This mirrors other studies in Pakistan pre-pandemic, where there was appreciable dispensing of ‘Watch’ and ‘Reserve’ antibiotics [24], as well as in Bangladesh [69]. This high use of ‘Watch’ antibiotics alongside any dispensing of ‘Reserve’ antibiotics needs to be urgently addressed if Pakistan is to reduce current high rates of AMR in line with the objectives of the NAP [43].
Similarly, patients with COVID-19 were frequently dispensed ceftriaxone, azithromycin, amoxicillin and ciprofloxacin. As a result, 68.3% of antibiotics dispensed for these patients were from the ‘Watch’ category, higher than those without COVID-19. Whilst our findings are comparable to those from Egypt, where ceftriaxone and azithromycin were again common antibiotics dispensed during COVID-19 [70], this also needs to be urgently addressed.
Of equal concern is that one-third of the total antibiotics dispensed by the surveyed outlets in our study were without a prescription from registered medical practitioners. Whilst this is an improvement from previous studies in the Hazara Division of Khyber Pakhtunkhwa Province, Pakistan, where more than 90% antibiotics were dispensed without prescriptions [71], as well as Lahore, where more than 95% of antibiotics were dispensed without a prescription [24], it is still against current regulations [72]. This is important for respiratory tract infections and gastrointestinal infections, common complaints where antibiotics were dispensed, for which antibiotics are often inappropriate [67].
We have seen a number of antimicrobial stewardship programs (ASPs) introduced in ambulatory care across LMICs to improve appropriate prescribing. These are described in Supplementary Table S1. There was a concern that ASPs were difficult to undertake in LMICs due to personnel and financial issues [73]. However, this no longer appears to be the case, with a number of exemplars discussed in Supplementary Table S1 providing direction to all key stakeholder groups in Pakistan. Active follow-up of any intervention is essential for the sustainability of any ASP (Supplementary Table S1). We are also aware of studies in other countries where ASPs have been successfully introduced in community pharmacies to reduce inappropriate dispensing of antibiotics (Supplementary Table S2). Alongside this, initiatives in LMICs that have been introduced but have failed to sustainably reduce inappropriate dispensing of antibiotics without a prescription. These latter initiatives typically failed due to a lack of follow-up and monitoring of community pharmacists’ activities. Consequently, any initiative introduced in Pakistan needs to be actively followed up or else the objectives will not be met. This is because it is essential that inappropriate dispensing of antibiotics is addressed to reduce AMR. Community pharmacists and pharmacy technicians will play a greater role in improving patient care across countries in the future, including LMICs, building on their increasing role during the COVID-19 pandemic [74,75,76,77,78]. In view of this, an increasingly important target group to improve antibiotic utilization is in ambulatory care. However, there are typically knowledge gaps among community pharmacists and pharmacy technicians regarding antibiotics and AMR in LMICs which urgently need to be addressed [39,79,80]. This starts in universities and other educational establishments, and continues post-qualification for maximum impact given current knowledge concerns among pharmacists and pharmacy technicians in Pakistan [79,81,82]. We have seen trained pharmacists, coupled with the use of guidelines suggesting alternatives to antibiotics, being used to good effect in LMICs to limit the dispensing of antibiotics for essentially self-limiting viral infections [78,83,84], and as a result providing future guidance to key stakeholder groups in Pakistan [78,83,84].
Another concern is that many of the drug outlets in our study did not have qualified pharmacists or pharmacy technicians to help give alternative advice for self-limiting conditions such as URTIs. Whilst this is similar to other countries [85], this also needs to be addressed in Pakistan going forward. In the first instance, this includes educational programs among all key dispensing personnel, along with guidelines and targets based on the AWaRe book with its multiple guidance on key infections in ambulatory care, to reduce inappropriate dispensing [67,83,84]. This is seen as more likely to succeed than introducing limited fines for community pharmacists for dispensing antibiotics without a prescription, as seen in Vietnam (Supplementary Table S2) [86].
We are aware of a number of limitations associated with our study. Firstly, we only conducted this study in four divisions of Punjab Province. However, we chose Punjab Province for this initial study for the reasons documented. In addition, we included an appreciable number of drug outlets and patients to help address concerns with bias. We also did not collect information regarding the signs and symptoms and laboratory findings of patients to fully assess the appropriateness of dispensed antibiotics. However, this was impractical given that the principal objective of this study was to assess actual antimicrobial dispensing practices. In addition, we only collected the number of antibiotics dispensed for particular conditions rather than the extent of multiple, as opposed to single, antibiotics for given patients for identified indications, as our principal objective was again to assess the actual extent of antibiotics dispensed for common infections. We will, however, be following this up in future studies. Despite these limitations, we believe our findings are robust and can provide guidance to health authorities, healthcare providers, including those working in drug sales outlets, as well as the general population in Pakistan, to reduce high rates of inappropriate dispensing of antibiotics in patients with or without COVID-19. This is important as Pakistan strives to reduce its high rate of AMR as part of its agreed NAP.
4. Materials and Methods
4.1. Study Design and Setting
This cross-sectional study was conducted in drug sale points (pharmacies/medical stores) in four divisions of the Punjab Province to evaluate the dispensing practices of antimicrobial agents during the current COVID-19 pandemic. Pharmacies are drug sale point/premises working under the supervision of a qualified pharmacist, i.e., a graduate in pharmacy which takes five years. Medical stores are run by pharmacy technicians (two-year diploma in pharmacy).
There are 11 divisions in Punjab and we conveniently selected 4 of these in this study, subsequently named D1 to D4. We chose the Punjab Province for this initial study as it covers more than half of the population of Pakistan; consequently, the findings can give good insight into the remainder of Pakistan [20].
Subsequent pharmacies and medical stores were conveniently selected to collect dispensing information in line with other studies. This included previous studies involving the co-authors [79,87,88]. A minimum of five pharmacies and ten medical stores were included from every division, with a minimum of 100 prescriptions included from each pharmacy and 50 from each medical store. There was no sample size calculation as this type of research has not been undertaken before in Pakistan among patients with COVID-19. However, we aimed for a substantial number of prescriptions and data to add robustness to our findings.
As per the drug law in Pakistan of 1976, medicines can only be sold at medical stores and pharmacies, and only following a prescription [72]. Medical stores are abundant, particularly in rural areas, where medical stores can be operated under the supervision of pharmacy technicians/dispensers. However, pharmacies are usually located in urban areas and these outlets work under the supervision of a pharmacist. The drug outlets provided medicines, including antimicrobials, vaccines and personal protective equipment (PPE), during the recent COVID-19 pandemic.
4.2. Study Variables
Information about the drug sale outlets included in the study, including pharmacies and medical stores, was collected by the investigators. This included their numbers, location, presence of qualified personnel at the pharmacy/medical store and whether the drug sales point was working independently or as a branch of a chain pharmacy/medical store.
The total number of patient encounters recorded and the encounters dispensed with at least one antimicrobial agent were recorded. The gender and age of the patients were documented alongside the type of antimicrobial dispensed. This included whether this was an antibiotic, antiviral, antifungal, antiprotozoal or anthelmintic. The route of administration was also documented.
Information about the number of encounters was collected by the investigators. We included all encounters recorded during the data collection period. We also included those encounters that included other medicines being dispensed alongside antibiotics. In addition, where antibiotics were being demanded from drug outlets without a prescription.
The dispensing practices surrounding antimicrobials were also recorded. This included the number of prescriptions that were dispensed with at least one antimicrobial out of total number of encounters, alongside the generic name of the antimicrobial (International Nonproprietary Name—INN [89]), the route of administration, the indications for any antibiotics dispensed as well as the type of antibiotic dispensed according to AWaRe classification [60,61]. Antibiotics dispensed for positive or suspected COVID-19 patients were also recorded alongside their categorization as per the AWaRe classification.
4.3. Data Collection Process
Investigators, including pharmacists and pharmacy technicians, visited drug sale outlets during the study period and requested the proprietor/owner or community pharmacist/pharmacy technicians of the concerned drug sale point to allow them to collect dispensing data after describing the study objectives. Those who were ready to facilitate this survey were briefed about the data collection process. Investigators were available at the drug sale point where medicines were being dispended and patients were counselled about the objectives of the study. Written informed consent was obtained from potential patient contributors prior to their enrollment into the study. No personal information, including the name of possible patients, their telephone number or national identity card number, was recorded to maintain confidentiality.
4.4. Statistical Analysis
The SPSS version 22.0 for Windows and Microsoft Excel version 2016 were used for analyzing the data of this study. Numbers and percentages were used to present categorical variables. Participants’ data were coded and stored in a password-protected file accessible only to the researchers to maintain confidentiality.
4.5. Study Approval
This study was approved by the ORIC, Lahore College for Women University (LCWU) Lahore, Pakistan. Written informed consent was obtained from all the study participants prior to their enrollment in the study. For children (age < 18 years), consent was obtained from the parent prior to data collection.
5. Conclusions
In conclusion, there are concerns regarding the appreciable dispensing of antimicrobials among drug sale outlets in Pakistan, including for patients with COVID-19, alongside the appreciable dispensing of ‘Watch’ versus ‘Access’ antibiotics. In addition, the majority of the drug sale outlets in Punjab were working without the presence of a community pharmacist or pharmacy technicians where antibiotics were being dispensed without a prescription. This needs to be urgently addressed going forward. This includes greater education of community pharmacists, pharmacy technicians and the public.
ASPs can successfully be implemented in these drug sale outlets along with improved education among the dispensers and patients to reduce inappropriate requests and dispensing of antibiotics without a prescription, and hence reduce AMR. Subsequently, regular monitoring of the interventions among sales outlets is necessary to continue to reduce inappropriate dispensing. This is important to meet key goals of the current NAP on AMR.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12061018/s1, Table S1. Initiatives to improve antibiotic utilization among physicians in ambulatory care in LMICs; Table S2. Summary of ASPs and other activities to reduce inappropriate dispensing of antibiotics without a prescription among LMICs; Table S3. Questionnaire. This includes references [90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108].
Author Contributions
Conceptualization: B.G., Z.U.M., J.C.M., T.M.S. and B.B.G.; methodology: B.G. and Z.U.M.; validation: B.G., M.S. (Maria Sana), A.S., Z.U.M., M.S. (Muhammad Salman), Y.H.K. and T.H.M.; formal analysis: Z.U.M., J.C.M. and B.B.G.; investigation and data curation: B.G., M.S. (Maria Sana), A.S., Z.U.M., M.S. (Muhammad Salman), Y.H.K., T.H.M. and T.M.S.; resources: Z.U.M.; writing—original draft: Z.U.M., T.M.S. and B.B.G.; writing—review and editing: B.G., M.S. (Maria Sana), Z.U.M., M.S. (Muhammad Salman), Y.H.K., Y.H.K., T.M.S., J.C.M. and B.B.G.; project administration: Z.U.M. and B.B.G.; visualization: Z.U.M.; supervision: Z.U.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the ORIC, Lahore College for Women University (LCWU), Lahore, Pakistan.
Informed Consent Statement
Written informed consent was obtained from all the study participants prior to their enrollment in the study. For children age <18 years, consent was obtained from a parent prior to data collection.
Data Availability Statement
Further additional data are available from the corresponding authors on reasonable request.
Acknowledgments
We would like to acknowledge the support and co-ordination of all the staff working in the surveyed outlets for the completion of this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Abid, K.; Bari, Y.A.; Younas, M.; Javaid, S.T.; Imran, A. Progress of COVID-19 Epidemic in Pakistan. Asia Pac. J. Public Health 2020, 32, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Shafi, M.; Liu, J.; Ren, W. Impact of COVID-19 pandemic on micro, small, and medium-sized Enterprises operating in Pakistan. Res. Glob. 2020, 2, 100018. [Google Scholar] [CrossRef]
- Salman, M.; Mustafa, Z.U.; Khan, T.M.; Shehzadi, N.; Hussain, K. How Prepared Was Pakistan for the COVID-19 Outbreak? Disaster Med. Public Health Prep. 2020, 14, e44–e45. [Google Scholar] [CrossRef]
- Aheron, S.; Victory, K.R.; Imtiaz, A.; Fellows, I.; Gilani, S.I.; Gilani, B.; Reed, C.; Hakim, A.J. A Nationally Representative Survey of COVID-19 in Pakistan, 2021–2022. Emerg. Infect. Dis. 2022, 28, S69–S75. [Google Scholar] [CrossRef]
- Taimoor, M.; Ali, S.; Shah, I.; Muwanika, F.R. COVID-19 Pandemic Data Modeling in Pakistan Using Time-Series SIR. Comput. Math. Methods Med. 2022, 2022, 6001876. [Google Scholar] [CrossRef]
- Abbas, A.; Mannan, A. Reasons behind declining of cases during the COVID-19 wavelets in Pakistan: Public healthcare system or government smart lockdown policy? Cien. Saude. Colet. 2022, 27, 2973–2984. [Google Scholar] [CrossRef]
- Akhtar, H.; Afridi, M.; Akhtar, S.; Ahmad, H.; Ali, S.; Khalid, S.; Awan, S.M.; Jahangiri, S.; Khader, Y.S. Pakistan’s Response to COVID-19: Overcoming National and International Hypes to Fight the Pandemic. JMIR Public Health Surveill. 2021, 7, e28517. [Google Scholar] [CrossRef]
- Kamran, K.; Ali, A. Challenges and Strategies for Pakistan in the Third Wave of COVID-19: A Mini Review. Front. Public Health 2021, 9, 690820. [Google Scholar] [CrossRef]
- Rasheed, R.; Rizwan, A.; Javed, H.; Sharif, F.; Zaidi, A. Socio-economic and environmental impacts of COVID-19 pandemic in Pakistan—An integrated analysis. Environ. Sci. Pollut. Res. 2021, 28, 19926–19943. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 25 February 2023).
- WHO. COVID-19. Pakistan. 2023. Available online: https://covid19.who.int/region/emro/country/pk (accessed on 10 April 2023).
- Mustafa, Z.U.; Khan, A.H.; Harun, S.N.; Salman, M.; Godman, B. Antibiotic Overprescribing among Neonates and Children Hospitalized with COVID-19 in Pakistan and the Implications. Antibiotics 2023, 12, 646. [Google Scholar] [CrossRef]
- Ramzan, K.; Shafiq, S.; Raees, I.; Mustafa, Z.U.; Salman, M.; Khan, A.H.; Meyer, J.C.; Godman, B. Co-Infections, Secondary Infections, and Antimicrobial Use in Patients Hospitalized with COVID-19 during the First Five Waves of the Pandemic in Pakistan; Findings and Implications. Antibiotics 2022, 11, 789. [Google Scholar] [CrossRef]
- Khan, E.A. COVID-19 in children: Epidemiology, presentation, diagnosis and management. J. Pak. Med. Assoc. 2020, 70 (Suppl. 3), S108–S112. [Google Scholar] [CrossRef]
- Mustafa, Z.U.; Kow, C.S.; Salman, M.; Kanwal, M.; Riaz, M.B.; Parveen, S.; Hasan, S.S. Pattern of medication utilization in hospitalized patients with COVID-19 in three District Headquarters Hospitals in the Punjab province of Pakistan. Explor. Res. Clin. Soc. Pharm. 2021, 5, 100101. [Google Scholar] [CrossRef]
- Iqtadar, S.; Khan, A.; Mumtaz, S.U.; Pascual-Figal, D.A.; Livingstone, S.; Abaidullah, S. Tocilizumab therapy for severely-ill COVID-19 pneumonia patients: A single-centre retrospective study. J. Physiol. Pharmacol. 2022, 73, 547–553. [Google Scholar]
- Kamran, S.H.; Mustafa, Z.U.; Rao, A.Z.; Hasan, S.S.; Zahoor, F.; Sarwar, M.U.; Khan, S.; Butt, S.; Rameez, M.; Asif Abbas, M. SARS-CoV-2 infection pattern, transmission and treatment: Multi-center study in low to middle-income districts hospitals in Punjab, Pakistan. Pak. J. Pharm. Sci. 2021, 34 (Suppl. 3), 1135–1142. [Google Scholar]
- Mustafa, Z.U.; Salman, M.; Aldeyab, M.; Kow, C.S.; Hasan, S.S. Antimicrobial consumption among hospitalized patients with COVID-19 in Pakistan. SN ComPract. Clin. Med. 2021, 3, 1691–1695. [Google Scholar] [CrossRef]
- Mustafa, Z.U.; Saleem, M.S.; Ikram, M.N.; Salman, M.; Butt, S.A.; Khan, S.; Godman, B.; Seaton, R.A. Co-infections and antimicrobial use among hospitalized COVID-19 patients in Punjab, Pakistan: Findings from a multicenter, point prevalence survey. Pathog. Glob. Health 2022, 116, 421–427. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.-P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Alshaikh, F.S.; Godman, B.; Sindi, O.N.; Seaton, R.A.; Kurdi, A. Prevalence of bacterial coinfection and patterns of antibiotics prescribing in patients with COVID-19: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0272375. [Google Scholar] [CrossRef]
- Saleem, Z.; Hassali, M.A.; Godman, B.; Fatima, M.; Ahmad, Z.; Sajid, A.; Rehman, I.U.; Nadeem, M.U.; Javaid, Z.; Malik, M.; et al. Sale of WHO AWaRe groups antibiotics without a prescription in Pakistan: A simulated client study. J. Pharm. Policy Pract. 2020, 13, 26. [Google Scholar] [CrossRef]
- Torres, N.F.; Chibi, B.; Kuupiel, D.; Solomon, V.P.; Mashamba-Thompson, T.P.; Middleton, L.E. The use of non-prescribed antibiotics; prevalence estimates in low-and-middle-income countries. A systematic review and meta-analysis. Arch. Public Health 2021, 79, 2. [Google Scholar] [CrossRef]
- Aslam, A.; Zin, C.S.; Jamshed, S.; Ab Rahman, N.S.; Ahmed, S.I.; Pallós, P.; Gajdács, M. Self-Medication with Antibiotics: Prevalence, Practices and Related Factors among the Pakistani Public. Antibiotics 2022, 11, 795. [Google Scholar] [CrossRef]
- Bilal, M.; Haseeb, A.; Khan, M.H.; Arshad, M.H.; Ladak, A.A.; Niazi, S.K.; Musharraf, M.D.; Manji, A.A. Self-Medication with Antibiotics among People Dwelling in Rural Areas of Sindh. J. Clin. Diagn. Res. 2016, 10, OC08–OC13. [Google Scholar] [CrossRef]
- Asghar, S.; Atif, M.; Mushtaq, I.; Malik, I.; Hayat, K.; Babar, Z.-U. Factors associated with inappropriate dispensing of antibiotics among non-pharmacist pharmacy workers. Res. Soc. Adm. Pharm. 2019, 16, 805–811. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Auta, A.; Hadi, M.A.; Oga, E.; Adewuyi, E.O.; Abdu-Aguye, S.N.; Adeloye, D.; Strickland-Hodge, B.; Morgan, D.J. Global access to antibiotics without prescription in community pharmacies: A systematic review and meta-analysis. J. Infect. 2019, 78, 8–18. [Google Scholar] [CrossRef]
- Belachew, S.A.; Hall, L.; Selvey, L.A. Non-prescription dispensing of antibiotic agents among community drug retail outlets in Sub-Saharan African countries: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control. 2021, 10, 13. [Google Scholar] [CrossRef]
- Ocan, M.; Obuku, E.A.; Bwanga, F.; Akena, D.; Richard, S.; Ogwal-Okeng, J.; Obua, C. Household antimicrobial self-medication: A systematic review and meta-analysis of the burden, risk factors and outcomes in developing countries. BMC Public Health 2015, 15, 742. [Google Scholar] [CrossRef]
- Nepal, G.; Bhatta, S. Self-medication with Antibiotics in WHO Southeast Asian Region: A Systematic Review. Cureus 2018, 10, e2428. [Google Scholar] [CrossRef]
- Yeika, E.V.; Ingelbeen, B.; Kemah, B.L.; Wirsiy, F.S.; Fomengia, J.N.; van der Sande, M.A.B. Comparative assessment of the prevalence, practices and factors associated with self-medication with antibiotics in Africa. Trop. Med. Int. Health 2021, 26, 862–881. [Google Scholar] [CrossRef]
- Torres, N.; Chibi, B.; Middleton, L.; Solomon, V.; Mashamba-Thompson, T. Evidence of factors influencing self-medication with antibiotics in low and middle-income countries: A systematic scoping review. Public Health 2019, 168, 92–101. [Google Scholar] [CrossRef]
- Belachew, S.A.; Hall, L.; Erku, D.A.; Selvey, L.A. No prescription? No problem: Drivers of non-prescribed sale of antibiotics among community drug retail outlets in low and middle income countries: A systematic review of qualitative studies. BMC Public Health 2021, 21, 1056. [Google Scholar] [CrossRef]
- Bert, F.; Previti, C.; Calabrese, F.; Scaioli, G.; Siliquini, R. Antibiotics Self Medication among Children: A Systematic Review. Antibiotics 2022, 11, 1583. [Google Scholar] [CrossRef]
- Lubwama, M.; Onyuka, J.; Ayazika, K.T.; Ssetaba, L.J.; Siboko, J.; Daniel, O.; Mushi, M.F. Knowledge, attitudes, and perceptions about antibiotic use and antimicrobial resistance among final year undergraduate medical and pharmacy students at three universities in East Africa. PLoS ONE 2021, 16, e0251301. [Google Scholar] [CrossRef]
- Edessa, D.; Sisay, M.; Hagos, B.; Amare, F. Antimicrobial Use and Management of Childhood Diarrhea at Community Drug Retail Outlets in Eastern Ethiopia: A Matched Questionnaire-Based and Simulated Patient-Case Study. Pediatr. Health Med. Ther. 2022, 13, 63–79. [Google Scholar] [CrossRef]
- Sulis, G.; Sayood, S.; Katukoori, S.; Bollam, N.; George, I.; Yaeger, L.H.; Chavez, M.A.; Tetteh, E.; Yarrabelli, S.; Pulcini, C.; et al. Exposure to World Health Organization’s AWaRe antibiotics and isolation of multidrug resistant bacteria: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 1193–1202. [Google Scholar] [CrossRef]
- Sulis, G.; Sayood, S.; Gandra, S. Antimicrobial resistance in low- and middle-income countries: Current status and future directions. Expert Rev. Anti-Infect. Ther. 2021, 20, 147–160. [Google Scholar] [CrossRef]
- Lai, C.-C.; Chen, S.-Y.; Ko, W.-C.; Hsueh, P.-R. Increased antimicrobial resistance during the COVID-19 pandemic. Int. J. Antimicrob. Agents 2021, 57, 106324. [Google Scholar] [CrossRef]
- Saleem, Z.; Hassali, M.A.; Hashmi, F.K. Pakistan’s national action plan for antimicrobial resistance: Translating ideas into reality. Lancet Infect. Dis. 2018, 18, 1066–1067. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: A univariate and multivariable analysis. Lancet Planet. Health 2018, 2, e398–e405. [Google Scholar] [CrossRef]
- Gandra, S.; Alvarez-Uria, G.; Turner, P.; Joshi, J.; Limmathurotsakul, D.; van Doorn, H.R. Antimicrobial Resistance Surveillance in Low- and Middle-Income Countries: Progress and Challenges in Eight South Asian and Southeast Asian Countries. Clin. Microbiol. Rev. 2020, 33, e00048-19. [Google Scholar] [CrossRef]
- WHO. Call to Action on Antimicrobial Resistance (AMR)-2021. Available online: https://www.un.org/pga/75/wp-content/uploads/sites/100/2021/04/Call-to-Action-on-Antimicrobial-Resistance-AMR-2021.pdf (accessed on 9 April 2023).
- Godman, B.; Egwuenu, A.; Haque, M.; Malande, O.O.; Schellack, N.; Kumar, S.; Saleem, Z.; Sneddon, J.; Hoxha, I.; Islam, S.; et al. Strategies to Improve Antimicrobial Utilization with a Special Focus on Developing Countries. Life 2021, 11, 528. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, e3463–e3470. [Google Scholar] [CrossRef]
- Bilal, H.; Khan, M.N.; Rehman, T.; Hameed, M.F.; Yang, X. Antibiotic resistance in Pakistan: A systematic review of past decade. BMC Infect. Dis. 2021, 21, 244. [Google Scholar] [CrossRef]
- Arshad, F.; Saleem, S.; Tahir, R.; Ghazal, A.; Khawaja, A.; Jahan, S. Four year trend of antimicrobial susceptibility of methicillin-resistant Staphylococcus aureus in a tertiary care hospital, Lahore. J. Pak. Med. Assoc. 2022, 72, 296–299. [Google Scholar]
- WHO. Global Action Plan on Antimicrobial Resistance-Report by the Secretariat. 2016. Available online: https://apps.who.int/gb/ebwha/pdf_files/WHA69/A69_24-en.pdf (accessed on 8 April 2023).
- OECD Health Policy Studies. Stemming the Superbug Tide. 2018. Available online: https://www.oecd-ilibrary.org/sites/9789264307599-en/index.html?itemId=/content/publication/9789264307599-en&mimeType=text/html. (accessed on 8 April 2023).
- World Bank Group. Pulling Together to Beat Superbugs Knowledge and Implementation Gaps in Addressing Antimicrobial Resistance. 2019. Available online: https://openknowledge.worldbank.org/bitstream/handle/10986/32552/Pulling-Together-to-Beat-Superbugs-Knowledge-and-Implementation-Gaps-in-Addressing-Antimicrobial-Resistance.pdf?sequence=1&isAllowed=y (accessed on 8 April 2023).
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2021. Available online: https://www.who.int/publications/i/item/9789240027336 (accessed on 8 April 2023).
- Saleem, Z.; Godman, B.; Azhar, F.; Kalungia, A.C.; Fadare, J.; Opanga, S.; Markovic-Pekovic, V.; Hoxha, I.; Saeed, A.; Al-Gethamy, M.; et al. Progress on the national action plan of Pakistan on antimicrobial resistance (AMR): A narrative review and the implications. Expert Rev. Anti-Infect. Ther. 2021, 20, 71–93. [Google Scholar] [CrossRef]
- Saleem, Z.; Haseeb, A.; Godman, B.; Batool, N.; Altaf, U.; Ahsan, U.; Khan, F.U.; Mustafa, Z.U.; Nadeem, M.U.; Farrukh, M.J.; et al. Point Prevalence Survey of Antimicrobial Use during the COVID-19 Pandemic among Different Hospitals in Pakistan: Findings and Implications. Antibiotics 2022, 12, 70. [Google Scholar] [CrossRef]
- Yau, J.W.; Thor, S.M.; Tsai, D.; Speare, T.; Rissel, C. Antimicrobial stewardship in rural and remote primary health care: A narrative review. Antimicrob. Resist Infect. Control. 2021, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.; Apisarnthanarak, A.; Schellack, N.; Cornistein, W.; Al Maani, A.; Adnan, S.; Stevens, M.P. Global Antimicrobial Stewardship with a Focus on Low- and Middle-Income Countries: A position statement for the international society for infectious diseases. Int. J. Infect. Dis. 2020, 96, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Sharland, M.; Pulcini, C.; Harbarth, S.; Zeng, M.; Gandra, S.; Mathur, S.; Magrini, N. Classifying antibiotics in the WHO Essential Medicines List for optimal use—Be AWaRe. Lancet Infect. Dis. 2018, 18, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Sharland, M.; Gandra, S.; Huttner, B.; Moja, L.; Pulcini, C.; Zeng, M.; Mendelson, M.; Cappello, B.; Cooke, G.; Magrini, N.; et al. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use—The new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect. Dis. 2019, 19, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Bednarčuk, N.; Golić Jelić, A.; Stoisavljević Šatara, S.; Stojaković, N.; Marković Peković, V.; Stojiljković, M.P.; Popović, N.; Škrbić, R. Antibiotic Utilization during COVID-19: Are We Over-Prescribing? Antibiotics 2023, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Daria, S.; Islam, M.R. Indiscriminate Use of Antibiotics for COVID-19 Treatment in South Asian Countries is a Threat for Future Pandemics Due to Antibiotic Resistance. Clin Pathol. 2022, 15, 2632010X221099889. [Google Scholar] [CrossRef]
- Malik, S.S.; Mundra, S. Increasing Consumption of Antibiotics during the COVID-19 Pandemic: Implications for Patient Health and Emerging Anti-Microbial Resistance. Antibiotics 2022, 12, 45. [Google Scholar] [CrossRef]
- Satria, Y.A.A.; Utami, M.S.; Prasudi, A. Prevalence of antibiotics prescription amongst patients with and without COVID-19 in low- and middle-income countries: A systematic review and meta-analysis. Ann. Trop. Med. Parasitol. 2022, 1–13. [Google Scholar] [CrossRef]
- Nandi, A.; Pecetta, S.; Bloom, D.E. Global antibiotic use during the COVID-19 pandemic: Analysis of pharmaceutical sales data from 71 countries, 2020–2022. Eclinicalmedicine 2023, 57, 101848. [Google Scholar] [CrossRef]
- WHO. The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book. 2022. Available online: https://www.who.int/publications/i/item/9789240062382 (accessed on 8 April 2023).
- Rachina, S.; Kozlov, R.; Kurkova, A.; Portnyagina, U.; Palyutin, S.; Khokhlov, A.; Reshetko, O.; Zhuravleva, M.; Palagin, I.; on behal of Russian Working Group of the Project. Antimicrobial Dispensing Practice in Community Pharmacies in Russia during the COVID-19 Pandemic. Antibiotics 2022, 11, 586. [Google Scholar] [CrossRef]
- Islam, A.; Akhtar, Z.; Hassan, Z.; Chowdhury, S.; Rashid, M.; Aleem, M.A.; Ghosh, P.K.; Mah-E-Muneer, S.; Parveen, S.; Ahmmed, K.; et al. Pattern of Antibiotic Dispensing at Pharmacies According to the WHO Access, Watch, Reserve (AWaRe) Classification in Bangladesh. Antibiotics 2022, 11, 247. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.A.; Darwish, S.F.; Zewail, M.B.; Mohammed, M.; Saeed, H.; Rabea, H. Antibiotic misuse and compliance with infection control measures during COVID-19 pandemic in community pharmacies in Egypt. Int. J. Clin. Pract. 2021, 75, e14081. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Khan, F.U.; Ali, S.; Rahman, A.U.; Khan, S.A. Assessment of without prescription antibiotic dispensing at community pharmacies in Hazara Division, Pakistan: A simulated client’s study. PLoS ONE 2022, 17, e0263756. [Google Scholar] [CrossRef]
- Punjab, GO. Punjab Drug Rules 2007. Available online: https://punjablaws.punjab.gov.pk/uploads/articles/drug-rules-pdf.pdf. (accessed on 10 May 2023).
- Cox, J.A.; Vlieghe, E.; Mendelson, M.; Wertheim, H.; Ndegwa, L.; Villegas, M.V.; Gould, I.; Levy Hara, G. Antibiotic stewardship in low- and middle-income countries: The same but different? Clin. Microbiol. Infect. 2017, 23, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Hamid, H.; Masood, R.A.; Tariq, H.; Khalid, W.; Rashid, M.A.; Munir, M.U. Current pharmacy practices in low- and middle-income countries; recommendations in response to the COVID-19 pandemic. Drugs Ther. Perspect. 2020, 36, 355–357. [Google Scholar] [CrossRef]
- Hedima, E.W.; Adeyemi, M.S.; Ikunaiye, N.Y. Community pharmacists: On the frontline of health service against COVID-19 in LMICs. Res. Soc. Adm. Pharm. 2021, 17, 1964–1966. [Google Scholar] [CrossRef]
- Sami, S.A.; Marma, K.K.S.; Chakraborty, A.; Singha, T.; Rakib, A.; Uddin, G.; Hossain, M.K.; Uddin, S.M.N. A comprehensive review on global contributions and recognition of pharmacy professionals amidst COVID-19 pandemic: Moving from present to future. Futur. J. Pharm. Sci. 2021, 7, 119. [Google Scholar] [CrossRef]
- Cadogan, C.A.; Hughes, C.M. On the frontline against COVID-19: Community pharmacists’ contribution during a public health crisis. Res. Soc. Adm. Pharm. 2020, 17, 2032–2035. [Google Scholar] [CrossRef]
- Kibuule, D.N.L.; Sefah, I.A.; Kurdi, A.; Phuong, T.N.T.; Kwon, H.-Y.; Godman, B. Activities in Namibia to limit the prevalence and mortality from COVID-19 including community pharmacy activities and the implications. Sch. Acad. J. Pharm. 2021, 5, 82–92. [Google Scholar] [CrossRef]
- Mustafa, Z.U.; Nazir, M.; Majeed, H.K.; Salman, M.; Hayat, K.; Khan, A.H.; Meyer, J.C.; Godman, B. Exploring Knowledge of Antibiotic Use, Resistance, and Stewardship Programs among Pharmacy Technicians Serving in Ambulatory Care Settings in Pakistan and the Implications. Antibiotics 2022, 11, 921. [Google Scholar] [CrossRef]
- Abdu-Aguye, S.N.; Barde, K.G.; Yusuf, H.; Lawal, B.K.; Shehu, A.; Mohammed, E. Investigating Knowledge of Antibiotics, Antimicrobial Resistance and Antimicrobial Stewardship Concepts Among Final Year Undergraduate Pharmacy Students in Northern Nigeria. Integr. Pharm. Res. Pract. 2022, 11, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Hayat, K.; Jamshed, S.; Rosenthal, M.; Haq, N.U.; Chang, J.; Rasool, M.F.; Malik, U.R.; Rehman, A.U.; Khan, K.M.; Fang, Y. Understanding of Pharmacy Students towards Antibiotic Use, Antibiotic Resistance and Antibiotic Stewardship Programs: A Cross-Sectional Study from Punjab, Pakistan. Antibiotics 2021, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Atif, M.; Asghar, S.; Mushtaq, I.; Malik, I. Community pharmacists as antibiotic stewards: A qualitative study exploring the current status of Antibiotic Stewardship Program in Bahawalpur, Pakistan. J. Infect. Public Health 2019, 13, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Godman, B.; Mukokinya, M.M.A.; Opanga, S.; Oluka, M. Dispensing of antimicrobials in Kenya: A cross-sectional pilot study and its implications. J. Res. Pharm. Pract. 2018, 7, 77–82. [Google Scholar] [CrossRef]
- Marković-Peković, V.; Grubiša, N.; Burger, J.; Bojanić, L.; Godman, B. Initiatives to Reduce Nonprescription Sales and Dispensing of Antibiotics: Findings and Implications. J. Res. Pharm. Pract. 2017, 6, 120–125. [Google Scholar] [CrossRef]
- Babar, Z.-U. Ten recommendations to improve pharmacy practice in low and middle-income countries (LMICs). J. Pharm. Policy Pract. 2021, 14, 6. [Google Scholar] [CrossRef]
- Nguyen, T.T.P.; Do, T.X.; Nguyen, H.A.; Nguyen, C.T.T.; Meyer, J.C.; Godman, B.; Skosana, P.; Nguyen, B.T. A National Survey of Dispensing Practice and Customer Knowledge on Antibiotic Use in Vietnam and the Implications. Antibiotics 2022, 11, 1091. [Google Scholar] [CrossRef]
- Setia, M.S. Methodology series module 5: Sampling strategies. Indian J. Dermatol. 2016, 61, 505–509. [Google Scholar] [CrossRef]
- Bornstein, M.H.; Jager, J.; Putnick, D.L. Sampling in developmental science: Situations, shortcomings, solutions, and standards. Dev. Rev. 2013, 33, 357–370. [Google Scholar] [CrossRef]
- WHO. Anatomical Therapeutic Chemical (ATC) Classification. 2021. Available online: https://www.who.int/tools/atc-ddd-toolkit/atc-classification (accessed on 5 March 2023).
- Teng, C.L.; Achike, F.I.; Phua, K.L.; Nurjahan, M.I.; Mastura, I.; Asiah, H.N.; Mariam, A.M.; Narayanan, S.; Norsiah, A.; Sabariah, I.; et al. Modifying antibiotic prescribing: The effectiveness of academic detailing plus information leaflet in a Malaysian primary care setting. Med. J. Malays. 2006, 61, 323–331. [Google Scholar]
- Awad, A.I.; Eltayeb, I.B.; Baraka, O.Z. Changing antibiotics prescribing practices in health centers of Khartoum State, Sudan. Eur. J. Clin. Pharmacol. 2006, 62, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.K.A.; Khan, O.F.; Matin, M.A.; Begum, K.; Galib, M.A. Effect of standard treatment guidelines with or without prescription audit on prescribing for acute respiratory tract infection (ARI) and diarrhoea in some thana health complexes (THCs) of Bangladesh. Bangladesh Med. Res. Counc. Bull. 2007, 33, 21–30. [Google Scholar] [PubMed]
- Kafle, K.K.; Bhuju, G.B.; Karkee, S.B.; Prasad, R.R.; Shrestha, N.; Shrestha, A.D.; Das, P.L.; Chataut, B.D.; Daud, M. An intervention improving prescribing practices and monitoring drugs availability in a district. Nepal Med. Coll. J. NMCJ 2009, 11, 217–221. [Google Scholar]
- Boonyasiri, A.; Thamlikitkul, V. Effectiveness of multifaceted interventions on rational use of antibiotics for patients with upper respiratory tract infections and acute diarrhea. J. Med. Assoc. Thail. 2014, 97 (Suppl 3), S13–S19. [Google Scholar]
- Egger, J.R.; Stankevitz, K.; Korom, R.; Angwenyi, P.; Sullivan, B.; Wang, J.; Hatfield, S.; Smith, E.; Popli, K.; Gross, J. Evaluating the effects of organizational and educational interventions on adherence to clinical practice guidelines in a low-resource primary-care setting in Kenya. Health Policy Plan. 2017, 32, 761–768. [Google Scholar] [CrossRef]
- Lunn, A.D. Reducing inappropriate antibiotic prescribing in upper respiratory tract infection in a primary care setting in Kolkata, India. BMJ Open Qual. 2018, 7, e000217. [Google Scholar] [CrossRef]
- Tay, K.H.; Ariffin, F.; Sim, B.L.; Chin, S.Y.; Sobry, A.C. Multi-Faceted Intervention to Improve the Antibiotic Prescriptions among Doctors for Acute URI and Acute Diarrhoea Cases: The Green Zone Antibiotic Project. Malays. J. Med. Sci. 2019, 26, 101–109. [Google Scholar] [CrossRef]
- Chowdhury, F.; Sturm-Ramirez, K.; Al Mamun, A.; Iuliano, A.D.; Chisti, M.J.; Ahmed, M.; Bhuiyan, M.U.; Hossain, K.; Haider, M.S.; Aziz, S.A.; et al. Effectiveness of an educational intervention to improve antibiotic dispensing practices for acute respiratory illness among drug sellers in pharmacies, a pilot study in Bangladesh. BMC Health Serv. Res. 2018, 18, 676. [Google Scholar] [CrossRef]
- Chang, J.; Xu, S.; Zhu, S.; Li, Z.; Yu, J.; Zhang, Y.; Zu, J.; Fang, Y.; Ross-Degnan, D. Assessment of non-prescription antibiotic dispensing at community pharmacies in China with simulated clients: A mixed cross-sectional and longitudinal study. Lancet Infect. Dis. 2019, 19, 1345–1354. [Google Scholar] [CrossRef]
- Opanga, S.R.N.; Wamaitha, A.; Abebrese Sefah, I.; Godman, B.B. Availability of Medicines in Community Pharmacy to Manage Patients with COVID-19 in Kenya; Pilot Study and Implications. Sch. Acad. J. Pharm. 2021, 3, 36–42. [Google Scholar] [CrossRef]
- Kimathi, G.; Kiarie, J.; Njarambah, L.; Onditi, J.; Ojakaa, D. A cross-sectional study of antimicrobial use among self-medicating COVID-19 cases in Nyeri County, Kenya. Antimicrob. Resist. Infect. Control. 2022, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Muloi, D.; Fèvre, E.M.; Bettridge, J.; Rono, R.; Ong’Are, D.; Hassell, J.M.; Karani, M.K.; Muinde, P.; van Bunnik, B.; Street, A.; et al. A cross-sectional survey of practices and knowledge among antibiotic retailers in Nairobi, Kenya. J. Glob. Health 2019, 9, 010412. [Google Scholar] [CrossRef] [PubMed]
- WHO. Kenya National Action Plan on Antimicrobial Resistance: Review of Progress in the Human Health Sector. 2022. Available online: https://www.who.int/publications/i/item/9789240062689 (accessed on 18 March 2023).
- Godman, B.; Kamati, M.; Kibuule, D. Prevalence of self-medication for acute respiratory infections in young children in namibia: Findings and implications. J. Res. Pharm. Pract. 2019, 8, 220–224. [Google Scholar] [CrossRef]
- Marković-Peković, V.; Grubiša, N. Self-medication with antibiotics in the Republic of Srpska community pharmacies: Pharmacy staff behavior. Pharmacoepidemiol. Drug Saf. 2012, 21, 1130–1133. [Google Scholar] [CrossRef]
- Kitutu, F.E.; Kalyango, J.N.; Mayora, C.; Selling, K.E.; Peterson, S.; Wamani, H. Integrated community case management by drug sellers influences appropriate treatment of paediatric febrile illness in South Western Uganda: A quasi-experimental study. Malar. J. 2017, 16, 425. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, V.; Herrera-Patino, J.J.; Santa-Ana-Tellez, Y.; Dreser, A.; Elseviers, M.; Stichele, R.V. Analysing policy interventions to prohibit over-the-counter antibiotic sales in four Latin American countries. Trop. Med. Int. Health 2013, 18, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Vacca, C.P.; Nino, C.Y.; Reveiz, L. Restriction of antibiotic sales in pharmacies in Bogota, Colombia: A descriptive study. Rev. Panam. De Salud Publica 2011, 30, 586–591. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).