Abstract
Indole, a metabolite of the amino acid tryptophan, has been proven to act as a signal molecule in bacteria, acting in different aspects of biofilm formation. The oral biofilm is a type of biofilm that has consequences for human health. It is a complex, three-dimensional structure that develops on the surface of teeth via the attachment of primary microbial colonizers. Many oral infections are caused by an imbalance occurring in the microorganisms naturally found in oral biofilms and are considered major public health concerns. In this study, we test the effect of a natural bis-indole, 3,3′-Diindolylmethane (DIM), in mitigating the pathogenicity of the oral biofilm inhabiting bacterium Streptococcus mutans, a bacterium that is considered to be a principal etiological agent in dental caries. Our study found that DIM was able to attenuate S. mutans biofilm formation by 92%. Additionally, treatment with DIM lowered extracellular polymeric substance (EPS) production and decreased its durability significantly under acidic conditions. Therefore, the anti-biofilm and anti-virulence properties of DIM against S. mutans bacteria in an “oral setting” provides evidence for its usefulness in reducing biofilm formation and potentially for caries attenuation.
1. Introduction
Oral microbial biofilms are known to contribute to infectious diseases and are a major public health concern. Oral biofilms are complex three-dimensional structures that develop from a conditioning saliva-derived film, also known as a pellicle, forming on the surface of the teeth. The formation of the pellicle is followed by the attachment of primary microbial colonizers, which are characterized by receptor molecules that are present in the developing pellicle. These early colonizers promote subsequent interactions with secondary colonizers that join the growing biofilm, creating a mature multispecies microbial community [1,2]. One key bacterial component of these biofilms, Streptococcus mutans, is considered a principal etiological agent of dental caries [3]. Dental caries is associated with the bacterial metabolism of carbohydrates, leading to prolonged periods of plaque acidification and demineralization of the tooth enamel [4]. Bacterial virulence factors that contribute to dental caries result in stable biofilm formation, acid tolerance, and acid production from carbohydrate metabolism [3].
The major virulence factor for S. mutans is its ability to form a biofilm framework that is mainly composed of extracellular polymeric substance (EPS) produced via sugar metabolism [5]. Once S. mutans participates in the differentiated community, EPS production promotes a cohesive three-dimensional network. The EPS is a glue-like structure consisting of polysaccharides, extracellular DNA, acids, and proteins [6]. The secretion of EPS by oral bacterial species, including S. mutans, mediates bacterial adherence onto the tooth surfaces, thus contributing to the formation of dental plaque biofilms [7]. Another virulence factor includes a membrane-bound F1Fo-ATPase system that pumps protons from cells, while maintaining the internal bacteria’s pH value, resulting in S. mutans’ acid tolerance [8].
Since biofilm formation by S. mutans plays a crucial role in caries promotion, its disruption and removal may be crucial in oral hygiene. Currently, several strategies have been developed against each stage of biofilm formation, including tooth coatings that prevent bacterial attachment and EPS production in addition to mechanical elimination and/or chemical controls [9]. The most common strategy to treat cariogenic biofilms is based on non-specific mechanical brushing and flossing in concert with the use of toothpastes and mouthwashes [10,11] containing antiseptic compounds such as chlorhexidine, fluorides, essential oils, and cetylpyridinium chloride. These treatments result in non-selective oral flora eradication [9,12].
A novel strategy to prevent or diminish dental plaque derived from S. mutans biofilms is the use of natural anti-biofilm agents. Indole is known as a metabolite of the amino acid tryptophan produced by many gut and environmental bacteria [13]. Indole is a key player that has been proven to participate in interspecies and interkingdom signaling as well as bacterial pathogenesis [13,14,15]. It affects biofilm formation [16], virulence [17], and the antibiotic resistance of a number of pathogens [18]. A number of indole derivatives, including naturally occurring and synthetic ones, have been reported to act as signaling molecules that affect the behavior of different bacteria [14]. In addition, several indole derivatives have been reported to have more potent antimicrobial and anti-biofilm activities than a basic indole such as 3,3′-Diindolylmethane (DIM) [19,20,21,22]. Furthermore, recent evaluations of a number of natural compounds that affect S. mutans biofilm and virulence factors have suggested that these indole compounds may have therapeutic properties [23,24,25,26]. Therefore, the purpose of this study is to evaluate the possibility of harnessing DIM as a therapeutic anti-biofilm and anti-virulent compound against S. mutans, allowing evaluation of its potential implementation in mitigating pathogenic biofilm and caries in the oral cavity.
2. Materials and Methods
2.1. Bacterial Culture and Growth Conditions
Streptococcus mutans (ATCC 25175) was cultivated for 24 h in Brain Heart Infusion (BHI) medium (HiMedia) with shaking (140 rpm) at a constant temperature of 37 °C. For acid tolerance assays, a tryptone–yeast extract medium containing 20 mM glucose (TYEG) was applied.
2.2. Pre-Coating with Saliva for Biofilm Assay
An amount of 2 mL of whole saliva was collected on ice from a healthy volunteer. Saliva was mixed 1:1 ratio v/v with AB buffer (50 mM KCl, 1 mM potassium phosphate (0.35 mM K2HPO4 plus 0.65 mM KH2PO4), 1 mM CaCl2 and 0.1 mM MgCl2). An amount of 1 μL of 0.2 M phenylmethyl-sulfonyl-fluoride (PMSF) was added to the mixture and then centrifuged (5500× g, 4 °C, 10 min). The supernatant (clarified whole saliva) was collected and filtered through a 0.22 μm PES low protein-binding filter [27]. An amount of 50 μL of clarified saliva was placed in a 96-well for about 18 h at 37 °C. Unbound saliva was removed by blotting the plate on a clean absorbent paper.
2.3. Biofilm Growth under Static Conditions
To assess the potential efficacy of DIM against biofilm formation, the S. mutans strain was tested under static growth conditions. An overnight culture of S. mutans suspensions was diluted 1:100 and incubated for ~3 h until it reached an early exponential phase (OD600~0.2). Subsequently, the culture was further diluted 1:100 and 200 μL of the suspension was placed in a glass bottom 96-well plate, which was previously pre-coated with human saliva as specified above (Thermo Fisher, Waltham, MA, USA). DIM (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in DMSO and supplemented in different concentration ranges (50, 5, 0.5, and 0.05 μM). The cultures were statically incubated at 37 °C for 24 h. For confocal laser scanning microscope (CLSM) visualization, biofilms were stained using a LIVE/DEAD BacLight viability staining kit (Molecular Probes Inc., Eugene, OR, USA) according to the manufacturer’s instructions. As for scanning electron microscope (SEM) imaging, biofilms were allowed to form on a glass slide (1 × 1 cm) in the same condition as mentioned above.
2.4. Microscopy and Image Analysis by CSLM
After 24 h, planktonic cells were removed by washing twice with PBS before staining. Biofilms were visualized using a FV1000 CLSM (Olympus, Tokyo, Japan) equipped with a 60 × 1.35 NA lens. Image scanning was carried out using the 488 and 541 nm lasers for the detection of SYTO 9 and PI, respectively. The emission of green-live dye SYTO9 was collected using a 505–525 band pass filter, whereas for the red dead PI stain, the emission was collected using a 560–660 band pass filter. Biofilm 3D images were reconstructed using IMARIS software (Bitplane AG, Zürich, Switzerland) along with quantitative structural calculations by IMARIS Measurement Pro. For each experiment, at least six random positions in each of the three independent cultures were chosen for microscopic analysis.
2.5. Dynamic Biofilm Model
In order to attain a greater understanding of DIM efficacy to prevent biofilm formation under shear flow conditions, a continuous culture flow cell was employed. S. mutans biofilm was allowed to form in the presence of DIM (0.5 μM) or an equivalent concentration of DMSO as a control on the inner surface of channels of the Ibidi flow cell (µ-slide sizes of 17 × 3.8 × 0.4 mm) for 48 h at 37 °C [19]. To initiate biofilm growth, the cells were inoculated with an overnight culture of S. mutans diluted with 0.9% NaCl to an OD600 of 0.05. Following 1 h of incubation under static conditions to allow bacterial adhesion, the flow was introduced with a constant flow rate of 3 mL h−1 (Masterflex L/S peristaltic pump, Cole-Parmer, Vernon Hills, IL, USA) of 10% BHI supplemented with 1% sucrose (w/v) for 48 h. The resulting biofilms were macroscopically visualized using CLSM and 3D processed by IMARIS software as stated previously.
2.6. Scanning Electron Microscope (SEM) Biofilm Analysis
The morphological properties of the biofilm were analyzed using scanning electron microscopy (SEM Quanta 200, FEI). Bacterial cultures were discarded after 24 h of incubation and the samples were prepared for SEM studies as follows. After fixation in 2.5% buffered glutaraldehyde, the samples were subsequently dehydrated via an ascending, serial ethanol gradient (50, 70, 80, 90, 95, and 100%) and immersed in a hexamethyldisilazane (HMDS)/ethanol gradient solution. The treated and control specimens were air-dried for 4 h, followed by sputter coating with a 20 nm layer of gold using the EMITECH K575× sputtering device (Emitech Ltd., South Petherton, UK). The images were observed under an FEI ESEM Quanta 200 instrument at magnifications ranging from × 2500 to 5000, using an accelerating voltage of 20 kV.
2.7. Extracellular Polymeric Substances (EPS) Measurement
For EPS quantification, biofilms of S. mutans were produced with different concentrations of DIM in 2 mL BHI, supplemented with 1% sucrose (w/v) in 24-well plates. The EPS of biofilms was determined by the “Anthrone method” [24]. Briefly, biofilms were collected by sonication in PBS buffer; then, the precipitate was obtained by centrifugation of 200 μL of the supernatant mixed with 600 μL of “Anthrone reagent” (Sigma-Aldrich) at 95 °C for 5 min. The absorbance at 625 nm was monitored and the concentrations of EPS were calculated using standard curves of dextran. The experiments were repeated three times independently.
2.8. Acid Tolerance Assay
The effect of DIM on the acid tolerance of S. mutans was evaluated by measuring the viability of the bacteria after 120 min exposure of pH 5.0 [28]. S. mutans was grown overnight in TYEG broth and then diluted by 1:20 until mid-logarithmic phase (OD = 0.4–0.5). After centrifugation, the cells were resuspended in TYEG broth buffered with 40 mM phosphate/citrate buffer (pH 5.0) containing 0.5 µM DIM, and incubated at 37 °C for 2 h. The control mixture was DMSO. Samples were removed before and after incubation at pH 5.0 for viable counts. The experiments were repeated three times independently.
3. Results and Discussion
Dental caries is one of the most common oral diseases, imposing a large economic burden on many segments of the population worldwide [29]. Dental caries is considered a chronic infectious disease that continues throughout life with high incidence and prevalence. Indeed, up to 90% of the world population has experienced one or more carious lesions during their lifetime [30]. Dental caries is formed through a dysbiosis of the oral microbiota found in the biofilm on the tooth surface [31,32]. This dysbiosis occurs via a shift of the microbial community towards cariogenic bacteria with characteristics of acidogency and acidurity due to high carbohydrate intake [5]. Although S. mutans is not the most abundant species in the oral microbiome, it is one of the most prominent cariogenic biofilm producers due to its durability in a high sugar and low pH environment [33]. Therefore, in order to progress towards the eradication of dental caries, alternative therapeutic approaches have been fostered. Recently, we explored the effects of a bis-indole derivative, DIM, on the biofilm formation of different Gram-negative pathogens. This compound was found to be a potential candidate for anti-biofilm properties and virulent attenuation [19]. In general, indole plays important roles as a signal molecule participating in biofilm formation and in multiple physiological pathways in many bacteria [13]. In previous studies, Oh et al. (2012) and Manoharan et al. (2018) showed that indole or indole derivatives attenuate Candida albicans, an important member of the flora in the oral cavity [34,35]. Fungal virulence is regulated by indole, mediating filamentation and biofilm formation. Based on these observations, DIM was assessed for its ability to attenuate a single species biofilm of S. mutans on glass surfaces.
3.1. Effects of DIM on Biofilm Formation of S. mutans
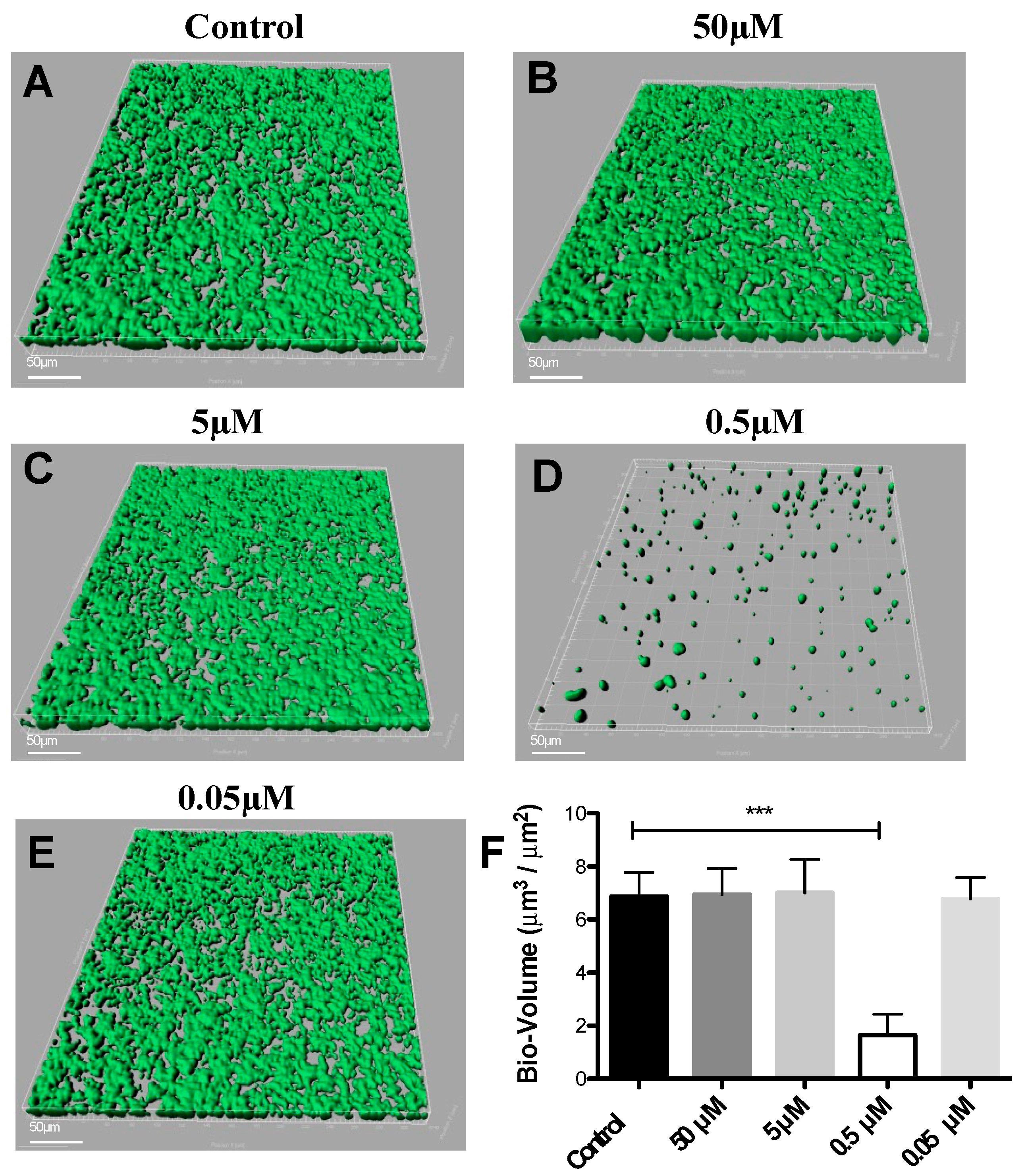
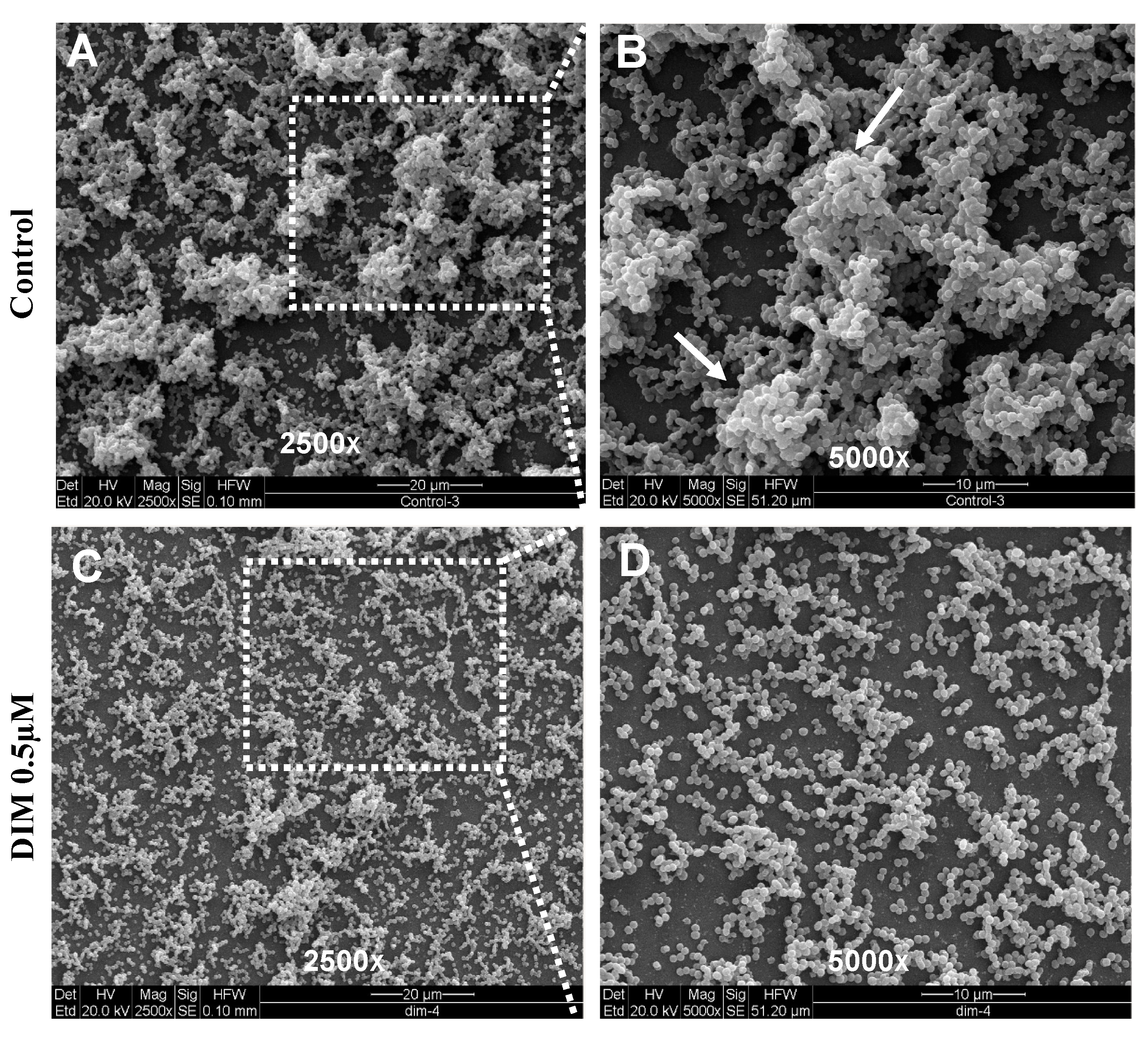
DIM was tested in a dose-dependent manner (50 μM, 5 μM, 0.5 μM, and 0.05 μM), (Figure 1B–E). Treatment with 0.5 μM DIM under static conditions demonstrated considerably thinner biofilms, being inhibited by 92% (Figure 1D). When compared with the thicker 3D morphology of the control biofilm using SEM imaging (Figure 2A,B), 0.5 µM of DIM also showed a major inhibition of biofilm formation as scattered bacterial growth was observed (Figure 2C,D). This observation was further supported by live bio-volume quantification. In the control, the live bio-volume of S. mutans (6.87 μm3/μm2) was significantly higher than in those under the DIM treatment (1.64 μm3/μm2) (Figure 1F). Interestingly, neither higher nor lower concentrations showed inhibition, and only 0.5 µM was an effective concentration at retaining apparent anti-biofilm properties against S. mutans. Dead bio-volumes in all dosages were not detected, eliminating the possibility of antimicrobial activity elicited by DIM treatment. These findings are in the line with a recent study by Kim et al. that reported the prominent anti-biofilm activity of DIM against acne-causing bacteria, Cutibacterium acnes [20]. Out of 20 indole derivatives, both natural and synthetic 7-azaindole, 7-benzyloxyindole, indole-3-carbinol, and DIM significantly inhibited biofilm formation. The latter was found as a highly potential candidate to treat multispecies biofilm-associated infections [20].
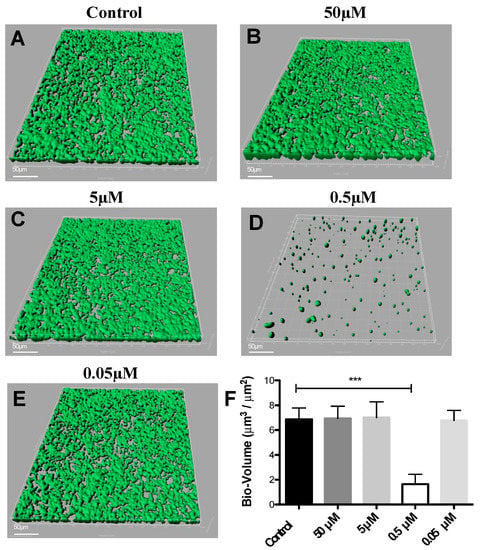
Figure 1.
Dose response of 3,3′-Diindolylmethane on biofilm formation. CLSM images of biofilm formed by S. mutans after 24 h of treatment by different concentrations of (B–E) DIM or (A) untreated (DMSO control). Biofilm formed on a glass bottom 96-well plate after static incubation coated with clarified saliva. CLSM images visualize viable cells stained green and dead cells stained red with the BacLight® LIVE/DEAD Kit, scanned areas of ~318 μm × 318 μm. (F) Statistical analysis showing the means ± SDs of biofilm volume generated from three independent sets of experiments (t-test; *** p < 0.001). Differences were also analyzed for their significance by using one-way ANOVA with Tukey’s test.
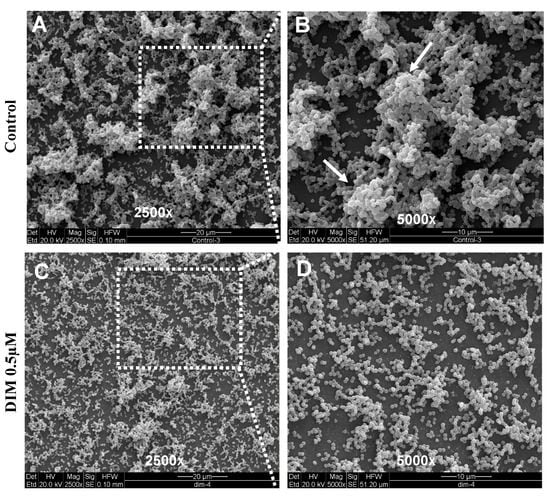
Figure 2.
Surface morphology of biofilm formation. SEM images of the biofilm formed by S. mutans after 24 h (A,B) untreated (DMSO control) or (C,D) treatment with 0.5 µM of DIM. Biofilm formed on a glass coated with clarified saliva after static incubation. White arrows indicate the presence of mature microcolonies in the control group. Images are shown at 250× and 5000× magnification.
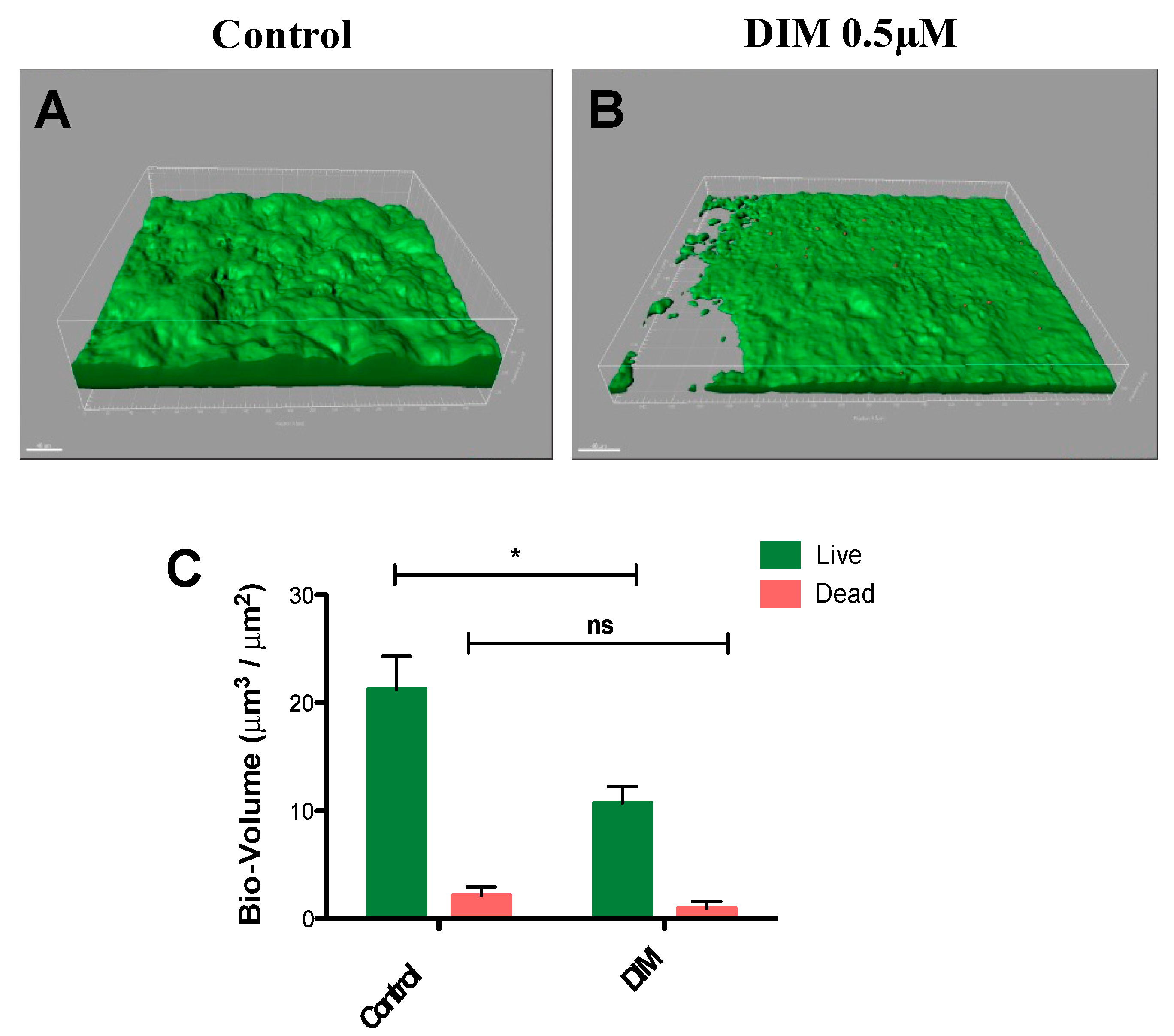
In common caries prevention and management treatments, most of the chemotherapeutic strategies are based on suppressing the levels of cariogenic bacteria by using antimicrobials [36,37]. However, the lack of selectivity found in these therapeutics harms both the pathogenic and natural and beneficial microflora. Moreover, mature differentiated biofilms become recalcitrant and difficult to remove by these antimicrobial agents [37]. Thus, natural molecules such as DIM which are devoid of selection pressure properties provide potential candidates in treating caries. To further this premise, we tested the prominent activity of DIM against biofilms of S. mutans under dynamic conditions similar to those found in the oral cavity. Flow cells provide a tool for in vitro cultivation and evaluation of bacterial biofilms under hydrodynamic conditions of flow [38]. The flow cell is designed to enable biofilms to grow in an environment that mimics the hydrodynamic conditions of the oral cavity [39]. Furthermore, since the oral cavity salivary flow (and amount) may affect adherence of bacteria to the substrate [40], the flow cell results are likely to provide a better reflection of what occurs in the mouth. Therefore, we examined the effect of DIM on S. mutans biofilm formation in a flow cell model for a period of 48 h (Figure 3A,B) and demonstrated that DIM provided a 50% inhibition of live biofilm bacteria and no significant difference in the dead biofilm bacteria (Figure 3C). Furthermore, dead staining by using propidium iodide did not show an increase in bacterial death as a result of the treatment, suggesting that biofilm inhibition was most likely not due to an antimicrobial effect.
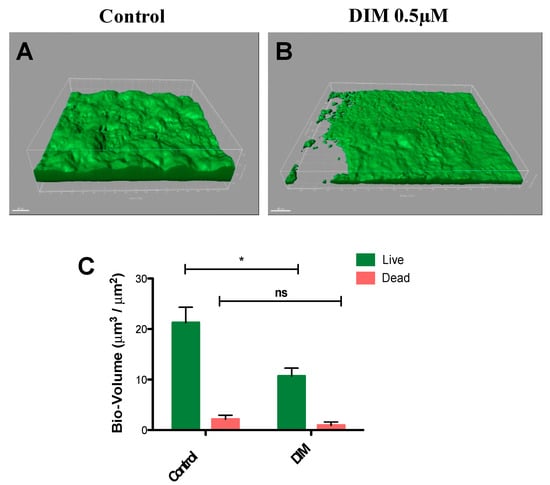
Figure 3.
Biofilm dispersal in dynamic culture. S. mutans biofilm (A) control (equivalent concentration of DMSO) or (B) 0.5µM DIM continuously supplemented through the inlet media. Biofilms were stained with the LIVE/DEAD bacterial viability kit and subsequently imaged by CSLM 48 h post inoculation. (C) Bio-volume (μm3/μm2) quantification is based on image analysis by IMARIS software. Images were acquired from three different areas for each treatment (means ± SDs), with at least three independent repetitions. Asterisks indicate significant differences compared to control (t-test; * p < 0.05).
3.2. Assessing Anti-Virulence Properties of DIM
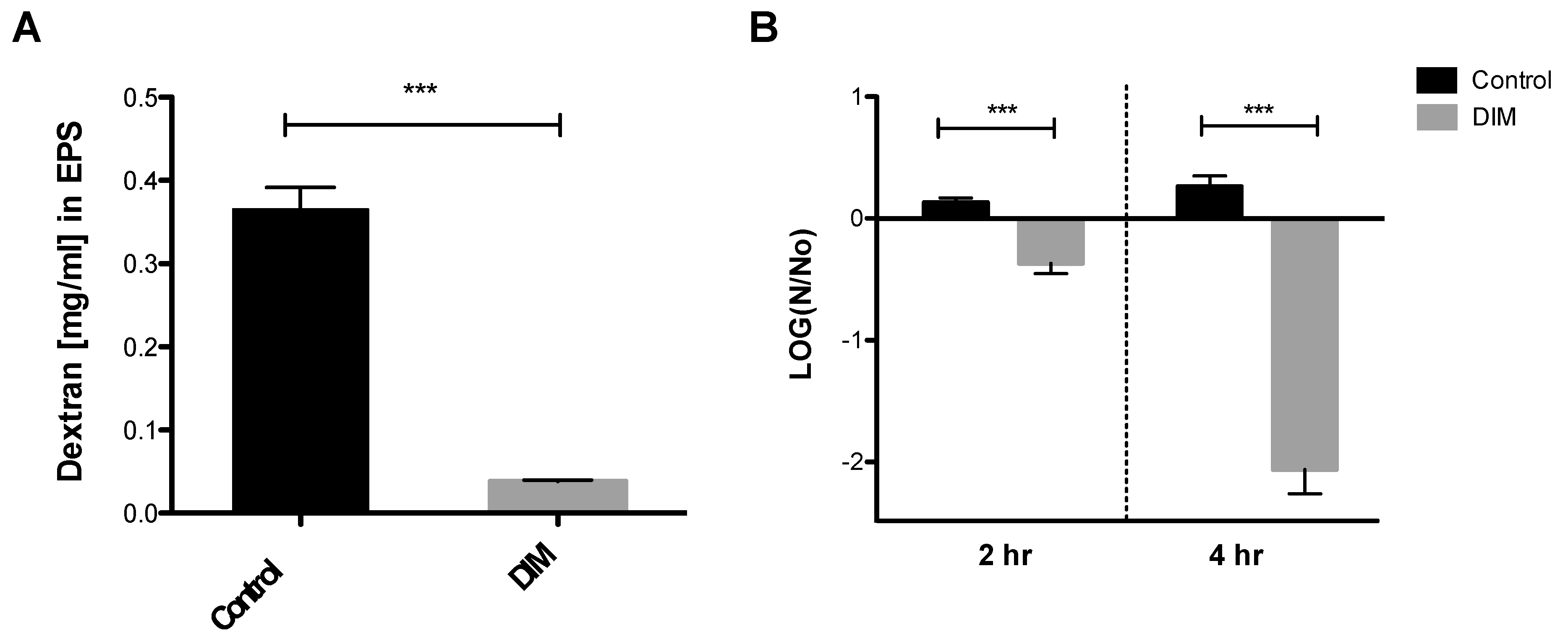
The oral biofilm bacterial residents are embedded in a self-produced extracellular polymeric matrix (EPS) that provides a fundamental scaffold for cariogenic biofilms, and are recognized as essential virulence factors associated with dental caries [41,42]. Tooth surface colonization takes place once the S. mutans cells produce a glue-like extracellular polymer glucan that promotes the buildup of biofilms. The foremost substrate for glucan synthesis, sucrose, has long been regarded as the most cariogenic of all carbohydrates [43]. Our results indicated that 0.5 µM of DIM disrupted the ability of S. mutans to synthesize EPS and to form a biofilm (Figure 4A). Indeed, we showed that S. mutans treated with DIM markedly reduced the covering of the EPS matrix by 90%. In this context, biofilm attenuation by DIM probably occurred due to a reduction in EPS synthesis that resulted in less adhesion of cells to the surface.
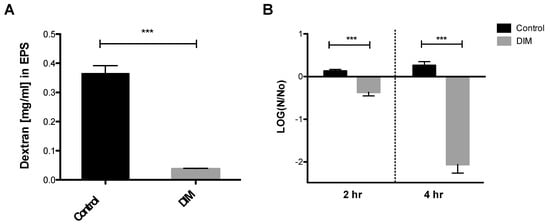
Figure 4.
Quantitative EPS production and aciduricity determination. (A) Biofilm’s EPS of S. mutans assessment after 24 h treated with 0.5 µM of DIM or an equivalent amount of DMSO as the control using Anthrone method [24]. Standard curve was prepared with dextran standard with various concentrations (data not shown). (B) Acid tolerance was determined by measuring the survival rate of S. mutans at pH 5.0. in the presence of 0.5 µM DIM compared with the untreated control (DMSO). N0 and N represent CFU counts before and after 2 h and 4 h treatments in pH 5.0 culture, respectively. Asterisks indicate significant differences compared to control (t-test; *** p < 0.001).
In the oral cavity, sucrose and other carbohydrates in the biofilm matrix are fermented to produce acid, which in turn converts the milieu into a highly acidic microenvironment [43]. The acidic stress increases, thereby creating a favorable niche for other acidogenic and aciduric species to thrive, resulting in the reduction of microbial diversity [44]. Subsequently, the deposition of this acidic biofilm results in the degeneration of the tooth enamel and to dental caries. The mechanism whereby caries is formed is by oral microflora producing lactic acid that causes demineralization of the calcium and phosphate present in the crystal form of hydroxyapatite that comprises the enamel of the teeth [45]. Examination and characterization of the virulence properties expressed by S. mutans reveal how this characteristic may actually favor bacterial propagation and explain its ability to thrive in a place that many competing oral bacteria find lethal [46]. S. mutans’ ability to tolerate this acidic milieu is considered a major virulence factor. Therefore, we tested the S. mutans survival rate after 2 and 4 h at pH 5.0 with and without DIM treatment (Figure 4B). Treatment with 0.5 µM DIM decreased S. mutans viability significantly after 2 and 4 h, when compared to the control where bacterial viability did not change significantly. Thus, in an acidic environment similar to what is found in the oral cavity, fewer S. mutans bacteria survived in the presence of DIM (Figure 4B).
This study demonstrates the possible usefulness of DIM in mitigating pathogenic oral biofilms and consequently chronic dental caries. It is important to note that DIM also has been classified as an anti-cancer agent involved in eradicating various solid malignancies [47] without imposing toxicity to normal cells [48,49]. Therefore, these reports of its low toxicity hold an important advantage in therapeutics as its use in treatment entails minimal complications.
The prevention of the ability of S. mutans to produce EPS using this compound may help to change the environment for the development of caries, thus improving oral disease management for the whole population. Additional studies regarding structure and function should be carried out in order to further clarify the possible mechanism of action of DIM. In addition, despite its low toxicity, the possible systemic absorption of DIM should be minimized and a target formulation should be developed for local application.
Author Contributions
Conceptualization, A.K., Q.S., K.Y.-H.G. and R.S.M.; data curation, Y.B.; investigation, Y.B. and K.G.; methodology, Y.B. and K.G.; supervision, A.K. and R.S.M.; validation, K.G., A.K. and R.S.M.; visualization, Y.B.; writing original draft, Y.B. and K.G.; funding acquisition, A.K., Q.S., K.Y.-H.G. and R.S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the International Research and Development Program of Sichuan (2019YFH0113) and SMART innovation grant ING-000398 (Singapore).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Shimona Geresh award. We also thank the professional staff at the Ilse Katz Center for Nanoscale Science and Technology, Ben Gurion University of the Negev, Israel, for their help in SEM and CLSM.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chawhuaveang, D.D.; Yu, O.Y.; Yin, I.X.; Lam, W.Y.-H.; Mei, M.L.; Chu, C.-H. Acquired salivary pellicle and oral diseases: A literature review. J. Dent. Sci. 2021, 16, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Nabert-Georgi, C.; Rodloff, A.C.; Jentsch, H.; Reissmann, D.R.; Schaumann, R.; Stingu, C.S. Influence of oral bacteria on adhesion of Streptococcus mutans and Streptococcus sanguinis to dental materials. Clin. Exp. Dent. Res. 2018, 4, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.A.; Burne, R.A. A model of efficiency: Stress tolerance by Streptococcus mutans. Microbiology 2008, 154, 3247–3255. [Google Scholar] [CrossRef]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm matrixome: Extracellular components in structured microbial communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Falsetta, M.; Klein, M. The exopolysaccharide matrix: A virulence determinant of cariogenic biofilm. J. Dent. Res. 2013, 92, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Dashper, S.; Reynolds, E. pH regulation by Streptococcus mutans. J. Dent. Res. 1992, 71, 1159–1165. [Google Scholar] [CrossRef]
- Takenaka, S.; Ohsumi, T.; Noiri, Y. Evidence-based strategy for dental biofilms: Current evidence of mouthwashes on dental biofilm and gingivitis. Jpn. Dent. Sci. Rev. 2019, 55, 33–40. [Google Scholar] [CrossRef]
- Mazhari, F.; Boskabady, M.; Moeintaghavi, A.; Habibi, A. The effect of toothbrushing and flossing sequence on interdental plaque reduction and fluoride retention: A randomized controlled clinical trial. J. Periodontol. 2018, 89, 824–832. [Google Scholar] [CrossRef]
- Zilm, P.; Weyrich, L.S.; Bright, R.; Gatej, S.; Rossi-Fedele, G.; Selbach, S.; Ketagoda, D.H.K.; Alani, A.; Lekkas, D.; Vasilev, K. Current and Future Applications to Control Polymicrobial Biofilms Associated with Oral Disease. In Antibiofilm Strategies: Current and Future Applications to Prevent, Control and Eradicate Biofilms; Springer: Berlin/Heidelberg, Germany, 2022; pp. 399–440. [Google Scholar]
- Zhang, G.; Lu, M.; Liu, R.; Tian, Y.; Vu, V.H.; Li, Y.; Liu, B.; Kushmaro, A.; Li, Y.; Sun, Q. Inhibition of Streptococcus mutans biofilm formation and virulence by Lactobacillus plantarum K41 isolated from traditional Sichuan pickles. Front. Microbiol. 2020, 11, 774. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 2010, 34, 426–444. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhang, C.; Mu, Y.; Shen, Q.; Feng, Y. Indole affects biofilm formation in bacteria. Indian J. Microbiol. 2010, 50, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Wood, T.K.; Lee, J. Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol. 2015, 23, 707–718. [Google Scholar] [CrossRef]
- Lee, J.; Jayaraman, A.; Wood, T.K. Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol. 2007, 7, 1–15. [Google Scholar] [CrossRef]
- Bommarius, B.; Anyanful, A.; Izrayelit, Y.; Bhatt, S.; Cartwright, E.; Wang, W.; Swimm, A.I.; Benian, G.M.; Schroeder, F.C.; Kalman, D. A family of indoles regulate virulence and Shiga toxin production in pathogenic E. coli. PLoS ONE 2013, 8, e54456. [Google Scholar] [CrossRef]
- Vega, N.M.; Allison, K.R.; Khalil, A.S.; Collins, J.J. Signaling-mediated bacterial persister formation. Nat. Chem. Biol. 2012, 8, 431–433. [Google Scholar] [CrossRef]
- Golberg, K.; Markus, V.; Kagan, B.-e.; Barzanizan, S.; Yaniv, K.; Teralı, K.; Kramarsky-Winter, E.; Marks, R.S.; Kushmaro, A. Anti-Virulence Activity of 3, 3′-Diindolylmethane (DIM): A Bioactive Cruciferous Phytochemical with Accelerated Wound Healing Benefits. Pharmaceutics 2022, 14, 967. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Lee, J.-H.; Park, S.; Lee, J. The Anticancer Agent 3, 3′-Diindolylmethane Inhibits Multispecies Biofilm Formation by Acne-Causing Bacteria and Candida albicans. Microbiol. Spectr. 2022, 10, e02021–e02056. [Google Scholar] [CrossRef] [PubMed]
- Raorane, C.J.; Lee, J.-H.; Lee, J. Rapid killing and biofilm inhibition of multidrug-resistant Acinetobacter baumannii strains and other microbes by iodoindoles. Biomolecules 2020, 10, 1186. [Google Scholar] [CrossRef]
- Sethupathy, S.; Sathiyamoorthi, E.; Kim, Y.-G.; Lee, J.-H.; Lee, J. Antibiofilm and antivirulence properties of indoles against Serratia marcescens. Front. Microbiol. 2020, 11, 584812. [Google Scholar] [CrossRef]
- Park, B.-I.; Kim, B.-S.; Kim, K.-J.; You, Y.-O. Sabinene suppresses growth, biofilm formation, and adhesion of Streptococcus mutans by inhibiting cariogenic virulence factors. J. Oral Microbiol. 2019, 11, 1632101. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Jiang, W.; Wang, K.; Luo, J.; Li, W.; Zhou, X.; Zhang, L. Antimicrobial peptide GH12 suppresses cariogenic virulence factors of Streptococcus mutans. J. Oral Microbiol. 2018, 10, 1442089. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Wang, Y.; Duan, Z.; Ling, Z.; Wu, W.; Tong, S.; Wang, H.; Deng, S. Theaflavin-3, 3′-digallate suppresses biofilm formation, acid production, and acid tolerance in Streptococcus mutans by targeting virulence factors. Front. Microbiol. 2019, 10, 1705. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, X.D.; Wu, C.D. The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrob. Agents Chemother. 2011, 55, 1229–1236. [Google Scholar] [CrossRef]
- Lemos, J.A.; Abranches, J.; Koo, H.; Marquis, R.E.; Burne, R.A. Protocols to study the physiology of oral biofilms. Oral Biol. Mol. Tech. Appl. 2010, 666, 87–102. [Google Scholar]
- Svensäter, G.; Larsson, U.B.; Greif, E.; Cvitkovitch, D.; Hamilton, I. Acid tolerance response and survival by oral bacteria. Oral Microbiol. Immunol. 1997, 12, 266–273. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, L.; Yue, L.; Ling, J.; Fan, M.; Yang, D.; Huang, Z.; Niu, Y.; Liu, J.; Zhao, J. Expert consensus on dental caries management. Int. J. Oral Sci. 2022, 14, 17. [Google Scholar] [CrossRef]
- Bader, J.; Shugars, D.; Rozier, G.; Lohr, K.; Bonito, A.; Nelson, J.; Jackman, A. Diagnosis and management of dental caries: Summary. In AHRQ Evidence Report Summaries; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2001. [Google Scholar]
- Kolenbrander, P.E.; London, J. Adhere today, here tomorrow: Oral bacterial adherence. J. Bacteriol. 1993, 175, 3247–3252. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Marsh, P.D. Are dental diseases examples of ecological catastrophes? Microbiology 2003, 149, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Go, G.; Mylonakis, E.; Kim, Y. The bacterial signalling molecule indole attenuates the virulence of the fungal pathogen Candida albicans. J. Appl. Microbiol. 2012, 113, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, R.K.; Lee, J.H.; Lee, J. Efficacy of 7-benzyloxyindole and other halogenated indoles to inhibit Candida albicans biofilm and hyphal formation. Microb. Biotechnol. 2018, 11, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Daliri, E.B.-M.; Kim, N.; Kim, J.-R.; Yoo, D.; Oh, D.-H. Microbial etiology and prevention of dental caries: Exploiting natural products to inhibit cariogenic biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef]
- Marsh, P.D.; Moter, A.; Devine, D.A. Dental plaque biofilms: Communities, conflict and control. Periodontology 2000 2011, 55, 16. [Google Scholar] [CrossRef]
- Crusz, S.A.; Popat, R.; Rybtke, M.T.; Cámara, M.; Givskov, M.; Tolker-Nielsen, T.; Diggle, S.P.; Williams, P. Bursting the bubble on bacterial biofilms: A flow cell methodology. Biofouling 2012, 28, 835–842. [Google Scholar] [CrossRef]
- Palmer, R.J., Jr.; Caldwell, D.E. A flowcell for the study of plaque removal and regrowth. J. Microbiol. Methods 1995, 24, 171–182. [Google Scholar] [CrossRef]
- Whittaker, C.J.; Klier, C.M.; Kolenbrander, P.E. Mechanisms of adhesion by oral bacteria. Annu. Rev. Microbiol. 1996, 50, 513–552. [Google Scholar] [CrossRef]
- Mattos-Graner, R.; Smith, D.; King, W.; Mayer, M.P.A. Water-insoluble glucan synthesis by mutans streptococcal strains correlates with caries incidence in 12-to 30-month-old children. J. Dent. Res. 2000, 79, 1371–1377. [Google Scholar] [CrossRef]
- Smith, A.V.; Scott-Anne, K.; Whelehan, M.; Berkowitz, R.; Feng, C.; Bowen, W. Salivary glucosyltransferase B as a possible marker for caries activity. Caries Res. 2007, 41, 445–450. [Google Scholar] [CrossRef]
- Lemos, J.; Palmer, S.; Zeng, L.; Wen, Z.; Kajfasz, J.; Freires, I.; Abranches, J.; Brady, L. The biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 1–7. [Google Scholar] [CrossRef]
- Baker, J.L.; Edlund, A. Exploiting the oral microbiome to prevent tooth decay: Has evolution already provided the best tools? Front. Microbiol. 2019, 9, 3323. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization–remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743. [Google Scholar] [CrossRef] [PubMed]
- Matsui, R.; Cvitkovitch, D. Acid tolerance mechanisms utilized by Streptococcus mutans. Future Microbiol. 2010, 5, 403–417. [Google Scholar] [CrossRef]
- Amare, D.E. Anti-cancer and other biological effects of a dietary compound 3, 3′-diindolylmethane supplementation: A systematic review of human clinical trials. Nutr. Diet. Suppl. 2020, 12, 123–137. [Google Scholar] [CrossRef]
- Lu, L.; Jiang, M.; Zhu, C.; He, J.; Fan, S. Amelioration of whole abdominal irradiation-induced intestinal injury in mice with 3, 3′-Diindolylmethane (DIM). Free Radic. Biol. Med. 2019, 130, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Morales-Prieto, D.M.; Herrmann, J.; Osterwald, H.; Kochhar, P.S.; Schleussner, E.; Markert, U.R.; Oettel, M. Comparison of dienogest effects upon 3, 3′–diindolylmethane supplementation in models of endometriosis and clinical cases. Reprod. Biol. 2018, 18, 252–258. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).