Facile Implementation of Antimicrobial Coatings through Adhesive Films (Wraps) Demonstrated with Cuprous Oxide Coatings
Abstract
1. Introduction
2. Results
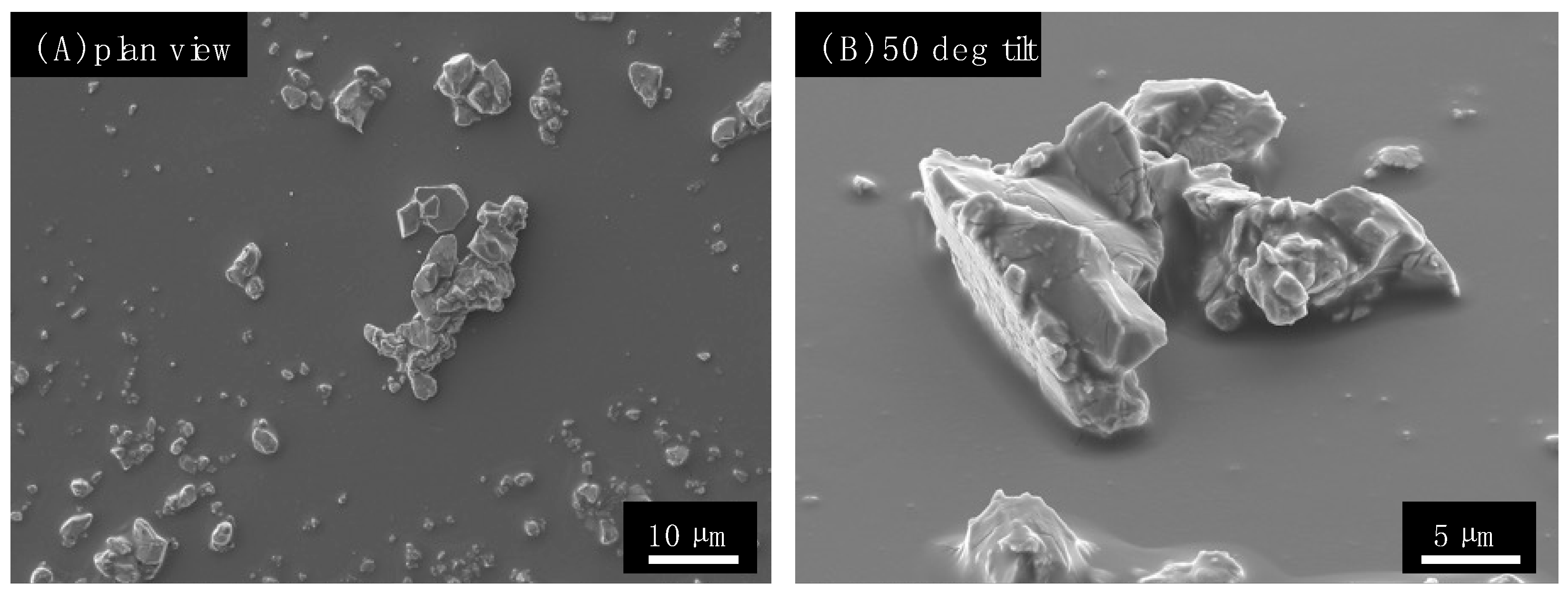
2.1. Preparation and Characterization of the Antimicrobial Wraps
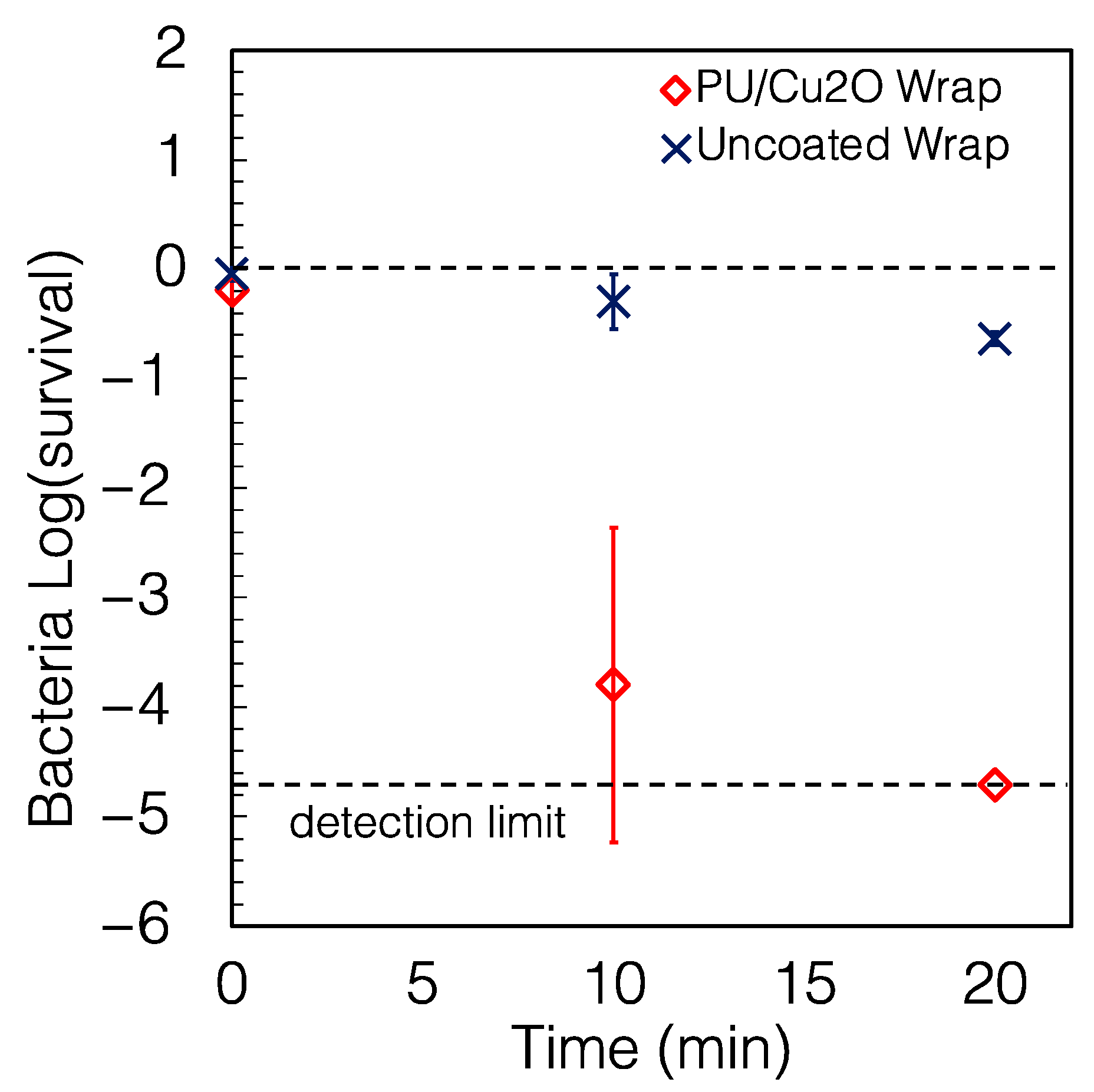
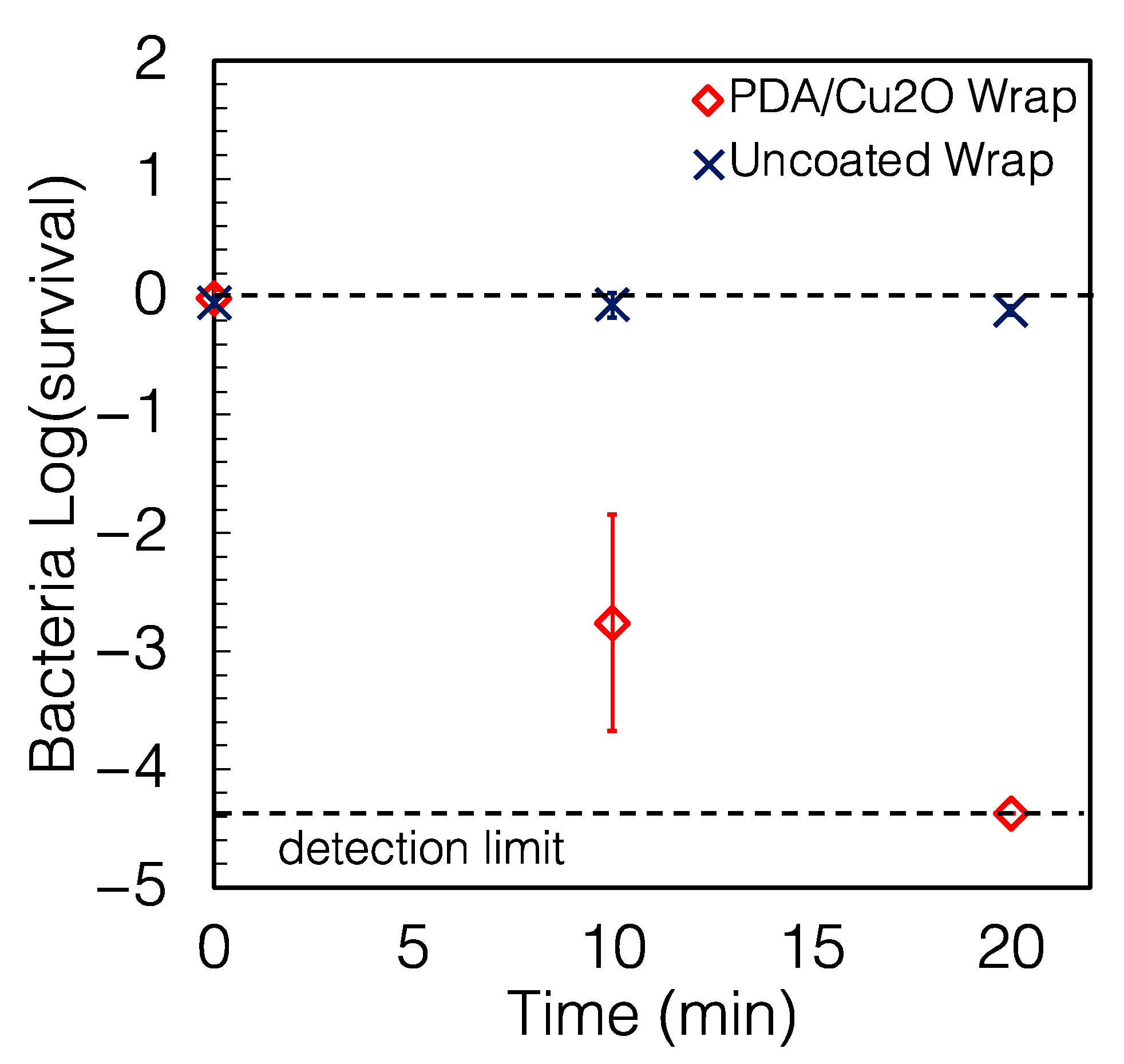
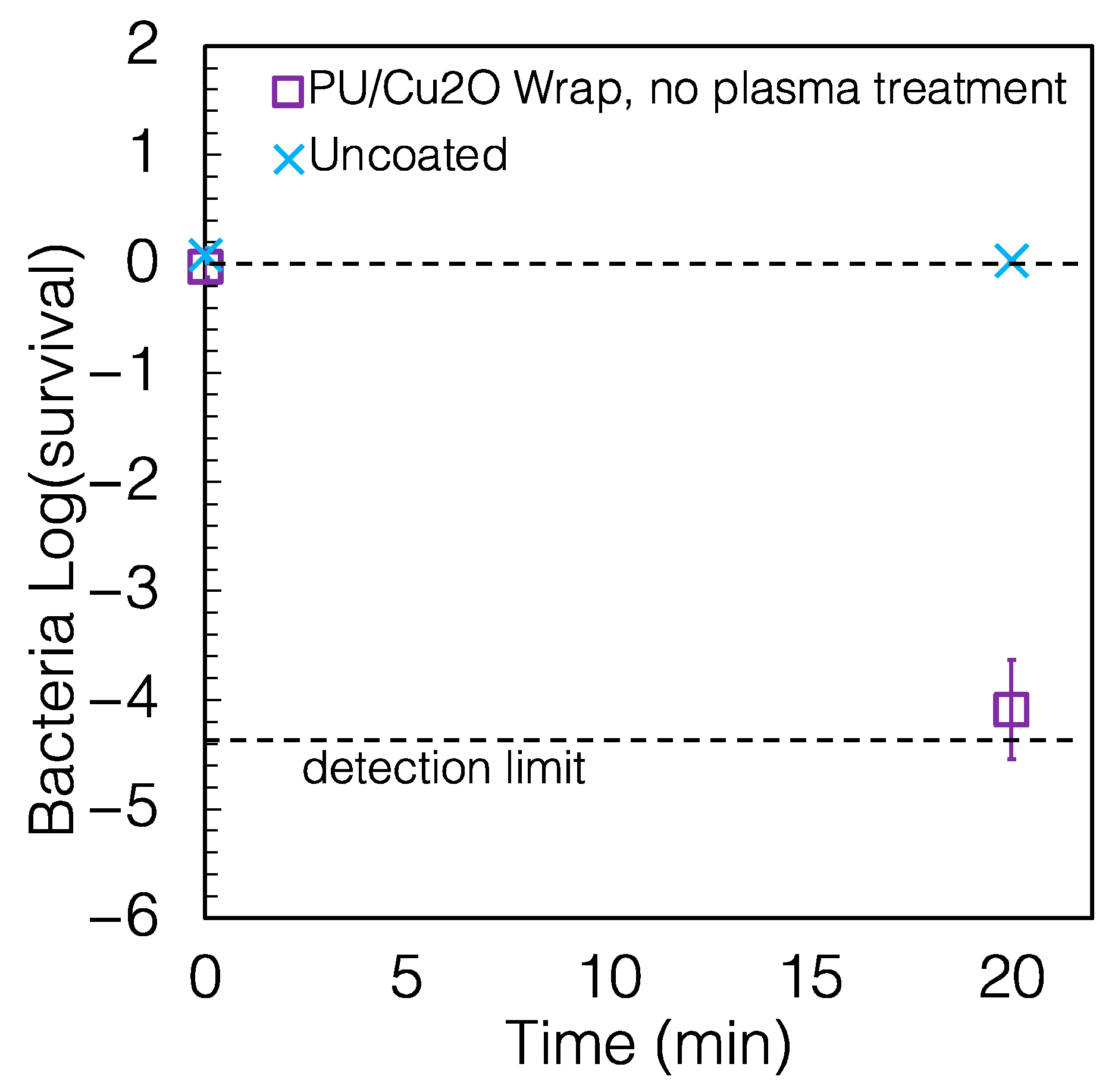
2.2. The Wraps Are Highly Antimicrobial
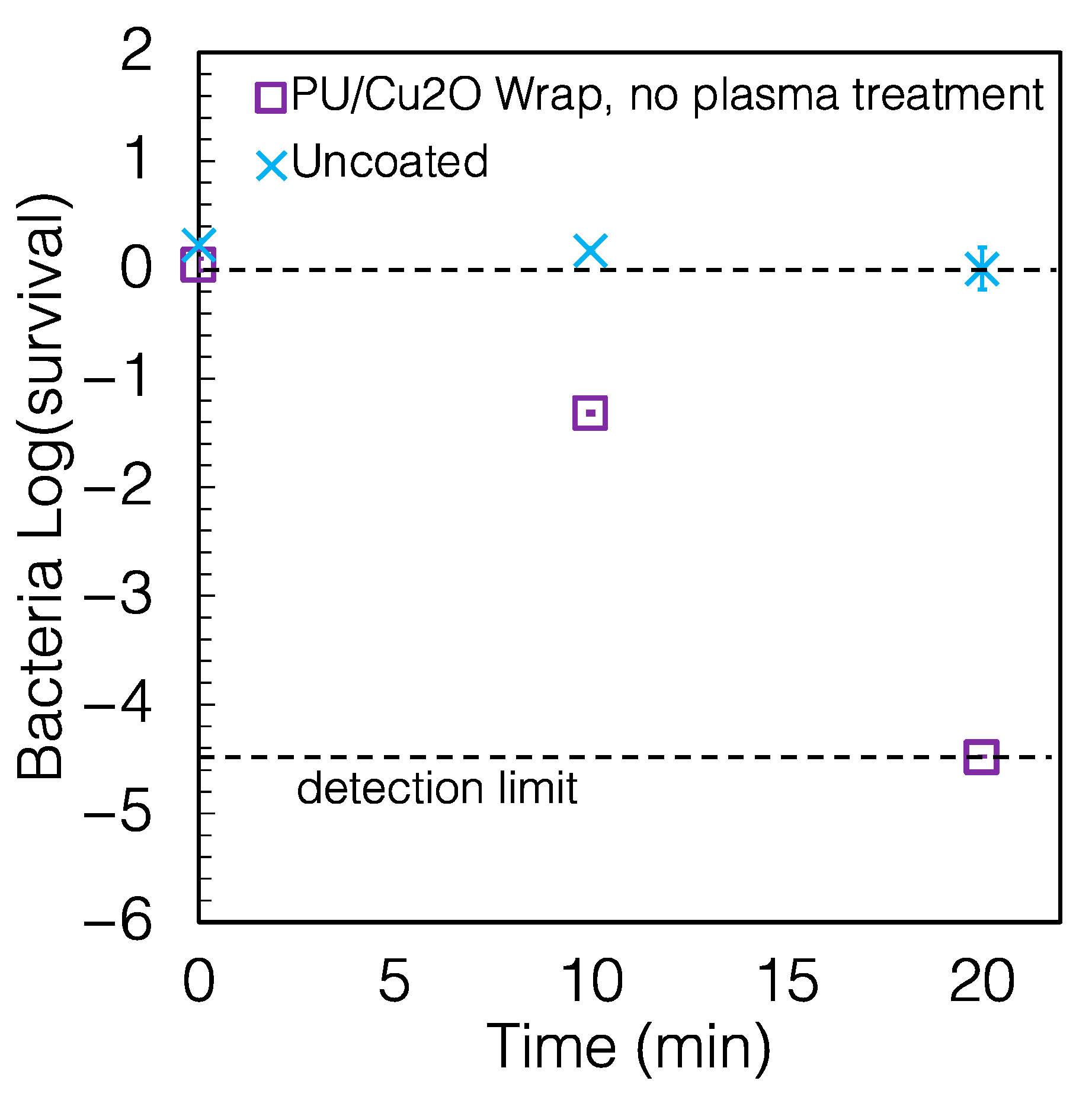
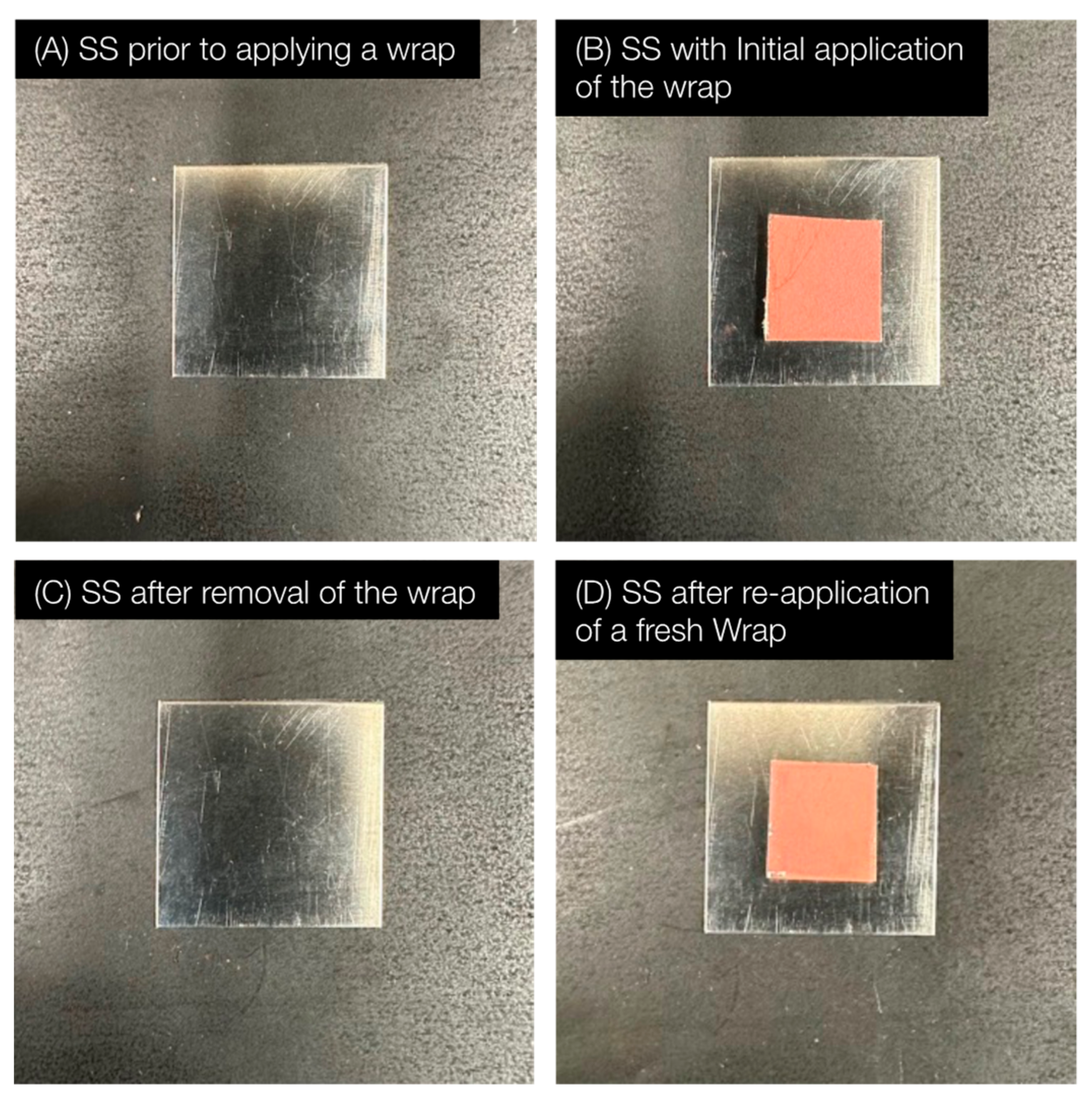
2.3. The Antimicrobial Wraps Are Easy to Replace
2.4. Other Characteristics of the Coating
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Fabrication of Antimicrobial PU/Cu2O Wrap
4.3. Fabrication of Antimicrobial PDA/Cu2O Wrap
4.4. Characterization of Test Solids
4.5. Antibacterial Assay
4.5.1. Choice of Microbial Strain
4.5.2. Growth of Microbial Strains
4.5.3. Preparation of Microbial Strains for Testing
4.5.4. Measurement of Viable Cell Number
4.5.5. Measurement of Surface-Killing
4.6. Statistical Analysis
4.7. Calculation of Microbial Survival and Reduction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piret, J.; Boivin, G. Pandemics throughout history. Front. Microbiol. 2021, 11, 631736. [Google Scholar] [CrossRef]
- Cutler, D.M.; Summers, L.H. The COVID-19 pandemic and the $16 trillion virus. JAMA 2020, 324, 1495–1496. [Google Scholar] [CrossRef]
- Fonkwo, P.N. Pricing infectious disease: The economic and health implications of infectious diseases. EMBO Rep. 2008, 9, S13–S17. [Google Scholar] [CrossRef]
- Hoffmann, S.; Ahn, J.-W. Economic cost of major foodborne illnesses increased $2 billion from 2013 to 2018. In Amber Waves: The Economics of Food, Farming, Natural Resources, and Rural America; United States Department of Agriculture: Washington, DC, USA, 2021; Volume 2021. [Google Scholar]
- Benedict, K.; Whitham, H.K.; Jackson, B.R. Economic Burden of Fungal Diseases in the United States. Open Forum. Infect. Dis. 2022, 9, ofac097. [Google Scholar] [CrossRef]
- Jit, M.; Ng, D.H.L.; Luangasanatip, N.; Sandmann, F.; Atkins, K.E.; Robotham, J.V.; Pouwels, K.B. Quantifying the economic cost of antibiotic resistance and the impact of related interventions: Rapid methodological review, conceptual framework and recommendations for future studies. BMC Med. 2020, 18, 38. [Google Scholar] [CrossRef]
- ECDPC. 33000 People Die Every Year Due to Infections with Antibiotic-Resistant Bacteria. Available online: https://www.ecdc.europa.eu/en/news-events/33000-people-die-every-year-due-infections-antibiotic-resistant-bacteria (accessed on 20 January 2023).
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Mahoney, A.R.; Safaee, M.M.; Wuest, W.M.; Furst, A.L. The silent pandemic: Emergent antibiotic resistances following the global response to SARS-CoV-2. Iscience 2021, 24, 102304. [Google Scholar]
- Centers for Disease Control and Prevention. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022. Available online: https://www.cdc.gov/drugresistance/pdf/covid19-impact-report-508.pdf (accessed on 25 March 2023).
- Centers for Disease Control and Prevention. COVID-19 & Antibiotic Resistance. Available online: https://www.cdc.gov/drugresistance/covid19.html (accessed on 28 March 2023).
- Centers for Disease Control and Prevention. HAI and Antibiotic Use Prevalence Survey. Available online: https://www.cdc.gov/hai/eip/antibiotic-use.html (accessed on 24 March 2023).
- Stephens, B.; Azimi, P.; Thoemmes, M.S.; Heidarinejad, M.; Allen, J.G.; Gilbert, J.A. Microbial exchange via fomites and implications for human health. Curr. Pollut. Rep. 2019, 5, 198–213. [Google Scholar] [CrossRef]
- Behzadinasab, S.; Chin, A.W.H.; Hosseini, M.; Poon, L.L.M.; Ducker, W.A. SARS-CoV-2 virus transfers to skin through contact with contaminated solids. Sci. Rep. 2021, 11, 22868. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Uses of inorganic hypochlorite (bleach) in health-care facilities. Clin. Microbiol. Rev. 1997, 10, 597–610. [Google Scholar] [CrossRef]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial coatings: Challenges, perspectives, and opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Salwiczek, M.; Qu, Y.; Gardiner, J.; Strugnell, R.A.; Lithgow, T.; McLean, K.M.; Thissen, H. Emerging rules for effective antimicrobial coatings. Trends Biotechnol. 2014, 32, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Behzadinasab, S.; Hosseini, M.; Williams, M.; Ivester, H.; Allen, I.; Falkinham, J., III; Ducker, W. Antimicrobial activity of cuprous oxide-coated and cupric oxide-coated surfaces. J. Hosp. Infect. 2022, 129, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Behzadinasab, S.; Williams, M.D.; Hosseini, M.; Poon, L.L.; Chin, A.W.; Falkinham, J.O., III; Ducker, W.A. Transparent and Sprayable Surface Coatings that Kill Drug-Resistant Bacteria Within Minutes and Inactivate SARS-CoV-2 Virus. ACS Appl. Mater. Interfaces 2021, 13, 54706–54714. [Google Scholar] [CrossRef]
- Behzadinasab, S.; Chin, A.; Hosseini, M.; Poon, L.; Ducker, W.A. A surface coating that rapidly inactivates SARS-CoV-2. ACS Appl. Mater. Interfaces 2020, 12, 34723–34727. [Google Scholar] [CrossRef]
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mat. 2021, 2, 53. [Google Scholar] [CrossRef]
- Bharadishettar, N.; Bhat, K.U.; Bhat Panemangalore, D. Coating technologies for copper based antimicrobial active surfaces: A perspective review. Metals 2021, 11, 711. [Google Scholar] [CrossRef]
- Singha, P.; Locklin, J.; Handa, H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2017, 50, 20–40. [Google Scholar] [CrossRef]
- Liu, G.; Li, K.; Wang, H.; Ma, L.; Yu, L.; Nie, Y. Stable fabrication of zwitterionic coating based on copper-phenolic networks on contact lens with improved surface wettability and broad-spectrum antimicrobial activity. ACS Appl. Mater. Interfaces 2020, 12, 16125–16136. [Google Scholar] [CrossRef]
- Adlhart, C.; Verran, J.; Azevedo, N.F.; Olmez, H.; Keinänen-Toivola, M.M.; Gouveia, I.; Melo, L.F.; Crijns, F. Surface modifications for antimicrobial effects in the healthcare setting: A critical overview. J. Hosp. Infect. 2018, 99, 239–249. [Google Scholar] [CrossRef]
- Iarikov, D.D.; Kargar, M.; Sahari, A.; Russel, L.; Gause, K.T.; Behkam, B.; Ducker, W.A. Antimicrobial surfaces using covalently bound polyallylamine. Biomacromolecules 2014, 15, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Behzadinasab, S.; Williams, M.D.; Aktuglu, M.; Falkinham, J.O., III; Ducker, W.A. Porous Antimicrobial Coatings for Killing Microbes within Minutes. ACS Appl. Mater. Interfaces 2023, 15, 15120–15128. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, K.D.; Pogreba-Brown, K.; Gerba, C.P.; Elliott, S.P. Impact of a novel antimicrobial surface coating on health care–associated infections and environmental bioburden at 2 urban hospitals. Clin. Infect. Dis. 2020, 71, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.; Shankaran, S.; King, L. The effect of copper-oxide-treated soft and hard surfaces on the incidence of healthcare-associated infections: A two-phase study. J. Hosp. Infect. 2020, 105, 265–271. [Google Scholar] [CrossRef]
- Minoshima, M.; Lu, Y.; Kimura, T.; Nakano, R.; Ishiguro, H.; Kubota, Y.; Hashimoto, K.; Sunada, K. Comparison of the antiviral effect of solid-state copper and silver compounds. J. Hazard. Mater. 2016, 312, 1–7. [Google Scholar] [CrossRef]
- Sunada, K.; Minoshima, M.; Hashimoto, K. Highly Efficient Antiviral and Antibacterial Activities of Solid-State Cuprous Compounds. J. Hazard. Mater. 2012, 235, 265–270. [Google Scholar] [CrossRef]
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mücklich, F. Role of copper oxides in contact killing of bacteria. Langmuir 2013, 29, 16160–16166. [Google Scholar] [CrossRef]
- Javadhesari, S.M.; Alipour, S.; Mohammadnejad, S.; Akbarpour, M. Antibacterial activity of ultra-small copper oxide (II) nanoparticles synthesized by mechanochemical processing against S. aureus and E. coli. Mater. Sci. Eng. C 2019, 105, 110011. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Multidrug-Resistant Pseudomonas Aeruginosa. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/pseudomonas-aeruginosa-508.pdf (accessed on 20 March 2023).
- Kerr, K.G.; Snelling, A.M. Pseudomonas aeruginosa: A formidable and ever-present adversary. J. Hosp. Infect. 2009, 73, 338–344. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.R.; Isabella, V.M.; Lewis, K. Pseudomonas aeruginosa biofilms in disease. Microb. Ecol. 2014, 68, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine surface chemistry: A decade of discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency. Antimicrobial Testing Methods & Procedures: Interim Method for Evaluating the Efficacy of Antimicrobial Surface Coatings. Available online: https://www.epa.gov/pesticide-analytical-methods/antimicrobial-testing-methods-procedures-interim-method-evaluating (accessed on 2 September 2022).
- Environmental Protection Agency. Protocol for the Evaluation of Bactericidal Activity of Hard, Non-porous Copper Containing Surface Products. Available online: https://www.epa.gov/sites/production/files/2016-02/documents/copper_and_copper-alloy_surface_protocol_revised_012916.pdf (accessed on 2 September 2022).
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behzadinasab, S.; Williams, M.D.; Falkinham, J.O., III; Ducker, W.A. Facile Implementation of Antimicrobial Coatings through Adhesive Films (Wraps) Demonstrated with Cuprous Oxide Coatings. Antibiotics 2023, 12, 920. https://doi.org/10.3390/antibiotics12050920
Behzadinasab S, Williams MD, Falkinham JO III, Ducker WA. Facile Implementation of Antimicrobial Coatings through Adhesive Films (Wraps) Demonstrated with Cuprous Oxide Coatings. Antibiotics. 2023; 12(5):920. https://doi.org/10.3390/antibiotics12050920
Chicago/Turabian StyleBehzadinasab, Saeed, Myra D. Williams, Joseph O. Falkinham, III, and William A. Ducker. 2023. "Facile Implementation of Antimicrobial Coatings through Adhesive Films (Wraps) Demonstrated with Cuprous Oxide Coatings" Antibiotics 12, no. 5: 920. https://doi.org/10.3390/antibiotics12050920
APA StyleBehzadinasab, S., Williams, M. D., Falkinham, J. O., III, & Ducker, W. A. (2023). Facile Implementation of Antimicrobial Coatings through Adhesive Films (Wraps) Demonstrated with Cuprous Oxide Coatings. Antibiotics, 12(5), 920. https://doi.org/10.3390/antibiotics12050920






