Efficacy of Plasma-Treated Water against Salmonella Typhimurium: Antibacterial Activity, Inhibition of Invasion, and Biofilm Disruption
Abstract
:1. Introduction
2. Results
2.1. Minimum Inhibitory and Minimum Bactericidal Concentrations of Plasma-Treated Water
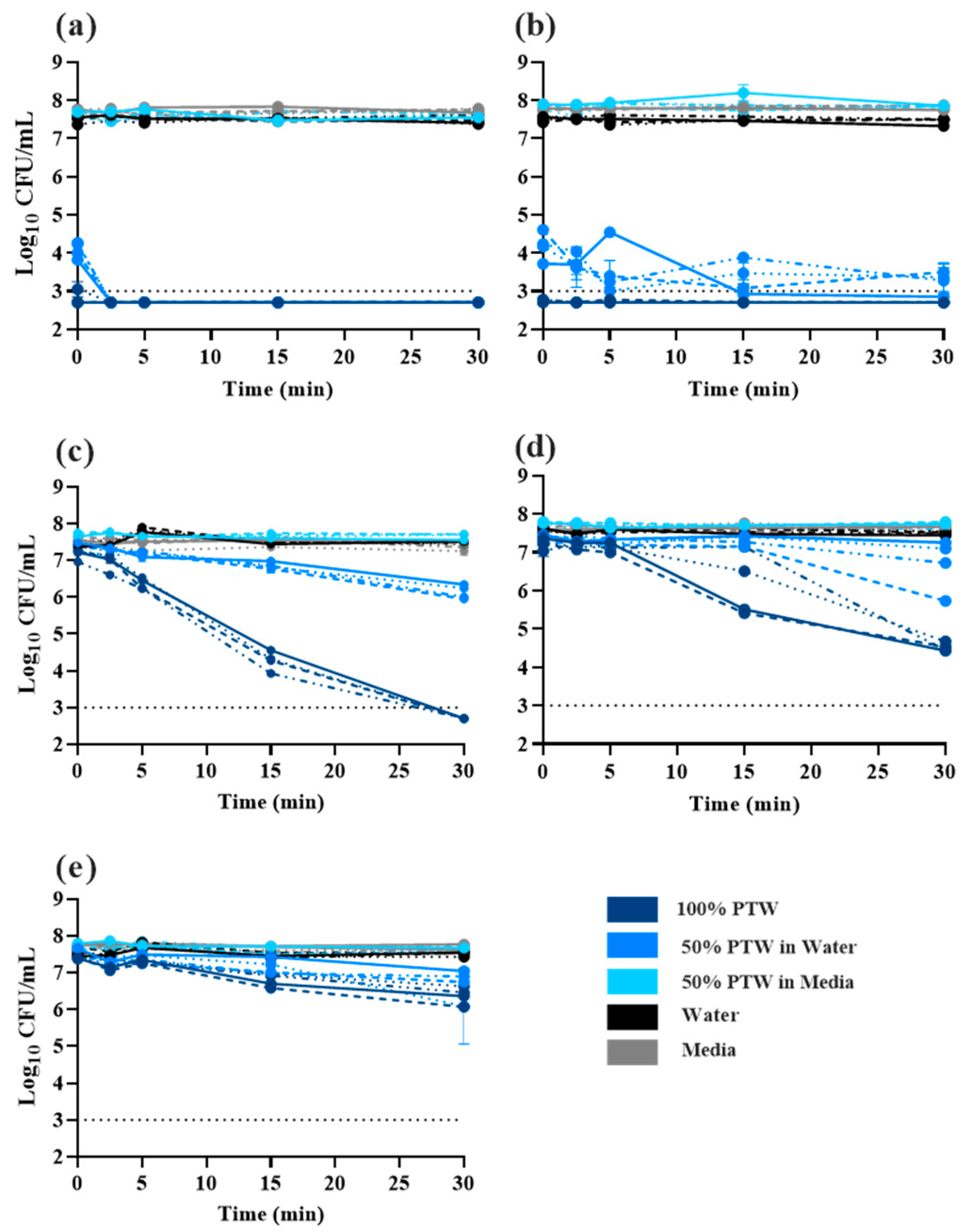
2.2. Time-Dependent Killing of Salmonella Typhimurium by Plasma-Treated Water
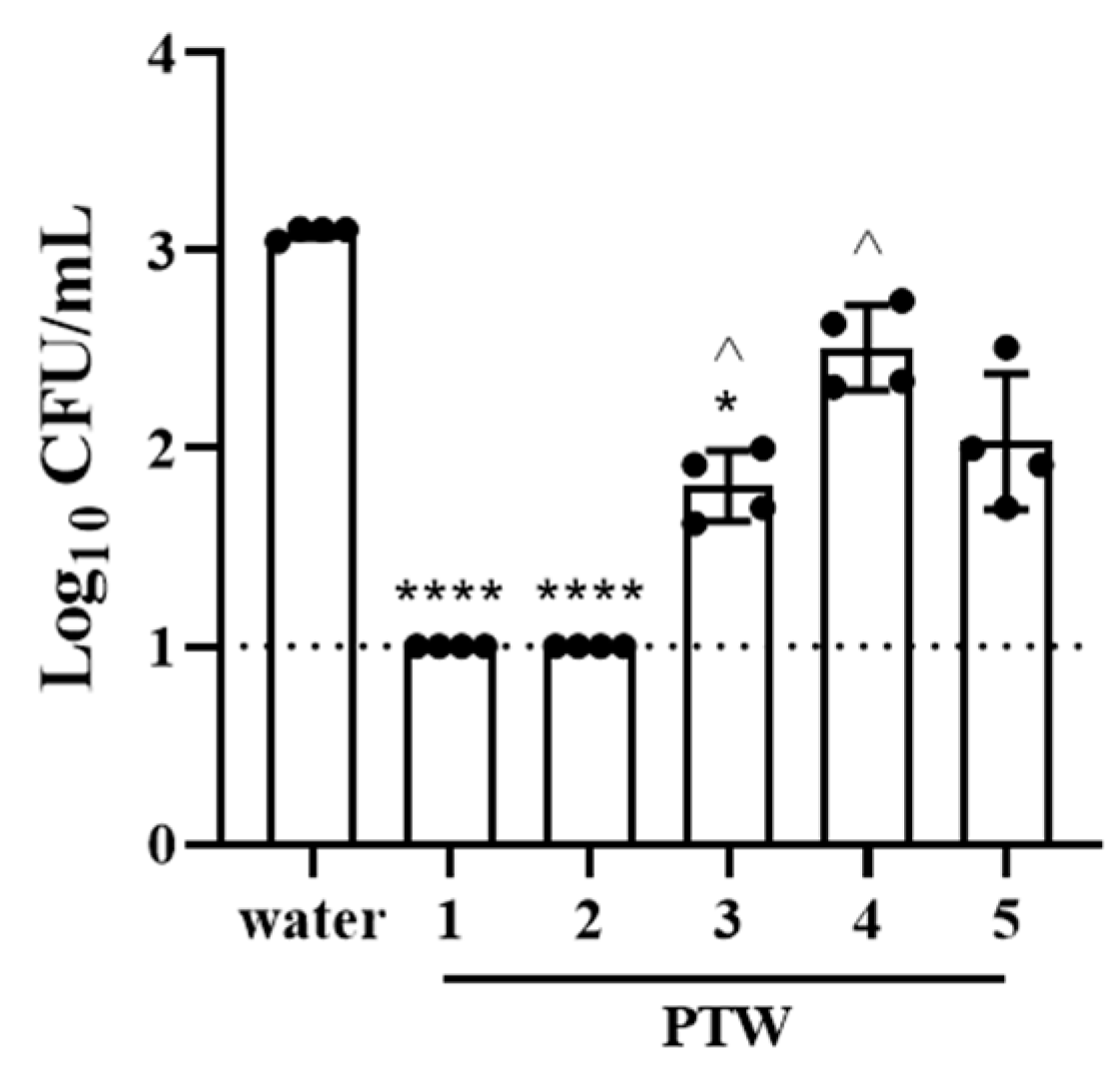
2.3. Effects of Plasma-Treated Water on Bacterial Invasion into Intestinal Epithelial Cells
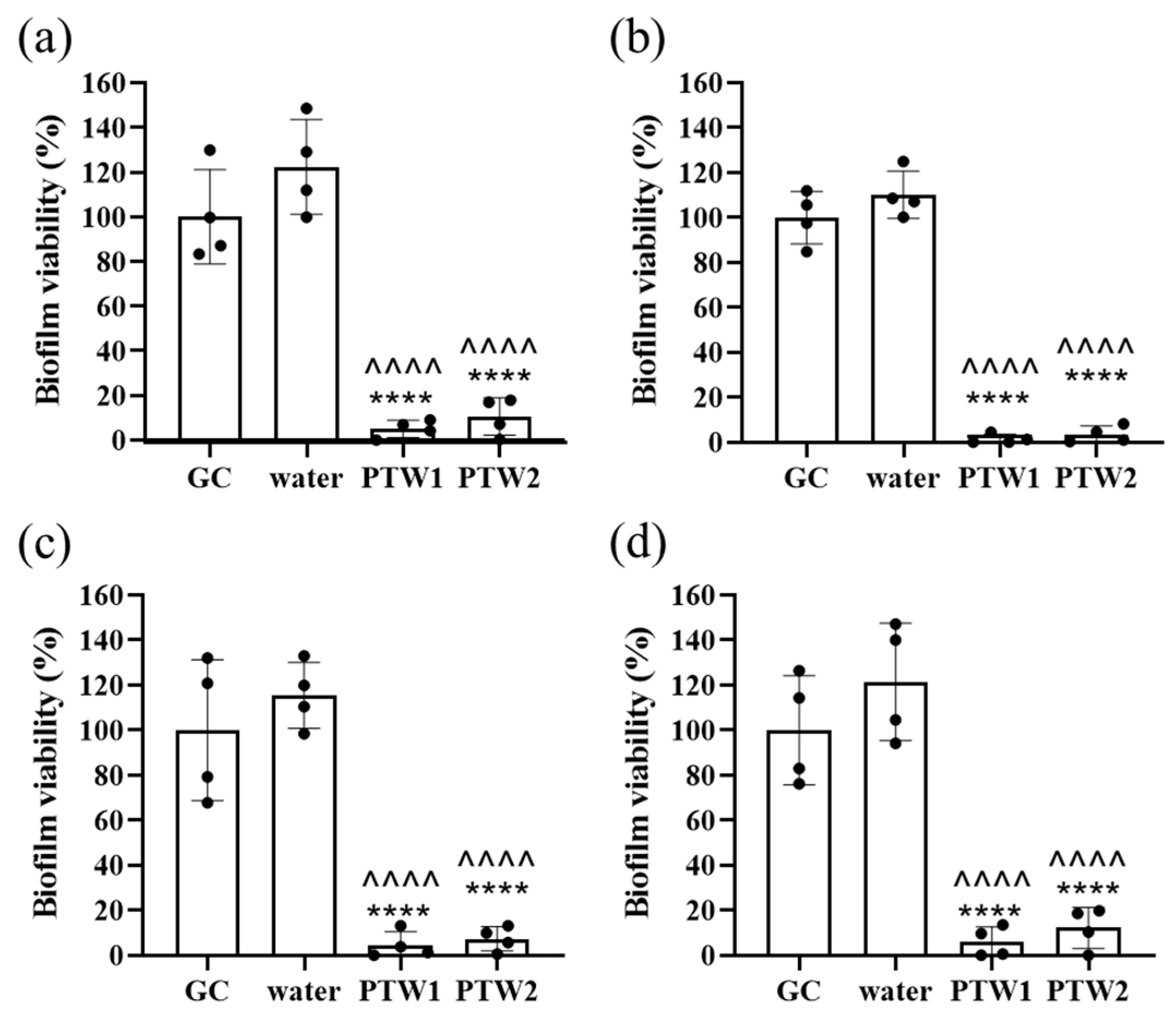
2.4. Biofilm Cell Viability
2.5. Bioflux
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plasma-Treated Water Types
4.3. Bacterial Strains
4.4. Minimum Inhibitory and Minimum Bactericidal Concentrations of PTW Types
4.5. Time-Dependent Killing Assay
4.6. Salmonella Invasion into Culture Human Intestinal Epithelial Cells (Caco-2)
4.7. Culture, Biofilm Formation, and PTW Treatment of Salmonella Typhimurium
4.8. Bacterial Viability Measured by Alamar Blue Assay
4.9. Biofilm Formation Measured by Crystal Violet Assay
4.10. Bioflux Experiment
4.11. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef]
- Le Minor, L.; Popoff, M.Y. Designation of Salmonella enterica sp. nov., nom. Rev., as the type and only species of the genus Salmonella. Int. J. Syst. Bact 1987, 37, 465–467. [Google Scholar] [CrossRef]
- Brenner, F.; Villar, R.G.; Angulo, F.J.; Tauxe, R.; Swaminathan, B. Salmonella nomenclature. J. Clin. Microbiol. 2000, 38, 2465–2467. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; Devleesschauwer, B.; Aspinall, W.; Cooke, R.; Corrigan, T.; Havelaar, A.; Angulo, F.; Gibb, H.; Kirk, M.; Lake, R.; et al. Attribution of global foodborne disease to specific foods: Findings from a World Health Organization structured expert elicitation. PLoS ONE 2017, 12, e0183641. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA J. 2015, 13, 4329. [Google Scholar]
- Food Standards Australia New Zealand. Application Handbook; Food Standards Australia New Zealand: Kingston, Australia, 2011.
- FSIS, USDA (United States Department of Agriculture-Food Safety and Inspection Service). Pathogen Reduction: Hazard Analysis and Critical Control Point (HACCP) Systems; Final Rule; Federal Register 61. 1996. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2020-08/93-016F_0.pdf (accessed on 30 May 2023).
- Møretrø, T.; Schirmer, B.C.; Heir, E.; Fagerlund, A.; Hjemli, P.; Langsrud, S. Tolerance to quaternary ammonium compound disinfectants may enhance growth of Listeria monocytogenes in the food industry. Int. J. Food Microbiol. 2017, 241, 215–224. [Google Scholar] [CrossRef]
- Hazards, E.P.o.B. Scientific Opinion on the public health hazards to be covered by inspection of meat (swine). EFSA J. 2011, 9, 2351. [Google Scholar] [CrossRef]
- Wang, X.; Biswas, S.; Paudyal, N.; Pan, H.; Li, X.; Fang, W.; Yue, M. Antibiotic Resistance in Salmonella Typhimurium Isolates Recovered From the Food Chain Through National Antimicrobial Resistance Monitoring System Between 1996 and 2016. Front. Microbiol. 2019, 10, 985. [Google Scholar] [CrossRef]
- Obe, T.; Richards, A.K.; Shariat, N.W. Differences in biofilm formation of Salmonella serovars on two surfaces under two temperature conditions. J. Appl. Microbiol. 2021, 132, 2410–2420. [Google Scholar] [CrossRef]
- Foster, J.E. Plasma-based water purification: Challenges and prospects for the future. Phys. Plasmas 2017, 24, 055501. [Google Scholar] [CrossRef]
- Puač, N.; Gherardi, M.; Shiratani, M. Plasma agriculture: A rapidly emerging field. Plasma Process. Polym. 2017, 15, e1700174. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Patange, A.; Sun, D.-W.; Tiwari, B. Plasma-activated water: Physicochemical properties, microbial inactivation mechanisms, factors influencing antimicrobial effectiveness, and applications in the food industry. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3951–3979. [Google Scholar] [CrossRef]
- Abdo, A.I.; Schmitt-John, T.; Richter, K. Cold Plasma Therapy as a Physical Antibiofilm Approach. In Antibiofilm Strategies: Current and Future Applications to Prevent, Control and Eradicate Biofilms; Richter, K., Kragh, K.N., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2022; pp. 225–261. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Zhou, R.; Zhang, T.; Ostrikov, K.; Mugunthan, S.; Rice, S.A.; Cullen, P.J. Interactions of plasma-activated water with biofilms: Inactivation, dispersal effects and mechanisms of action. Npj Biofilms Microbiomes 2021, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, J.; Judée, F.; Yousfi, M.; Vicendo, P.; Merbahi, N. Analysis of reactive oxygen and nitrogen species generated in three liquid media by low temperature helium plasma jet. Sci. Rep. 2017, 7, 4562. [Google Scholar] [CrossRef] [PubMed]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: A data synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef]
- Kurtz, J.R.; Goggins, J.A.; McLachlan, J.B. Salmonella infection: Interplay between the bacteria and host immune system. Immunol. Lett. 2017, 190, 42–50. [Google Scholar] [CrossRef]
- Crum-Cianflone, N.F. Salmonellosis and the gastrointestinal tract: More than just peanut butter. Curr. Gastroenterol. Rep. 2008, 10, 424–431. [Google Scholar] [CrossRef] [PubMed]
- National Research Council; Committee on the Review of the Use of Scientific Criteria and Performance Standards for Safe Food. Scientific Criteria to Ensure Safe Food; National Academies Press: Washington, DC, USA, 2003. [Google Scholar] [CrossRef]
- Weerasooriya, G.; Khan, S.; Chousalkar, K.K.; McWhorter, A.R. Invasive potential of sub-lethally injured Campylobacter jejuni and Salmonella Typhimurium during storage in chicken meat juice. Food Control 2022, 135, 108823. [Google Scholar] [CrossRef]
- Shen, J.; Tian, Y.; Li, Y.; Ma, R.; Zhang, Q.; Zhang, J.; Fang, J. Bactericidal Effects against S. aureus and Physicochemical Properties of Plasma Activated Water stored at different temperatures. Sci. Rep. 2016, 6, 28505. [Google Scholar] [CrossRef]
- Ikawa, S.; Tani, A.; Nakashima, Y.; Kitano, K. Physicochemical properties of bactericidal plasma-treated water. J. Phys. D Appl. Phys. 2016, 49, 425401. [Google Scholar] [CrossRef]
- Tiganitas, A.; Zeaki, N.; Gounadaki, A.S.; Drosinos, E.H.; Skandamis, P.N. Study of the effect of lethal and sublethal pH and aw stresses on the inactivation or growth of Listeria monocytogenes and Salmonella Typhimurium. Int. J. Food Microbiol. 2009, 134, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Shadbolt, C.; Ross, T.; McMeekin, T. Differentiation of the effects of lethal pH and water activity: Food safety implications. Lett. Appl. Microbiol. 2001, 32, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- McWhorter, A.R.; Chousalkar, K.K. Comparative phenotypic and genotypic virulence of Salmonella strains isolated from Australian layer farms. Front. Microbiol. 2015, 6, 12. [Google Scholar] [CrossRef]
- Pande, V.V.; McWhorter, A.R.; Chousalkar, K.K. Salmonella enterica isolates from layer farm environments are able to form biofilm on eggshell surfaces. Biofouling 2016, 32, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Smet, C.; Govaert, M.; Kyrylenko, A.; Easdani, M.; Walsh, J.L.; Van Impe, J.F. Inactivation of Single Strains of Listeria monocytogenes and Salmonella Typhimurium Planktonic Cells Biofilms With Plasma Activated Liquids. Front. Microbiol. 2019, 10, 1539. [Google Scholar] [CrossRef]
- Li, Y.; Pan, J.; Wu, D.; Tian, Y.; Zhang, J.; Fang, J. Regulation of Enterococcus faecalis Biofilm Formation and Quorum Sensing Related Virulence Factors with Ultra-low Dose Reactive Species Produced by Plasma Activated Water. Plasma Chem. Plasma Process. 2018, 39, 35–49. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, X.; Yang, W.; Zhang, Y.; Ye, Z.; Hu, S.; Ye, C.; Li, Y.; Lan, Y.; Shen, J.; et al. In vitro antimicrobial effects and mechanism of air plasma-activated water on Staphylococcus aureus biofilm. Plasma Process. Polym. 2020, 17, 1900270. [Google Scholar] [CrossRef]
- Rothwell, J.G.; Alam, D.; Carter, D.A.; Soltani, B.; McConchie, R.; Zhou, R.; Cullen, P.J.; Mai-Prochnow, A. The antimicrobial efficacy of plasma-activated water against Listeria and E. coli is modulated by reactor design and water composition. J. Appl. Microbiol. 2021, 132, 2490–2500. [Google Scholar] [CrossRef]
- Wang, Q.; Salvi, D. Evaluation of plasma-activated water (PAW) as a novel disinfectant: Effectiveness on Escherichia coli and Listeria innocua, physicochemical properties, and storage stability. LWT 2021, 149, 111847. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, Y.; Feng, H.; Ma, R.; Tian, Y.; Zhang, J.; Fang, J. A study of oxidative stress induced by non-thermal plasma-activated water for bacterial damage. Appl. Phys. Lett. 2013, 102, 203701. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, R.; Zhang, Q.; Feng, H.; Liang, Y.; Zhang, J.; Fang, J. Assessment of the Physicochemical Properties and Biological Effects of Water Activated by Non-thermal Plasma Above and Beneath the Water Surface. Plasma Process. Polym. 2014, 12, 439–449. [Google Scholar] [CrossRef]
- Weinstein, M.P.; Lewis, J.S. The Clinical and Laboratory Standards Institute Subcommittee on Antimicrobial Susceptibility Testing: Background, Organization, Functions, and Processes. J. Clin. Microbiol. 2020, 58, 10–1128. [Google Scholar] [CrossRef]
- Kaul, L.; Abdo, A.I.; Coenye, T.; Krom, B.P.; Hoogenkamp, M.A.; Zannettino, A.C.W.; Süss, R.; Richter, K. The combination of diethyldithiocarbamate and copper ions is active against Staphylococcus aureus and Staphylococcus epidermidis biofilms in vitro and in vivo. Front. Microbiol. 2022, 13, 999893. [Google Scholar] [CrossRef] [PubMed]


| ID | Resistivity (μΩ × m) | Conductivity (S/m) | ORP (mV) |
|---|---|---|---|
| PTW1 | 2200 | 4.55 × 10−4 | 626 |
| PTW2 | 806 | 1.24 × 10−4 | 582 |
| PTW3 | 1122 | 8.91 × 10−4 | 594 |
| PTW4 | 3890 | 2.57 × 10−4 | 593 |
| PTW5 | 826 | 1.21 × 10−4 | 592 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdo, A.; McWhorter, A.; Hasse, D.; Schmitt-John, T.; Richter, K. Efficacy of Plasma-Treated Water against Salmonella Typhimurium: Antibacterial Activity, Inhibition of Invasion, and Biofilm Disruption. Antibiotics 2023, 12, 1371. https://doi.org/10.3390/antibiotics12091371
Abdo A, McWhorter A, Hasse D, Schmitt-John T, Richter K. Efficacy of Plasma-Treated Water against Salmonella Typhimurium: Antibacterial Activity, Inhibition of Invasion, and Biofilm Disruption. Antibiotics. 2023; 12(9):1371. https://doi.org/10.3390/antibiotics12091371
Chicago/Turabian StyleAbdo, Adrian, Andrea McWhorter, Daniel Hasse, Thomas Schmitt-John, and Katharina Richter. 2023. "Efficacy of Plasma-Treated Water against Salmonella Typhimurium: Antibacterial Activity, Inhibition of Invasion, and Biofilm Disruption" Antibiotics 12, no. 9: 1371. https://doi.org/10.3390/antibiotics12091371
APA StyleAbdo, A., McWhorter, A., Hasse, D., Schmitt-John, T., & Richter, K. (2023). Efficacy of Plasma-Treated Water against Salmonella Typhimurium: Antibacterial Activity, Inhibition of Invasion, and Biofilm Disruption. Antibiotics, 12(9), 1371. https://doi.org/10.3390/antibiotics12091371







