Genomic Characterization of Antibiotic-Resistant Campylobacterales Isolated from Chilean Poultry Meat
Abstract
1. Introduction
2. Results
2.1. Prevalence and Distribution of Campylobacterales
2.2. Antimicrobial Resistance
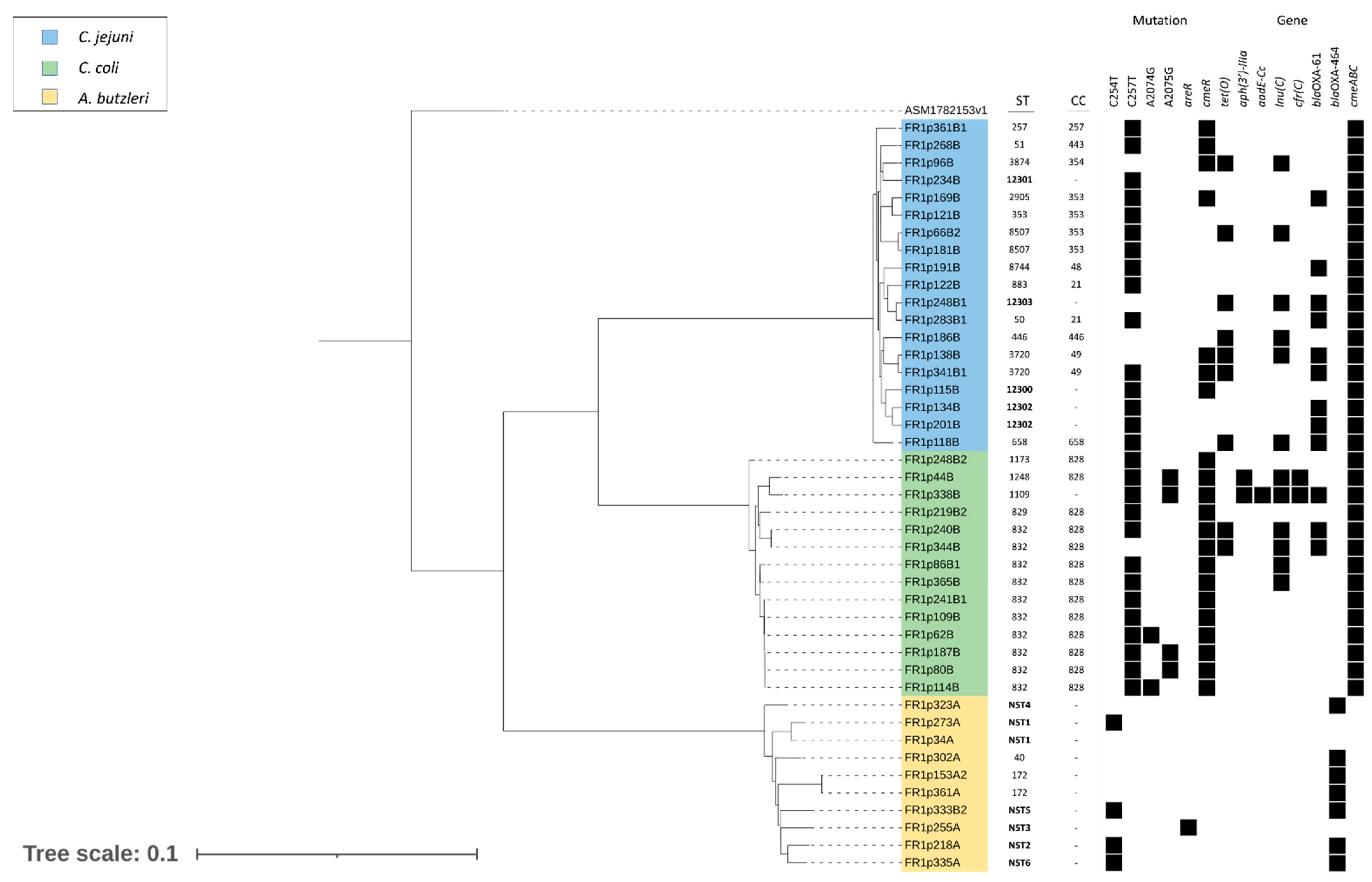
2.3. Molecular Mechanisms of Antibiotic Resistance
2.4. Virulence Genes
2.5. Genotyping
3. Discussion
3.1. Prevalence and Distribution of Campylobacterales
3.2. Antimicrobial Resistance and Virulence
3.3. Epidemiology
4. Materials and Methods
4.1. Sample Collection
4.2. Isolation of Campylobacterales
4.3. Identification
4.4. Antimicrobial Susceptibility Testing
4.5. Whole-Genome Sequencing
4.6. Molecular Detection of Helicobacter Pullorum
4.7. Phylogenomic Tree
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waite, D.W.; Vanwonterghem, I.; Rinke, C.; Parks, D.H.; Zhang, Y.; Takai, K.; Sievert, S.; Simon, J.; Campbell, B.; Hanson, T.; et al. Comparative genomic analysis of the class Epsilonproteobacteria and proposed reclassification to Epsilonbacteraeota (phyl. nov.). Front. Microbiol. 2017, 8, 682. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, L.; Gatica, M.A.; Riquelme, V.; Vergara, C.; Yañez, J.M.; San Martin, B.; Sáenz, L.; Vidal, M.; Martínez, M.C.; Araya, P.; et al. Characterization of antimicrobial susceptibility and its association with virulence genes related to adherence, invasion, and cytotoxicity in Campylobacter jejuni and Campylobacter coli isolates from animals, meat, and humans. Microb. Drug Resist. 2016, 22, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Nachamkin, I.; Allos, B.M.; Ho, T. Campylobacter Species and Guillain-Barré Syndrome. Clin. Microbiol. Rev. 1998, 11, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, O.; Dediste, A.; Houf, K.; Ibekwem, S.; Souayah, H.; Cadranel, S.; Douat, N.; Zissis, G.; Butzler, J.P.; Vandamme, P. Arcobacter species in humans. Emerg. Infect. Dis. 2004, 10, 1863–1867. [Google Scholar] [CrossRef] [PubMed]
- Collado, L.; Figueras, M.J. Taxonomy, Epidemiology, and Clinical Relevance of the Genus Arcobacter. Clin. Microbiol. Rev. 2011, 24, 174–192. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.; Warren, J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 323, 1311–1315. [Google Scholar] [CrossRef]
- Ochoa, S.; Collado, L. Enterohepatic Helicobacter Species—Clinical Importance, Host Range and Zoonotic Potential. Crit. Rev. Microbiol. 2021, 47, 728–761. [Google Scholar] [CrossRef]
- Thépault, A.; Rose, V.; Queguiner, M.; Chemaly, M.; Rivoal, K. Dogs and Cats: Reservoirs for Highly Diverse Campylobacter jejuni and a Potential Source of Human Exposure. Animals 2020, 10, 838. [Google Scholar] [CrossRef]
- Man, S.M. The clinical importance of emerging Campylobacter species. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 669–685. [Google Scholar] [CrossRef]
- Epps, S.V.; Harvey, R.B.; Hume, M.E.; Phillips, T.D.; Anderson, R.C.; Nisbet, D.J. Foodborne Campylobacter: Infections, metabolism, pathogenesis and reservoirs. Int. J. Environ. Res. Public Health 2013, 10, 6292–6304. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.; Thomson, J.M.; Fox, J.G.; El-Omar, E.M.; Hold, G.L. Could Helicobacter organisms cause inflammatory bowel disease? FEMS Microbiol. Rev. 2011, 61, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Endtz, H.P. Campylobacter Infections. In Hunter’s Tropical Medicine and Emerging Infectious Diseases, 10th ed.; Ryan, E., Hill, D., Solomon, T., Aronson, N.E., Endy, T.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 507–511. [Google Scholar] [CrossRef]
- Van den Abeele, A.M.; Vogelaers, D.; Vanlaere, E.; Houf, K. Antimicrobial susceptibility testing of Arcobacter butzleri and Arcobacter cryaerophilus strains isolated from Belgian patients. J. Antimicrob. Chemother. 2016, 71, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Son, I.; Englen, M.D.; Berrang, M.E.; Fedorka-Cray, P.J.; Harrison, M.A. Prevalence of Arcobacter and Campylobacter on broiler carcasses during processing. Int. J. Food Microbiol. 2007, 113, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Magrini, N.; Kahlmeter, G.; Singh, N. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. WHO 2017, 27, 318–327. [Google Scholar]
- Collado, L.; Muñoz, N.; Porte, L.; Ochoa, S.; Varela, C.; Muñoz, I. Genetic diversity and clonal characteristics of ciprofloxacin-resistant Campylobacter jejuni isolated from Chilean patients with gastroenteritis. Infect. Genet. Evol. 2018, 58, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Collado, L. Microbial diagnosis and epidemiological surveillance of campylobacteriosis in Chile: Present state and further challenges. Rev. Chilena Infectol. 2020, 37, 244–251. [Google Scholar] [CrossRef]
- Lopez-Cantillo, M.; Opazo-Capurro, A.; Lopez-Joven, C.; Vidal-Veuthey, B.; Collado, L. Campylobacter jejuni and other emerging Campylobacteraceae in retail beef liver–an underestimated potential source? Lett. Appl. Microbiol. 2022, 75, 1505–1514. [Google Scholar] [CrossRef]
- Iannetti, S.; Calistri, P.; Di Serafino, G.; Marotta, F.; Alessiani, A.; Antoci, S.; Neri, D.; Perilli, M.; Iannitto, G.; Iannetti, L.; et al. Campylobacter jejuni and Campylobacter coli: Prevalence, contamination levels, genetic diversity and antibiotic resistance in Italy. Vet. Ital. 2020, 56, 23–34. [Google Scholar] [CrossRef]
- Hansson, K. To narrate danger: Antibiotics and resistant bacteria’s. In Proceedings of the SANT2021-the Swedish Anthropological Association, Stockholm, Sweden, 22–23 April 2021; Lunds Universitet: Lund, Sweden, 2021. [Google Scholar]
- Popa, S.A.; Morar, A.; Ban-Cucerzan, A.; Tîrziu, E.; Herman, V.; Sallam, K.I.; Morar, D.; Acaroz, U.; Imre, M.; Florea, T.; et al. Occurrence of Campylobacter spp. and Phenotypic Antimicrobial Resistance Profiles of Campylobacter jejuni in Slaughtered Broiler Chickens in North-Western Romania. Antibiotics 2022, 11, 1713. [Google Scholar] [CrossRef]
- Zhang, Q.; Beyi, A.F.; Yin, Y. Zoonotic and antibiotic-resistant Campylobacter: A view through the One Health lens. One Health Adv. 2023, 1, 4. [Google Scholar] [CrossRef]
- Pettan-Brewer, C.; Martins, A.F.; Abreu, D.P.B.D.; Brandão, A.P.D.; Barbosa, D.S.; Figueroa, D.P.; Cediel, N.; Kahn, L.H.; Brandespim, D.F.; Velásquez, J.C.C.; et al. From the approach to the concept: One health in Latin America-Experiences and perspectives in Brazil, Chile, and Colombia. Front. Public Health 2021, 9, 687110. [Google Scholar] [CrossRef] [PubMed]
- Zbrun, M.V.; Rossler, E.; Romero-Scharpen, A.; Soto, L.P.; Berisvil, A.; Zimmermann, J.A.; Fusari, M.L.; Signorini, M.L.; Frizzo, L.S. Worldwide meta-analysis of the prevalence of Campylobacter in animal food products. Res. Vet. Sci. 2020, 132, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Atanassova, V.; Kessen, V.; Reich, F.; Klein, G. Incidence of Arcobacter spp. in poultry: Quantitative and qualitative analysis and PCR differentiation. J. Food Prot. 2008, 71, 2533–2536. [Google Scholar] [CrossRef]
- Silva, W.C.; Targino, B.N.; Mendonça, R.S.; Sant’Ana, A.S.; Hungaro, H.M. Campylobacter: An overview of cases, occurrence in food, contamination sources, and antimicrobial resistance in Brazil. Food Rev. Int. 2018, 34, 364–389. [Google Scholar] [CrossRef]
- Zbrun, M.V.; Rossler, E.; Soto, L.P.; Rosmini, M.R.; Sequeira, G.J.; Frizzo, L.S.; Signorini, M.L. Molecular epidemiology of Campylobacter jejuni isolates from the broiler production chain: First report of MLST profiles in Argentina. Rev. Argent. Microbiol. 2021, 53, 59–63. [Google Scholar] [CrossRef]
- Lapierre, L.; Cornejo, J.; Zavala, S.; Galarce, N.; Sánchez, F.; Benavides, M.B.; Gúzman, M.; Sáenz, L. Phenotypic and genotypic characterization of virulence factors and susceptibility to antibiotics in Salmonella Infantis strains isolated from chicken meat: First findings in Chile. Animals 2020, 10, 1049. [Google Scholar] [CrossRef]
- Geissler, A.L.; Bustos Carrillo, F.; Swanson, K.; Patrick, M.E.; Fullerton, K.E.; Bennett, C.; Barrett, K.; Mahon, B.E. Increasing Campylobacter infections, outbreaks, and antimicrobial resistance in the United States, 2004–2012. Clin. Infect. Dis. 2017, 65, 1624–1631. [Google Scholar] [CrossRef]
- Ministerio de Salud, Departamento de Epidemiología. Informe Epidemiológico Anual, Diarrea Aguda en Menores de 5 años 2014–2018. Gobierno de Chile. 2018. Available online: http://epi.minsal.cl/wp-content/uploads/2020/03/DIARREA_INFORME_2014_2018.pdf (accessed on 5 August 2022).
- Dekker, D.; Eibach, D.; Boahen, K.G.; Akenten, C.W.; Pfeifer, Y.; Zautner, A.E.; Mentens, E.; Krumkamp, R.; Jaeger, A.; Flieger, A.; et al. Fluoroquinolone-Resistant Salmonella enterica, Campylobacter spp.; and Arcobacter butzleri from Local and Imported Poultry Meat in Kumasi, Ghana. Foodborne Pathog. Dis. 2019, 16, 352–358. [Google Scholar] [CrossRef]
- Vidal-Veuthey, B.; Jara, R.; Santander, K.; Mella, A.; Ruiz, S.; Collado, L. Antimicrobial resistance and virulence genes profiles of Arcobacter butzleri strains isolated from back yard chickens and retail poultry meat in Chile. Lett. Appl. Microbiol. 2021, 72, 126–132. [Google Scholar] [CrossRef]
- Ohnishi, T.; Hara-Kudo, Y. Presence and quantification of pathogenic Arcobacter and Campylobacter species in retail meats available in Japan. Lett. Appl. Microbiol. 2021, 73, 81–87. [Google Scholar] [CrossRef] [PubMed]
- ICMSF. Microorganisms in Foods 7-Microbiological Testing in Food Safety Management; International Commission on Microbiological Specifications for Foods; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002. [Google Scholar]
- Collado, L.; Gutiérrez, M.; González, M.; Fernández, H. Assessment of the prevalence and diversity of emergent campylobacteria in human stool samples using a combination of traditional and molecular methods. Diagn. Microbiol. Infect. Dis. 2013, 75, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Salas-Massó, N.; Pérez-Cataluña, A.; Collado, L.; Levican, A.; Figueras, M.J. Chapter 23: Arcobacter. In Handbook of Foodborne Diseases, 1st ed.; Dongyou, L., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 243–260. [Google Scholar]
- Ochoa, S.; Martínez, O.A.; Fernández, H.; Collado, L. Comparison of media and growth conditions for culturing enterohepatic Helicobacter species. Lett. Appl. Microbiol. 2019, 69, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Habib, I.; Uyttendaele, M.; De Zutter, L. Evaluation of ISO 10272: 2006 standard versus alternative enrichment and plating combinations for enumeration and detection of Campylobacter in chicken meat. Food Microbiol. 2011, 28, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Jebellijavan, A.; Emadi Chashmi, S.H.; Staji, H.; Akhlaghi, H. Comparison of the Culture and PCR Methods to Determine the Prevalence and Antibiotic Resistance of Helicobacter pullorum Isolated from Chicken Thigh Samples in Semnan, Iran. J. Hum. Environ. Health Promot. 2020, 6, 167–172. [Google Scholar]
- Akhlaghi, H.; Chashmi, S.E.; Javan, A.J. Development of a novel and specialized cultivation method for isolating Helicobacter pullorum from chicken meat. Iran. J. Vet. Res. 2021, 22, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G.; Dewhirst, F.E.; Shen, Z.; Feng, Y.; Taylor, N.S.; Paster, B.J.; Ericson, E.L.; Lau, C.N.; Correa, P.; Araya, J.C.; et al. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology 1998, 114, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Aleksić, E.; Miljković-Selimović, B.; Tambur, Z.; Aleksić, N.; Biočanin, V.; Avramov, S. Resistance to antibiotics in thermophilic Campylobacters. Front. Med. 2021, 8, 763434. [Google Scholar] [CrossRef]
- Luo, N.; Pereira, S.; Sahin, O.; Lin, J.; Huang, S.; Michel, L.; Zhang, Q. Enhanced in vivo fitness of fluoroquinolone-resistant Campylobacter jejuni in the absence of antibiotic selection pressure. Proc. Natl. Acad. Sci. USA 2005, 102, 541–546. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Sahin, O.; Shen, Z.; Guo, B.; Shen, J.; Zhang, Q. A fluoroquinolone resistance associated mutation in gyrA Affects DNA supercoiling in Campylobacter jejuni. Front. Cell. Infect. Microbiol. 2012, 2, 21. [Google Scholar] [CrossRef]
- Béjaoui, A.; Gharbi, M.; Bitri, S.; Nasraoui, D.; Ben Aziza, W.; Ghedira, K.; Rfaik, M.; Marzougui, L.; Ghram, A.; Maaroufi, A. Virulence Profiling, Multidrug Resistance and Molecular Mechanisms of Campylobacter Strains from Chicken Carcasses in Tunisia. Antibiotics 2022, 11, 830. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Pu, S.; Wang, F.; Meng, J.; Ge, B. Fitness cost of macrolide resistance in Campylobacter jejuni. Int. J. Antimicrob. Agents 2009, 34, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Luangtongkum, T.; Shen, Z.; Seng, V.W.; Sahin, O.; Jeon, B.; Liu, P.; Zhang, Q. Impaired fitness and transmission of macrolide-resistant Campylobacter jejuni in its natural host. Antimicrob. Agents Chemother. 2012, 56, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Anampa, D.; Benites, C.; Lázaro, C.; Espinoza, J.; Angulo, P.; Díaz, D.; Manchego, A.; Rojas, M. Detección del gen ermB asociado a la resistencia a macrólidos en cepas de Campylobacter aisladas de pollos comercializados en Lima, Perú. Rev. Panam. Salud Pública 2020, 44, e60. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhao, W.; Li, S.; Yao, H.; Zhang, Q.; Yang, L. Characterization of erm(B)-carrying Campylobacter spp. of retail chicken meat origin. J. Glob. Antimicrob. Resist. 2022, 30, 173–177. [Google Scholar] [CrossRef]
- Lin, J.; Sahin, O.; Michel, L.O.; Zhang, Q. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infect. Immun. 2003, 71, 4250–4259. [Google Scholar] [CrossRef] [PubMed]
- Gibreel, A.; Wetsch, N.M.; Taylor, D.E. Contribution of the CmeABC efflux pump to macrolide and tetracycline resistance in Campylobacter jejuni. Antimicrob. Agents Chemother. 2007, 51, 3212–3216. [Google Scholar] [CrossRef] [PubMed]
- Bravo, V.; Katz, A.; Porte, L.; Weitzel, T.; Varela, C.; Gonzalez-Escalona, N.; Blondel, C.J. Genomic analysis of the diversity, antimicrobial resistance and virulence potential of clinical Campylobacter jejuni and Campylobacter coli strains from Chile. PLoS Negl. Trop. Dis. 2021, 15, e0009207. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cagliero, C.; Guo, B.; Barton, Y.W.; Maurel, M.C.; Payot, S.; Zhang, Q. Bile salts modulate expression of the CmeABC multidrug efflux pump in Campylobacter jejuni. J. Bacteriol. 2005, 187, 7417–7424. [Google Scholar] [CrossRef]
- Achard, A.; Villers, C.; Pichereau, V.; Leclercq, R. New lnu(C) gene conferring resistance to lincomycin by nucleotidylation in Streptococcus agalactiae UCN36. Antimicrob. Agents Chemother. 2005, 49, 2716–2719. [Google Scholar] [CrossRef]
- Tang, Y.; Dai, L.; Sahin, O.; Wu, Z.; Liu, M.; Zhang, Q. Emergence of a plasmid-borne multidrug resistance gene cfr(C) in foodborne pathogen Campylobacter. J. Antimicrob. Chemother. 2017, 72, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Tyson, G.H.; Chen, Y.; Li, C.; Mukherjee, S.; Young, S.; Lam, C.; Folster, J.P.; Whichard, J.M.; McDermott, P.F. Whole-genome sequencing analysis accurately predicts antimicrobial resistance phenotypes in Campylobacter spp. Appl. Environ. Microbiol. 2016, 82, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhu, D.; Lai, H.; Zeng, H.; Zhou, K.; Zou, L.; Wu, C.; Han, G.; Liu, S. Prevalence, antimicrobial resistance profiling and genetic diversity of Campylobacter jejuni and Campylobacter coli isolated from broilers at slaughter in China. Food Control 2016, 69, 160–170. [Google Scholar] [CrossRef]
- Suman Kumar, M.; Ramees, T.P.; Dhanze, H.; Gupta, S.; Dubal, Z.B.; Kumar, A. Occurrence and antimicrobial resistance of Campylobacter isolates from broiler chicken and slaughter house environment in India. Anim. Biotechnol. 2021, 34, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Paravisi, M.; Laviniki, V.; Bassani, J.; Kunert Filho, H.C.; Carvalho, D.; Wilsmann, D.E.; Borges, K.A.; Furian, T.Q.; Salle, C.T.P.; Moraes, H.L.S.; et al. Antimicrobial resistance in Campylobacter jejuni isolated from Brazilian poultry slaughterhouses. Braz. J. Poult. Sci. 2020, 22, 02. [Google Scholar] [CrossRef]
- Ferreira, S.; Luis, A.; Oleastro, M.; Pereira, L.; Domingues, F.C. A meta-analytic perspective on Arcobacter spp. antibiotic resistance. J. Glob. Antimicrob. Resist. 2019, 16, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Martins, S.; Oleastro, M.; Domingues, F.C.; Ferreira, S. Arcobacter spp. at retail food from Portugal: Prevalence, genotyping and antibiotics resistance. Food Control 2018, 85, 107–112. [Google Scholar] [CrossRef]
- Jehanne, Q.; Bénéjat, L.; Ducournau, A.; Bessède, E.; Lehours, P. Molecular Cut-off Values for Aliarcobacter butzleri Susceptibility Testing. Microbiol. Spectr. 2022, 10, e01003-22. [Google Scholar] [CrossRef]
- Parisi, A.; Capozzi, L.; Bianco, A.; Caruso, M.; Latorre, L.; Costa, A.; Giannico, A.; Ridolfi, D.; Bulzacchelli, C.; Santagada, G. Identification of virulence and antibiotic resistance factors in Arcobacter butzleri isolated from bovine milk by Whole Genome Sequencing. Ital. J. Food Saf. 2019, 8, 7840. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhou, G.L.; Li, Y.; Gu, Y.X.; He, M.; Zhang, S.; Ji, G.Q.; Yang, J.; Wang, M.; Ma, H.M.; et al. Genetic characteristics and antimicrobial susceptibility of Arcobacter butzleri isolates from raw chicken meat and patients with diarrhea in China. Biomed. Environ. Sci. 2021, 34, 1024–1028. [Google Scholar]
- Kadam, U.S.; Lossie, A.C.; Schulz, B.; Irudayaraj, J. Gene expression analysis using conventional and imaging methods. In DNA and RNA Nanobiotechnologies in Medicine: Diagnosis and Treatment of Diseases; Springer: Berlin/Heidelberg, Germany, 2013; pp. 141–162. [Google Scholar] [CrossRef]
- Isidro, J.; Ferreira, S.; Pinto, M.; Domingues, F.; Oleastro, M.; Gomes, J.P.; Borges, V. Virulence and antibiotic resistance plasticity of Arcobacter butzleri: Insights on the genomic diversity of an emerging human pathogen. Infect. Genet. Evol. 2020, 80, 104213. [Google Scholar] [CrossRef] [PubMed]
- Bonifácio, M.; Mateus, C.; Alves, A.R.; Maldonado, E.; Duarte, A.P.; Domingues, F.; Oleastro, M.; Ferreira, S. Natural transformation as a mechanism of horizontal gene transfer in Aliarcobacter butzleri. Pathogens 2021, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Palma, E.; Tilocca, B.; Roncada, P. Antimicrobial resistance in veterinary medicine: An overview. Int. J. Mol. Sci. 2020, 21, 1914. [Google Scholar] [CrossRef] [PubMed]
- McDermott, P.F.; Bodeis, S.M.; English, L.L.; White, D.G.; Walker, R.D.; Zhao, S.; Simjee, S.; Wagner, D.D. Ciprofloxacin resistance in Campylobacter jejuni evolves rapidly in chickens treated with fluoroquinolones. J. Infect. Dis. 2002, 185, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Ladely, S.R.; Harrison, M.A.; Fedorka-Cray, P.J.; Berrang, M.E.; Englen, M.D.; Meinersmann, R.J. Development of macrolide-resistant Campylobacter in broilers administered subtherapeutic or therapeutic concentrations of tylosin. J. Food Prot. 2007, 70, 1945–1951. [Google Scholar] [CrossRef]
- Lin, J.; Yan, M.; Sahin, O.; Pereira, S.; Chang, Y.J.; Zhang, Q. Effect of macrolide usage on emergence of erythromycin-resistant Campylobacter isolates in chickens. Antimicrob. Agents Chemother. 2007, 51, 1678–1686. [Google Scholar] [CrossRef]
- Avrain, L.; Humbert, F.; L’Hospitalier, R.; Sanders, P.; Vernozy-Rozand, C.; Kempf, I. Antimicrobial resistance in Campylobacter from broilers: Association with production type and antimicrobial use. Vet. Microbiol. 2003, 96, 267–276. [Google Scholar] [CrossRef]
- Pardo-Este, C.; Lorca, D.; Castro-Severyn, J.; Krüger, G.; Alvarez-Thon, L.; Zepeda, P.; Sulbaran-Bracho, Y.; Hidalgo, A.; Tello, M.; Molina, F.; et al. Genetic characterization of Salmonella infantis with multiple drug resistance profiles isolated from a poultry-farm in Chile. Microorganisms 2021, 9, 2370. [Google Scholar] [CrossRef]
- Frosth, S.; Karlsson-Lindsjö, O.; Niazi, A.; Fernström, L.L.; Hansson, I. Identification of transmission routes of Campylobacter and on-farm measures to reduce Campylobacter in chicken. Pathogens 2020, 9, 363. [Google Scholar] [CrossRef]
- Ramonaite, S.; Tamuleviciene, E.; Alter, T.; Kasnauskyte, N.; Malakauskas, M. MLST genotypes of Campylobacter jejuni isolated from broiler products, dairy cattle and human campylobacteriosis cases in Lithuania. BMC Infect. Dis. 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Zhang, T.; Dong, J.; Cheng, Y.; Lu, Q.; Luo, Q.; Wen, G.; Liu, G.; Shao, H. Genotypic diversity, antimicrobial resistance and biofilm-forming abilities of Campylobacter isolated from chicken in Central China. Gut Pathog. 2017, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mäesaar, M.; Meremäe, K.; Ivanova, M.; Roasto, M. Antimicrobial resistance and multilocus sequence types of Campylobacter jejuni isolated from Baltic broiler chicken meat and Estonian human patients. Poult. Sci. 2018, 97, 3645–3651. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, S.K.; Maiden, M.C. The evolution of Campylobacter jejuni and Campylobacter coli. Cold Spring Harb. Perspect. Biol. 2015, 7, a018119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, X.; Liu, Y.; Jiang, J.; Shen, Z.; Chen, Q.; Ma, X. Multilocus sequence types and antimicrobial resistance of Campylobacter jejuni and C. coli isolates of human patients from Beijing, China, 2017–2018. Front. Microbiol. 2020, 11, 554784. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.G.; Wesley, I.V.; On, S.L.; Houf, K.; Mégraud, F.; Wang, G.; Yee, E.; Srijan, A.; Mason, C.J. First multi-locus sequence typing scheme for Arcobacter spp. BMC Microbiol. 2009, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Steele, T.W.; McDermott, S.N. The use of membrane filters applied directly to the surface of agar plates for the isolation of Campylobacter jejuni from feces. Pathology 1984, 16, 263–265. [Google Scholar] [CrossRef]
- Houf, K.; De Zutter, L.; Van Hoof, J.; Vandamme, P. Assessment of the genetic diversity among arcobacters isolated from poultry products by using two PCR-based typing methods. Appl. Environ. Microbiol. 2002, 68, 2172–2178. [Google Scholar] [CrossRef]
- Yamazaki-Matsune, W.; Taguchi, M.; Seto, K.; Kawahara, R.; Kawatsu, K.; Kumeda, Y.; Kitazato, M.; Nukina, M.; Misawa, N.; Tsukamoto, T. Development of a multiplex PCR assay for identification of Campylobacter coli, Campylobacter fetus, Campylobacter hyointestinalis subsp. hyointestinalis, Campylobacter jejuni, Campylobacter lari and Campylobacter upsaliensis. J. Med. Microbiol. 2007, 56, 1467–1473. [Google Scholar] [CrossRef]
- Harmon, K.M.; Wesley, I.V. Identification of Arcobacter isolates by PCR. Lett. Appl. Microbiol. 1996, 23, 241–244. [Google Scholar] [CrossRef]
- Douidah, L.; De Zutter, L.; Vandamme, P.; Houf, K. Identification of five human and mammal associated Arcobacter species by a novel multiplex-PCR assay. J. Microbiol. Methods 2010, 80, 281–286. [Google Scholar] [CrossRef]
- CLSI. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria. In Approved Guideline, CLSI Guideline M45, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, CLSI Supplement M100, 30th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 August 2022).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [PubMed]
- Molina-Mora, J.A.; Campos-Sánchez, R.; Rodríguez, C.; Shi, L.; García, F. High quality 3C de novo assembly and annotation of a multidrug resistant ST-111 Pseudomonas aeruginosa genome: Benchmark of hybrid and non-hybrid assemblers. Sci. Rep. 2020, 10, 1392. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Dingle, K.E.; Colles, F.M.; Wareing, D.R.A.; Ure, R.; Fox, A.J.; Bolton, F.E.; Bootsma, H.J.; Willems, R.J.; Urwin, R.; Maiden, M.C.J. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 2001, 39, 14–23. [Google Scholar] [CrossRef]
- Rocha, M.; Avenaud, P.; Menard, A.; Le Bail, B.; Balabaud, C.; Bioulac-Sage, P.; Magalhaes, D.M.; Megraud, F. Association of Helicobacter species with hepatitis C cirrhosis with or without hepatocellular carcinoma. Gut 2005, 54, 396–401. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed]
| Isolated Species | Positive Sample | |
|---|---|---|
| N° | % | |
| C. jejuni | 43 | 11.3 |
| C. coli | 25 | 6.5 |
| A. butzleri | 96 | 25.1 |
| A. cryaerophilus | 7 | 1.8 |
| A. skirrowii | 4 | 1 |
| C. jejuni + C. coli | 2 | 0.5 |
| C. jejuni + A. butzleri | 27 | 7.1 |
| C. jejuni + A. cryaerophilus | 1 | 0.3 |
| C. coli + A. butzleri | 12 | 3.1 |
| C. coli + A. cryaerophilus | 1 | 0.3 |
| A. butzleri + A. cryaerophilus | 4 | 1 |
| A. butzleri + A. skirrowii | 1 | 0.3 |
| C. jejuni + C. coli + A. butzleri | 2 | 0.5 |
| C. coli + A. butzleri + A. cryaerophilus | 1 | 0.3 |
| Total | 226 | 59.2 |
| Isolation Protocols | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | A | B | C1 | C2 | Total (n = 382) | |||||
| N° | % | N° | % | N° | % | N° | % | N° | % * | |
| C. jejuni | 75 | 19.6 | 75 | 19.6 | ||||||
| C. coli | 43 | 11.3 | 43 | 11.3 | ||||||
| Total Campylobacter | 114 | 29.8 | 114 ** | 29.8 | ||||||
| A. butzleri | 130 | 34 | 45 | 11.8 | 3 | 0.8 | 143 | 37.4 | ||
| A. cryaerophilus | 8 | 2.1 | 4 | 1 | 1 | 0.3 | 1 | 0.3 | 14 | 3.7 |
| A. skirrowii | 4 | 1 | 1 | 0.3 | 5 | 1.3 | ||||
| Total Arcobacter | 142 | 37.2 | 49 | 12.8 | 1 | 0.3 | 5 | 1.3 | 156 ** | 40.8 |
| Antimicrobial Agent | Species | n° | Disk Difussion Method | Test Strip | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Strains | Distribution of MIC (µg/ml) | ||||||||||||||||||||||||
| S | I | R | %R | 0.032 | 0.75 | 1.0 | 1.5 | 2 | 3 | 4 | 6 | 8 | 12 | 16 | 24 | 32 | 48 | 64 | 96 | 128 | >256 | %R | |||
| Ciprofloxacin | C. jejuni | 75 | 47 | 28 | 37.3 | 1 | 27 | 37.3 | |||||||||||||||||
| C. coli | 43 | 19 | 24 | 55.8 | 1 | 23 | 55.8 | ||||||||||||||||||
| A. butzleri | 143 | 135 | 8 | 5.6 | 1 | 3 | 1 | 1 | 1 | 1 | 2.8 | ||||||||||||||
| A. cryaerophilus | 15 | 15 | |||||||||||||||||||||||
| A. skirrowii | 4 | 4 | |||||||||||||||||||||||
| Erythromycin | C. jejuni | 75 | 75 | ||||||||||||||||||||||
| C. coli | 43 | 36 | 7 | 16.3 | 7 | 16.3 | |||||||||||||||||||
| A. butzleri | 143 | 140 | 2 | 1 | 1 | 1 | 1 | 1 | 0.7 | ||||||||||||||||
| A. cryaerophilus | 15 | 15 | |||||||||||||||||||||||
| A. skirrowii | 4 | 4 | |||||||||||||||||||||||
| Tetracycline | C. jejuni | 75 | 60 | 15 | 20 | 1 | 5 | 5 | 2 | 1 | 1 | 20 | |||||||||||||
| C. coli | 43 | 41 | 2 | 4.7 | 1 | 1 | 4.7 | ||||||||||||||||||
| A. butzleri | 143 | 59 | 32 | 52 | 36.4 | 1 | 1 | 16 | 9 | 22 | 14 | 11 | 6 | 4 | 2.8 | ||||||||||
| A. cryaerophilus | 15 | 12 | 2 | 1 | 6.7 | 1 | 1 | 1 | |||||||||||||||||
| A. skirrowii | 4 | 2 | 2 | 2 | |||||||||||||||||||||
| Gentamicin | C. jejuni | 75 | 75 | ||||||||||||||||||||||
| C. coli | 43 | 43 | |||||||||||||||||||||||
| A. butzleri | 143 | 143 | |||||||||||||||||||||||
| A. cryaerophilus | 15 | 15 | |||||||||||||||||||||||
| A. skirrowii | 4 | 4 | |||||||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Concha-Toloza, M.; Lopez-Cantillo, M.; Molina-Mora, J.A.; Collado, L. Genomic Characterization of Antibiotic-Resistant Campylobacterales Isolated from Chilean Poultry Meat. Antibiotics 2023, 12, 917. https://doi.org/10.3390/antibiotics12050917
Concha-Toloza M, Lopez-Cantillo M, Molina-Mora JA, Collado L. Genomic Characterization of Antibiotic-Resistant Campylobacterales Isolated from Chilean Poultry Meat. Antibiotics. 2023; 12(5):917. https://doi.org/10.3390/antibiotics12050917
Chicago/Turabian StyleConcha-Toloza, Macarena, Mónica Lopez-Cantillo, Jose Arturo Molina-Mora, and Luis Collado. 2023. "Genomic Characterization of Antibiotic-Resistant Campylobacterales Isolated from Chilean Poultry Meat" Antibiotics 12, no. 5: 917. https://doi.org/10.3390/antibiotics12050917
APA StyleConcha-Toloza, M., Lopez-Cantillo, M., Molina-Mora, J. A., & Collado, L. (2023). Genomic Characterization of Antibiotic-Resistant Campylobacterales Isolated from Chilean Poultry Meat. Antibiotics, 12(5), 917. https://doi.org/10.3390/antibiotics12050917








