Regional Perspective of Antimicrobial Stewardship Programs in Latin American Pediatric Emergency Departments
Abstract
1. Introduction
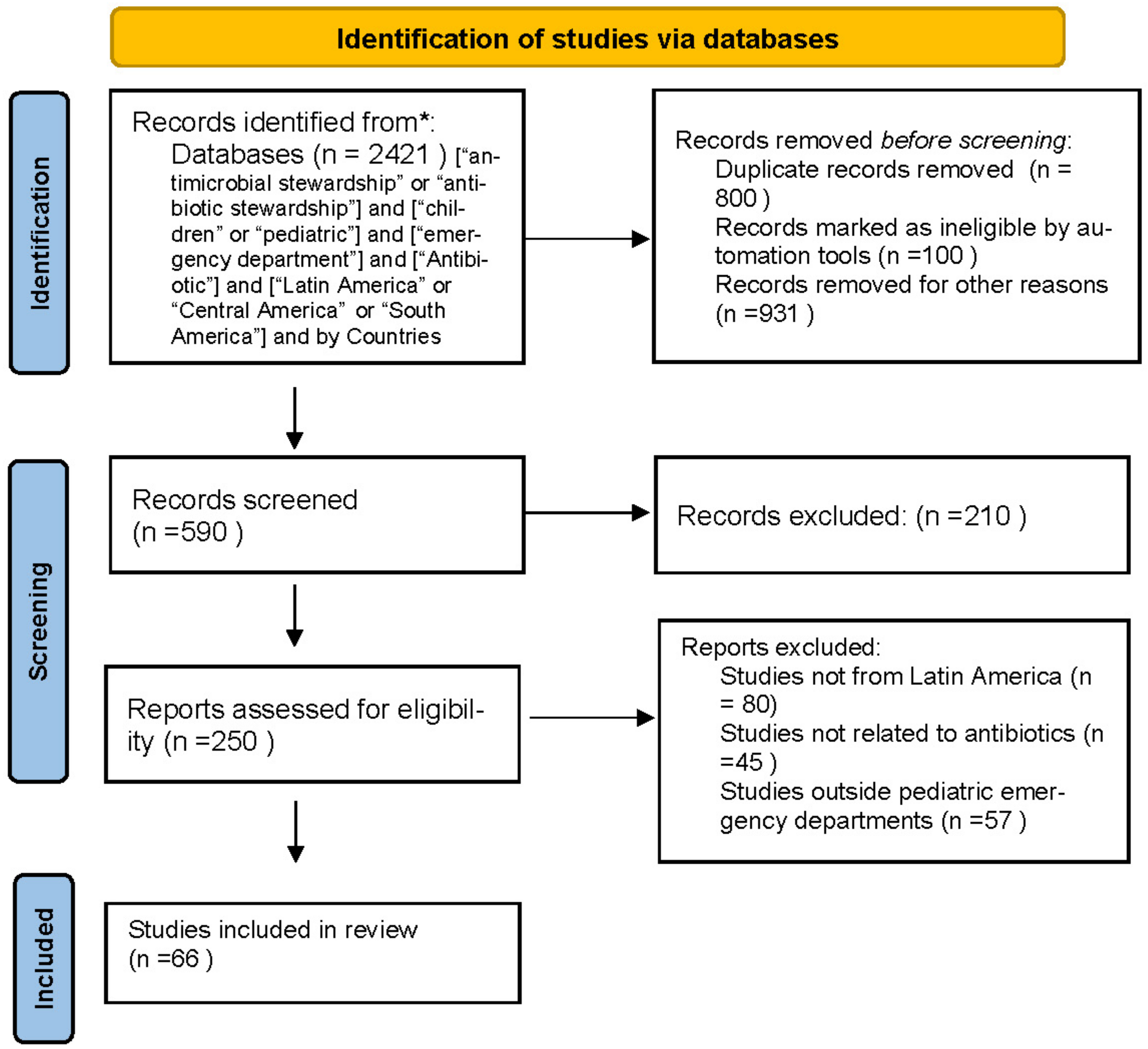
2. Methods
3. Results
3.1. Antibiotic Stewardship in Pediatric Emergency Departments
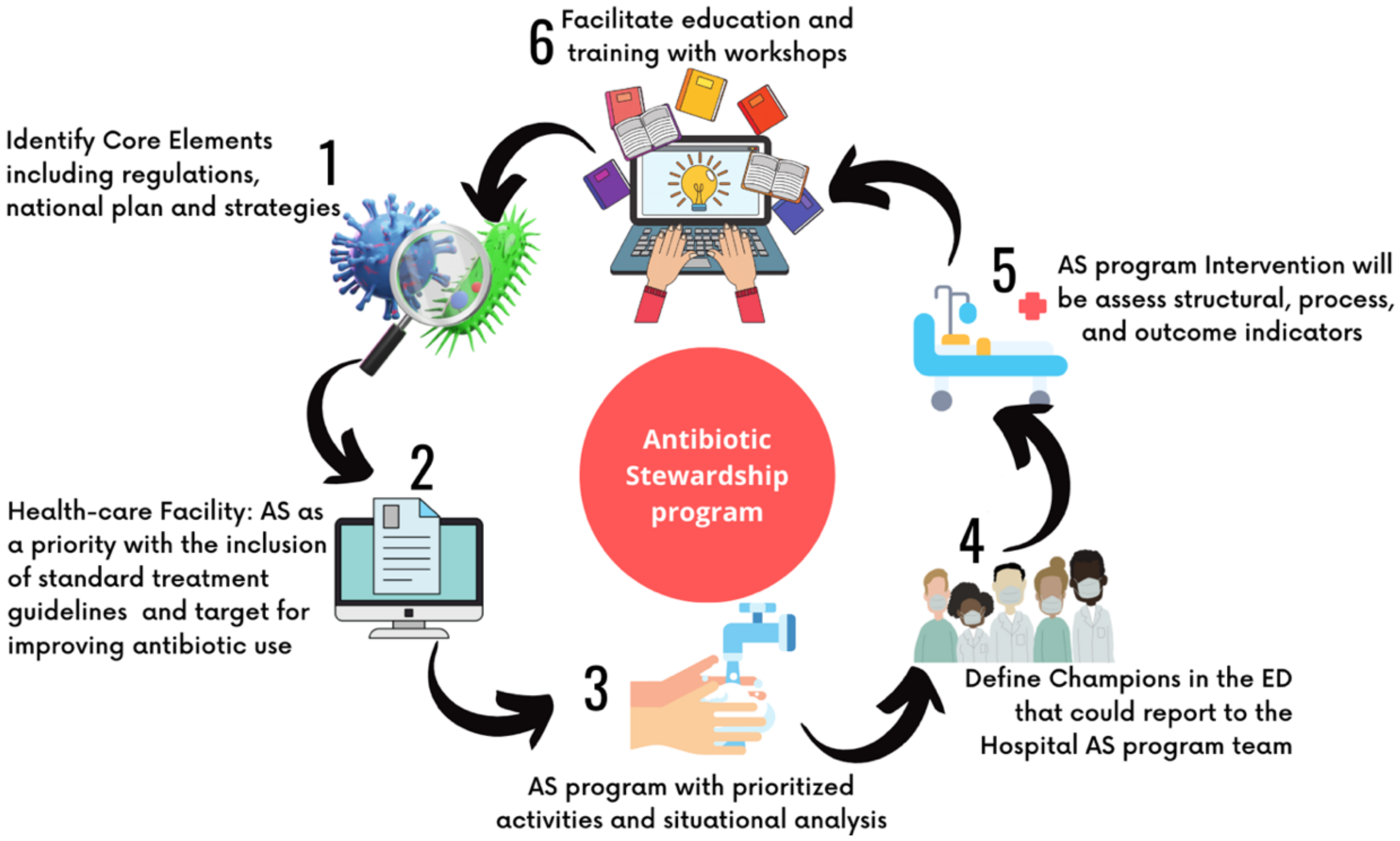
3.2. How to Implement a Stewardship Program in a Pediatric Emergency Department?
3.3. Antibiotic Stewardship in Latin America
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jackson, C.; Hsia, Y.; Bielicki, J.A.; Ellis, S.; Stephens, P.; Wong, I.C.K.; Sharland, M. Estimating global trends in total and childhood antibiotic consumption, 2011–2015. BMJ Glob. Health 2019, 4, e001241. [Google Scholar] [CrossRef] [PubMed]
- Bacteria FTFoCA-R. National Action Plan for Combating Antibiotic-Resistant Bacteria; U.S. Government: Washington, DC, USA, 2020. Available online: https://aspe.hhs.gov/sites/default/files/migrated_legacy_files//196436/CARB-National-Action-Plan-2020-2025.pdf (accessed on 21 January 2023).
- Ozkaynak, M.; Wu, D.T.Y.; Hannah, K.; Dayan, P.S.; Mistry, R.D. Examining Workflow in a Pediatric Emergency Department to Develop a Clinical Decision Support for an Antimicrobial Stewardship Program. Appl. Clin. Inform. 2018, 9, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Brigadoi, G.; Rossin, S.; Visentin, D.; Barbieri, E.; Giaquinto, C.; Da Dalt, L.; Dona, D. The impact of Antimicrobial Stewardship Programmes in paediatric emergency departments and primary care: A systematic review. Ther. Adv. Infect. Dis. 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Pulia, M.; Redwood, R.; May, L. Antimicrobial Stewardship in the Emergency Department. Emerg. Med. Clin. N. Am. 2018, 36, 853–872. [Google Scholar] [CrossRef]
- Pourmand, A.; Mazer-Amirshahi, M.; Jasani, G.; May, L. Emerging trends in antibiotic resistance: Implications for emergency medicine. Am. J. Emerg. Med. 2017, 35, 1172–1176. [Google Scholar] [CrossRef]
- Klatte, J.M. Pediatric Antimicrobial Stewardship Programs: Current Perspectives. Pediatr. Health Med. Ther. 2020, 11, 245–255. [Google Scholar] [CrossRef]
- Dellit, T.H.; Owens, R.C.; McGowan, J.E., Jr.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Fishman, N.O.; Carpenter, C.F.; et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar] [CrossRef]
- Newland, J.G.; Hersh, A.L. Purpose and design of antimicrobial stewardship programs in pediatrics. Pediatr. Infect. Dis. J. 2010, 29, 862–863. [Google Scholar] [CrossRef]
- Howard, P.; Pulcini, C.; Levy Hara, G.; West, R.M.; Gould, I.M.; Harbarth, S.; Nathwani, D. An international cross-sectional survey of antimicrobial stewardship programmes in hospitals. J. Antimicrob. Chemother. 2015, 70, 1245–1255. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Yock-Corrales, A.; Lenzi, J.; Ulloa-Gutierrez, R.; Gomez-Vargas, J.; Antunez-Montes, O.Y.; Rios Aida, J.A.; Del Aguila, O.; Arteaga-Menchaca, E.; Campos, F.; Uribe, F.; et al. High rates of antibiotic prescriptions in children with COVID-19 or multisystem inflammatory syndrome: A multinational experience in 990 cases from Latin America. Acta Paediatr. 2021, 110, 1902–1910. [Google Scholar] [CrossRef]
- Perez-Corrales, C.; Leandro-Sandi, K. Diarrheagenic Escherichia coli in Costa Rican children: A 9-year retrospective study. BMC Res. Notes 2019, 12, 297. [Google Scholar] [CrossRef]
- Chacon-Gonzalez, C.; Rivera-Salgado, D.; Brenes-Chacon, H.; Naranjo-Zuniga, G.; Avila-Aguero, M.L. Use of Meropenem in a Tertiary Pediatric Hospital in Costa Rica and Its Role in the Era of Antimicrobial Stewardship. Cureus 2021, 13, e15809. [Google Scholar]
- Huerta-Gutierrez, R.; Braga, L.; Camacho-Ortiz, A.; Diaz-Ponce, H.; Garcia-Mollinedo, L.; Guzman-Blanco, M.; Valderrama-Beltran, S.; Landaeta-Nezer, E.; Moreno-Espinosa, S.; Morfin-Otero, R.; et al. One-day point prevalence of healthcare-associated infections and antimicrobial use in four countries in Latin America. Int. J. Infect. Dis. 2019, 86, 157–166. [Google Scholar] [CrossRef]
- Levy Hara, G.; Rojas-Cortes, R.; Molina Leon, H.F.; Dreser Mansilla, A.; Alfonso Orta, I.; Rizo-Amezquita, J.N.; Santos Herrera, R.G.; Mendoza de Ayala, S.; Arce Villalobos, M.; Mantilla Ponte, H.; et al. Point prevalence survey of antibiotic use in hospitals in Latin American countries. J. Antimicrob. Chemother. 2022, 77, 807–815. [Google Scholar] [CrossRef]
- Ecker, L.; Ruiz, J.; Vargas, M.; Del Valle, L.J.; Ochoa, T.J. Prevalence of purchase of antibiotics without prescription and antibiotic recommendation practices for children under five years of age in private pharmacies in peri-urban areas of Lima, Peru. Rev. Peru. Med. Exp. Salud Publica 2016, 33, 215–223. [Google Scholar] [CrossRef]
- Ecker, L.; Ochoa, T.J.; Vargas, M.; Del Valle, L.J.; Ruiz, J. Preferences of antibiotic use in children less than five in physicians working health centers of primary level in peri-urban areas of Lima, Peru. Rev. Peru. Med. Exp. Salud Publica 2013, 30, 181–189. [Google Scholar]
- Ruvinsky, S.; Monaco, A.; Perez, G.; Taicz, M.; Inda, L.; Epelbaum, C.; Kijko, I.; Constanzo, P.; Bologna, R. Effectiveness of a program to improve antibiotic use in children hospitalized in a children’s tertiary care facility in Argentina. Arch. Argent Pediatr. 2014, 112, 124–131. [Google Scholar]
- Wolfson, L.J.; Castillo, M.E.; Giglio, N.; Meszner, Z.; Molnar, Z.; Vazquez, M.; Wysocki, J.; Altland, A.; Kuter, B.J.; Stutz, M.; et al. The use of antibiotics in the treatment of pediatric varicella patients: Real-world evidence from the multi-country MARVEL study in Latin America & Europe. BMC Public Health 2019, 19, 826. [Google Scholar]
- Hernandez-Gomez, C.; Hercilla, L.; Mendo, F.; Perez-Lazo, G.; Contreras, E.; Ramirez, E.; Flores, W.; Julca, A.; Chuquiray, N.; Arenas, B.; et al. Antimicrobial Stewardship programs in Peru: A fundamental agreement. Rev. Chil. Infectol. 2019, 36, 565–575. [Google Scholar]
- Zintgraff, J.; Gagetti, P.; Napoli, D.; Sanchez Eluchans, N.; Irazu, L.; Moscoloni, M.; Argentina Spn Working, G.; Regueira, M.; Lara, C.S.; Corso, A. Invasive Streptococcus pneumoniae isolates from pediatric population in Argentina for the period 2006–2019. Temporal progression of serotypes distribution and antibiotic resistance. Vaccine 2022, 40, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, F.; Colston, J.M.; Paredes Olortegui, M.; Rengifo Pinedo, S.; Zamora Babilonia, M.; Ramal Asayag, C.; Penataro Yori, P.; Kosek, M.N. Antibiotic Use and Stewardship Practices in a Pediatric Community-Based Cohort Study in Peru: Shorter Would be Sweeter. Clin. Infect. Dis. 2022, 76, e1054–e1061. [Google Scholar] [CrossRef] [PubMed]
- Christian, J.; Pallares, J.C.C. Impacto del uso racional de antimicrobianos en una clínica de tercer nivel en Colombia. Rev. Chil. Infectol. 2017, 34, 205–211. [Google Scholar]
- O’Neal, L.; Alvarez, D.; Mendizabal-Cabrera, R.; Ramay, B.M.; Graham, J. Community-Acquired Antimicrobial Resistant Enterobacteriaceae in Central America: A One Health Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 7622. [Google Scholar] [CrossRef] [PubMed]
- Dreser, A.; Wirtz, V.J.; Corbett, K.K. Uso de antibióticos en México: Revisión de problemas y políticas. Salud Pública México 2008, 50, S480–S487. [Google Scholar] [CrossRef]
- Aguilera-Alonso, D. It is time for pediatric antimicrobial stewardship programs. Enferm. Infecc. Microbiol. Clin. 2021, 39, 113–114. [Google Scholar] [CrossRef]
- Lipsett, S.C.; Hall, M.; Ambroggio, L.; Hersh, A.L.; Shah, S.S.; Brogan, T.V.; Gerber, J.S.; Williams, D.J.; Grijalva, C.G.; Blaschke, A.J.; et al. Antibiotic Choice and Clinical Outcomes in Ambulatory Children with Community-Acquired Pneumonia. J. Pediatr. 2021, 229, 207–215.e1. [Google Scholar] [CrossRef]
- Fishman, N.; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America; Pediatric Infectious Diseases Society. Policy Statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect. Control. Hosp. Epidemiol. 2012, 33, 322–327. [Google Scholar] [CrossRef]
- Gerber, J.S.; Jackson, M.A.; Tamma, P.D.; Zaoutis, T.E.; AAP Committee on Infectious Diseases; Pediatric Infectious Diseases Society. Policy Statement: Antibiotic Stewardship in Pediatrics. J. Pediatr. Infect. Dis. Soc. 2021, 10, 641–649. [Google Scholar] [CrossRef]
- Dona, D.; Barbieri, E.; Daverio, M.; Lundin, R.; Giaquinto, C.; Zaoutis, T.; Sharland, M. Implementation and impact of pediatric antimicrobial stewardship programs: A systematic scoping review. Antimicrob. Resist. Infect. Control 2020, 9, 3. [Google Scholar] [CrossRef]
- Dyar, O.J.; Beovic, B.; Pulcini, C.; Tacconelli, E.; Hulscher, M.; Cookson, B.; ESCMID generic competencies working group. ESCMID generic competencies in antimicrobial prescribing and stewardship: Towards a European consensus. Clin. Microbiol. Infect. 2019, 25, 13–19. [Google Scholar] [CrossRef]
- Turner, R.B.; Valcarlos, E.; Loeffler, A.M.; Gilbert, M.; Chan, D. Impact of an Antimicrobial Stewardship Program on Antibiotic Use at a Nonfreestanding Children’s Hospital. J. Pediatr. Infect. Dis. Soc. 2017, 6, e36–e40. [Google Scholar] [CrossRef]
- Tannous, R.; Haddad, R.N.; Torbey, P.H. Management of Community-Acquired Pneumonia in Pediatrics: Adherence to Clinical Guidelines. Front. Pediatr. 2020, 8, 302. [Google Scholar] [CrossRef]
- Woods, C.R.; Bradley, J.S.; Chatterjee, A.; Copley, L.A.; Robinson, J.; Kronman, M.P.; Arrieta, A.; Fowler, S.L.; Harrison, C.; Carrillo-Marquez, M.A.; et al. Clinical Practice Guideline by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America: 2021 Guideline on Diagnosis and Management of Acute Hematogenous Osteomyelitis in Pediatrics. J. Pediatr. Infect. Dis. Soc. 2021, 10, 801–844. [Google Scholar] [CrossRef]
- Tien, I. Update on the management of skin, soft-tissue, and osteoarticular infections in children. Curr. Opin. Pediatr. 2006, 18, 254–259. [Google Scholar] [CrossRef]
- Kronman, M.P.; Banerjee, R.; Duchon, J.; Gerber, J.S.; Green, M.D.; Hersh, A.L.; Hyun, D.; Maples, H.; Nash, C.B.; Parker, S.; et al. Expanding Existing Antimicrobial Stewardship Programs in Pediatrics: What Comes Next. J. Pediatr. Infect. Dis. Soc. 2018, 7, 241–248. [Google Scholar] [CrossRef]
- May, L.; Cosgrove, S.; L’Archeveque, M.; Talan, D.A.; Payne, P.; Jordan, J.; Rothman, R.E. A call to action for antimicrobial stewardship in the emergency department: Approaches and strategies. Ann. Emerg. Med. 2013, 62, 69–77.e2. [Google Scholar] [CrossRef]
- Covino, M.; Buonsenso, D.; Gatto, A.; Morello, R.; Curatole, A.; Simeoni, B.; Franceschi, F.; Chiaretti, A. Determinants of antibiotic prescriptions in a large cohort of children discharged from a pediatric emergency department. Eur. J. Pediatr. 2022, 181, 2017–2030. [Google Scholar] [CrossRef]
- Mistry, R.D.; May, L.S.; Pulia, M.S. Improving Antimicrobial Stewardship in Pediatric Emergency Care: A Pathway Forward. Pediatrics 2019, 143, e20182972. [Google Scholar] [CrossRef]
- Poole, N.M.; Shapiro, D.J.; Fleming-Dutra, K.E.; Hicks, L.A.; Hersh, A.L.; Kronman, M.P. Antibiotic Prescribing for Children in United States Emergency Departments: 2009–2014. Pediatrics 2019, 143, e20181056. [Google Scholar] [CrossRef]
- Hersh, A.L.; De Lurgio, S.A.; Thurm, C.; Lee, B.R.; Weissman, S.J.; Courter, J.D.; Brogan, T.V.; Shah, S.S.; Kronman, M.P.; Gerber, J.S.; et al. Antimicrobial stewardship programs in freestanding children’s hospitals. Pediatrics 2015, 135, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ramos, J.; Vallve Alcon, E.; Moreno Ramos, F.; Santolaya-Perrin, R.; Guardiola Tey, J.M. Antimicrobial stewardship programs in emergency departments: How do we measure antimicrobial use? A systematic review. Rev. Esp. Quim. 2021, 34, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Mistry, R.D.; Newland, J.G.; Gerber, J.S.; Hersh, A.L.; May, L.; Perman, S.M.; Kuppermann, N.; Dayan, P.S. Current State of Antimicrobial Stewardship in Children’s Hospital Emergency Departments. Infect. Control Hosp. Epidemiol. 2017, 38, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, M.; Morris, A.M.; Thursky, K.; Pulcini, C. How to start an antimicrobial stewardship programme in a hospital. Clin. Microbiol. Infect. 2020, 26, 447–453. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Stewardship Programmes in Health-Care Facilities in Low- and Middle-Income Countries. A Practical Toolkit. 2019. Available online: https://www.who.int/publications/i/item/9789241515481 (accessed on 12 February 2023).
- Cox, J.A.; Vlieghe, E.; Mendelson, M.; Wertheim, H.; Ndegwa, L.; Villegas, M.V.; Gould, I.; Levy Hara, G. Antibiotic stewardship in low- and middle-income countries: The same but different? Clin. Microbiol. Infect. 2017, 23, 812–818. [Google Scholar] [CrossRef]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef]
- Naylor, N.R.; Zhu, N.; Hulscher, M.; Holmes, A.; Ahmad, R.; Robotham, J.V. Is antimicrobial stewardship cost-effective? A narrative review of the evidence. Clin. Microbiol. Infect. 2017, 23, 806–811. [Google Scholar] [CrossRef]
- Quiros, R.E.; Bardossy, A.C.; Angeleri, P.; Zurita, J.; Aleman Espinoza, W.R.; Carneiro, M.; Guerra, S.; Medina, J.; Castaneda Luquerna, X.; Guerra, A.; et al. Antimicrobial stewardship programs in adult intensive care units in Latin America: Implementation, assessments, and impact on outcomes. Infect. Control Hosp. Epidemiol. 2022, 43, 181–190. [Google Scholar] [CrossRef]
- Fabre, V.; Cosgrove, S.E.; Secaira, C.; Tapia Torrez, J.C.; Lessa, F.C.; Patel, T.S.; Quiros, R. Antimicrobial stewardship in Latin America: Past, present, and future. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e68. [Google Scholar] [CrossRef]
- Hegewisch-Taylor, J.; Dreser-Mansilla, A.; Romero-Monico, J.; Levy-Hara, G. Antimicrobial stewardship in hospitals in Latin America and the Caribbean: A scoping review. Rev. Panam. Salud Publica 2020, 44, e68. [Google Scholar] [CrossRef]
- Muñoz, J.S.; Motoa, G.; Escandón-Vargas, K.; Bavestrello, L.; Quiros, R.; Hernandez, C.; Pallares, C.; Villegas, M.V. Current Antimicrobial Stewardship Practices in Latin America: Where Are we? Open Forum Infect. Dis. 2015, 2, 192. [Google Scholar] [CrossRef]
- Morgan, D.J.; Okeke, I.N.; Laxminarayan, R.; Perencevich, E.N.; Weisenberg, S. Non-prescription antimicrobial use worldwide: A systematic review. Lancet Infect. Dis. 2011, 11, 692–701. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Rottingen, J.A.; Klugman, K.; Davies, S. Access to effective antimicrobials: A worldwide challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
- Pan American Health Organization. Fighting Antimicrobial Resistance in the Americas AMR Special Programme 2018–2020. 2020. Available online: https://www.paho.org/en/file/84741/download?token=Wqs5fMqi (accessed on 15 December 2022).
- Organización Panamericana de la Salud. La Red Latinoamericana de Vigilancia de la Resistencia a los Antimicrobianos (ReLAVRA). OPS. 2022. Available online: https://www.paho.org/es/relavra (accessed on 10 January 2023).
- Mota Curiel, C.; Yock-Corrales, A.; Contreras, C.; Corona, L.; Pavlichiv, V.; Alvarez, E.; Gonzalez Del Rey, J.; Committee, S.E. Pediatric Emergency Medicine Training: A Survey of Current Status in Latin America. Pediatr. Emerg. Care 2022, 38, e766–e770. [Google Scholar] [CrossRef]
- Ana Laura Guerrero, R.A.; Saavedra, M.; Casuriaga, A.; Notejane, M.; Giachetto, G. Prescripción de antibióticos en salas de cuidados moderados del Hospital Pediátrico, Centro Hospitalario Pereira Rossell, Uruguay. Arch. Pediatr. Urug. 2021, 92, e204. [Google Scholar]
- INCIENSA. Guía: Estrategia Para la Vigilancia de Laboratorio de la Resistencia a los Antimicrobianos de Microorganismos de Importancia en Salud Pública. 2021. Available online: https://www.inciensa.sa.cr/vigilancia_epidemiologica/informes_vigilancia/2021/cnr_bacteriologiaGuia%20Estrategia%20para%20la%20vigilancia%20RAM%202021.pdf (accessed on 2 February 2023).
- La Salud es de Todos Minsalud. Lineamientos Técnicos Para la Implementación de Programas de Optimización de Antimicrobianos en el Escenario Hospitalario y Ambulatorio. 2019. Available online: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/PP/ET/lineamientos-optimizacion-uso-antimicrobianos.pdf (accessed on 5 February 2023).
- Pearson, M.A.J.; Galas, M.; Corso, A.; Hormazábal, J.C.; Valderrama, C.D.; Marcano, N.S.; Ramón-Pardo, P.; Melano, R.G. Consenso latinoamericano para definir, categorizar y notificar patógenos multirresistentes, con resistencia extendida o panresistentes. Rev. Panam. Salud Pública 2019, 43, e65. [Google Scholar]
- Forster, C.S.; Almaazi, A.; Hamdy, R.; Harik, N. Predictors of Empiric Antibiotic Use in the Emergency Department in Children without Urinary Tract Infections: Erratum. Pediatr. Emerg. Care 2022, 38, 341. [Google Scholar] [CrossRef]
- da Cunha, A.J.; Amaral, J.; e Silva, M.A. Inappropriate antibiotic prescription to children with acute respiratory infection in Brazil. Indian Pediatr. 2003, 40, 7–12. [Google Scholar]
- Rodolfo Quirós, E.E.F.; Tapia, J.C.; Fabre, V.; Soloaga, R. PROAnet Optimizando el uso de Antimicrobianos. Argentina. 2019. Available online: https://s1.proanet.org/ords/f?p=12101:17:14932868786878::NO::: (accessed on 5 February 2023).
- Goycochea-Valdivia, W.A.; Pérez, S.M.; Aguilera-Alonso, D.; Escosa-Garcia, L.; Campos, L.M.; Baquero-Artigao, F.; Grupo de Trabajo PROA de la Sociedad Española de Infectología Pediátrica (SEIP). Position statement of the Spanish Society of Paediatric Infectious Diseases on the introduction, implementation and assessment of antimicrobial stewardship programmes in paediatric hospitals. An. Pediatría 2022, 9, 351.e1–351.e12. [Google Scholar] [CrossRef]
| Authors | Country | Population | Study Type | Primary or Secondary Outcomes |
|---|---|---|---|---|
| Yock et al. (2021) [12] | Costa Rica, México, Perú, Colombia, Argentina | 990 children | Observational prospective study | Rate of antibiotic prescription |
| Pérez et al. (2019) [13] | Costa Rica | All stool diarrheic samples (46,906), from outpatients under 13 years old, between January 2008 and December 2016 | Retrospective study | Co-infections and resistance to antimicrobials |
| Chacón et al. (2021) [14] | Costa Rica | 181 children | Retrospective observational study | Meropenem use |
| Huerta-Gutiérrez et al. (2019) [15] | Brazil, Venezuela, México, and Colombia | 2740 patients | Point prevalence study | Prevalence of patients with at least one antimicrobial |
| Levy Hara et al. (2022) [16] | Cuba, Paraguay, El Salvador, México and Perú | 5444 patients | Point prevalence study | Prevalence of antibiotic use |
| Ecker et al. (2016) [17] | Perú | 263 adults who purchased antibiotics for children under 5 years old | Transversal survey | Prevalence of purchase of antibiotics without a prescription |
| Ecker et al. (2013) [18] | Perú | 218 general practitioners | Survey | Preferences of antibiotic use in children |
| Ruvinsky et al. (2014) [19] | Argentina | 376 children | Prospective, longitudinal, before and after study | To assess the effectiveness of an AMS program |
| Wolfson et al. (2019) [20] | Argentina, Hungary, Mexico, Peru, Poland | 386 children | Multicenter retrospective study | Rate, appropriateness, and patterns of prescribing antibiotics for management of varicella-associated complications |
| Hernandez-Gomez et al. (2019) [21] | Perú | 3 hospitals | Quasi-experimental, multicenter study | To evaluate an AMS program |
| Zintgraff et al. (2021) [22] | Argentina | 2908 S. pneumoniae isolates | Retrospective study | Epidemiology of invasive pneumococcal disease in children <5 years old |
| Schiaffino et al. (2022) [23] | Perú | 303 children | Prospective study | To evaluate the incidence, duration of therapy, and appropriateness of antibiotic prescriptions by five main antibiotic prescribers |
| Christian J. et al. (2017) [24] | Colombia | Quasi-experimental study in a third-level clinic in the city of Medellin, evaluation of two time periods (pre-intervention and post intervention) | Retrospective Study | Evaluation of the impact of an antimicrobial stewardship program in terms of antibiotic consumption and bacterial ecology |
| O’Neal L. et al. (2020) [25] | Central America | Systematic Review on Community-Acquired Antimicrobial Resistant Enterobacteriaceae (CA-ARE) in Central America | Systematic Review | Describing gaps in the current understanding of CA-ARE in Central America |
| Dreser at al. (2008) [26] | México | Review on inappropriate use of antibiotics | Review | Describes the use of antimicrobials in Mexico |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yock-Corrales, A.; Naranjo-Zuñiga, G. Regional Perspective of Antimicrobial Stewardship Programs in Latin American Pediatric Emergency Departments. Antibiotics 2023, 12, 916. https://doi.org/10.3390/antibiotics12050916
Yock-Corrales A, Naranjo-Zuñiga G. Regional Perspective of Antimicrobial Stewardship Programs in Latin American Pediatric Emergency Departments. Antibiotics. 2023; 12(5):916. https://doi.org/10.3390/antibiotics12050916
Chicago/Turabian StyleYock-Corrales, Adriana, and Gabriela Naranjo-Zuñiga. 2023. "Regional Perspective of Antimicrobial Stewardship Programs in Latin American Pediatric Emergency Departments" Antibiotics 12, no. 5: 916. https://doi.org/10.3390/antibiotics12050916
APA StyleYock-Corrales, A., & Naranjo-Zuñiga, G. (2023). Regional Perspective of Antimicrobial Stewardship Programs in Latin American Pediatric Emergency Departments. Antibiotics, 12(5), 916. https://doi.org/10.3390/antibiotics12050916









