Aegle marvels (L.) Correa Leaf Essential Oil and Its Phytoconstituents as an Anticancer and Anti-Streptococcus mutans Agent
Abstract
1. Introduction
2. Results
2.1. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
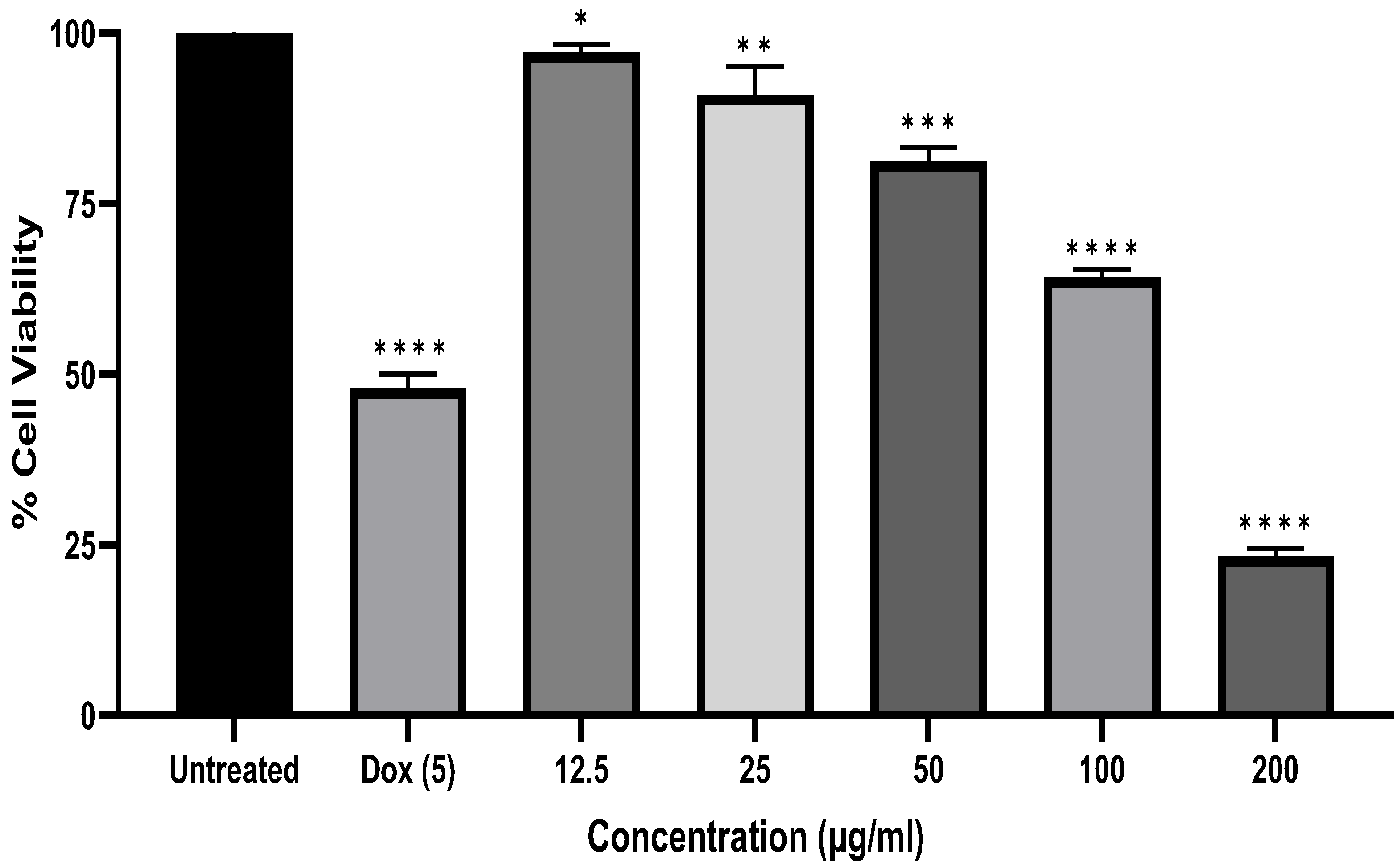
2.2. Anti-Carcinoma Effects of A. marmelos Leaves Essential Oil
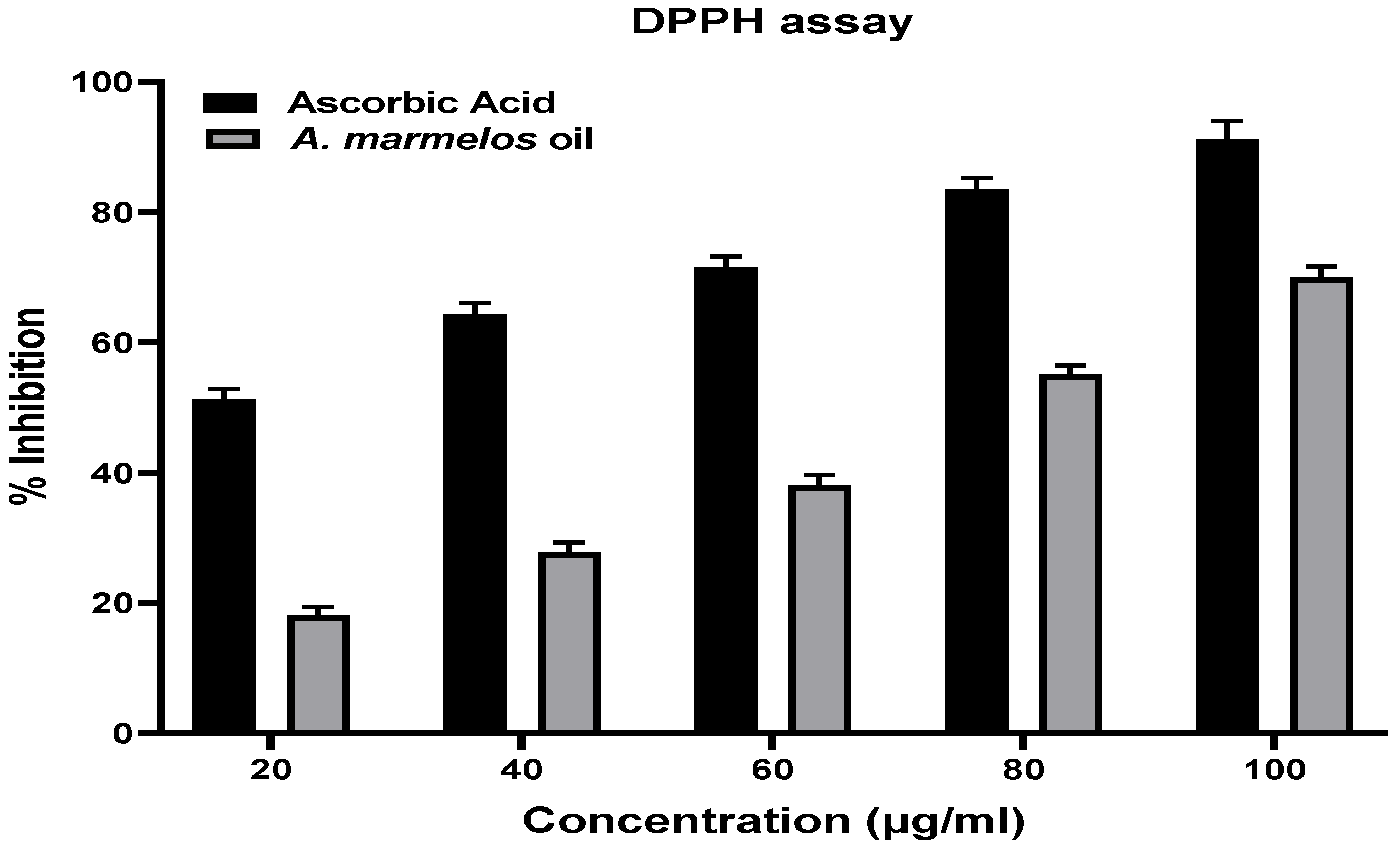
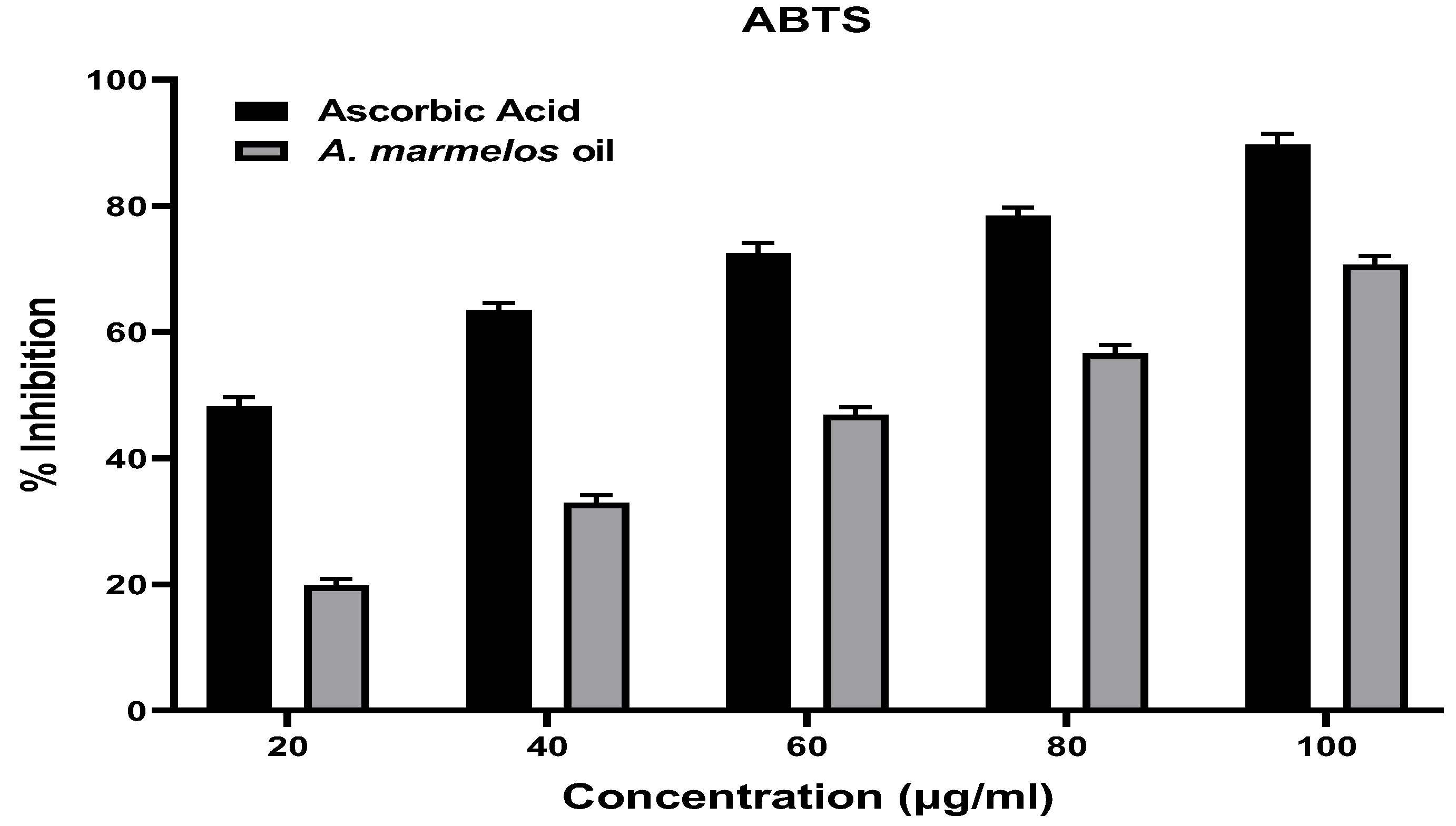
2.3. Antioxidant Activity of A. marmelos Leaves Essential Oil
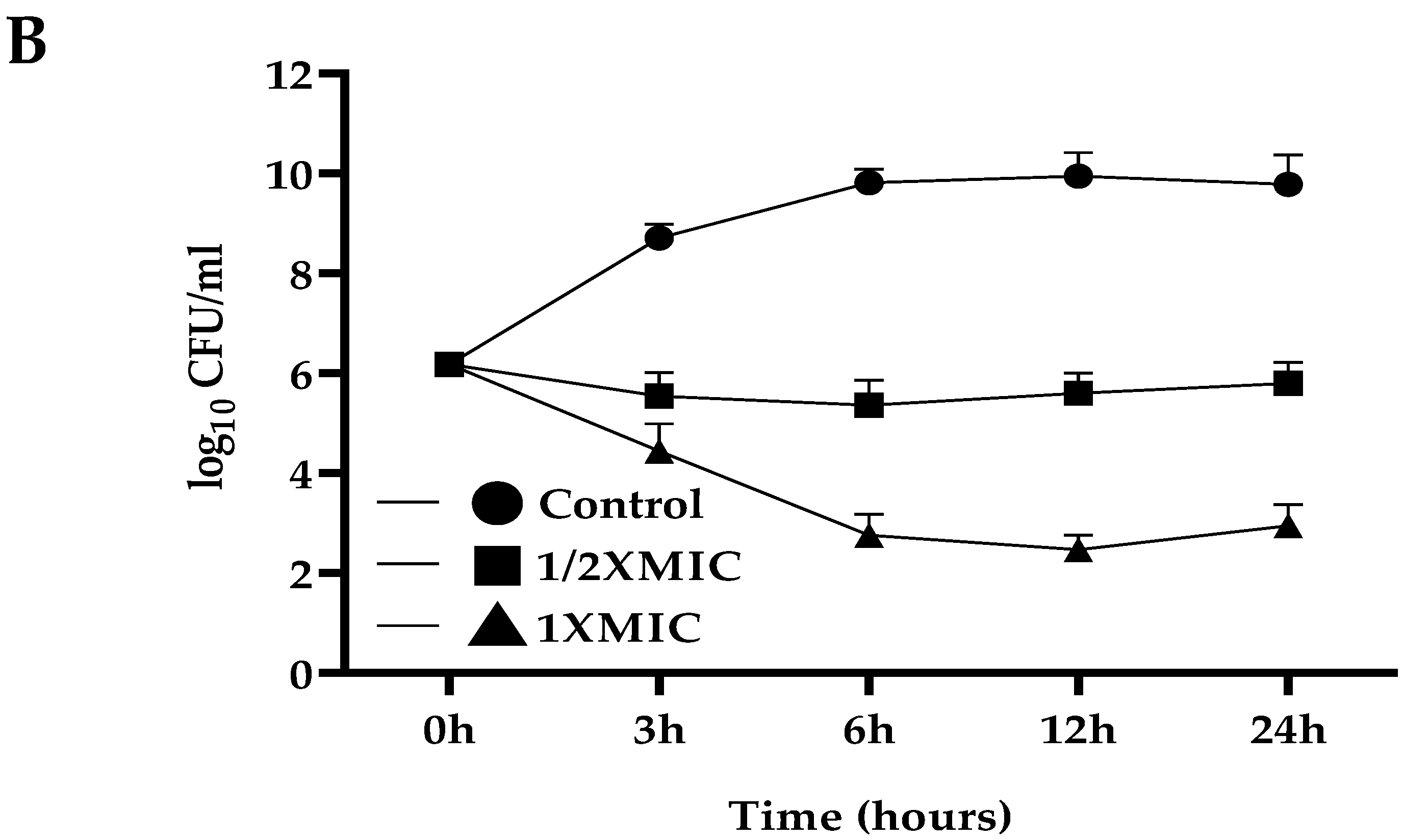
2.4. Anti-S. mutans Activity of A. marmelos Leaves Essential Oil
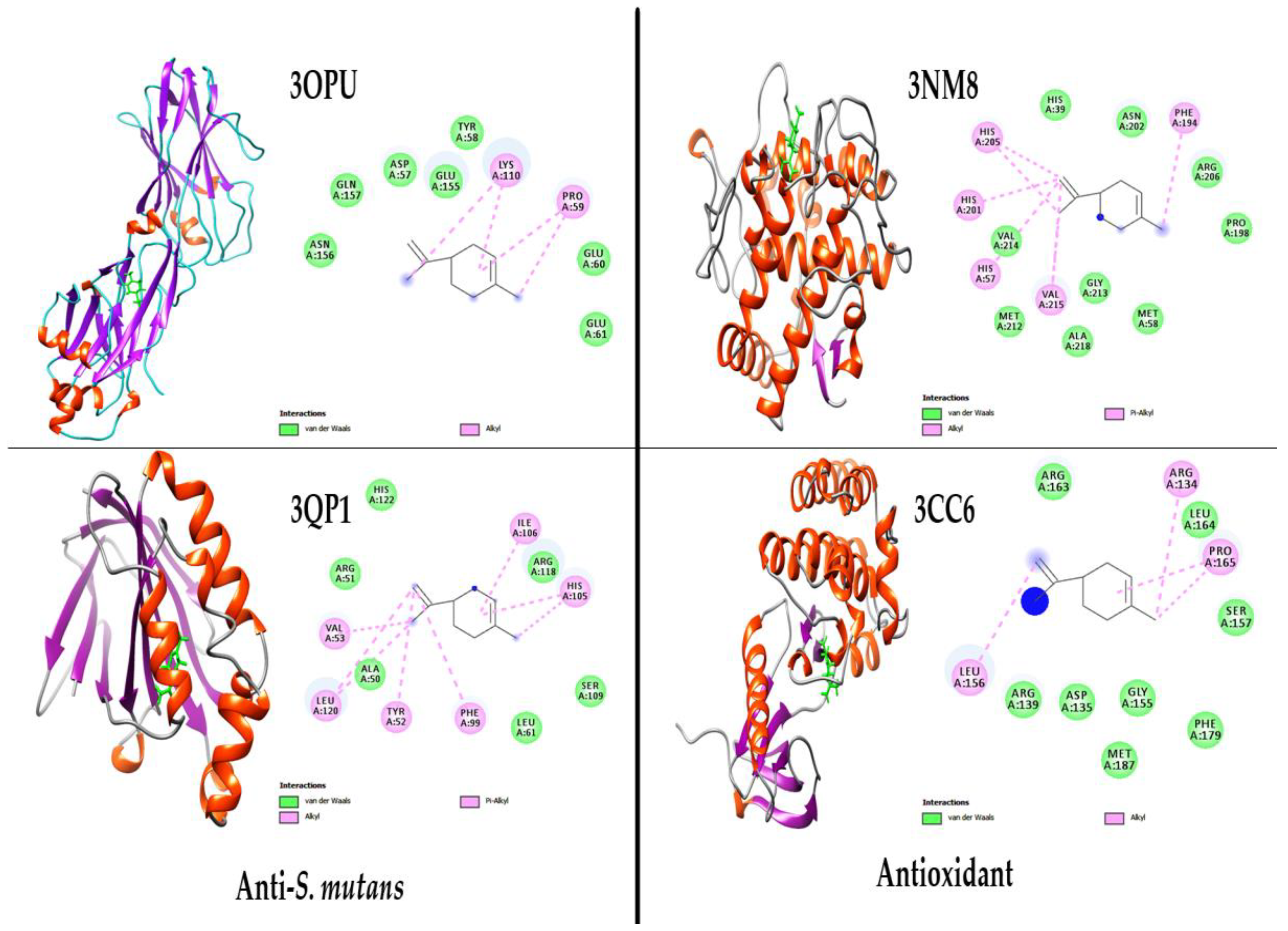
2.5. Molecular Docking Study of the Major Compound Limonene
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Methods
4.2.1. Extraction of Essential Oil
4.2.2. Gas-Chromatography/Mass Spectrometry (GC-MS) Analysis of Essential Oil
4.2.3. Cytotoxicity Assay of Essential Oil
4.2.4. Free Radical Scavenging Activity of Essential Oil
4.2.5. Anti-S. mutans Activity of Essential Oil
4.2.6. Molecular Docking Effects of Major Compound Limonene
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brijesh, S.; Daswani, P.; Tetali, P.; Antia, N.; Birdi, T. Studies on the antidiarrhoeal activity of Aegle marmelos unripe fruit: Validating its traditional usage. BMC Complement. Altern. Med. 2009, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, C.K.; Madhujith, T.; Eeswara, J. Bael (Aegle marmelos L. Corrêa), a Medicinal Tree with Immense Economic Potentials. Adv. Agric. 2020, 2020, 8814018. [Google Scholar] [CrossRef]
- Rahman, S.; Parvin, R. Therapeutic potential of Aegle marmelos (L.)-An overview. Asian Pacific J. Trop. Dis. 2014, 4, 71–77. [Google Scholar] [CrossRef]
- Sarkar, T.; Salauddin, M.; Chakraborty, R. In-depth pharmacological and nutritional properties of bael (Aegle marmelos): A critical review. J. Agric. Food Res. 2020, 2, 100081. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Venkatesh, P.; Baliga, M.S. Aegle marmelos (L.) Correa inhibits the proliferation of transplanted Ehrlich ascites carcinoma in mice. Biol. Pharm. Bull. 2005, 28, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Dhankhar, S.; Ruhil, S.; Balhara, M.; Dhankhar, S.; Chhillar, A.K. Aegle marmelos (Linn.) Correa: A potential source of Phytomedicine. J. Med. Plants Res. 2011, 5, 1497–1507. [Google Scholar]
- Iqbal, T.; Hussain, A.I.; Chatha, S.A.S.; Naqvi, S.A.R.; Bokhari, T.H. Antioxidant activity and volatile and phenolic profiles of essential oil and different extracts of wild mint (Mentha longifolia) from the Pakistani flora. J. Anal. Methods Chem. 2013, 2013, 536490. [Google Scholar] [CrossRef]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Lugo-Flores, M.A.; Quintero-Cabello, K.P.; Palafox-Rivera, P.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; Ortega-Ramirez, L.A.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Plant-derived substances with antibacterial, antioxidant, and flavoring potential to formulate oral health care products. Biomedicines 2021, 9, 1669. [Google Scholar] [CrossRef]
- Salman, B.N.; Darvish, S.; Goriuc, A.; Mazloomzadeh, S.; Hossein Poor Tehrani, M.; Luchian, I. Salivary oxidative stress markers’ relation to oral diseases in children and adolescents. Antioxidants 2021, 10, 1540. [Google Scholar] [CrossRef]
- Pytko-Polończyk, J.; Stawarz-Janeczek, M.; Kryczyk-Poprawa, A.; Muszyńska, B. Antioxidant-rich natural raw materials in the prevention and treatment of selected oral cavity and periodontal diseases. Antioxidants 2021, 10, 1848. [Google Scholar] [CrossRef] [PubMed]
- Pant, P.; Sut, S.; Castagliuolo, I.; Gandin, V.; Maggi, F.; Gyawali, R.; Dall’Acqua, S. Sesquiterpene rich essential oil from Nepalese Bael tree (Aegle marmelos (L.) Correa) as potential antiproliferative agent. Fitoterapia 2019, 138, 104266. [Google Scholar] [CrossRef]
- Poonkodi, K.; Vimaladevi, K.; Suganthi, M.; Gayathri, N. Essential Oil Composition and Biological Activities of Aegle marmelos (L.) Correa Grown in Western Ghats Region-South India. J. Essent. Oil-Bear. Plants 2019, 22, 1013–1021. [Google Scholar] [CrossRef]
- Nocini, R.; Lippi, G.; Mattiuzzi, C. The worldwide burden of smoking-related oral cancer deaths. Clin. Exp. Dent. Res. 2020, 6, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Pires, F.R.; Ramos, A.B.; de Oliveira, J.B.C.; Tavares, A.S.; de Luz, P.S.R.; dos Santos, T.C.R.B. Oral squamous cell carcinoma: Clinicopathological features from 346 cases from a single oral pathology service during an 8-year period. J. Appl. Oral Sci. 2013, 21, 460–467. [Google Scholar] [CrossRef]
- Rivera, C.; Venegas, B. Histological and molecular aspects of oral squamous cell carcinoma. Oncol. Lett. 2014, 8, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Oluwaseun Ademiluyi, A.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Lok, B.; Babu, D.; Tabana, Y.; Dahham, S.S.; Adam, M.A.A.; Barakat, K.; Sandai, D. The Anticancer Potential of Psidium guajava (Guava) Extracts. Life 2023, 13, 346. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque as a biofilm and a microbial community—Implications for health and disease. BMC Oral Health 2006, 6, S14. [Google Scholar] [CrossRef]
- Qiu, W.; Zhou, Y.; Li, Z.; Huang, T.; Xiao, Y.; Cheng, L.; Peng, X.; Zhang, L.; Ren, B. Application of Antibiotics/Antimicrobial Agents on Dental Caries. BioMed Res. Int. 2020, 2020, 5658212. [Google Scholar] [CrossRef]
- Kumar, M.; Prakash, S.; Radha; Kumari, N.; Pundir, A.; Punia, S.; Saurabh, V.; Choudhary, P.; Changan, S.; Dhumal, S.; et al. Beneficial role of antioxidant secondary metabolites from medicinal plants in maintaining oral health. Antioxidants 2021, 10, 1061. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Costa, R.C.; Barão, V.A.R.; Cunha Villar, C.; Retamal-Valdes, B.; Feres, M.; Silva Souza, J.G. Oral Microorganisms and Biofilms: New Insights to Defeat the Main Etiologic Factor of Oral Diseases. Microorganisms 2022, 10, 2413. [Google Scholar] [CrossRef] [PubMed]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Thosar, N.; Basak, S.; Bahadure, R.N.; Rajurkar, M. Antimicrobial efficacy of five essential oils against oral pathogens: An in vitro study. Eur. J. Dent. 2013, 7, S071–S077. [Google Scholar] [CrossRef]
- Zomorodian, K.; Ghadiri, P.; Saharkhiz, M.J.; Moein, M.R.; Mehriar, P.; Bahrani, F.; Golzar, T.; Pakshir, K.; Fani, M.M. Antimicrobial activity of seven essential oils from Iranian aromatic plants against common causes of oral infections. Jundishapur J. Microbiol. 2015, 8, e17766. [Google Scholar] [CrossRef]
- Tambur, Z.; Miljković-Selimović, B.; Opačić, D.; Vuković, B.; Malešević, A.; Ivančajić, L.; Aleksić, E. Inhibitory effects of propolis and essential oils on oral bacteria. J. Infect. Dev. Ctries. 2021, 15, 1027–1031. [Google Scholar] [CrossRef]
- Bardají, D.K.R.; Reis, E.B.; Medeiros, T.C.T.; Lucarini, R.; Crotti, A.E.M.; Martins, C.H.G. Antibacterial activity of commercially available plant-derived essential oils against oral pathogenic bacteria. Nat. Prod. Res. 2016, 30, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Jawaid, T.; Alsanad, S.M.; Kamal, M.; Balaha, M.F. Composition, Antibacterial Efficacy, and Anticancer Activity of Essential Oil Extracted from Psidium guajava (L.) Leaves. Plants 2023, 12, 246. [Google Scholar] [CrossRef]
- Jamal, M.A.H.M.; Rahman, M.S.; Hossain, M.B.; Sharma, S.P.; Chung, H.J.; Kim, H.J.; Hong, S.T. Antibacterial Properties and Chemical Composition of Essential Oil from Aegle marmelos (L.) Corr. Leaves Growing in Bangladesh. J. Essent. Oil-Bear. Plants 2017, 20, 155–174. [Google Scholar] [CrossRef]
- Balakumar, S.; Rajan, S.; Thirunalasundari, T.; Jeeva, S. Antifungal activity of Aegle marmelos (L.) Correa (Rutaceae) leaf extract on dermatophytes. Asian Pac. J. Trop. Biomed. 2011, 1, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.A.; El-Sakhawy, F.M.; Mohammed, M.M.D.; Farid, M.A.; Abdel-Wahed, N.A.M.; Deabes, D.A.H. Chemical composition, antimicrobial and antifungal activities of essential oils of the leaves of Aegle marmelos (L.) Correa growing in Egypt. J. Appl. Pharm. Sci. 2015, 5, 001–005. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, A.; Dubey, N.K.; Gupta, R. Essential Oil of Aegle marmelos as a Safe Plant-Based Antimicrobial Against Postharvest Microbial Infestations and Aflatoxin Contamination of Food Commodities. J. Food Sci. 2009, 74, M302–M307. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.S.; Padalia, R.C.; Chauhan, A. Essential oil composition of Aegle marmelos (L.) Correa: Chemotypic and seasonal variations. J. Sci. Food Agric. 2014, 94, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Ekins, S.; Mestres, J.; Testa, B. In silico pharmacology for drug discovery: Methods for virtual ligand screening and profiling. Br. J. Pharmacol. 2007, 152, 9–20. [Google Scholar] [CrossRef]
- Alam, A.; Jawaid, T.; Alam, P. In vitro antioxidant and anti-inflammatory activities of green cardamom essential oil and in silico molecular docking of its major bioactives. J. Taibah Univ. Sci. 2021, 15, 757–768. [Google Scholar] [CrossRef]
- Kaur, H.P.; Garg, S.N.; Sashidhara, K.V.; Yadav, A.; Naqvi, A.A.; Khanuja, S.P.S. Chemical composition of the essential oil of the twigs and leaves of Aegle marmelos (L.) correa. J. Essent. Oil Res. 2006, 18, 288–289. [Google Scholar] [CrossRef]
- Fawzi Mahomoodally, M.; Mollica, A.; Stefanucci, A.; Zakariyyah Aumeeruddy, M.; Poorneeka, R.; Zengin, G. Volatile components, pharmacological profile, and computational studies of essential oil from Aegle marmelos (Bael) leaves: A functional approach. Ind. Crops Prod. 2018, 126, 13–21. [Google Scholar] [CrossRef]
- Asma, S.T.; Acaroz, U.; Imre, K.; Morar, A.; Shah, S.R.A.; Hussain, S.Z.; Arslan-Acaroz, D.; Demirbas, H.; Hajrulai-Musliu, Z.; Istanbullugil, F.R.; et al. Natural Products/Bioactive Compounds as a Source of Anticancer Drugs. Cancers 2022, 14, 6203. [Google Scholar] [CrossRef]
- Chimsook, T. Phytochemical screening, total phenolic content, antioxidant activities and cytotoxicity of Dendrobium signatum leaves. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2016; Volume 62. [Google Scholar]
- Lampronti, I.; Martello, D.; Bianchi, N.; Borgatti, M.; Lambertini, E.; Piva, R.; Jabbar, S.; Choudhuri, M.S.K.; Khan, M.T.H.; Gambari, R. In vitro antiproliferative effects on human tumor cell lines of extracts from the Bangladeshi medicinal plant Aegle marmelos Correa. Phytomedicine 2003, 10, 300–308. [Google Scholar] [CrossRef]
- Veerappan, A.; Miyazaki, S.; Kadarkaraisamy, M.; Ranganathan, D. Acute and subacute toxicity studies of Aegle marmelos Corr., an Indian medicinal plant. Phytomedicine 2007, 14, 209–215. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. TR-347 Toxicology and Carcinogenesis Studies of d-Limonene (CAS No. 5989-27-5) in F344/N Rats and B6C3F1Mice (Gavage Studies). Available online: https://pubmed.ncbi.nlm.nih.gov/12704437/ (accessed on 15 March 2023).
- Zhou, J.; Azrad, M.; Kong, L. Effect of Limonene on Cancer Development in Rodent Models: A Systematic Review. Front. Sustain. Food Syst. 2021, 5, 725077. [Google Scholar] [CrossRef]
- Mandal, D.; Patel, P.; Verma, S.K.; Sahu, B.R.; Parija, T. Proximal discrepancy in intrinsic atomic interaction arrests G2/M phase by inhibiting Cyclin B1/CDK1 to infer molecular and cellular biocompatibility of d-limonene. Sci. Rep. 2022, 12, 18184. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P.; Urooj, A. Antioxidant properties and stability of aegle marmelos leaves extracts. J. Food Sci. Technol. 2013, 50, 135–140. [Google Scholar] [CrossRef]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic Clin. Pharmacol. Toxicol. 2010, 106, 38–44. [Google Scholar] [CrossRef]
- Ahmadi, H.; Ebrahimi, A.; Ahmadi, F. Antibiotic Therapy in Dentistry. Int. J. Dent. 2021, 2021. [Google Scholar] [CrossRef]
- Wang, H.; Ren, D. Controlling Streptococcus mutans and Staphylococcus aureus biofilms with direct current and chlorhexidine. AMB Express 2017, 7, 204. [Google Scholar] [CrossRef]
- Miyazawa, M.; Hashimoto, Y. Antimicrobial and bactericidal activities of esters of 2-endo-hydroxy-1,8-cineole as new aroma chemicals. J. Agric. Food Chem. 2002, 50, 3522–3526. [Google Scholar] [CrossRef]
- Soković, M.D.; Vukojević, J.; Marin, P.D.; Brkić, D.D.; Vajs, V.; Van Griensven, L.J.L.D. Chemical composition of essential oils of Thymus and Mentha species and their antifungal activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef]
- Madi, Y.F.; Choucry, M.A.; Meselhy, M.R.; El-Kashoury, E.S.A. Essential oil of Cymbopogon citratus cultivated in Egypt: Seasonal variation in chemical composition and anticholinesterase activity. Nat. Prod. Res. 2021, 35, 4063–4067. [Google Scholar] [CrossRef]
- Benzaid, C.; Belmadani, A.; Tichati, L.; Djeribi, R.; Rouabhia, M. Effect of Citrus aurantium L. Essential oil on Streptococcus mutans growth, biofilm formation and virulent genes expression. Antibiotics 2021, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.N. Terpenoids Against Human Diseases, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Foudah, A.I.; Alqarni, M.H.; Alam, A.; Salkini, M.A.; Ross, S.A.; Yusufoglu, H.S. Phytochemical Screening, In Vitro and In Silico Studies of Volatile Compounds from Petroselinum crispum (Mill) Leaves Grown in Saudi Arabia. Molecules 2022, 27, 934. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.P.S.; Seito, L.N.; Eberlin, S.; Dieamant, G.C.; Nogueira, C.; Pereda, M.C.V.; Di Stasi, L.C. Photoprotective and antioxidant effects of Rhubarb: Inhibitory action on tyrosinase and tyrosine kinase activities and TNF-α, IL-1α and α-MSH production in human melanocytes. BMC Complement. Altern. Med. 2013, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- De Dormael, R.; Bastien, P.; Sextius, P.; Gueniche, A.; Ye, D.; Tran, C.; Chevalier, V.; Gomes, C.; Souverain, L.; Tricaud, C. Vitamin C prevents ultraviolet-induced pigmentation in healthy volunteers: Bayesian meta-analysis results from 31 randomized controlled versus vehicle clinical studies. J. Clin. Aesthet. Dermatol. 2019, 12, E53. [Google Scholar]
- Liao, Y.; Zhang, M.; Lin, X.; Yan, F. Diaryl Urea Derivative Molecule Inhibits Cariogenic Streptococcus mutans by Affecting Exopolysaccharide Synthesis, Stress Response, and Nitrogen Metabolism. Front. Cell. Infect. Microbiol. 2022, 12, 904488. [Google Scholar] [CrossRef]
- Ghannay, S.; Aouadi, K.; Kadri, A.; Snoussi, M. GC-MS Profiling, Vibriocidal, Antioxidant, Antibiofilm, and Anti-Quorum Sensing Properties of Carum carvi L. Essential Oil: In Vitro and In Silico Approaches. Plants 2022, 11, 1072. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Pub. Corp.: Carol Stream, IL, USA, 2007; Volume 8. [Google Scholar]
- Manosroi, J.; Dhumtanom, P.; Manosroi, A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006, 235, 114–120. [Google Scholar] [CrossRef]
- Alam, A.; Singh, V. Composition and pharmacological activity of essential oils from two imported Amomum subulatum fruit samples. J. Taibah Univ. Med. Sci. 2021, 16, 231–239. [Google Scholar] [CrossRef]
- Xiao, Z.; He, L.; Hou, X.; Wei, J.; Ma, X.; Gao, Z.; Yuan, Y.; Xiao, J.; Li, P.; Yue, T. Relationships between structure and antioxidant capacity and activity of glycosylated flavonols. Foods 2021, 10, 849. [Google Scholar] [CrossRef]
- Zhang, S.; Krumberger, M.; Morris, M.A.; Parrocha, C.M.T.; Kreutzer, A.G.; Nowick, J.S. Structure-based drug design of an inhibitor of the SARS-CoV-2 (COVID-19) main protease using free software: A tutorial for students and scientists. Eur. J. Med. Chem. 2021, 218, 113390. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, M.; Singh, V.; Misra, T.K.; Roy, D.N. In silico studies on structural inhibition of SARS-CoV-2 main protease Mpro by major secondary metabolites of Andrographis paniculata and Cinchona officinalis. Biologia 2022, 77, 1373–1389. [Google Scholar] [CrossRef] [PubMed]
- Rocchitta, G.; Rozzo, C.; Pisano, M.; Fabbri, D.; Dettori, M.A.; Ruzza, P.; Honisch, C.; Dallocchio, R.; Dessì, A.; Migheli, R.; et al. Inhibitory Effect of Curcumin-Inspired Derivatives on Tyrosinase Activity and Melanogenesis. Molecules 2022, 27, 7942. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.S.; Chang, J.H.; Hung, W.Y.; Yang, Y.C.; Chien, M.H. The interplay of reactive oxygen species and the epidermal growth factor receptor in tumor progression and drug resistance. J. Exp. Clin. Cancer Res. 2018, 37, 61. [Google Scholar] [CrossRef] [PubMed]
- Alafeef, M.; Dighe, K.; Moitra, P.; Pan, D. Monitoring the Viral Transmission of SARS-CoV-2 in Still Waterbodies Using a Lanthanide-Doped Carbon Nanoparticle-Based Sensor Array. ACS Sustain. Chem. Eng. 2022, 10, 245–258. [Google Scholar] [CrossRef]
- Martins, F.G.; Melo, A.; Sousa, S.F. Identification of new potential inhibitors of quorum sensing through a specialized multi-level computational approach. Molecules 2021, 26, 2600. [Google Scholar] [CrossRef]
- Burley, S.K.; Berman, H.M.; Duarte, J.M.; Feng, Z.; Flatt, J.W.; Hudson, B.P.; Lowe, R.; Peisach, E.; Piehl, D.W.; Rose, Y.; et al. Protein Data Bank: A Comprehensive Review of 3D Structure Holdings and Worldwide Utilization by Researchers, Educators, and Students. Biomolecules 2022, 12, 1425. [Google Scholar] [CrossRef]
- Steffen, C.; Thomas, K.; Huniar, U.; Hellweg, A.; Rubner, O.; Schroer, A. CHARMM: The Biomolecular Simulation Program. J. Comput. Chem. 2010, 31, 1545–1614. [Google Scholar]
- Wróbel, A.; Baradyn, M.; Ratkiewicz, A.; Drozdowska, D. Synthesis, biological activity, and molecular dynamics study of novel series of a trimethoprim analogs as multi-targeted compounds: Dihydrofolate reductase (dhfr) inhibitors and dna-binding agents. Int. J. Mol. Sci. 2021, 22, 3685. [Google Scholar] [CrossRef]
- Terefe, E.M.; Ghosh, A. Molecular Docking, Validation, Dynamics Simulations, and Pharmacokinetic Prediction of Phytochemicals Isolated from Croton dichogamus Against the HIV-1 Reverse Transcriptase. Bioinform. Biol. Insights 2022, 16. [Google Scholar] [CrossRef]
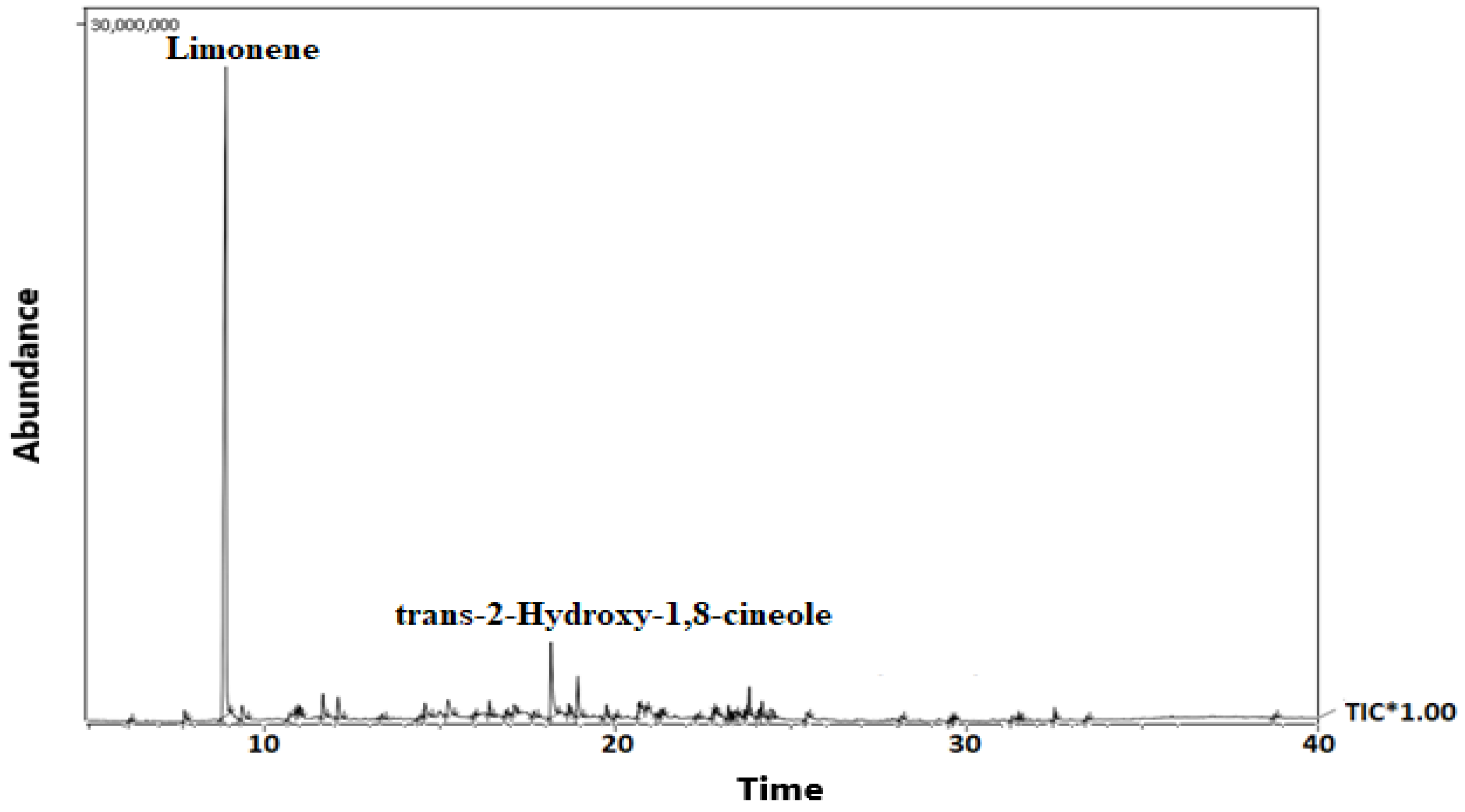

| Composition | RI (Lit) | RI (Obs) | Area % |
|---|---|---|---|
| Monoterpenes | |||
| α-Pinene | 932 | 948 | 0.19 |
| Myrcene | 988 | 958 | 0.73 |
| Limonene | 972 | 976 | 63.71 |
| β-Ocimene | 1022 | 1018 | 1.73 |
| cis-Sabinene hydrate | 1059 | 1041 | 0.35 |
| Linalool | 1082 | 1082 | 0.45 |
| p-Menth-2,8-dien-1-ol | 1138 | 1140 | 2.29 |
| D-Carvone | 1210 | 1190 | 1.76 |
| cis-Carveol | 1209 | 1206 | 1.10 |
| (-)-Myrtenol, TMS derivative | 1221 | 1221 | 1.06 |
| trans-2-Hydroxy-1,8-cineole | 1247 | 1247 | 6.85 |
| Menthyl acetate | 1294 | 1304 | 2.83 |
| trans-p-menth-8-ene-1,2-diol | 1340 | 1346 | 0.95 |
| Sesquiterpenes | |||
| γ-Elemene | 1430 | 1431 | 0.19 |
| trans-β-Caryophyllene | 1417 | 1414 | 1.05 |
| α-Bisabolene | 1521 | 1518 | 0.66 |
| Globulol | 1548.1 | 1530 | 0.71 |
| Ledene alcohol | 1570 | 1561 | 0.54 |
| Caryophyllene oxide | 1582 | 1587 | 2.04 |
| Humulene-1,2-epoxide | 1608 | 1592 | 0.34 |
| Rosifoliol | 1600 | 1598 | 0.48 |
| Diterpene | |||
| Geranyl-α-terpinene | 1960 | 1962 | 0.28 |
| Other compounds | |||
| 3-Methyl-2-(2-methyl-2-butenyl)-furan | 1099 | 1109 | 0.45 |
| Trimethyl[2-(phenylthio)ethoxy]silane | 1214 | 1228 | 0.94 |
| 6-Allyl-2-cresol | 1316 | 1316 | 0.40 |
| Pentanoic acid, 2-methyl-, anhydride | 1347.5 | 1339 | 2.92 |
| (+)-3-Carene, 2-(acetylmethyl)- | 1380 | 1384 | 0.29 |
| (2E)-7-Ethoxy-3,7-dimethyl-2-octen-1-ol | 1463 | 1431 | 0.24 |
| beta-Ionone epoxide | 1456 | 1436 | 0.48 |
| 10,12-Hexadecadien-1-ol | 1880 | 1870 | 0.39 |
| 9,12,15-Octadecatrienoic acid, ethyl ester, (Z,Z,Z)- | 2145 | 2201 | 0.25 |
| 9,12-Octadecadiynoic acid, trimethylsilyl ester | 2201 | 2221 | 0.53 |
| 13-Hexyloxacyclotridec-10-en-2-one | 2071.2 | 2325 | 0.58 |
| Cholesta-3,5-diene | 2580 | 2390 | 0.23 |
| Monoterpenes (84.02%) | |||
| Sesquiterpenes (6.01%) | |||
| Diterpene (0.28%) | |||
| Non-terpene compounds (8.01%) | |||
| Total % Area (98.32%) | |||
| Enzymes | PDB: ID | Binding Energy ΔG (kcal/mol) | Inhibition Constant (µM) | Hydrogen Bond Interaction |
|---|---|---|---|---|
| Tyrosinase | 3NM8 | −7.73 | 898.74 | PHE A: 194, VAL A: 215, HIS A: 57, HIS A: 201, HIS A: 205 |
| Protein tyrosine kinase 2 beta (PTK2B) | 3CC6 | −7.40 | 859.54 | ARG A: 134, PRO A: 165, LEU A: 156 |
| C-terminal domain of S. mutans surface protein | 3OPU | −7.31 | 987.29 | PRO A: 59, LYS A: 110 |
| CviR ligand-binding domain bound to the native ligand C6-HSL | 3QP1 | −7.89 | 764.34 | ILE A: 106, HIS A: 105, PHE A: 99, TYR A: 52, LEU A: 120, VAL A: 53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aodah, A.H.; Balaha, M.F.; Jawaid, T.; Khan, M.M.; Ansari, M.J.; Alam, A. Aegle marvels (L.) Correa Leaf Essential Oil and Its Phytoconstituents as an Anticancer and Anti-Streptococcus mutans Agent. Antibiotics 2023, 12, 835. https://doi.org/10.3390/antibiotics12050835
Aodah AH, Balaha MF, Jawaid T, Khan MM, Ansari MJ, Alam A. Aegle marvels (L.) Correa Leaf Essential Oil and Its Phytoconstituents as an Anticancer and Anti-Streptococcus mutans Agent. Antibiotics. 2023; 12(5):835. https://doi.org/10.3390/antibiotics12050835
Chicago/Turabian StyleAodah, Alhussain H., Mohamed F. Balaha, Talha Jawaid, Mohammed Moizuddin Khan, Mohammad Javed Ansari, and Aftab Alam. 2023. "Aegle marvels (L.) Correa Leaf Essential Oil and Its Phytoconstituents as an Anticancer and Anti-Streptococcus mutans Agent" Antibiotics 12, no. 5: 835. https://doi.org/10.3390/antibiotics12050835
APA StyleAodah, A. H., Balaha, M. F., Jawaid, T., Khan, M. M., Ansari, M. J., & Alam, A. (2023). Aegle marvels (L.) Correa Leaf Essential Oil and Its Phytoconstituents as an Anticancer and Anti-Streptococcus mutans Agent. Antibiotics, 12(5), 835. https://doi.org/10.3390/antibiotics12050835









